Growth characteristics and reproductive output of dwarf mistletoeinfected Juniperus polycarpos in Iran
Abolfazl Daneshvar · Mulualem Tigabu · Asaddollah Karimidoost Mostafa Farhadi · Per Christer Odén
ORIGINAL PAPER
Growth characteristics and reproductive output of dwarf mistletoeinfected Juniperus polycarpos in Iran
Abolfazl Daneshvar · Mulualem Tigabu · Asaddollah Karimidoost Mostafa Farhadi · Per Christer Odén
Received: 2013-04-01; Accepted: 2013-04-14
? Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2014
Dwarf mistletoes are parasitic flowering plants that infect conifers, resulting in substantial loss of growth and mortality. Recently, forest managers in Iran are contemplating whether infection of Juniperus polycarpos C. Koch forests by dwarf mistletoe, Arceuthobium oxycedri (DC.) M. Bieb, influences tree vigor and contributes to insufficient natural regeneration. The present study aimed at assessing the severity of infection and its impact on growth and reproductive output of J. polycarpos. Infected and uninfected trees (n =20 each) were selected for assessment of diameter, height, crown area, and crown volume as well as quantity and quality of cones and seeds. The severity of infection of trees was determined by Hawksworth’s 6-class dwarf mistletoe rating (DMR) system. The DMR system revealed that 40% of the infected sample trees were lightly infected (DMR =1?2) and 60% were moderately infected (DMR =3?4). Growth characteristics did not differ significantly (p >0.05) between infected and uninfected trees. However, moderate infection affected the reproductive output of J. polycarpos by significantly (p<0.05) reducing the mean number of cones per unit area of the crown, increasing the number of damaged seeds, and reducing seed size and seedgermination capacity. We conclude that reproductive output of J. polycarpos is more sensitive than growth characters to moderate infection by juniper dwarf mistletoe, and this might partly account for poor natural regeneration.
conifers, Greek juniper, forest health, parasitic plant, seed size
Introduction
The genus Juniperus (Cupressaceae) consists of approximately 67 species that are mostly distributed over the northern hemisphere and Africa (Farjon 2005). Juniperus polycarpos C. Koch is one of six native juniper species in Iran that are naturally distributed along the southern slopes of the Alborz mountain chain, Arassbaran, and Northern part of Khorasan (Korouri et al. 2012). The juniper forest is highly valued for its economic and ecological importance: the wood is suitable for manufacturing of pencils; the essential oil is used in several pharmaceutical preparations and for traditional treatment of various diseases (Akkol et al. 2009). Juniper forest also provides habitats for wildlife and protection against soil erosion (Korouri et al. 2012). Although commercial harvesting of J. polycarpos in Iran has been prohibited since 1989, the population size of juniper forests continues to decline due to illegal cutting and overgrazing as well as insufficient natural regeneration due to poor seed production (Ahani et al. 2013). Recently, forest managers are contemplating whether infection of juniper forests by dwarf mistletoe, particularly at elevations from 1800 to 2700 m.a.s.l., influences tree vigor and reproductive output, thereby contributing to insufficient natural regeneration of juniper forests in Iran.
Dwarf mistletoes are parasitic flowering plants that infect conifers, producing characteristic yellow to orange or green to brown leafless aerial shoots on the host plant. The dwarf mistletoe associated with juniper (hereafter referred to as juniper dwarf mistletoe) is Arceuthobium oxycedri (DC.) M. Bieb, Viscaceae, which has a wide geographic distribution, ranging from NorthAfrica, Southern Europe to Central and Western Asia as far as near East and Western China (Hawksworth and Weins 1996; Sarangzai et al. 2010). Dwarf mistletoes depend on their host trees for their nutrients and water. Infections cause branch swelling, reduced growth, dieback, and abnormal proliferation of host branches known as “witches’ brooms”, all of which vary with infection severity and the vigor of the host. Generally, severe infection is associated with growth loss and mortality (Geils and Hawksworth 2002; Stanton 2006; Scott and Mathiasen 2012), alteration of physiological processes of the hosts (Glatzel and Geils 2009) and predisposition of host trees to other pests (Kenaley et al. 2006) and climate factors (Stanton 2007).
Impacts of infection by dwarf mistletoe on the reproductive outputs (cone production, seed quantity and quality, and seedling survival) of its coniferous hosts in general have not been a subject of many studies (Geils and Hawksworth 2002). To our knowledge, no detailed studies have been carried out regarding the extent of damage incurred by dwarf mistletoe on J. polycarpos. Such information is useful for forest managers who rely on natural regeneration to mitigate the damage to juniper forests that results from infection of this dwarf mistletoe. Thus, the main objective of the study was to investigate the effect of juniper dwarf mistletoe infection on growth characteristics and reproductive output of its host, J. polycarpos. The specific research questions addressed were: (1) How severe is dwarf mistletoe infection and to what extent has this infection influenced growth characteristics of J. polycarpos? (2) Does infection by dwarf mistletoe reduce cone production, seed quality or seed quantity? To answer these questions, infected and uninfected trees (n = 20 each) were sampled in J. polycarpos stands at Chaharbagh, northeastern Iran, and their growth characteristics were measured. The severity of dwarf mistletoe infection was assessed using Hawksworth’s six-class dwarf mistletoe rating system (Hawksworth 1977) coupled with quantitative estimate of the dwarf mistletoe colonies and witches’ brooms. The number of cones produced by each tree was assessed using the frame method (Lamien et al. 2006), and cones were collected for characterization of cone and seed characters.
Materials and methods
Study site
The study was conducted in a J. polycarpos stand at Chaharbagh in northeastern Iran, located between 36° 36′?36° 41′ N and 54° 28′?54° 35′ E at an elevation range of 2150?3150 m a.s.l. (Fig. 1). The climate is characterized as semi-dry with average annual precipitation of 305 mm, which appears mostly in the form of snow. The study area has cold winters and moderately warm summers with the minimum temperature (-14°C) occurring in December and the maximum (31°C) in July. Relative humidity varies during the year from 46% to 69.9%, the frost period is 120 days, starts in November and continues until February. The site has shallow sandy soils (45?71.8% sand, 8.3?26.6% clay, 19.8?28.4% silt, pH 8.3 and electrical conductivity 0.15?0.18) with stone outcrops and is prone to low nutrient availability (Korouri et al. 2012).
Fig. 1: Geographic distribution of J. polycarpos and J. excelsa and location of the study area, Charbagh, Golestan province, Iran.
Sample tree selection
A total of 40 sample trees (n = 20 for infected and uninfected trees each) in the same altitudinal range were marked for subsequent assessment of dwarf mistletoe infection and collection of cones. Near every infected tree, one uninfected tree with comparable growth characteristics and morphological features was chosen as a control. As we were interested to evaluate stress reactions only from dwarf mistletoe infection, thereby avoiding potential confounding effects of other factors on cone and seed characters, trees free from decay in the trunk, pest attack, dis-eases and other environmental stresses were judiciously selected as controls.
Assessment of dwarf mistletoe infection
The severity of a dwarf mistletoe-infected tree was assessed using dwarf mistletoe rating (DMR) system, where ratings ranged from 0 to 6 (Hawksworth 1977). With this method, the tree crown was first divided into three parts (Fig. 2), and the severity of infection in each third was rated as 0 if there was no visible dwarf mistletoe infection, 1 if less than half of the branches were infected or 2 if more than half of the branches were infected. Ratings for each third of the crown were then summed to obtain a DMR for the whole tree. Finally, trees with a DMR of 1 to 2 were considered lightly infected, trees with a DMR of 3 to 4 were considered moderately infected, and trees with a DMR of 5 to 6 were considered heavily infected (Hawksworth 1977). Presence of infection was most easily determined from the ground by the presence of witches’ broom-like growth. Mistletoe brooms are infected host branches with excessive branching and shortened/lengthened internodes.
To get a quantitative estimate of the infection level, the number of witches’ brooms was also counted in each vertical third of the crown. In addition, the numbers of dwarf mistletoe colonies were counted in the middle part of the crown along the four sides of the crown (north, south, east and west) using 1 m × 1 m wooden-frame mounted on a long pole (Fig. 2). This frame method is commonly applied in flower and fruit assessment (Lamien et al. 2006). The middle part of the crown was selected as point of assessment because most cones and dwarf mistletoe colonies appear around this part of the crown of J. polycarpos.
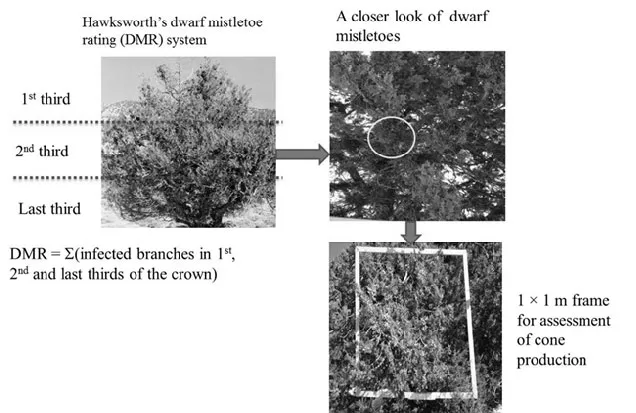
Fig. 2: An illustration of dwarf mistletoe rating system (DMR) and assessment of cone production using the frame method.
Assessment of cone production and cone characters
Prior to collection of cones, the number of cones produced by infected and uninfected trees was counted to the main stem using 1 × 1 m wooden-frame in the same way as the assessment of the mistletoe colonies above (Fig. 2). Thereafter, 2-year-old mature cones with dark blue or dark blue with a white and waxy coating were picked from each infected and uninfected sample tree. From each sample tree, one kg of cones was collected along the four sides of the crown to get representative cone samples. After thoroughly mixing the cones from the four sides of the crown of each sample tree, four samples, 25 g each, were randomly drawn and the numbers of cones in each 25 g sample were counted. From each 25 g sample, 40 cones were randomly sampled and cone diameter and number of seeds per cone were assessed and recorded.
Seed extraction and assessment of seed characters
Seeds were extracted manually by rubbing macerated cones (for 48 hours) between two pieces of screening mesh. Seeds were soaked and stirred in lime water to separate the pulp and remove the resin from seeds. Seeds were then air-dried at room temperature for one week. Thereafter, the following seed characters were measured: 1000-seed weight, seed length, seed width, and seedlot quality. For 1000-seed weight, 4 replications of 100 seeds from each tree seed lot were randomly sampled and weighed using a sensitive balance (with precision 0.0001 g). Seed length and width were measured on individual seeds (4 replicates of 25 seeds each) using a digital micro caliper.
Seed lot quality was assessed by determining the proportion of empty, damaged and filled seeds followed by germination and cutting tests. The proportion of filled and damaged seeds was determined using a floatation technique, and seed viability was assessed by a germination test. First, a random sample of 100 seeds, replicated four times, was drawn from each seed lot, placed in a bowl containing water (Millipore-filtered water, ca. 10 oC), and stirred to facilitate the separation process. The floating and sunken fractions were separately collected after five minutes and counted to quantify the floating and sunken fractions. Thereafter, the floating and sunken seeds were cut and examined to quantify filled and damaged seeds. Seeds with visible embryonic axis and megagametophyte were recognized as filled seeds while damaged seeds were empty, insect-damaged and degenerated seeds. Seeds were considered to be empty if the megagametophyte and embryo were absent, insect-damaged if visible exit holes made by the emerging insect were observed, and degenerated if black wrinkled internal tissues were observed.
For the germination test, sunken seeds (which were mostly filled seeds) were first cold-stratified in moist sand for eight weeks at 5°C to break dormancy, based on experiences from other juniper species (Tigabu et al. 2007). Thereafter, germination tests were carried out on Jacobsen’s apparatus at a constant temperature of 20±1°C with an illumination of c. 20 μE m-2s-1(Fluorescent lamp F 40 W / 33 RS cool white light) for 30 days. Four replicates of 50 seeds from each seed lot were sown on standard germination papers (Munktell analytical filter paper, ? 75 mm), and the germination process was monitored every day. Germinated seeds were counted when the radicle reached 2 mm long and had a normal appearance. Seeds that remained ungerminated were further cut and examined for their viability. Germination capacity (GC) was computed as the proportion of germinated seeds and seeds confirmed filled-viable by cutting test to that of sown seeds, expressed as a percentage. It should be noted that the optimal dormancy breaking treatment is still not known for this species, and work is in progress to develop a suitable dormancy breaking method. As an index of seed vigor, the mean germination time (MGT in days) was computed as follows:
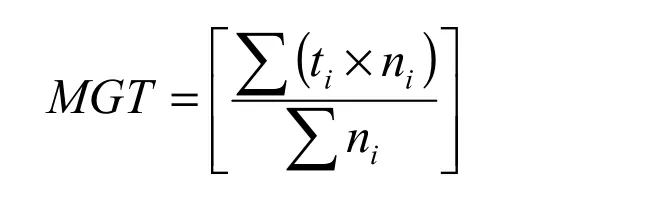
where n is number of seeds completing germination on day t and t is the time in days starting from day 0, the day of sowing.
Statistical analysis
To compare differences in mean numbers of dwarf mistletoe colonies per m2area and witches’ brooms per tree between infected trees, two-sample T-test, after testing for equality of variance, was performed. One-way ANOVA was performed to compare differences in growth characteristics (DBH, total height, crown surface area and crown volume), cone characteristics (number of cones per unit area, number of cones per kg of cones, cone diameter and mean number of seeds per cone) and seed characters (seed length, seed width, 1000-seed weight, number of filled and damaged seeds, and seed viability) across infection classes. Crown surface area and volume were calculated from crown width and height assuming a cone shape, which is a typical crown shape of J. polycarpos. Prior to ANOVA, germination capacity was arcsine transformed to meet the normality assumption for the analysis of variance. A Chi-square analysis of a 2 × 6 contingency table was also performed to test the null hypothesis that the proportion of cones with varying number of seeds per cone was independent of the health status of trees (infected versus uninfected trees). All data analyses were performed with Minitab 16.0 statistical software (Minitab Inc., State College, PA, USA).
Results
Severity of infection and its impact on growth characteristics
The DMR system revealed that 40% of the sample trees were lightly infected and the remaining 60% were moderately infected with dwarf mistletoe (Table 1). The mean number of witches’brooms did not vary significantly between low and moderate infection severity classes (t[0.05,11]=- 1.94, p =0.078), but it tended to be higher in moderately infected than lightly infected trees (Table 1). Mean numbers of dwarf mistletoe colonies per m2also did not differ significantly (t[0.05,18]=- 0.87, p =0.398) between lightly and moderately infected trees. With regard to growth characteristics, no significant differences (p >0.05) in diameter, total height, crown volume, and crown area were detected between uninfected, lightly and moderately infected trees (Table 2).
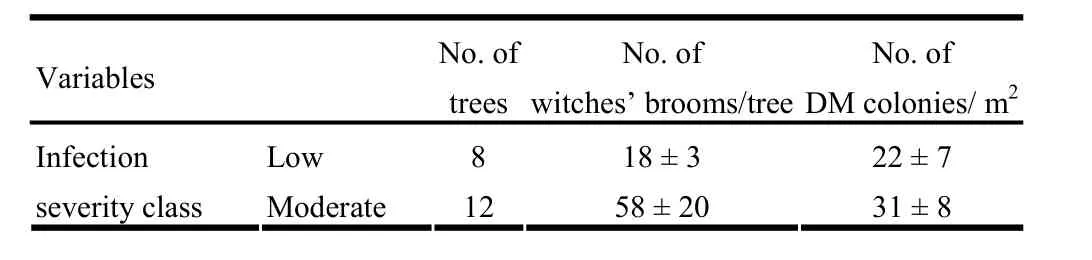
Table 1: Number of infected trees, dwarf mistletoe (DM) colonies per unit area and witches’ broom per tree across different infection severity classes (mean ± SE, except for No. of trees).
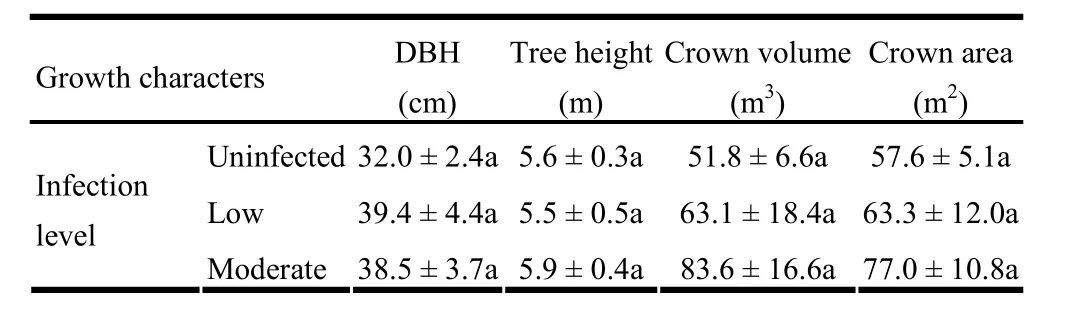
Table 2: Growth characteristics of J. polycarpos trees across three levels of infection (mean ± SE). For each growth character, means followed by the same letter across the row are not significantly different.
Impact of dwarf mistletoe infection on cone and seed characteristics
For cone characters, significant differences in mean number of cones per unit area (p =0.001) and number of cones/kg (p =0.024) were detected between uninfected, lightly infected and moderately infected trees, but mean cone diameters were similar (p = 0.095). Uninfected trees produced nearly twice as many cones per unit area as infected trees, but both lightly and moderately infected trees produced similar numbers of cones per unit area (Table 3). On the contrary, moderately infected trees produced 1.1 times more cones/kg than uninfected trees. Although the mean number of seeds per cone was similar (p =0.503) for uninfected, lightly infected, and moderately infected trees (Table 3), the proportion of cones with varying numbers of seeds per cone differed significantly between uninfected and infected trees (χ2= 12.859, d.f. =5, p =0.025). Uninfected trees produced slightly more cones with one, two and five seeds per cone than infected trees, whereas infected trees produced slightly more cones with three and four seeds per cone than their uninfected counterparts (Fig. 3).
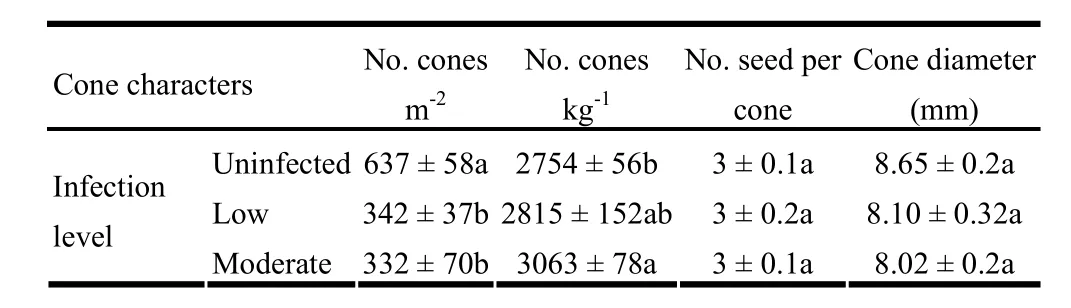
Table 3: Cone characters of J. polycarpos trees across three levels of infection (mean ± SE). For each cone character, means followed by different letter across the row are significantly different.
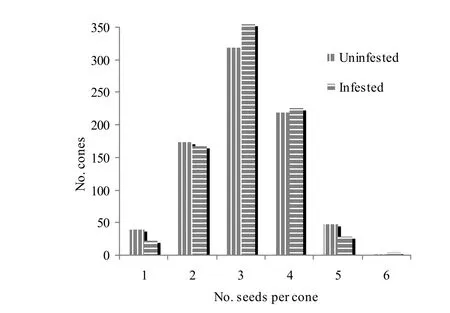
Fig. 3: Number cones with varying number of seeds per cone of dwarf mistletoe infected and uninfected trees of J. polycarpos (n = 800 cones for each group of trees).
For seed characters, significant differences in 1000-seed weight (p =0.001), seed width (p <0.001), seed length (p <0.001), number of damaged seeds/kg (p =0.008) and total number of seeds/kg (p =0.021) were detected between uninfected, lightly infected and moderately infected trees, but not for number of filled seeds (p =0.640). Seeds from uninfected trees were heavier than seeds from moderately infected trees, and were longer and wider than seeds from both lightly and moderately infected trees (Fig. 4). The numbers of filled seeds were generally low for both uninfected and infected trees compared to damaged seeds, which were more for moderately infected trees than for uninfected trees (Table 4). As a whole, infected trees produced more seeds than uninfected trees. With regard to seed viability, significant differences in germination capacity (t[0.05,18]= 3.68, p = 0.002) and mean germination time (t[0.05,18]= - 4.17, p = 0.001) were detected. Germination capacity was higher for uninfected than infected trees, and seeds from uninfected trees germinated faster than seeds from infected trees (Table 5).
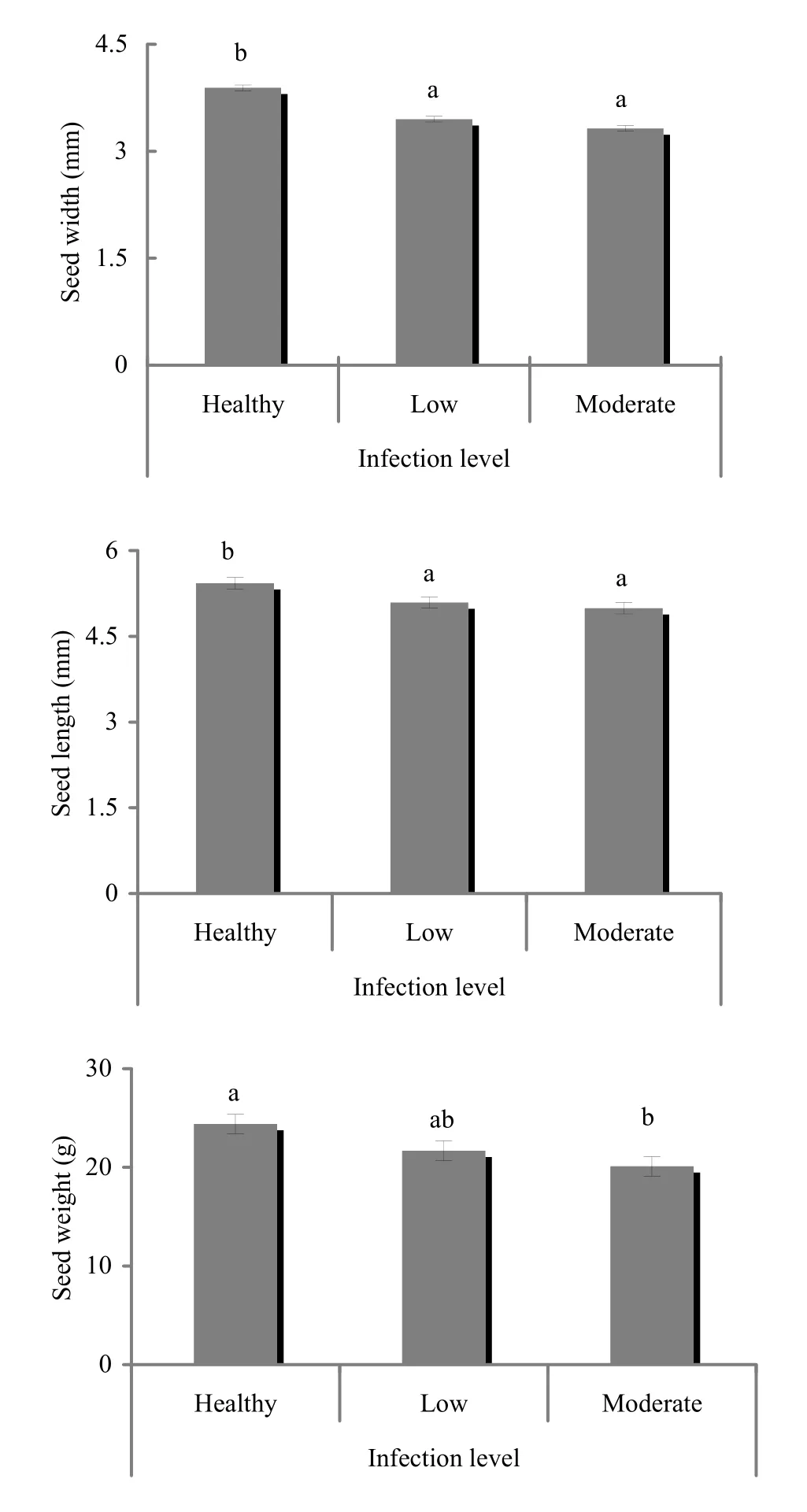
Fig. 4: Comparison of seed size characters between uninfected and infected trees of J. polycarpos (mean ± SE).
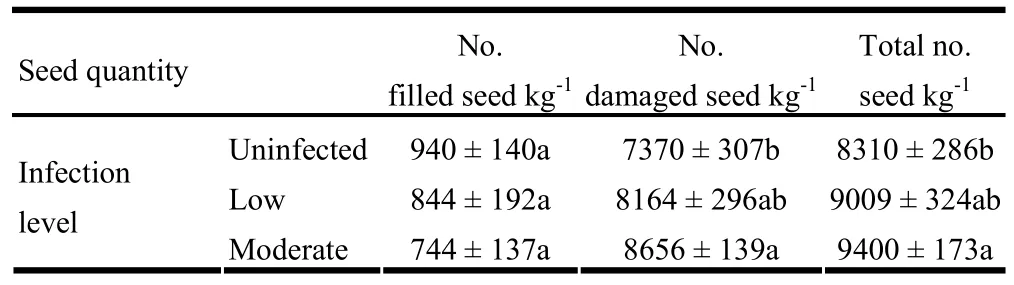
Table 4: Comparison of seed quantity across three levels of infection (mean ± SE). For each variable, means followed by different letter across the row are significantly different.
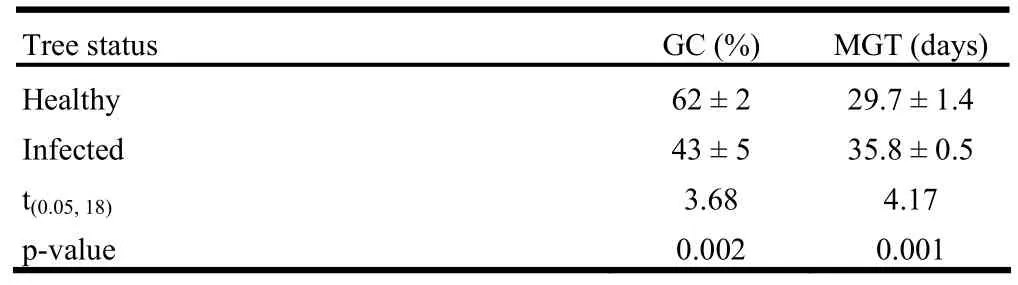
Table 5: Comparison of germination capacity (GC) and mean germination time (MGT) between healthy and dwarf mistletoe infected trees of J. polycarpos (mean ± SE).
Discussion
The severity of J. polycarpos infection by juniper dwarf mistletoe was low to moderate. The severity of infection generally depends on host quality and dispersal agents. According to differential parasitism theory, mistletoes selectively parasitize or survive better on hosts with high nutrient (Panivi and Eickmeier 1993; Dean et al. 1994) and water status (Gregg and Eleringer 1990). Previous studies have shown positive correlation between mistletoe growth and host nitrogen status (Seel et al. 1993; Dean et al. 1994), and host nitrogen and mistletoe canopy size (Bowie and Ward 2004). Furthermore, Bowie and Ward (2004) observed a negative correlation between host water status and flower production of mistletoes, i.e., mistletoes produce fewer flowers as hosts experience more water stress. The poor growth conditions in the natural habitat of J. polycarpos (semi-dry climate and poor soil quality) may predispose the trees to high water stress and nutrient deficiency, which in turn, affect the fitness (growth and flower production) of juniper dwarf mistletoes.
The spread of the parasite within the crown of individual trees and sites is influenced by the activity of seed dispersal vectors (frugivorous birds). Previous studies have shown that mistletoes are more abundant on hosts whose fruiting phenology is synchronized with that of the mistletoes (Aukema and Martínez del Rio 2002; Green et al. 2009; Okubamichael et al. 2011), since the hosts themselves are a rich source of food for frugivorous birds. For instance, Carlo and Aukema (2005) observed more infection on female hosts with fruits than male hosts with no fruits. As J. polycarpos is a unisexual species that produces ripened cones every two years, asynchronized fruiting with that of the juniper dwarf mistletoe coupled with limited availability of food for frugivores might explain the low to moderate level of infection observed in the present study.
The negative impact of infection by mistletoe on growth of its host is usually associated with high infection severity and time since infection. Compared to low and moderate infection, significant growth losses and less annual variation in growth were observed for severely infected bristlecone pines (Scott and Mathiasen 2012), western hemlock (Shaw et al. 2008) and ponderosa pine (Stanton 2006). Moreover, Stanton (2006) observed that witch’s broom abundance does not uniquely impact recent radial growth. We also found weak insignificant correlations between growth characteristics and numbers of witches’ brooms and numbers of dwarf mistletoe colonies per unit area (data not shown). In addition, time since infection determines the severity of infection and the associated impact on growth of the host. At the beginning, infection occurs at a slow rate with little effect, but it increases progressively over time and culminates in a large damaging effect (Geils and Hawksworth 2002). Although there is no record of time since infection of J. polycarpos by dwarf mistletoe in Iran, the infection is apparently a recent phenomenon as can be inferred from the low to moderate level of infection observed in the present study. As a whole, the lack of significant differences in growth characteristics between infected and uninfected J. polycarpos trees in our study can be related to the severity of infection, which was low to moderate.
Infection by juniper dwarf mistletoe has a significant effect on the reproductive output of its host by significantly reducing the mean number of cones per unit area in the host crown, and increasing the number of damaged seeds. Generally, reproductive output is governed by changes in resource allocation patterns (Bazzaz et al. 2000). Mistletoes drain nutrients (particularly nitrogen) from hosts by both active and passive uptake mechanisms (Okubamichael et al. 2011), which offer the mistletoes competitive advantage over the neighboring uninfected branches, thereby perturbing resource allocation for reproduction at the individual tree level. In addition, under conditions of limited resource availability a plant might allocate the available resource to the production of many smaller seeds. This is evident in the present study where infected trees (subjected to resource competition with the dwarf mistletoe) produced 1.1 times more cones/kg but smaller seeds than did uninfected trees.
It is interesting to note that the proportion of damaged seeds was slightly higher for infected than uninfected trees, and most of the damaged seeds were empty seeds in both infected and uninfected trees. Poor seed production is typical of many conifers due to lack of pollination and fertilization, pollination by non-viable pollen grain, perturbations in the pollination mechanism, or degeneration of ovules or early embryos (e.g. Slobotník and Gutternberger 2000). However, infection by juniper dwarf mistletoe appears to aggravate this problem. We also recorded a significant difference in the number of seeds per cone between infected and uninfected trees. Uninfected trees produced slightly more cones with one or two large seeds per cone while infected trees produce more cones with three or four small seeds per cone, indicating a resource trade-off that can be interpreted as evidence of ovule competition (Wulff 1995).
With regard to germination, infection by juniper dwarf mistletoe resulted in reduced overall germination and reduced germination rates compared to seeds from uninfected trees. The difference in rates of germination might be related to the amount ofreserve compounds (seeds from infected trees were significantly lighter than those from uninfected trees), and their subsequent conversion into energy to trigger radicle protrusion. Vigorous seeds exhibit increased fumarase activity, a key respiratory enzyme in the tricarboxylic acid cycle (Shen and Oden 2000) that fuels rapid germination. As a whole, our results are consistent with previous studies that have reported fewer cones, fewer seeds, lighter seeds and lower germination for dwarf mistletoe infected than uninfected conifer species (Singh 1981; Singh and Carew 1989; Schaffer et al. 1983; Sproule 1996).
Conclusions and recommendations
In this study, the severity of infection by juniper dwarf mistletoe on its host, J. polycarpos, and the associated impact on growth characteristics and reproductive output were investigated to gain insight into the contribution of juniper dwarf mistletoe to poor natural regeneration. We offer the following conclusions and recommendations:
The severity of infection by juniper dwarf mistletoe on J. polycarpos was low to moderate, and such level of infection did not induce marked differences in growth characteristics compared to uninfected trees.
Moderate infection by juniper dwarf mistletoe had a negative effect on the reproductive output of J. polycarpos by significantly reducing the mean number of cones per unit area of the host crown, increasing the number of damaged seeds, reducing seed size and reducing seed germination.
Reproductive output appeared to be more sensitive to moderate infection by juniper dwarf mistletoe than growth characters of its host; which might partly account for poor natural regeneration.
As this study is the first attempt to systematically assess interaction between juniper dwarf mistletoe and its host in Central and Southwest Asia (c.f. Sarangzai et al. 2010), we recommend stand level dwarf mistletoe inventory to confirm whether the observed severity of infection in the present study is a stand-level phenomenon. Further study is also recommended to understand the mechanisms of selection of hosts and the distribution of infection within individual trees and across the landscape. Forest managers relying on natural regeneration should consider enrichment planting because the production of filled seeds was low irrespective of the intensity of parasitism of the tree. For reforestation purposes, it is advisable to collect seeds from uninfected trees to ensure fairly good germination. In addition, an efficient method is needed for separating the large number of non-viable seeds from the seed lots.
Acknowledgements
We thank the employees of Iranian Agricultural and Natural Resources Research Center (Golestan Province) for their help during the fieldwork. We also express our gratitude to Mohsen Ghezelsofla for preparing the distribution map of J. excelsa and J. polycarpos in Iran.
Ahani H, Jalilvand H, Hosseini-Nasr SM, Soltani KH, Ghazi MR, Mohammadzadeh H. 2013. Reproduction of Juniperus polycarpos in Khorasan Razavi, Iran. Forest Science and Practice, 15(3): 231?237.
Akkol EK, Guvenc A, Yesilada E. 2009. A comparative study on the antinociceptive and anti-inflammatory activities of five Juniperus taxa. Journal of Ethnopharmacology, 125(2): 330?336.
Aukema JE, Martinez del Rio C. 2002. Mistletoes as parasites and seeddispersing birds as disease vectors: current understanding, challenges, and opportunities. In: Levey DJ, Silva WR, Galetti M. (eds), Seed Dispersal and Frugivory: Ecology, Evolution and Conservation. Oxfordshire, UK: CAB International Press, pp. 99?110.
Bazzaz FA, Ackerly DD, Reekie EG. 2000. Reproductive allocation in plants. In: Fenner M. (ed), Seeds: the Ecology of Regeneration in Plant Communities. Wallingford/New York: CABI Publishing, pp. 1?29.
Bowie M, Ward D. 2004. Water and nutrient status of the mistletoe Plicosepalus acaciae parasitic on isolated Negev Desert populations of Acacia raddiana differing in level of mortality. Journal of Arid Environments, 56: 487–508.
Carlo TA, Aukema JE. 2005. Female-directed dispersal and facilitation between a tropical mistletoe and a dioecious host. Ecology, 86(12): 3245?3251.
Dean WRJ, Midgley JJ, Stock WD. 1994. The distribution of mistletoes in South Africa: patterns of species richness and host choice. Journal of Biogeography, 21(5): 503?510.
Farjon A. 2005. A monograph of Cupressaceae and Sciadopitys. Kew: Royal Botanic Gardens Press, 648 p.
Geils BW, Hawksworth FG. 2002. Damage, effects, and importance of dwarf mistletoes. In: Geils BW, Tovar JC & Moody B (eds), Mistletoes of North American Conifers, p 1-5. USDA, Forest Service General Technical Report, RMRS, GTR.
Glatzel G, Geils BW. 2009. Mistletoe Ecophysiology: host parasite interactions. Botany, 87: 10?15.
Green AK, Ward D, Griffiths ME. 2009. Directed dispersal of mistletoe (Plicosepalus acaciae) by Yellow-vented Bulbuls (Pycnonotus xanthopygos). Journal of Ornithology, 150: 167?173.
Gregg JW, Ehleringer JR. 1990. Is mistletoe presence dependent on host quality? Bulletin of the Ecological Society of America, 71: 173 p.
Hawksworth FG. 1977. The 6-class dwarf mistletoe rating system. USDA Forest Service, Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colorado, General Technical Report RM-48, 7 pp.
Hawksworth FG, Wiens D. 1996. Dwarf mistletoe: biology, pathology and systematics. USDA Forest Service Agricultural Handbook 709.
Kenaley SC, Mathiasen RL, Daugherty CM. 2006. Selection of dwarf mistletoe-infected ponderosa pines by Ips species (Coleoptera: Scolytidae) in northern Arizona. Western North American Naturalist, 66(3): 279?284.
Korouri AAS, Khoshnevis M, Matinizadeh M. 2012. Comprehensive studies of Juniperus species in Iran. Forest, Range and Watershed Management Organization, 450 p. (In Persian with English Abstract).
Lamien N, Boussim JI, Nygard R, Ouédraogo JS, Odén PC, Guinko S. 2006. Mistletoe impact on Shea tree (Vitellaria paradoxa C.F. Gaertn.) flowering and fruiting behaviour in savanna area from Burkina Faso. Environmental and Experimental Botany, 55: 142?148.
Okubamichael DY, Griffiths ME, Ward D. 2011. Host specificity, nutrient and water dynamics of the mistletoe (Viscum rotundifolium) potential host species in the Kalahari of South Africa. Journal of Arid Environments, 75: 643–649.
Panvini AD, Eickmeier WG. 1993. Nutrient and water relations of the mistletoe Phoradendron leucarpum (Viscaceae): How tightly are they integrated? American Journal of Botany, 80(8): 872?878.
Sarangzai AM, Khan N, Wahab M, Kakar A. 2010. New spread of dwarf mistletoe (arceuthobium oxycedri) in Juniper forests, Ziarat, Balochistan, Pakistan. Pakistan Journal of Botany, 42(6): 3709?3714.
Schaffer B, Hawksworth FG, Jacobi WR. 1983. Effects of Comandra blister rust and dwarf Mistletoe on cone and seed production of Lodgepole pine. Plant Disease, 67(2): 215–217.
Scott JM, Mathiasen RL. 2012. Assessing growth and mortality of Bristlecone pine infected by dwarf mistletoe using dendrochronology. Journal of Forest Science, 58(4): 366?376.
Seel WE, Cooper RE, Press MC. 1993. Growth, gas exchange and water use efficiency of the facultative hemi-parasite Rhinanthus minor associated with hosts differing in foliar nitrogen concentration. Physiologia Plantarum, 89: 64?70.
Shaw DC, Huso M, Bruner H. 2008. Basal area growth impacts of dwarf mistletoe on western hemlock in an old-growth forest. Canadian Journal of Forest Research, 38: 576?583.
Shen TY, Odén PC. 2000. Fumarase activity as a quicker vigor test for Scots pine (Pinus sylvestris L.) seeds. Seed Science and Technology, 28: 825?835.
Singh P. 1981. Eastern Dwarf Mistletoe Arceuthobium-Pusillum on Black Spruce in Newfoundland Canada. Phytopathology, 71. Contact: Singh P. New Found Land Forest Res. Centre, Canadian Forestry Service, St John's, NFLD, CANADA.
Singh P, Carew GC. 1989. Impact of eastern dwarf mistletoe in black spruce forests of Newfoundland. European Journal of Forest Pathology, 19: 305?322.
Slobodnik B, Gutternberger H. 2000. Ovule, megaspores and female gametophyte formation in Larix decidua Mill. (Pinaceae). Acta Biologica Cracoviensia Serie Botanique, 42(2): 93?100.
Sproule A. 1996. Impact of dwarf mistletoe on some aspects of the reproductive biology of jack pine. The Forestry Chronicle, 72(3): 303?306.
Stanton S. 2006. The differential effects of dwarf mistletoe infection and broom abundance on the radial growth of managed ponderosa pine. Forest Ecology and Management, 223: 318?326.
Stanton S. 2007. Effects of dwarf mistletoe on climate response of mature ponderosa pine trees. Tree-Ring Research, 63: 69?80.
Tigabu M, Fjellstr?m J, Odén PC, Teketay D. 2007. Germination of Juniperus procera seeds in response to stratification and smoke treatments, and detection of insect-damaged seeds with VIS + NIR spectroscopy. New Forests, 33: 155–169.
Wulff RD. 1995. Environmental maternal effects on seed quality and germination. In: Kigel J, Galili G. (eds), Seed development and germination. New York/Basel/Hong Kong: Marcel Dekker Inc., pp.491?505.
DOI 10.1007/s11676-014-0530-6
Project funding: This study was supported by the Iran Government Science and Technology Scholarship Program.
The online version is available at http://www.springerlink.com
Abolfazl Daneshvar1,2, Mulualem Tigabu1,(), Mostafa Farhadi1
Per Christer Odén11Swedish University of Agricultural Sciences, Southern Swedish Forest Research Centre, P.O Box 49, SE-230 53, Alnarp, Sweden.2permanent address: Department of Natural Resources, Gonbad Kavous University, Shahid Fallahi Street, POBOX: 163, Gonbad, Iran. Email: Mulualem.Tigabu@slu.se.
Asaddollah Karimidoost
Agriculture and Natural Resources Research Center of Golestan Province, Beheshti Ave. PO Box 4915677555, Gorgan, IRAN. karimidoost@yahoo.com
Corresponding editor: Hu Yanbo
 Journal of Forestry Research2014年4期
Journal of Forestry Research2014年4期
- Journal of Forestry Research的其它文章
- Growth and yield of two grain crops on sites former covered with eucalypt plantations in Koga Watershed, northwestern Ethiopia
- Full length cDNA cloning and expression analysis of annexinA2 gene from deer antler tissue
- Bamboo resources of Sikkim Himalaya: diversity, distribution and utilization
- Implications of crude oil pollution on natural regeneration of plant species in an oil-producing community in the Niger Delta Region of Nigeria
- Enhancement of seed germination in Macaranga peltata for use in tropical forest restoration
- Bio-amelioration of alkali soils through agroforestry systems in central Indo-Gangetic plains of India
