Synthesis, Characterization and Evaluation of Sulfur Transfer Catalysts for FCC Flue Gas
Jiang Ruiyu; Shan Honghong; Zhang Jiling; Yang Chaohe; Li Chunyi
(1. Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province, Yancheng Institute of Technology, Yancheng 224051; 2. State Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580; 3. East-China Design Branch, China Petroleum Engineering Construction Corporation, Qingdao 266071)
Synthesis, Characterization and Evaluation of Sulfur Transfer Catalysts for FCC Flue Gas
Jiang Ruiyu1; Shan Honghong2; Zhang Jiling3; Yang Chaohe2; Li Chunyi2
(1. Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province, Yancheng Institute of Technology, Yancheng 224051; 2. State Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580; 3. East-China Design Branch, China Petroleum Engineering Construction Corporation, Qingdao 266071)
In this work, Zr-M (M=Cu, Mn, Ce) type sulfur transfer agent was prepared by impregnation method. Under the condition similar to that in the regenerator of FCC units, the influence of different active metal components and their contents on sulfur transfer agent were investigated. Moreover, the crystalline structure of sulfur transfer agent was characterized by X-ray diffraction (XRD) and Fourier transforms infrared spectroscopy (FT-IR). The result showed that the Zr-Mn sulfur transfer agent could effectively reduce the SO2content in FCC regenerator flue gas, featuring high SO2adsorption capacity. The sulfur transfer agent was inactivated in 40—60 min during the test. In the course of reduction reaction, after several reaction cycles, the formation of SO2ceased and only H2S was detected as the reduction product.
fluid catalytic cracking; sulfur-transfer catalyst; flue gas; zirconium
1 Introduction
The emission of SOx(SO2and SO3) from either motor vehicles or stationary units causes serious atmospheric pollution and destruction of the ozone layer[1-7]. Thus, their emission is increasingly restricted by environmental legislations. Although the contribution of refineries is relatively small (6%—10%) compared with the total amount of SOxemitted from other sources, it can become very significant in smog-filled areas that are ringed with heavy industry, metal smelters, and coal-fired power plants. It is well known that if the sulfur-containing feedstock is fed into the fluid catalytic cracker unit (FCCU) without pretreatment, about 45%—55% of the total sulfur compounds present are released as H2S, and another 35%—45% would remain in the liquid products, with the rest being deposited on the coke which is formed on the FCC catalysts and transformed to sulfur oxides (with more than 90% of SO2and less than 10% of SO3) when the coke deposits are burned off in the regenerator of FCC unit. Therefore, the emission of SOxderived from FCC units can be divided into two parts: one is originated from the combustion of fuel produced from FCC units and the other is from the combustion of the sulfur-containing coke deposited on the catalyst in the regenerator of FCC unit[4-5,8-16].
As far as the reduction of sulfur oxides emissions derived from the FCC unit is concerned, a variety of methods have been proposed either for treating the fuel before combustion or for disposing of the flue gas generated during fuel combustion. However, the least costly strategy is the addition of sulfur transfer additives to the FCC catalyst. By mixing the FCC catalysts with the additives, sulfur oxides can be fixed as sulfates in the regenerator in an oxidative atmosphere; then the sulfates formed on the additives move together with FCC catalysts to a riser where they are released as H2S. The discharged gaseous H2S is separated from the oil products and is subsequently reduced in the usual way known as the Clause process to form elemental sulfur. This technique is much less costly than the hydrodesulfurization or stack-gas scrubbing techniques, and, from the economical and technical viewpoint, it is a very practical and attractive technique[4-5,12-13,17-18]. The three steps that determine the performance of a SO2transfer catalyst are shown below:
(1) Oxidation of SO2to SO3in the FCC regenerator, typically at a reaction temperature in the range of from 700 ℃to 730 ℃, SO2(g)+1/2 O2(g)→SO3(g)
(2) Trapping of SO3on the catalyst to form sulfates, MO(s)+SO3(g)→MSO4(s)
(3) Reduction of sulfates to release sulfur as H2S in FCC riser, typically at a reaction temperature in the range of 500 ℃to 530 ℃, MSO4(s)+4H2(g)→MO(s)+3H2O(g)+H2S(g)
Many efforts have been made to improve the activity of SOxuptake catalysts. MgAl2O4-spinel or Mg/Almixed oxides derived from hydrotalcite-like compounds (HTLCs) have been attracting much attention because they offer a large capacity for adsorption of SO3. Different metallic oxides such as those of Ce, Cu, Co, V, Cr, and Fe are incorporated into HTLCs either via impregnation or coprecipitation, as the best way to prepare the solid solutions of mixed oxides/spinels with both basic and redox properties required for good performance in the De-SOx process[4-5,10,12-13,17,19]. Lately, Henriques[9,16]and co-workers demonstrated that Mg, Mn and Al-oxides with the spinel structure would be promising as additives for SO2removal in FCC unit. In addition to the spinel systems, we preliminary used zirconia as carrier loaded alkaline species functioning as a sulfur oxide transfer agent. Zirconia can be used as the catalyst and also the catalyst carrier thanks to its acid-base property, redox performance, good thermal stability and mechanical strength. As a support, zirconia favors the dispersion and stability of active component. Furthermore, it is also involved in catalytic reactions because the presence of zirconia could increase the mobility of lattice oxygen to improve the oxidationreduction property. In this experiment, the preliminary exploration of load-based FCC flue gas sulfur transfer agent was carried out under FCC conditions. The effect of different active metal components on the adsorption of SO2in flue gas was studied and characterized by X-ray diffractometry (XRD) and the Fourier transform infrared (FT-IR) spectroscopy.
2 Experimental
2.1 Preparation of ZrO2
ZrO2was prepared by means of the ammonia complexation method. Zirconium oxychloride octahydrate [ZrOCl2·8H2O] and ammonium hydroxide [NH3·H2O] were used as starting materials to prepare catalysts. Firstly, two precursors were completely dissolved in distilled water at a pH value of between 9—10. The precipitate was formed and filtered, washed with distilled water, and soaked in ethanol for 36 h and then filtered. Then, the filter cake was dried for 8 h to remove moisture at 120 ℃. The dried solid was calcined in a muffle furnace for 2 h at 700 ℃ and then ground. The solid particles with a size of 80—120 mesh were collected through sieving.
2.2 Preparation of ZrO2-M (M=Cu, Mn, Ce)
ZrO2-M catalysts were prepared by the impregnation method. Zirconia and metal nitrates were mixed in different proportions. The solution was dried for 2 h to remove moisture from the solid material at 120 ℃. The dried catalyst was calcined in a muffle furnace for 2 h at 700 ℃.
2.3 Characterization of samples
All samples were characterized by X-ray diffraction (XRD) on a DANalytical X'Pert PROMPD spectrometric diffractometer (using Cu-Kα radiation), and by FT-IR spectrometry on a Micromeritics Nexu STM spectrometer using the KBr-disk method.
2.4 Catalytic performance tests[7-8]
Experiments were performed in a tubular reactor placed in a vertically installed electric furnace filled with 0.5 g of composite catalysts. Figure 1 shows the schematic diagram of experimental setup for this study. Gas entered the top of reactor with its flow rate being controlled by a mass flowmeter. The concentration of SO2was 2 730 mL/L; in order to carry out SO2oxidative adsorption, a stream with a flow rate of 131 mL/min containing 2 730 mL/L of SO2and 1.2% of air was introduced through the catalyst bed at 700 ℃ in 10 min. A simulated FCC gas was used to investigate the effect of desulfurization. The gases exiting the reactor were automatically analyzed by a flue gas analyzer (Model Testo 350 M/X, made in Germany). The SO2content was calculated from the relevant data.

Figure 1 Homemade fixed-bed micro-reactor unit
3 Results and Discussion
3.1 Catalytic performance
3.1.1 Effect of the Zr/Cu ratios on desulfurization efficiency
To investigate the in fluence of the different Zr/Cu ratios on the desulfurization performance, a series of tests were conducted to measure their desulfurization efficiency. It can be seen from Table 1 that the group effect of sulfur transfer agent was not very good, but it showed that Cu had some effect on SO2adsorption. The catalyst with a Zr/Cu ratio of 2 worked relatively well probably because the role of ZrO2as well as Cu in the micro-surface reaction process showed a relatively unique performance. ZrO2not only played the role of support, but also favored the dispersion and stability of active component. Furthermore, ZrO2might be involved in catalytic reaction, and could shape up the active center with Cu co-catalyst.

Table 1 Effect of different Zr/Cu ratios on the desulfurization efficiency
3.1.2 Effect of Zr/Mn ratios on the desulfurization efficiency
Upon comparing the role of zirconia used as the Mn containing carrier, it is recognized that its performance was better than the catalyst containing Cu as depicted in Table 1. The effect of Zr/Mn ratio in the sulfur transfer agent on adsorption of SO2at 700 ℃ during the simulated catalytic cracking process is presented in Table 2. The sulfur capacity of sulfur transfer agent was smaller because Mn, which possessed a shorter atomic radius than that of Zr, already changed the structure of zirconium oxide. The introduction of Mn is good for the oxidative adsorption of SO2to form the related sulfates. Furthermore, with the continuous reduction of Mn species, the SO2concentration firstly increased and then declined. A Zr/Mn ratio of 2 was better because with increase in the metal amount, more active components could migrate into the metal carrier holes, which would cause deposition on the pore walls and fast deactivation of the catalyst. It is reasonable if we suppose that Mn (III) may function as the active species, which can promote the oxidation of SO2to SO3with itself being simultaneously reduced to Mn (II), while the excess O2will subsequently oxidize Mn (II) to Mn (III)[9,20].

Table 2 Effect of different Zr/Mn ratios on the desulfurization efficiency
3.1.3 Effect of the Zr/Ce ratios on the desulfurization efficiency
The data listed in Table 3 are in agreement with those depicted in Table 1. The group effect of sulfur transfer agent was not very good, but it also showed that Ce had some effect on SO2adsorption. A Zr/Ce ratio of 1 worked relatively well. This fact has confirmed that Ce atoms were the active sites for SO2oxidative adsorption in this series, although the formation of a crystalline phase could not be detected according to the thermodynamic study.
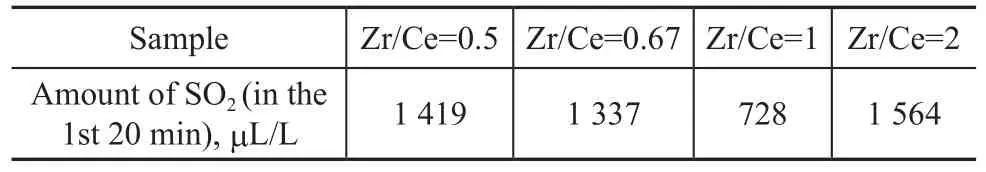
Table 3 Effect of different Zr/Ce ratios on the desulfurization efficiency
3.2 XRD analysis
The XRD spectrograms of the as-synthesized sample and the used sample are shown in Figure 2. With respect to the sample (a), small diffraction peaks corresponding to a Mn2O3-bixbyite C, the syn-type phase, were observed along with those of the crystal phase, indicating that a part of Mn2+cations were transformed into Mn3+duringthe synthesis process, as previously suggested by Velu, et al. With respect to the sample (b), a similar behavior was observed for the virgin and the used samples. Besides the Mn2O3-bixbyite C, the syn-type phase, diffraction lines were also observed, but it was difficult to identify whether there was a manganese sulfate phase. According to the redox potentials, the oxidation of Mn2+to Mn3+is highly favorable in a basic medium in the course of catalyst synthesis[9,20].

Figure 2 XRD patterns of different samples
3.3 FT-IR analysis
To identify the changes of virgin sulfur-transfer catalyst and sulfur species after SO2oxidative adsorption, FT-IR measurements of the samples such as virgin Zr/Cu, Zr/Mn, Zr/Ce sulfur-transfer catalysts with different metal contents were carried out with the results presented in Figure 3.

Figure 3 IR spectra of different samples
According to the literature information[14], a very wide band in the range of between 3 410 cm-1and 3 450 cm-1could be observed and it could be attributed to the interaction between the hydroxyl radicals and oxygen lattice in the framework due to the water adsorbed on the surface of the sample. This band shifted towards higher frequencies if the temperature was increased. Hereby, we mainly focused on the absorption bands ranging from 2 000 cm-1to 400 cm-1. After 20 min of adsorption, the samples (a) and (b) indicated intense absorption bands at 1 140 cm-1and 1 020 cm-1. However, with regard to the sample (d), only one inferior absorption band appeared at 1 140 cm-1. The above bands were assigned to the asymmetric and symmetric O=S=O and O-S-O stretching vibration of surface and bulk-like sulfates. The FT-IR study confirmed the existence of stable sulfur complexes formed from strong chemisorption or reaction of SO2with the active sites[14,21].
3.4 Analysis of adsorptive desulfurization mechanism of sulfur transfer agent
There are many mechanisms relating to flue gas desulfurization. Different researchers have proposed different desulfurization mechanisms according to experimental phenomena. By studying the absorptive property of Zr-Mn type spinels, the representative results show that SO2mainly forms MgSO4. Experimental results also indicate that the manganese-containing spinel is a promising sulfur transfer agent. After the reduction of the sulfated additives, when the reduction temperature reached 500 ℃, SO2was formed as the initial product during the reduction of sulfated species, which was different from that observed during the regeneration process starting at 530 ℃, where no SO2was detected at all. After several reaction cycles, the formation of SO2ceased and only H2S was detected as the reduction product.
4 Conclusions
Under conditions similar to those existing in the regenerator of FCC units, the influence of different active metal components and their contents were investigated. The results showed that the Zr-Mn based sulfur transfer agent could effectively reduce SO2content in the FCC regenerator flue gas, leading to higher rate for adsorption of SO2. The sulfur transfer agent was deactivated in 40—60 min. The FT-IR characterization outcome indicated that SO2was mainly fixed as the sulfate. The Zr-Mn sulfur transferagent is an active promoter to enhance the ability of SOxadditives in the regenerator of FCC units. Further studies must be done to improve the additive performance for simultaneous removal of SOx. In the course of reduction reaction, when the reduction temperature reached 500 ℃, SO2was formed as the initial product during the reduction of sulfated species. After several reaction cycles, the formation of SO2ceased and only H2S was detected as the reduction product.
Acknowledgements:The project was supported by the research fund of the National Natural Science Foundation of China (21306162), the National Basic Research Program “973” Project of China (2010CB226903) and Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province (AE201309).
[1] Lowell P S, Schwitzgebel K, Parsons T B, et al. Selection of metal oxides for removing SO2from flue gas[J]. Industrial & Engineering Chemistry Process Design and Development, 1971, 10(3): 384-390
[2] Jr Blanton W A, Flanders R L. Process for controlling sulfur oxides using an alumina-impregnated catalyst: The United States, US 4 332 672 [P], 1982
[3] Baron K, Wu A, Krenzke L. Origin and control of SOx emissions from FCC unit regenerators[M]. Am Chem Soc, Div Pet Chem Prepr; The United States, 1983
[4] Bhattacharyya A A, Woltermann G M, Yoo J S, et al. Catalytic SOx abatement: the role of magnesium aluminate spinel in the removal of SOx from fluid catalytic cracking (FCC) flue gas [J]. Industrial & Engineering Chemistry Research, 1988, 27(8): 1356-1360
[5] Centi G, Passarini N, Perathoner S, et al. Combined DeSOx/ DeNOx reactions on a copper on alumina sorbent-catalyst. 2. Kinetics of the DeSOx reaction[J]. Industrial & Engineering Chemistry Research, 1992, 31(8): 1956-1963
[6] Polato C M S, Henriques C A, Neto A A, et al. Synthesis, characterization and evaluation of CeO2/Mg, Al-mixed oxides as catalysts for SOx removal [J]. Journal of Molecular Catalysis A: Chemical, 2005, 241(1/2): 184-193
[7] Jiang Ruiyu, Shan Honghong, Li Chunyi, et al. Preparation and characterization of Mn/MgAlFe as transfer catalyst for SOx abatement [J]. Journal of Natural Gas Chemistry, 2011, 20(2): 191-197
[8] Jiang Ruiyu, Shan Honghong, Zhang Qiang, et al. The influence of surface area of De-SOx catalyst on its performance [J]. Separation and Purification Technology, 2012, 95: 144-148
[9] Pereira H B, Polato C, Monteiro J L F, et al. Mn/Mg/Alspinels as catalysts for SOx abatement: Influence of CeO2incorporation andcatalytic stability [J]. Catalysis Today, 2010, 149(3/4): 309-315
[10] Yoo J S, Bhattacharyya A, Radlowski C, et al. Advanced De-SOx catalyst: Mixed solid solution spinels with cerium oxide [J]. Applied Catalysis B: Environmental, 1992, 1: 169-189
[11] Kim G, Juskelis M V. Catalytic reduction of SO3stored in SOx transfer catalysts-A temperature programmed reaction study [J]. Studies in Surface Science and Catalysis, 1996, 101: 137-142
[12] Corma A, Palomares A, Rey F, et al. Simultaneous catalytic removal of SOx and NOx with hydrotalcite-derived mixed oxides containing copper, and their possibilities to be used in FCC units [J]. Journal of Catalysis, 1997, 170(1): 140-149
[13] Cheng W C, Kim G, Peters A, et al. Environmental fluid catalytic cracking technology [J]. Catalysis Reviews, 1998, 40(1/2): 39-79
[14] Wang J, Li C. SO2adsorption and thermal stability and reducibility of sulfates formed on the magnesium-aluminate spinel sulfur-transfer catalyst [J]. Applied Surface Science, 2000, 161(3/4): 406-416
[15] Centi G, Perathoner S. Dynamics of SO2adsorption-oxidation in SOx traps for the protection of NOx adsorbers in diesel engine emissions [J]. Catalysis Today, 2006, 112: 174-179
[16] Hernandez S, Fino D, Russo N. High performance sorbents for diesel oil desulfurization [J]. Chemical Engineering Science, 2010, 65: 603-609
[17] Chao K J, Lin L H, Ling Y C, et al. Vanadium passivation of cracking catalysts by imaging secondary ion mass spectrometry [J]. Applied Catalysis A: General, 1995, 121(2): 217-229
[18] Velu S, Shah N, Jyothi T, et al. Effect of manganese substitution on the physicochemical properties and catalytic toluene oxidation activities of Mg-Al layered double hydroxides [J]. Microporous and Mesoporous Materials, 1999, 33(1): 61-75
[19] Polato C M S, Henriques C A, Rodrigues A C C, et al. De-SOx additives based on mixed oxides derived from Mg, Al-hydrotalcite-like compounds containing Fe, Cu, Co or Cr [J]. Catalysis Today, 2008, 133: 534-540
[20] Polato C M S, Rodrigues A C C, Monteiro J L F, et al. High surface area Mn, Mg, Al-spinels as catalyst additives for SOx abatement in fluid catalytic cracking units[J]. Industrial & Engineering Chemistry Research, 2009, 49(3): 1252-1258
[21] Waqif M, Saur O, Lavalley J C, et al. Nature and mechanism of formation of sulfate specles on copper/alumina sorbent-catalysts for SO2removal [J]. The Journal of Physical Chemistry, 1991, 95(10): 4051-4058
Recieved date: 2013-09-09; Accepted date: 2014-04-20.
Jiang Ruiyu, Telephone: +86-515-88298923; E-mail: jiangry@ycit.cn.
- 中國煉油與石油化工的其它文章
- Synthesis of PE with Broad MWD Catalyzed by Supported Ziegler-Natta Catalyst Consisting of Cycloalkoxy Silane as IED
- Deep Extractive Desulfurization of Gasoline with Ionic Liquids Based on Metal Halide
- Synthesis of Macro-Mesostructured γ-Al2O3with Large Pore Volume and High Surface Area by a Facile Secondary Reforming Method
- Performance of FCC Catalyst Improved with Vanadium Trapping Components
- Catalytic Cracking of Cycloparaffins Admixed with Olefins: 2. Single-Event Microkinetic (SEMK) Assessment
- Selection of Chelated Fe (III)/Fe (II) Catalytic Oxidation Agents for Desulfurization Based on Iron Complexation Method

