Deep Extractive Desulfurization of Gasoline with Ionic Liquids Based on Metal Halide
Wang Haojie; He Jianxun; Yang Cairong; Zhang Hang
(School of Chemical Engineering, Northwest University, Xi’an 710069)
Deep Extractive Desulfurization of Gasoline with Ionic Liquids Based on Metal Halide
Wang Haojie; He Jianxun; Yang Cairong; Zhang Hang
(School of Chemical Engineering, Northwest University, Xi’an 710069)
Ionic liquid [Et3NH]Cl-FeCl3/CuCl was synthesized by mixing [Et3NH]Cl, anhydrous FeCl3and anhydrous CuCl, and the desulfurization activity of this ionic liquid was tested. It exhibited remarkable ability in effective desulfurization of model gasoline (thiophene inn-octane) and fluid catalytic cracking (FCC) gasoline, and the sulfur removal of thiophene in model oil (V(IL):V(oil)=0.08) could reach 93.9% in 50 min at 50 ℃. Low-sulfur (<10 μg/g) FCC gasoline could be obtained after three extraction runs at an ionic liquid/oil volume ratio of 0.1, with the yield of FCC gasoline reaching 94.3%. The ionic liquid could be recycled 5 times with merely a slight decrease in activity.
ionic liquid; metal halide; extractive desulfurization; FCC gasoline
1 Introduction
Deep desulfurization process for oils has attracted increasing attention, since the pollution caused by exhaust emissions (especially SOx) is one of the most serious environmental problems in the world[1]. With more and more stringent regulations on sulfur content, producing low-sulfur fuels (S content <10 μg/g) has become a common trend[2].
The traditional catalytic hydrodesulfurization (HDS) method is highly efficient in removing thiols, sulfides and disulfides. However, it is difficult to achieve the so-called“ultra-low sulfur” level by employing the traditional HDS method. Because aromatic sulfur compounds, such as thiophene and its derivatives existing in a high proportion in diesel fuel can hardly be desulfurized by HDS because of their steric hindrance[3-4]. Although thiophene and its derivatives are the main sulfur-containing components in FCC gasoline with relatively high reactivity for HDS, the major drawback is the loss in octane number, which is caused largely by undesired hydrogenation of olefins[5].
Therefore, many alternative desulfurization technologies for treating fuels have been developed in the last several decades, such as adsorption[6-7], extraction[8-10], oxidation[11-14]and bio-catalytic desulfurization[15]. Among these methods, extractive desulfurization (EDS) has been attracting much more attention because of its facile operation[16]. Many organic solvents, such as dimethyl sulfoxide, acetonitrile, 1-methyl-2-pyrrolidinone and dimethyl formamide have been used as extractants. However, the low desulfurization rate, poor selectivity, as well as use of flammable and volatile organic compounds can lead to further environmental and safety concerns[17].
Ionic liquids (ILs) are more competitive because of its non-volatility, non-flammability, high thermal stability and recyclability compared with organic solvents. Extraction of fuels with ILs to remove sulfur compounds has been recently reported. In previous studies, imidazolium compound-based ILs and pyridinium-based ILs have been most frequently employed in EDS, but some problems exist, such as low efficiency of sulfur removal, high cost for synthesis of imidazolium compound-based ILs and pyridinium-based ILs, and reduction in economic benefits caused by high ionic liquid/oil volume ratio.
In this paper, the Fe-containing and Cu-containing ionic liquids based on triethylamine hydrochloride salts, [Et3NH]Cl-FeCl3, [Et3NH]Cl-CuCl and [Et3NH]Cl-FeCl3/ CuCl were synthesized and used as extractants for desulfurization of the model gasoline and FCC gasoline. Because thiophene and its derivatives are the main sulfur-containing components in FCC gasoline, thiophene was employed as the model sulfur compound used in experiments.
2 Experimental
Ionic liquid [Et3NH]Cl-FeCl3were prepared via the reaction of [Et3NH]Cl on anhydrous FeCl3under stirring for two hours at 80 ℃. [Et3NH]Cl-CuCl and [Et3NH]Cl-FeCl3/CuCl were synthesized by the same method.
The model gasoline with a sulfur content of 1 484 μg/g was prepared by dissolving thiophene inn-octane. Commercial FCC gasoline with a sulfur content of 161 μg/g was provided by the Yan’an refinery. Desulfurization experiments were conducted in a 100-mL three-necked flask. The required amounts of IL and the model oil were added into the flask in turn and the mixture was stirred vigorously. After reaction, the upper oil phase was periodically withdrawn and the sulfur content of oil phase was analyzed using a micro-coulometer. IL was regenerated by evaporating the sulfur compounds.
3 Results and Discussion
3.1 Extractive desulfurization performance of IL for model oil
Desulfurization results of the model oil extracted by IL based on metal halide are shown in Table 1, some results of desulfurization using other ILs referred to in literature are also listed for comparison.
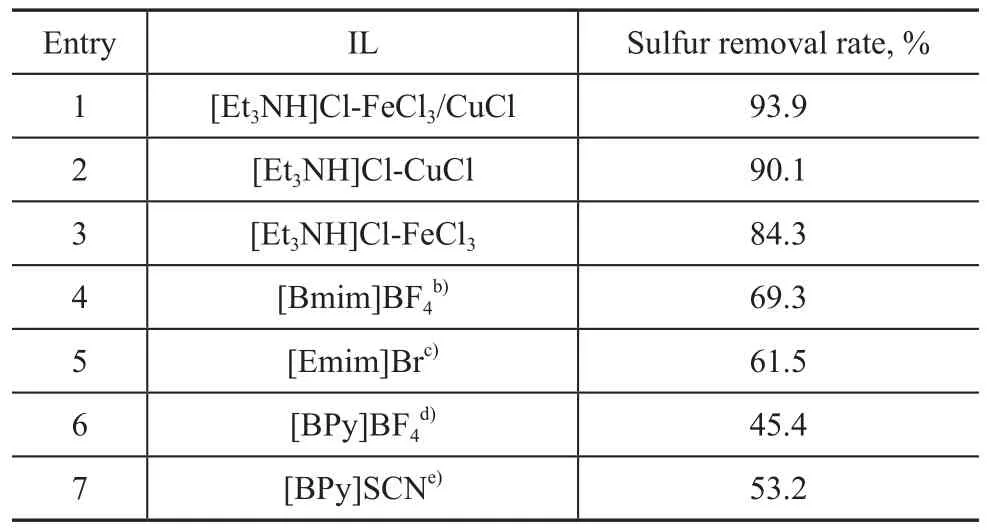
Table 1 Sulfur removal of thiophene using ILa)
It can be seen from Table 1 that all the ILs based on the metal halide show remarkable ability for sulfur removal. The sulfur removal rate from model oil could exceed 84% after extraction with a volume ratio of IL/oil equating to merely 0.08, whereas the sulfur removal rates of thiophene were all below 70% with imidazolium saltbased and pyridinium-based ILs at higher volume ratio of IL/oil. The metal halide-based ILs possessed greater ability for sulfur removal than the imidazolium saltbased and pyridinium-based ILs, which can be attributed to the interaction of Lewis acidic Fe3+and Cu+species with the Lewis basic thiophene. The extractive desulfurization mechanism of metal halide-based IL lies in the π-complexation between sulfur compounds and metals[20]. Comparatively, different metal salts containing various anions showed different desulfurization efficiencies, such as [Et3NH]Cl-FeCl3/CuCl (93.9%), [Et3NH]Cl-CuCl (90.1%) and [Et3NH]Cl-FeCl3(84.3%). The sulfur removal from model gasoline by [Et3NH]Cl-CuCl was higher than that by [Et3NH]Cl-FeCl3, which can be explained as follows. According to the law of acid-base reaction, since hard acid combines with hard base preferentially, and soft acid combines with soft base preferentially, compounds originating from reactions of soft acid with soft base or hard acid with hard base are more stable than compounds originating from reactions of soft acid (or base) with hard base (or acid). The Cu+ion is a soft acid and Fe3+ion is a hard acid, whereas the S atom of thiophene is a soft base, and the complexation between sulfur compounds and Cu+ions is more stable than the reaction products of sulfur compounds and Fe3+ions according to the law of acidbase reaction. It is clear that [Et3NH]Cl-FeCl3/CuCl has a better extraction ability than others judging from the complexation between sulfur compounds and [Et3NH] Cl-FeCl3/CuCl. Simultaneously, the Lewis acidity of IL [Et3NH]Cl-CuCl is weaker than that of [Et3NH]Cl-FeCl3/ CuCl and the viscosity of [Et3NH]Cl-FeCl3/CuCl (50 mPa·s, at 50 ℃) is lower than that of [Et3NH]Cl-CuCl (130.5 mPa·s, at 50 ℃), which enhances the affinity between IL and thiophene, leading to improved desulphurization rate.
3.2 Effect of volume ratio between IL and model oil on sulfur removal
In order to achieve the successful industrial application of IL, the dosage of IL is of vital importance. The amount of [Et3NH]Cl-FeCl3/CuCl is an important factor in EDS. Effects of various ratios of IL versus model oil on the re-moval of thiophene are given in Figure 1.
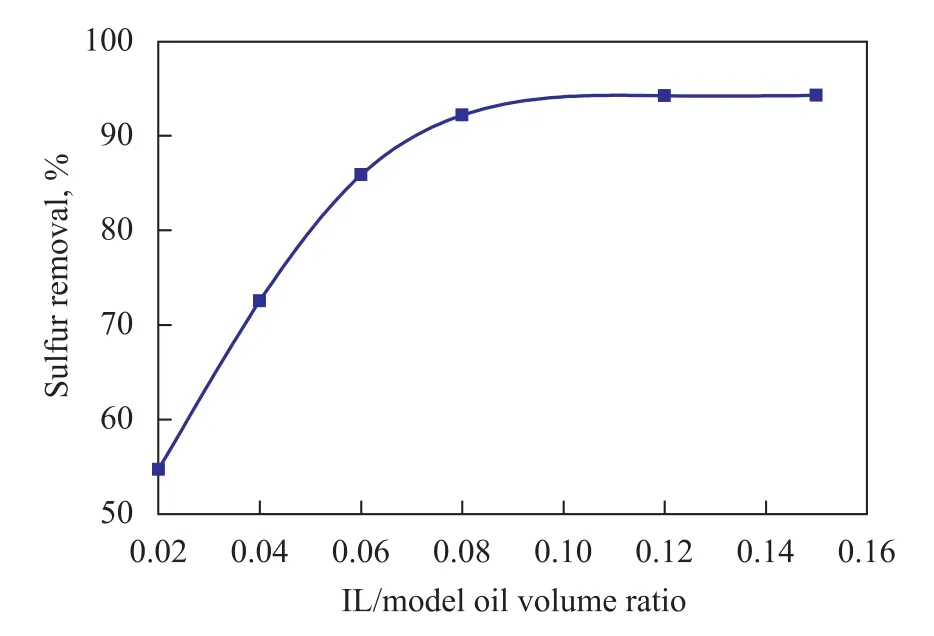
Figure 1 Effect of IL/model oil volume ratios on sulfur removal
It can be seen that, as expected, a higher volume ratio of IL/model oil resulted in a higher sulfur removal rate. The sulfur removal increased sharply when this volume ratio increased from 0.02 to 0.08, while the sulfur removal reached a relatively plateau of 92.17% when the volume ratio increased to 0.08, which might be related to an extraction equilibrium. When the dosage of IL was small, with the increase of volume ratio of IL/model oil, the sulfur removal also increased. Because thiophene had more contact opportunities with IL, following an increasing dosage of IL, the desulphurization rate was increased. However, when the volume ratio of IL/model oil increased to 0.08, the sulfur removal increased slightly with an increasing volume ratio of IL/model oil, because the dosage of IL is no longer a decisive factor of extraction equilibrium. Simultaneously, with an increasing dosage of IL, the ensuing separation of thiophene from IL became difficult, which would lead to increased cost of desulfurization process.
3.3 Effect of temperature on sulfur removal
Figure 2 shows the effect of temperature on sulfur removal and loss rate of model gasoline. Sulfur removal and loss rate of model gasoline increased with a rising temperature. When temperature increased from 25 ℃ to 50 ℃, the sulfur removal rate increased sharply from 58.65% to 95.83%, which might be ascribed to the reduction in viscosity of IL with an increasing temperature (with the viscosity equating to 90, 70, 50, 38, and 28 mPa·s at 30 ℃, 40 ℃, 50 ℃, 60 ℃, and 70 ℃, respectively), and the increase in solubility of thiophene in IL phase resulted from an increasing temperature. However the loss rate of model gasoline was relatively unchanged, which was almost maintained at zero. When the temperature was higher than 50 ℃, the sulfur removal almost no longer increased, while the loss rate of model gasoline rose. In addition, a high temperature would increase the energy consumption required.
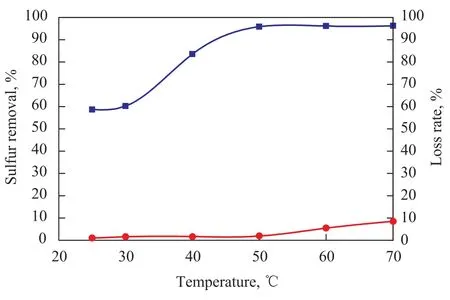
Figure 2 Effect of temperature on desulfurization rate
3.4 Effect of extraction time on sulfur removal
Figure 3 shows the effect of extraction time on sulfur removal. It can be seen that the sulfur removal increased from 42.05% to 93.56% when the extraction time was extended to 50 min from 10 min. However, the sulfur removal rate could hardly be improved in an extraction time of beyond 50 min. Since the extraction ability of IL would decrease slightly at high temperature in an extended extraction duration, the sulfur removal would decrease slightly beyond 50 min, and in the meantime an extended extraction time would lead to higher operating cost.
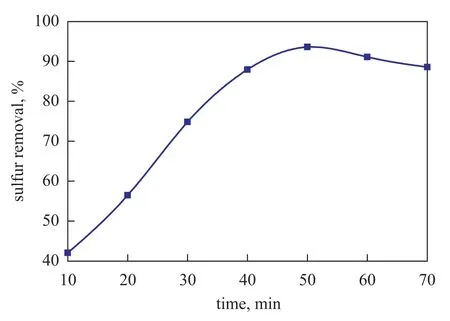
Figure 3 Effect of extraction time on the desulfurization rate
3.5 Gas chromatogram of model gasoline before and after desulfurization
Figure 4 shows the gas chromatogram of model gasoline before and after desulfurization with IL. It can be seen that the sulfur content of model gasoline dropped significantly after desulfurization, indicating that the IL has good desulfurization ability. Absence of new peaks appearing after desulfurization process explained that there was no other new substance existing in model gasoline, denoting that the IL did not dissolve in the model gasoline. Therefore, IL could be recycled almost without any loss. Figure 2 shows that the loss rate of model gasoline was 1.92% at 50 ℃, which demonstrated that the IL could extract thiophene almost without any effect on aromatic content. All these facts have proved that the IL has a good desulfurization performance accompanied with merely a slight loss of gasoline, and the IL does not dissolve in the model gasoline.
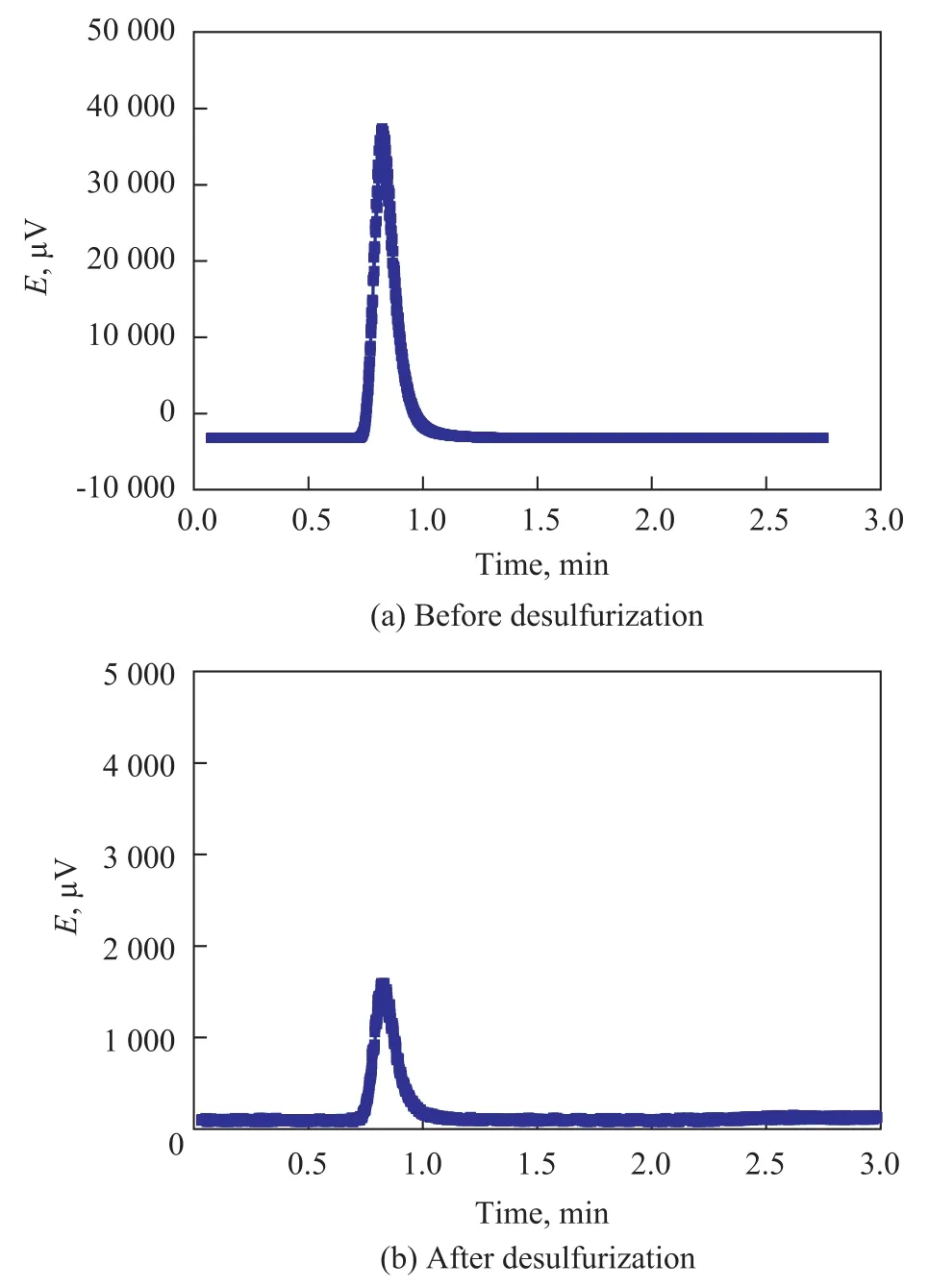
Figure 4 Gas chromatograms of model gasoline before and after desulfurization with IL
3.6 Recycling of used IL
From an industrial point of view, it is vitally important to recycle IL after the extraction of sulfur compounds. So the recycling of EDS system for IL [Et3NH]Cl-FeCl3/CuCl was investigated in the course of removal of thiophene from the model oil. After the reaction was terminated, the system was still a biphasic system consisting of the IL phase (the lower layer) and the oil phase (the upper layer). Therefore, the oil phase could be separated easily from the system, while the ionic liquid phase was recycled again through evaporating the sulfide. The ionic liquid was then placed in a vacuum oven and dried to constant weight. Then the fresh thiophene-containing model oil was directly added in for the next run. Data in Figure 5 indicated that the sulfur removal by fresh ionic liquid was 96.56%, while the sulfur content of model gasoline reduced to 51 μg/g from 1 484 μg/g after a single extraction. The sulfur removal can still reach 95.02% after five cycles, which verified that IL could be recycled for five times with a slightly decrease in extraction efficiency.
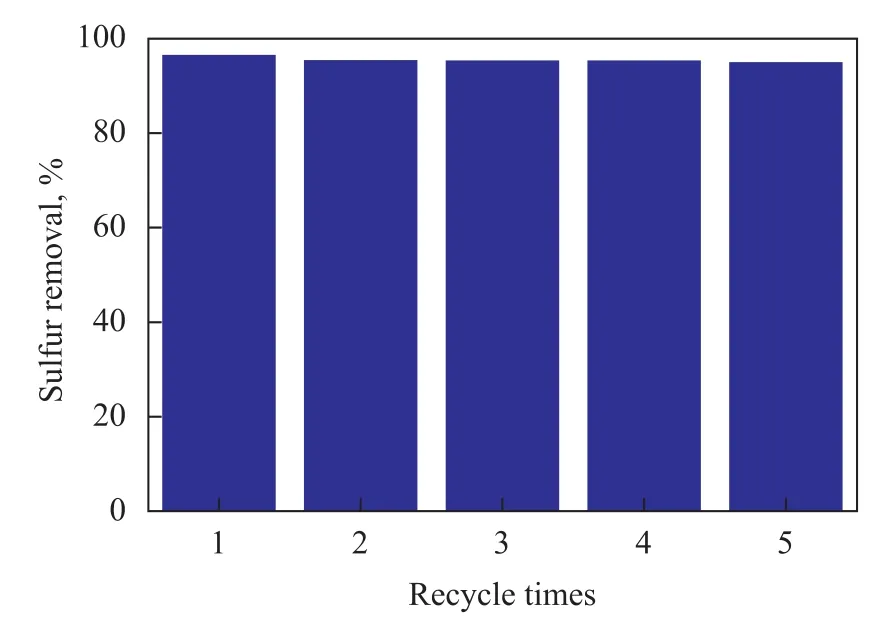
Figure 5 Desulfurization activity of recycled ionic liquids
3.7 Desulfurization of FCC gasoline
The desulfurization of FCC gasoline was investigated using an IL/oil volume ratio of 0.1 at a temperature of 50 ℃ and a reaction time of 50 min. The S-free oil cannot be obtained after only a single extraction. To meet the stringent sulfur specification, more extraction stages were necessary. That is, the desulfurized oil from the first extraction step was treated again with a fresh IL. It can be seen from Figure 6 that the removal of sulfur from real FCC gasoline is more difficult than treating the model oil, because many nitrogen and oxygen compounds and aromatic compounds exist in the FCC gasoline, which can decrease the extraction performance of IL. The sulfurcontent of FCC gasoline was 9.8 μg/g (at a sulfur removal rate of 93.8%) after three extraction runs, and could be classified as a low-sulfur gasoline. The yield of FCC gasoline was 94.3%.
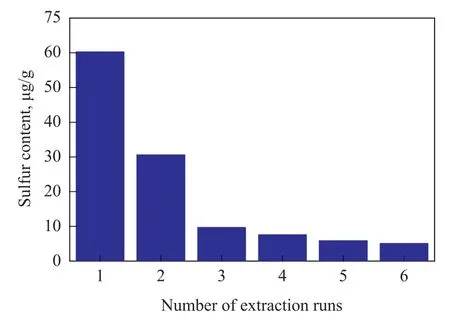
Figure 6 Effect of Multi-step on desulfurization rate
4 Conclusions
Ionic liquids based on metal salts [Et3NH]Cl-FeCl3, [Et3NH]Cl-CuCl and [Et3NH]Cl-FeCl3/CuCl were synthesized and employed in extractive desulfurization systems. The [Et3NH]Cl-FeCl3/CuCl showed a highest extractive efficiency. It is worthwhile to mention that [Et3NH]Cl-FeCl3/CuCl could be synthesized from cheap starting materials as compared to those imidazolium salt-based and pyridinium-based ILs. The sulfur removal of the thiophene-containing model oil could reach 93.9% by using [Et3NH]Cl-FeCl3/CuCl as the extractant at 50 ℃for 50 min, even when the IL/model oil ratio was 0.08 (by volume). Under the experimental conditions, the loss of model gasoline was 1.92% and the IL did not dissolve in gasoline. Low-sulfur (<10 μg/g) FCC gasoline could be obtained after extraction three times at an ionic liquid/oil volume ratio of 0.1, while the yield of FCC gasoline was 94.3%. The ionic liquid could be recycled 5 times with merely a slight decrease in its activity.
Acknowledgment:This work was supported by the National Natural Science Foundation of Shanxi Educational Committee (07JK384), the Whole Innovation of Science and Technology Project Plan of Shanxi Province (2012KTD01-01-04), the Graduate Innovation Project of Northwest University (YZZ13029)
[1] Hu Y, He Q, Zhang Z, et al. Oxidative desulfurization of dibenzothiophene with hydrogen peroxide catalyzed by selenium (IV)-containing peroxotungstate [J]. Chem Commun, 2011, 47(44): 12194-12196
[2] Zhu W, Li H, Jiang X, et al. Commercially available molybdic compound-catalyzed ultra-deep desulfurization of fuels in ionic liquids[J]. Green Chemistry, 2008, 10(6): 641-646
[3] Lü H, Gao J, Jiang Z, et al. Ultra-deep desulfurization of diesel by selective oxidation with [C18H37N (CH3)3]4[H2NaPW10O36] catalyst assembled in emulsion droplets[J]. Journal of Catalysis, 2006, 239(2): 369-375
[4] Ko N H, Lee J S, Huh E S, et al. Extractive desulfurization using Fe-containing ionic liquids[J]. Energy & Fuels, 2008, 22(3): 1687-1690
[5] Nie Y, Li C, Sun A, et al. Extractive desulfurization of gasoline using imidazolium-based phosphoric ionic liquids [J]. Energy & Fuels, 2006, 20(5): 2083-2087
[6] Kwon J M, Moon J H, Bae Y S, et al. Adsorptive desulfurization and denitrogenation of refinery fuels using mesoporous silica adsorbents [J]. Chem Sus Chem, 2008, 1(4): 307-309
[7] Srivastav A, Srivastava V C. Adsorptive desulfurization by activated alumina [J]. Journal of Hazardous Materials, 2009, 170(2): 1133-1140
[8] Zhai L, Zhong Q, He C, et al. Hydroxyl ammonium ionic liquids synthesized by water-bath microwave: Synthesis and desulfurization [J]. Journal of Hazardous Materials, 2010, 177(1): 807-813
[9] B?smann A, Datsevich L, Jess A, et al. Deep desulfurization of diesel fuel by extraction with ionic liquids [J]. Chemical Communications, 2001, 23: 2494-2495
[10] Asumana C, Yu G, Li X, et al. Extractive desulfurization of fuel oils with low-viscosity dicyanamide-based ionic liquids [J]. Green Chemistry, 2010, 12(11): 2030-2037
[11] Campos-Martin J M, Capel-Sanchez M C, Perez-Presas P, et al. Oxidative processes of desulfurization of liquid fuels [J]. Journal of Chemical Technology and Biotechnology, 2010, 85(7): 879-890
[12] He L, Li H, Zhu W, et al. Deep oxidative desulfurization of fuels using peroxophosphomolybdate catalysts in ionic liquids[J]. Industrial & Engineering Chemistry Research, 2008, 47(18): 6890-6895
[13] Dhir S, Uppaluri R, Purkait M K. Oxidative desulfurization: Kinetic modeling [J]. Journal of Hazardous Materials, 2009, 161(2): 1360-1368
[14] Wang B, Zhu J, Ma H. Desulfurization from thiophene bycatalytic oxidation at room temperature and atmospheric pressure [J]. Journal of Hazardous Materials, 2009, 164(1): 256-264
[15] Kirimura K, Furuya T, Nishii Y, et al. Biodesulfurization of dibenzothiophene and its derivatives through the selective cleavage of carbon-sulfur bonds by a moderately thermophilic bacterium Bacillus subtilis WU-S2B [J]. Journal of Bioscience and Bioengineering, 2001, 91(3): 262-266
[16] Li F, Liu R, Wen J, et al. Desulfurization of dibenzothiophene by chemical oxidation and solvent extraction with Me3NCH2C6H5Cl· 2ZnCl2ionic liquid [J]. Green Chemistry, 2009, 11(6): 883-888
[17] Seeberger A, Jess A. Desulfurization of diesel oil by selective oxidation and extraction of sulfur compounds by ionic liquids—a contribution to a competitive process design [J]. Green Chemistry, 2010, 12(4): 602-608
[18] Dharaskar S A, Wasewar K L, Varma M N, et al. Ionic liquids: The novel solvent for removal of dibenzothiophene from liquid fuel [J]. Procedia Engineering, 2013, 51: 314-317
[19] Zhao D, Wang Y, Duan E, et al. Oxidation desulfurization of fuel using pyridinium-based ionic liquids as phasetransfer catalysts [J]. Fuel Processing Technology, 2010, 91(12): 1803-1806
[20] Zhang S, Zhang Q, Zhang Z C. Extractive desulfurization and denitrogenation of fuels using ionic liquids [J]. Industrial & Engineering Chemistry Research, 2004, 43(2): 614-622
Recieved date: 2013-12-12; Accepted date: 2014-04-22.
Prof. He Jianxun, Telehone: +86-29-88303841; E-mail: hejx@nwu.edu.cn.
- 中國煉油與石油化工的其它文章
- Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
- Synthesis of Macro-Mesostructured γ-Al2O3with Large Pore Volume and High Surface Area by a Facile Secondary Reforming Method
- Performance of FCC Catalyst Improved with Vanadium Trapping Components
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 2. Single-Event Microkinetic (SEMK) Assessment
- Synthesis, Characterization and Evaluation of Sulfur Transfer Catalysts for FCC Flue Gas
- Selection of Chelated Fe (III)/Fe (II) Catalytic Oxidation Agents for Desulfurization Based on Iron Complexation Method

