WNT5A modulates cell cycle progression and contributes to the chemoresistance in pancreatic cancer cells
Tianjin,China
Introduction
Pancreatic cancer is one of the most lethal human cancers.The majority of the patients lost the chance to undergo curative resection.[1,2]Although gemcitabine is the first-line drug in the treatment of patients with advanced pancreatic cancer,the response rate is low because of the growing drug resistance.[3,4]Therefore,to clarify the molecular mechanisms of gemcitabine resistance may lead a way to increase the gemcitabine sensitivity of pancreatic cancer.
Gemcitabine is able to modulate the cell cycle process with a decrease in the G2-M-phase peak and a restoration of cells to the G1/early S-phase,which is consistent with its mechanism of action as an inhibitor of DNA elongation.[5,6]It was reported that some drugs/molecules had effects on inducing G1arrest,modulating restrictionpoint (R-point) and enhancing the gemcitabine chemosensitivity of pancreatic cancer.[7,8]Recent studies[7,9,10]also demonstrated that some molecules,such as NF-κB,PI3K/AKT and K-RAS,mediated gemcitabine resistance in tumor cells by regulating cell cycle progression,and targeting these signaling nodes that could enhance the cancer cell response to chemotherapy.
WNT5A is one of the WNT family proteins and a tumor autocrine/paracrine factor,which is highly expressed in pancreatic cancer.[11]WNT5A enhanced AKT phosphorylation is involved in the tumorigenesis of some cancers,[12-14]and the AKT activation was found in pancreatic cancer cell.[15]AKT was demonstrated to upregulate the expression of Cyclin D through promoting the transcription of c-Myc[16]and to inhibit the degradation of c-Myc's ubiquitin by blocking GSK3 activation.[17,18]Cyclin D plays a key role in promoting G1-S cell phase transition,and drives the cell towards the R-point by modulating retinoblastoma protein (pRb)-E2F complex function.The cell passes the R-point to enter S phase for implementation of DNA synthesis.The PI3K/AKT activation can phosphorylate pRb to induce pRb-E2F complex depolymerization and to activate G1-S phase transition.
Since WNT5A and p-AKT were overexpressed in pancreatic cancer and PI3K/AKT activity was found to play an important role in regulating cell cycle and contribute to drug resistance in pancreatic cancer,we hypothesized that WNT5A might enhance the G1-S phase transition by enhancing the PI3K/AKT activity and therefore,induce drug-resistance in pancreatic cancer cells.To test this hypothesis,we used immunohistochemistry to evaluate the expressions of WNT5A in pancreatic adenocarcinoma and paracarcinoma tissues.We analyzed the efficiency of gemcitabine cytotoxicity after knockdown of WNT5A in PANC-1 and MIAPaCa2 cell lines,and elucidated the mechanisms of WNT5A for cell cycle progression.
Methods
Patients and sample preparation
Fresh pancreatic adenocarcinoma and paracarcinoma tissues were obtained from 32 patients who received radical pancreatectomy for pancreatic adenocarcinoma without radiation or chemotherapy before the operation.The TNM stage was identified according to the standard of American Joint Committee on Cancer (AJCC,2010).Histologic slides were reviewed by two experienced pathologists blinded to the clinical data.The study was approved by the Human Research Committee of Nankai University and China Anti-Cancer Association (CACA)and had been performed in accordance with the Helsinki Declaration.Each pancreatic cancer surgical specimen was cut into pieces of 1 cm3sizes and promptlyfixed with 100 g/L formaldehyde solution and embedded in paraffin for further study.
Gene cloning and RNA interference
cDNA of human WNT5A (MGC:71588 IMAGE:30346200) was subcloned into the KpnI and SpeI restriction sites of the tetracyclin-inducible pBig2i expression vector,which was a gift from Dr.P.Michl(Department of Internal Medicine,Philipps University,Marburg,Germany).
Small interfering RNAs (siRNAs) targeting human WNT5A were synthesized by RiboBio (RiboBio,Guangzhou,China).Their sequences are:hWNT5A_1 siRNA:5'-GCA AGT TGG TAC AGT CAA-3',hWNT5A_2 siRNA:5'-GGT CGC TAG GTA TGA ATA A-3'.Two shRNAs targeting human WNT5A were generated and blasted using RNAi designer from Invitrogen website (https://rnaidesigner.invitrogen.com/rnaiexpress/index.jsp).The following sequences were used:shRNA1-WNT5A (5'-tcgagGCA AGT TGG TAC AGG TCA ATT CAA GAG ATT GAC CTG TAC CAA CTT GCT TTT TTa-3') and its scrambled control sequence:shRNA1-Ctl (5'-tcgagGTC AGA GAT CAC GTA GAT GTT CAA GAG ACA TCT ACG TGA TCT CTG ATT TTT Ta-3'),shRNA2-WNT5A:(5'-tcgagGGT CGC TAG GTA TGA ATA ATT CAA GAG ATT ATT CAT ACC TAG CGA CCT TTT TTa-3') and its scrambled control sequence:shRNA2-Ctl (5'-tcgagGTG GCT ATA TGA TAA ACG GTT CAA GAG ACC GTT TAT CAT ATA GCC ATT TTT Ta-3').The shRNAs were subcloned into the XhoI and HindIII restriction sites of doxycyclin (Dox)-inducible pSingletTS-shRNA expression vector (Invitrogen,Carlsbad,USA).
Cell cultures and transfection
Wild types PANC-1 and MIAPaCa2 cell lines were purchased from the Cancer Institute & Hospital of the Chinese Academy of Medical Sciences and cultured in RPMI-DMEM (GIBCO,Grand Island,NY,USA)with 10% fetal calf serum (GIBCO,Grand Island,NY,USA),containing 100 U/mL of penicillin-streptomycin(GIBCO,Grand Island,NY,USA).
Cells were transfected with the siRNA by using Lipofectamine 2000 (Invitrogen,Carlsbad,USA) according to the manufacturer's instructions.To optimize efficiency,the cells were transfected with siRNA twice with an interval of 24 hours.2×105cells were transfected with 5 μL siRNA.As non-silencing control,the silencer negative control siRNA from RiboBio was used.
WNT5A knockdown group:PANC-1 cells were transfected with pSingle-shRNA-WNT5A; WNT5A overexpression group:PANC-1 cells were transfected with pBig2i-WNT5A cDNA by using Lipofectamine 2000.For generation of stable WNT5A knockdown clones,the cells were cultured in the presence of G418 (250 μg/mL,Sigma-Aldrich,Shanghai,China) for 24 hours after transfection with pSingle-shRNA-WNT5A.Stable silencing of WNT5A was confirmed after incubation with 2 μg/mL Dox for 24 hours (Invitrogen,Carlsbad,USA).For control purposes,pSingle-shRNA-Ctl vector was transfect into PANC-1 with identical clonal selection procedures.For overexpression of WNT5A,hygromycin B (400 μg/mL,Sigma-Aldrich,Shanghai,China) was added into culture medium and Dox (5 μg/mL for 24 hours,Invitrogen,USA) was used to induce WNT5A's expression.Stable transfection of pBig2i-WNT5A vector without inducing by Dox was chosen as a control.All cell lines were grown at 37 ℃ in 5% CO2condition.
In the following experiments,recombinant human WNT5A (rhWNT5A) (Abnova,Taipei,China) was added for stimulation at the concentration of 0 ng/mL and 500 ng/mL for 24 hours when the cells were at the density of 2×105/well in the 6-well plate.The inhibitor of PI3K/AKT,ly294002 (Sigma-Aldrich,St.Louis,USA) was applied to the culture medium at thefinal concentration of 25 μmol/L for 1 hour.
Cytotoxicity assay and cell proliferation
For the cytotoxicity assay,PANC-1 cells were cultured with the same concentration of rhWNT5A and as the concentrations of gemcitabine (Lilly,Indianapolis,USA) were 0.01-1000 μg/mL for 24 hours.The cells were grown for an additional 24 hours in gemcitabinefree culture medium.The cytotoxicity of gemcitabine was examined by CCK-8 (Keygen,Nanjing,China)assay and was determined by the ratio of cell viability between gemcitabine treated and untreated groups.The 50% inhibitory concentration of cell growth (IC50) was calculated by non-linear least squares curvefitting.[19]
For the CCK-8 assay,cells were plated in 96-well plates at a density of 3×103cells per well in 100 μL culture medium with different treatment.Then 10 μL CCK-8 solution was added in each well and the cells were incubated for 90 minutes at 70 ℃.Optical density(OD) values were read at 450 nm.
Flow cytometry for cell cycle assay
The cell cycle assay was performed by propidium iodide (PI) DNA staining method (Keygen,Nanjing,China).Firstly,the supernatant of the cells was removed and attached cells (viable cells) were collected andfixed by ice-cold 70% ethanol PBS for 4 hours at 4 ℃.Then,1 ×106cells were resuspended in 1 mL PBS and incubated with 10 μL of PI (5 mg/mL) at room temperature in the dark for 30 minutes.Flow cytometry analysis was performed by an FACS Calibur cytometer (BD Biosciences,New York,USA).At least 20 000 cells were counted in each test.For BrdU-7-AAD cell cycle assay,the cells were cultured in fetal bovine serum (FBS)-free medium for 12 hours,for control group the cells were cultured for another 48 hours,for gemcitabine treatment group,the cells were cultured with gemcitabine (5 μg/mL) for 24 hours followed by another 24 hours culture in gemcitabine-free medium.For stable silencing of overexpression cell lines,Dox was given 24 hours before the BrdU "pulse" and ly294002 was given 1 hour before the BrdU "pulse" according to the experimental design.The cells were "pulsed" with 10 μmol 5-bromo-2-deoxyuridine (BrdU,Sigma-Aldrich,Shanghai,China)for 4 hours and stained with FITC-labeled anti-BrdU antibody and 7-amino-actinomycin D (7-AAD).The cytofix/cytoperm kit (BD Biosciences,New York,USA)was used to perform a cell cycle assay.
Immunohistochemistry and scoring
Paraffin sections of tumor and paracarcinoma tissues from all 32 patients were cut into 4 μm thickness,deparaffinized with xylene,and rehydrated with graded ethanol.Primary rabbit polyclonal anti-WNT5A 1:200(Abcam,Hong Kong,China) was used and the sections were incubated with biotinylated secondary antibody.The chromogen 3,3'-diaminobenzidine tetrachloride(Serva,Heidelberg,Germany) was applied as a substrate.Hematoxylin was used as the counterstain for nuclei.For the detection of AKT/p-AKT and Cyclin D1,tumor and paracarcinoma tissues from 4 patients in stage I and 4 patients in stage II were chosen randomly.Primary antibodies of rabbit monoclonal anti-AKT (1:150 Bioworld,St.Louis Park,USA),rabbit polyclonal antiphosphorylated-AKT (p-AKT,1:200,Santa Cruz,CA,USA) and mouse monoclonal anti-Cyclin D1 (1:200 Santa Cruz,CA,USA) were used.
The expression of WNT5A was scored according to the extent and intensity of the staining.The extent of positive staining was scored by the percentage of stained cells in region of interest:0 for a percentage <5%,1 for 5%-25%,2 for 25%-50%,3 for 50%-75%,and 4 for≥75%.The intensity of staining was scored as 0,1,2 and 3 for the representation of negative (no staining),mild(weak but detectable above control),moderate (distinct)and intense (strong).The percentage of stained cells and intensity of staining were multiplied to produce a weighted score.[20]The scoring was performed blindly by two independent evaluators without knowledge of the pathological and clinical characteristics.
Western blotting analysis
The protein extraction of whole cells from cell lines was prepared with lysis buffer (150 mmol/L NaCl,50 mmol/L Tris,5 mmol/L EDTA,5% glycerol,1%TritonX-100,25 mmol/L NaF,and 2 mmol/L NaVO4,pH 7.5) in the presence of phosphatase inhibitor cocktails I and II and 1× protease inhibitor cocktail (Roche,Basel,Switzerland).For proteins extracted from tissues sample,tumor and paracarcinoma tissues from 4 patients with stage I and 4 with stage II were chosen randomly.The fresh tissues were firstly cut into small pieces,per 100 mg tissue lysed with 1 mL lysis buffer (Sangon Biotech,Shanghai,China).After being homogenized manually,the lysis was centrifuged at 16 000 g.Protein concentrations were quantitated by the Bradford assay(Axygen,Tewksbury,USA).
Thirty μg of proteins were loaded and separated by 10% SDS-PAGE gels.Primary antibodies including rabbit polyclonal anti-WNT5A (Abcam,Hong Kong,China),rabbit monoclonal anti-AKT (Bioworld,St.Louis Park,USA),rabbit polyclonal anti-p-AKT (Santa Cruz,CA,USA),mouse monoclonal Cyclin D1 (Santa Cruz,CA,USA),rabbit polyclonal anti-E2F (Santa Cruz,USA),rabbit polyclonal anti-pRb (Santa Cruz,CA,USA),mouse monoclonal anti-β-actin antibody (ZSGB Biotech,Beijing,China) and horseradish peroxidaseconjugated secondary antibodies (ZSGB Biotech,Beijing,China) were used.Protein bands were stained by an ECL chemiluminescence kit (Millipore,MA,USA).The digital images were quantitated by MetaMorph software (MDS Analytical Technologies,Shanghai,China).
Real-time RT-PCR detection
Total mRNAs from PANC-1 were isolated by TRIzol (TianGen biotech,Beijing,China).cDNA was reverse-transcribed by TransScript First-Strand cDNA Synthesis (TransGen,Beijing,China).Real-time RTPCR was performed in 25 μL reaction volumes by using QuantiFast SYBR Green PCR kit (Qiagen,Valencia,CA,USA).Using standard curve method,we determined the result that the amplification efficiencies of Cyclin D1 and GAPDH were similar,so the relative mRNA folding changes were expressed by 2-ΔΔCt.The mRNA of human GAPDH was used as an internal control.Primer sequences of Cyclin D1 (forward primer 5'-GAG GAA CAG AAG TGC GAG GAG-3' and backward primer 5'-TGG AGT TGT CGG TGT AGA TGC-3') and GAPDH(forward primer 5'-TGA CGC TGG GGC TGG CAT TG-3' and backward primer 5'-GCT CTT GCT GGG GCT GGT GG-3') are used.The results were proved by three independent experiments.
Immune precipitation (IP)
1.5 mg Dynabeads Protein G (Novex,Carlsbad,USA) was incubated with 10 μg rabbit polycloal anti-E2F,rabbit polyclonal anti-pRb or preimmune serum in 200 μL PBS w/Tween 20 for 10 minutes.The supernatant was removed and the beads were washed three times with 200 μL PBS,then 1 mg fresh cells lysate was incubated with Dynabeads-Ab complex overnight at 4 ℃.After discarding the supernatant,the beads were washed by 200 μL PBS forfive times,the Dynabeads-Ab-Ag complex was heated for 10 minutes at 70 ℃.Then the supernatant of cell lysis was mixed with the SDS sample buffer and target proteins were detected with Western blotting using anti-pRb and anti-E2F.
Statistical analysis
SPSS 17.0 software (SPSS,Chicago,IL,USA) was used to perform statistical analyses.Scores for WNT5A staining between subgroups by patients' characteristics and tumor features were compared with the Kolmogorov-Smirnov test.Two-tailed Student's t test was used to assess the difference of proteins expression,IC50 values and cell percentage in cell cycle.A P value less than 0.05 was considered statistically significant.
Results
High expressions of WNT5A,p-AKT and Cyclin D1 in pancreatic cancer
The expression of WNT5A was significantly different among TNM stages (P<0.05) (Table).WNT5A,p-AKT and Cyclin D1 staining were more intensive in pancreatic cancer than in paracarcinoma tissues(Fig.1).Also intense staining of WNT5A was located in intracellular and stroma tissue,but that of p-AKT andCyclin D1 was localized only in the intracellular tissue.The protein levels of WNT5A/p-AKT/Cyclin D1 were higher in tumor tissues than those in paracarcinoma tissues (n=8) (Fig.1C).The results indicated that WNT5A/p-AKT/Cyclin D1 proteins play an important role in tumor progression of pancreatic cancer.
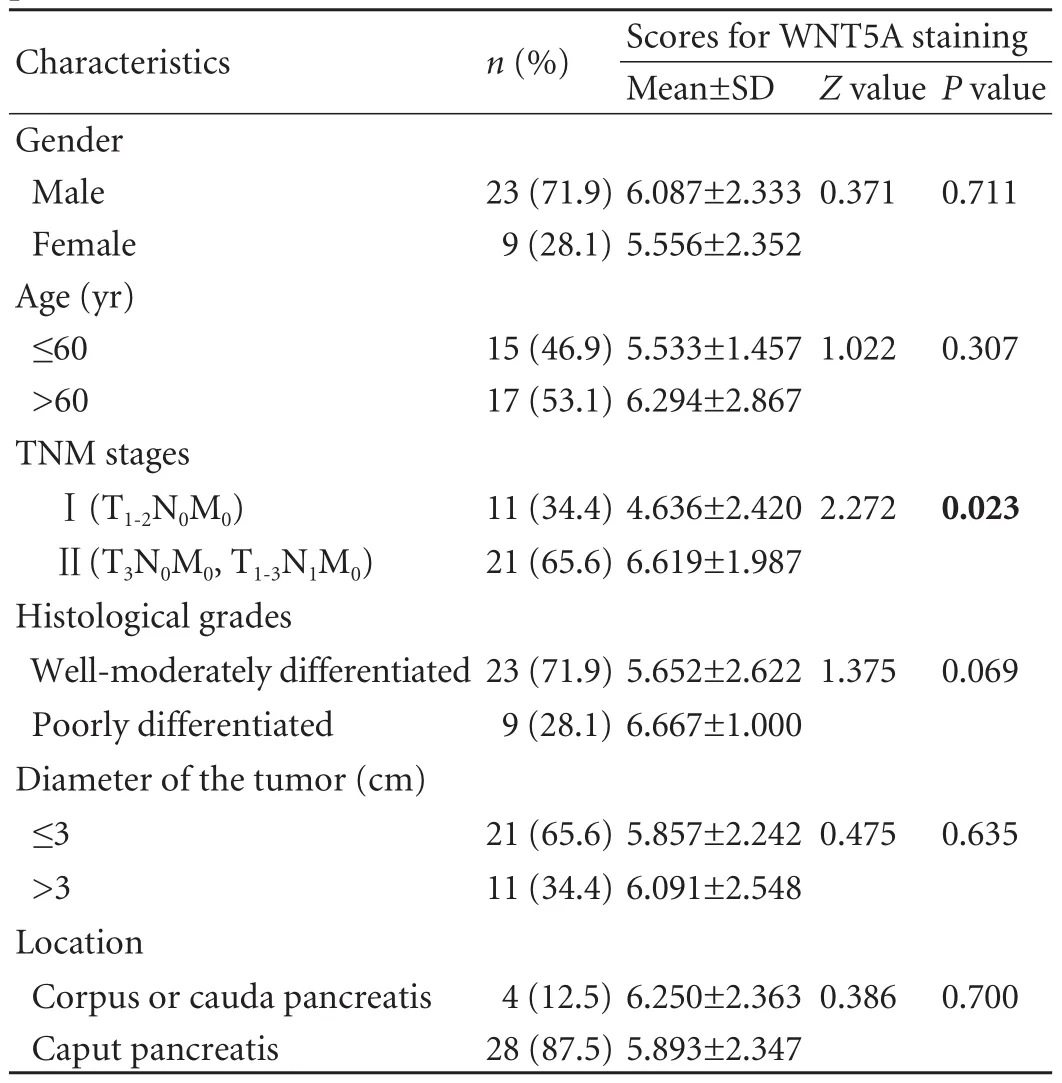
Table.The correlation between WNT5A and characteristics of patients
Gemcitabine sensitivity increased by knockdown of WNT5A protein
The knockdown efficiency of WNT5A by hWNT5A_siRNA was about 70% in PANC-1 and MIAPaCa2 cells(Fig.2A).The IC50 values for gemcitabine were about 0.35 fold-changes in PANC-1 and 0.58 fold-changes in MIAPaCa2 cells,respectively (Fig.2B).The two cell lines had similar expressions of WNT5A protein and siRNA knockdown efficiency,and the fold-change of IC50 in PANC-1 was greater after knockdown of WNT5A.Thus we selected PANC-1 to assess the cell sensitivity to gemcitabine.
Gemcitabine sensitivity decreased by WNT5A via Cyclin D1 activation and AKT phosphorylation

Fig.1.The expression of WNT5A/AKT/Cyclin D1 proteins in pancreatic adenocarcinoma and paracarcinoma tissues.Scatter plot evaluated the scores of WNT5A protein expressed in pancreatic adenocarcinoma and paracarcinoma tissues (A); Immunohistochemical staining of AKT,p-AKT,Cyclin D1 (n=8) and WNT5A proteins (n=32) (all brown) (original magnification ×40) (B); Western blotting results of WNT5A,AKT,p-AKT and Cyclin D1 (n=8) (C).*:P<0.05.

Fig.2.Knockdown of WNT5A enhance gemcitabine sensitivity in PANC-1 and MIAPaCa2.Knockdown efficiency of WNT5A in PANC-1 and MIAPaCa2 by transiently transfected with hWNT5A_1 siRNA (n=3) (A).Cytotoxicity effect of gemcitabine in PANC-1 and MIAPaCa2 with knockdown of WNT5A (n=3) (B).*:P<0.05.NS:no significance.

Fig.3.WNT5A's effects on chemoresistance and cell cycle in pancreatic cancer cells.Western blotting results of WNT5A after stable interference or overexpression of WNT5A (n=3) (A).Cytotoxicity effect of gemcitabine in PANC-1 with knockdown and overexpression of WNT5A (n=3) (B).WNT5A's effect on cell cycle (C):Schematic diagram for FACS results of cell cycle analysis (C1); Representative FACS analysis results of PANC-1 stained with BrdU-FITC and 7-AAD (C2); Statistical results (n=3) (C3).Real-time quantitative PCR results of Cyclin D1 and the productions of real-time RT-PCR (n=3) (D).*:P<0.05.
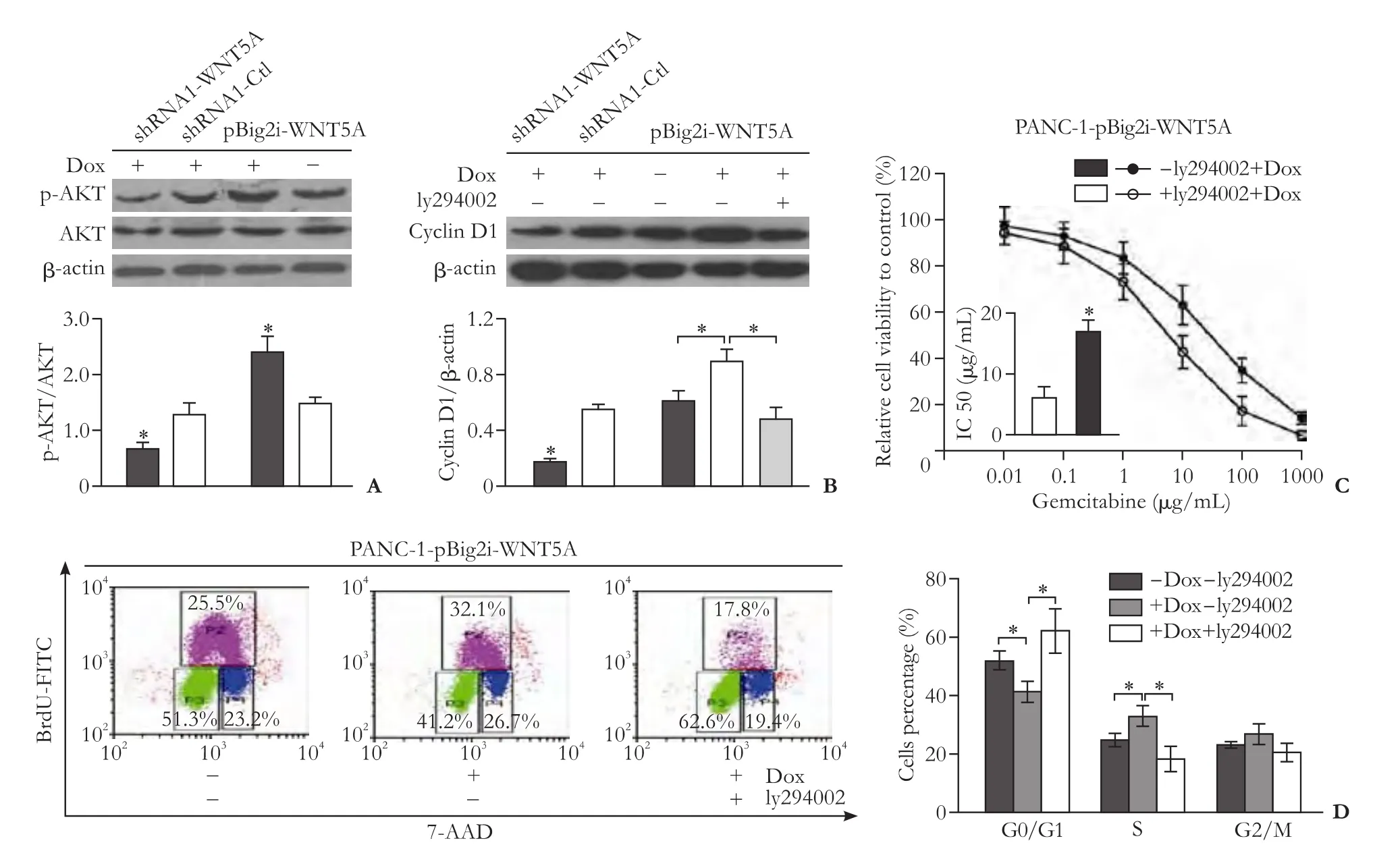
Fig.4.WNT5A modulates Cyclin D1 protein expression via regulation of p-AKT.Western blotting results of AKT and p-AKT in PANC-1 after knockdown or overexpression of WNT5A (n=3) (A); Western blotting results of Cyclin D1 in PANC-1 after knockdown and overexpression of WNT5A or inhibition of AKT with ly294002 (n=3) (B); Cytotoxicity effect of gemcitabine in PANC-1 after overexpression of WNT5A with or without treatment of ly294002 (n=3) (C); FACS analysis results of PANC-1-pBig2i-WNT5A with or without treatment of ly294002 (D).*:P<0.05.
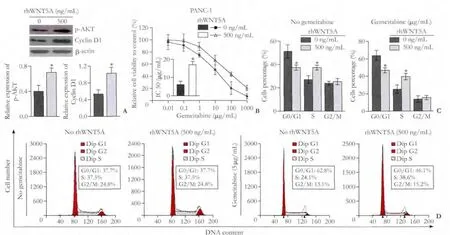
Fig.5.rhWNT5A's effect on cell cycle transition and chemoresistance in pancreatic cancer cells.Western blotting results of p-AKT and Cyclin D1 in PANC-1 with or without treatment of rhWNT5A (n=3) (A).Cytotoxicity effect of gemcitabine in PANC-1 with rhWNT5A at the concentration of 0 ng/mL and 500 ng/mL (n=3) (B).G0/G1 phase rate and S phase rate (n=3) (C).Flow cytometry cell cycle analyses of PANC-1 being treated by rhWNT5A additions with treatment of gemcitabine (5 μg/mL) or without gemcitabine (D).*:P<0.05.
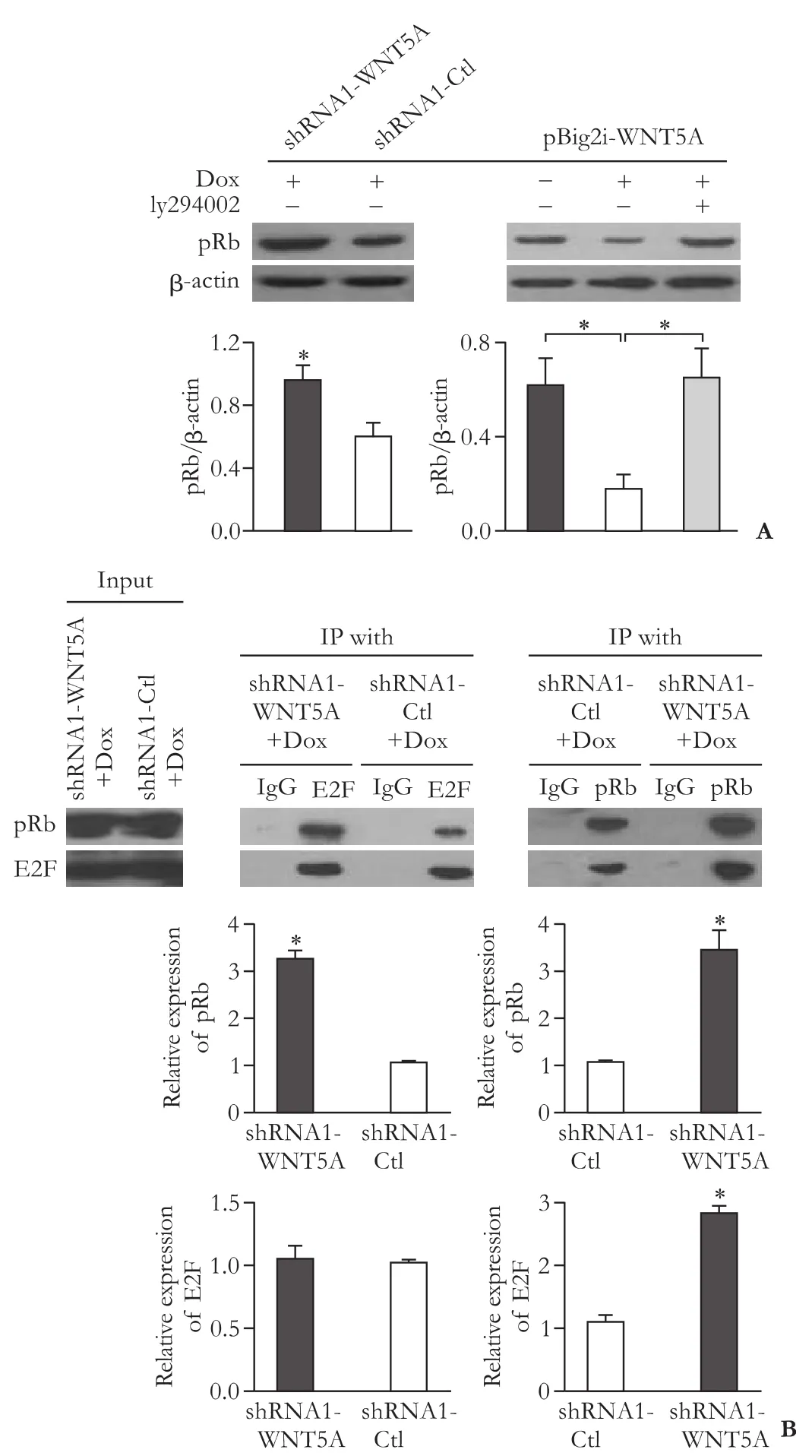
Fig.6.WNT5A enhanced cell G1/S phase transition.Western blotting of pRb when WNT5A was knocked down,overexpressed with or without inhibition of AKT (n=3) (A); Effects of WNT5A on regulating pRb-E2F complex.Fresh cells lysate of PANC-1-shRNA1-WNT5A immunoprecipitated with E2F or pRb (B).
The silence efficiency of WNT5A in PANC-1-shRNAWNT5A was about 60%.The expression of WNT5A was about 2 folds higher in PANC-1-pBig2i-WNT5A with Dox than in that without Dox (Fig.3A).The sensitivity to gemcitabine in PANC-1 was enhanced after downregulation of WNT5A.Vice versa,overexpression of WNT5A resulted in increasing chemoresistance (Fig.3B).The cell number in G0/G1 phase was increased and in S phase was decreased when WNT5A was knocked down (Fig.3C).
Real-time RT-PCR showed that WNT5A increased the transcription of Cyclin D1 (Fig.3D),and WNT5A activated AKT (Fig.4A).When AKT was inhibited,the level of Cyclin D1 transcription was decreased as well (Fig.4B).Cell cycle analysis found that the cell percentage was decreased in G0/G1 phase and increased in S phase when WNT5A was overexpressed.Inhibition of AKT blocked the process of G1-S transition (Fig.4D),and contributed to the chemotherapy sensitization in PANC-1 (Fig.4C).Moreover,the exogenous rhWNT5A induced gemcitabine resistance.rhWNT5A increased AKT phosphorylation,Cyclin D1 expression (Fig.5A)and gemcitabine resistance (Fig.5B).rhWNT5A (500 ng/mL) reduced the cell percentage in G0/G1 phase and increased that in S phase after treatment (Fig.5C,D).
WNT5A regulating pRb and driving cells toward R-point
As shown in Fig.6A,the level of phosphorylated Rb was increased after inhibition of WNT5A and decreased after the overexpression of WNT5A,which could be rescued by blocking of AKT.Using IP test to check the stability of pRb-E2F complex,we found the protein level of pRb in shRNA1-WNT5A was higher after IP with anti-E2F,which indicates that knockdown of WNT5A can inhibit the depolymerization of pRb-E2F (Fig.6B).
Discussion
The present study demonstrated that WNT5A was overexpressed in most of the pancreatic cancer tissues and WNT5A expression was correlated with the TNM stage,WNT5A might serve as a prognostic marker of pancreatic cancer and a marker to distinguish tumor from non-tumor tissues.It was reported that WNT5A plays a pathophysiological role in tumorigenesis and tumor progression.However,whether WNT5A is a tumor suppressor or promoter remains controversial.[21,22]One thing is clear that the overexpression of WNT5A protein could serve as a diagnostic marker of pancreatic cancer.In addition,it has been widely accepted that a more advanced cancer stage had a poor chemotherapy result for solid tumors.[23,24]Hence,as a secretory protein,WNT5A expression might indicate the chemoresistance response and tumor development.
We further revealed WNT5A effect on modulating cell cycle progression and mediating gemcitabine resistant.Previous studies[25,26]reported that some drugs/molecules/proteins display the anticancer effect on regulation of G0/G1 phase cell cycle and G0/G1 arrest implied chemoresistance in certain types of tumor.[27,28]As first-line chemotherapy for pancreatic cancer,gemcitabine mediates a G1 phase prolongation to conduct cytotoxic activity.[29,30]In the present study,after knockdown of WNT5A,the G0/G1 phase cell rate increased and the S phase rate decreased,which enhance the chemosensitivity of gemicitabine in pancreatic cancer cells.A recent study[31]reported that WNT5A contributed to the drug-resistance by enhancing anti-apoptosis ability in pancreatic cancer cells.Because gemcitabine was a cell cycle specific drug,however,our gemcitabine cytotoxicity assay did not show that WNT5A was involved in cell proliferation.We also found that WNT5A mediated gemcitabine chemoresistance was via the regulation of cell cycle.Consequently,we demonstrated that WNT5A might be a special predictor of gemcitabine chemoresistance and an effective target for chemotherapeutic response in pancreatic cancer.
Moreover,we proved that WNT5A enhanced G1-S transition in pancreatic cancer through activation of PI3K/AKT/Cyclin D1 signals and WNT5A induced the drug resistance.Previous reports[16-18]suggested that AKT was activated by WNT5A in some tumors including gastric cancer,dermalfibroblast and colon cancer,[12-14]and AKT promoted Cyclin D expression.Cyclin D-CDK phosphorylated pRb decreased the Rb protein and increased the depolymerization of pRb-E2F complex.Freed E2F protein enabled cells transfer from G1 to S phase.[32]Some studies[33,34]showed the linkage of drugresistance and R-point.Our study found that AKT activation was significantly inhibited after WNT5A knockdown,and that inhibition of AKT downregulated Cyclin D1 expression,stabilized the pRb-E2F complex,and induced G0/G1 arrest,thus contributing to gemcitabine sensitization.We also found that WNT5A activated AKT might play an important role in chemoresistance of gemcitabine.We assumed that AKT inhibitor could increase the gemcitabine sensitization of pancreatic cancer with highly expressed WNT5A protein.
In conclusion,chemoresistance induced by WNT5A is due to the regulation of cell cycle in pancreatic cancer.WNT5A could serve as a predictor of gemcitabine response and potentially as a target for chemotherapy of pancreatic cancer.
Contributors:SXH proposed the study.WW performed the research and wrote the first draft.WW and SHH analyzed the experimental data.LN,LHY and LX collected the data.LQ provided important advice.All authors contributed to the design and interpretation of the study and to further drafts.SXH is the guarantor.
Funding:This study was supported by a grant from Tianjin Natural Science Foundation (13JCZDJC31300).
Ethical approval:The study was approved by the Human Research Committee of Nankai University and China Anti-Cancer Association (CACA) and had been performed in accordance with the Helsinki Declaration.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Siegel R,Naishadham D,Jemal A.Cancer statistics,2013.CA Cancer J Clin 2013;63:11-30.
2 Raimondi S,Maisonneuve P,Lowenfels AB.Epidemiology of pancreatic cancer:an overview.Nat Rev Gastroenterol Hepatol 2009;6:699-708.
3 Stathis A,Moore MJ.Advanced pancreatic carcinoma:current treatment and future challenges.Nat Rev Clin Oncol 2010;7:163-172.
4 Arends JJ,Sleeboom HP,Leys MB,Ten Bokkel Huinink D,de Jong RS,Smit JM,et al.A phase II study of raltitrexed and gemcitabine in patients with advanced pancreatic carcinoma.Br J Cancer 2005;92:445-448.
5 Longley DB,Harkin DP,Johnston PG.5- fluorouracil:mechanisms of action and clinical strategies.Nat Rev Cancer 2003;3:330-338.
6 Ng SSW,Tsao MS,Chow S,Hedley DW.Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells.Cancer Res 2000;60:5451-5455.
7 Arora S,Bhardwaj A,Srivastava SK,Singh S,McClellan S,Wang B,et al.Honokiol arrests cell cycle,induces apoptosis,and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells.PLoS One 2011;6:e21573.
8 Wang Y,Zhou Y,Zhou H,Jia G,Liu J,Han B,et al.Pristimerin causes G1 arrest,induces apoptosis,and enhances the chemosensitivity to gemcitabine in pancreatic cancer cells.PLoS One 2012;7:e43826.
9 Wong HH,Lemoine NR.Pancreatic cancer:molecular pathogenesis and new therapeutic targets.Nat Rev Gastroenterol Hepatol 2009;6:412-422.
10 Motoshige H,Oyama K,Takahashi K,Sakurai K.Involvement of phosphatidylinositol 3-kinase/Akt pathway in gemcitabine-induced apoptosis-like cell death in insulinoma cell line INS-1.Biol Pharm Bull 2012;35:1932-1940.
11 Ripka S,K?nig A,Buchholz M,Wagner M,Sipos B,Kl?ppel G,et al.WNT5A--target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer.Carcinogenesis 2007;28:1178-1187.
12 Liu J,Zhang Y,Xu R,Du J,Hu Z,Yang L,et al.PI3K/Aktdependent phosphorylation of GSK3β and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration.Cell Signal 2013;25:447-456.
13 Kawasaki A,Torii K,Yamashita Y,Nishizawa K,Kanekura K,Katada M,et al.Wnt5a promotes adhesion of human dermalfibroblasts by triggering a phosphatidylinositol-3 kinase/Akt signal.Cell Signal 2007;19:2498-2506.
14 Kumar A,Pandurangan AK,Lu F,Fyrst H,Zhang M,Byun HS,et al.Chemopreventive sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3β pathway in colon cancer.Carcinogenesis 2012;33:1726-1735.
15 Schlieman MG,Fahy BN,Ramsamooj R,Beckett L,Bold RJ.Incidence,mechanism and prognostic value of activated AKT in pancreas cancer.Br J Cancer 2003;89:2110-2115.
16 Ahmed NN,Grimes HL,Bellacosa A,Chan TO,Tsichlis PN.Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase.Proc Natl Acad Sci U S A 1997;94:3627-3632.
17 Sears R,Nuckolls F,Haura E,Taya Y,Tamai K,Nevins JR.Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability.Genes Dev 2000;14:2501-2514.
18 Alt JR,Cleveland JL,Hannink M,Diehl JA.Phosphorylationdependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation.Genes Dev 2000;14:3102-3114.
19 Giovannetti E,Mey V,Danesi R,Mosca I,Del Tacca M.Synergistic cytotoxicity and pharmacogenetics of gemcitabine and pemetrexed combination in pancreatic cancer cell lines.Clin Cancer Res 2004;10:2936-2943.
20 Lu CD,Altieri DC,Tanigawa N.Expression of a novel antiapoptosis gene,survivin,correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas.Cancer Res 1998;58:1808-1812.
21 Dejmek J,Leandersson K,Manjer J,Bjartell A,Emdin SO,Vogel WF,et al.Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival.Clin Cancer Res 2005;11:520-528.
22 Fernandez-Cobo M,Zammarchi F,Mandeli J,Holland JF,Pogo BG.Expression of Wnt5A and Wnt10B in nonimmortalized breast cancer cells.Oncol Rep 2007;17:903-907.
23 Tobe SW,Noble-Topham SE,Andrulis IL,Hartwick RW,Skorecki KL,Warner E.Expression of the multiple drug resistance gene in human renal cell carcinoma depends on tumor histology,grade,and stage.Clin Cancer Res 1995;1:1611-1615.
24 Wilson TR,Longley DB,Johnston PG.Chemoresistance in solid tumours.Ann Oncol 2006;17:x315-324.
25 Qin R,Chen Z,Ding Y,Hao J,Hu J,Guo F.Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis.Neoplasma 2013;60:486-492.
26 Zhou X,Zheng M,Chen F,Zhu Y,Yong W,Lin H,et al.Gefitinib inhibits the proliferation of pancreatic cancer cells via cell cycle arrest.Anat Rec (Hoboken) 2009;292:1122-1127.
27 Zhang M,Wang J,Yao R,Wang L.Small interfering RNA (siRNA)-mediated silencing of the M2 subunit of ribonucleotide reductase:a novel therapeutic strategy in ovarian cancer.Int J Gynecol Cancer 2013;23:659-666.
28 Bu HQ,Luo J,Chen H,Zhang JH,Li HH,Guo HC,et al.Oridonin enhances antitumor activity of gemcitabine in pancreatic cancer through MAPK-p38 signaling pathway.Int J Oncol 2012;41:949-958.
29 Matsumoto K,Nagahara T,Okano J,Murawaki Y.The growth inhibition of hepatocellular and cholangiocellular carcinoma cells by gemcitabine and the roles of extracellular signal-regulated and checkpoint kinases.Oncol Rep 2008;20:863-872.
30 Yoshino K,Hiramatsu K,Enomoto T,Fujita M,Ueda Y,Kimura T,et al.Salvage chemotherapy using gemcitabine for taxane/platinum-resistant recurrent ovarian cancer:a single institutional experience.Anticancer Res 2012;32:4029-4033.
31 Griesmann H,Ripka S,Pralle M,Ellenrieder V,Baumgart S,Buchholz M,et al.WNT5A-NFAT signaling mediates resistance to apoptosis in pancreatic cancer.Neoplasia 2013;15:11-22.
32 Fan J,Bertino JR.Functional roles of E2F in cell cycle regulation.Oncogene 1997;14:1191-1200.
33 Xu H,Cheung IY,Wei XX,Tran H,Gao X,Cheung NK.Checkpoint kinase inhibitor synergizes with DNA-damaging agents in G1 checkpoint-defective neuroblastoma.Int J Cancer 2011;129:1953-1962.
34 Meinel FG,Mandl-Weber S,Baumann P,Leban J,Schmidmaier R.The novel,proteasome-independent NF-kappaB inhibitor V1810 induces apoptosis and cell cycle arrest in multiple myeloma and overcomes NF-kappaB-mediated drug resistance.Mol Cancer Ther 2010;9:300-310.
 Hepatobiliary & Pancreatic Diseases International2014年5期
Hepatobiliary & Pancreatic Diseases International2014年5期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Non-operative management of isolated liver trauma
- Lymphoepithelial cysts of the pancreas:a management dilemma
- Prognostic significance of epidermal growth factor-like domain 7 in pancreatic cancer
- Post-pancreaticoduodenectomy hemorrhage:risk factors,managements and outcomes
- Laparoscopic liver resection under hemihepatic vascular in flow occlusion using the lowering of hilar plate approach
- Long-term results of liver transplantation for over 60 years old patients with hepatitis B virus-related end-stage liver disease
