Identification of Five Sea Cucumber Species Through PCR-RFLP Analysis
LV Yingchun, ZHENG Rong, ZUO Tao, WANG Yuming, LI Zhaojie, XUE Yong, XUE Changhu, and TANG Qingjuan
College of Food Science and Engineering, Ocean University of China, Qingdao 266003, P. R. China
Identification of Five Sea Cucumber Species Through PCR-RFLP Analysis
LV Yingchun#, ZHENG Rong#, ZUO Tao, WANG Yuming, LI Zhaojie, XUE Yong, XUE Changhu, and TANG Qingjuan*
College of Food Science and Engineering, Ocean University of China, Qingdao 266003, P. R. China
Sea cucumbers are traditional marine food and Chinese medicine in Asia. The rapid expansion of sea cucumber market has resulted in various problems, such as commercial fraud and mislabeling. Conventionally, sea cucumber species could be distinguished by their morphological and anatomical characteristics; however, their identification becomes difficult when they are processed. The aim of this study was to develop a new convenient method of identifying and distinguishing sea cucumber species. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis of mitochondrial cytochrome oxidase I gene (COI) was used to identifing five sea cucumber species (Apostichopus japonicus, Cucumaria frondosa, Thelenota ananas, Parastichopus californicus and Actinopyga lecanora). A 692 bp fragment of COI was searched for BamHI, KpnI, PstI, XbaI and Eco31I restriction sites with DNAMAN 6.0, which were then used to PCR-RFLP analysis. These five sea cucumber species can be discriminated from mixed sea cucumbers. The developed PCR-RFLP assay will facilitate the identification of sea cucumbers, making their source tracing and quality controlling feasible.
sea cucumber; mtDNA; COI gene; species identification; PCR-RFLP
1 Introduction
Sea cucumber (Echinodermata: Holothuroidea), a traditional seafood in Asia, has recently become an expensive tonic food. Because of its various biological activities including anti-tumor, immunoregulatory, anti-atherosclerotic and anti-aging property, sea cucumber are believed to have high nutritional and medicinal value (Hirata et al., 2005). In the past two decades, sea cucumber have become one of the most valuable seafoods in Asian countries (FAO, 2008). Currently, there are several deepprocessing products, such as dried and canned sea cucumber and sea cucumber capsules. With the rapid expansion and intensification of sea cucumber market, a series of commercial frauds appeared, which included mislabeling and substitution of high-value species with low-value ones. The rapid identification of sea cucumber species is important for maintaining sea cucumber quality and protecting the right of the consumers.
About 1400 species of sea cucumber around the world belong to 25 families, 6 orders (Smiley, 1994). More than 140 species of sea cucumber have been described in China Sea, of them approximately 20 are believed to have high commercial value as food (Chen, 2003). The traditional methods of identifying sea cucumber to family or order generally based on their morphological characteristics, such as tentacles, tentacular retractor muscles, internal respiratory trees, trunk podia, tube feet, esophageal calcareous rings and dermal ossicles. However, to species, the difference in morphological characteristics is usually too subtle to be used (Arndt et al., 1996). As the ossicles of body walls, the main characteristics of a species, are highly variable, and the composition of body wall spicules are not applicable to the identification of sea cucumber species (Levin and Gudimova, 1997; Gudimova, 1991; Toral-Granda, 2005). In addition, visual authentication of sea cucumbers is often difficult as most of morphological characteristics will disappear during processing, particularly when sea cucumber are pulverized or cut into pieces. An alternative of sea cucumber identification is species-specific chemical constitution. Kalinin et al. (1994) have successfully applied the distribution of different triterpene glycosides to the taxonomy of sea cucumber in order Aspidochirotida. With glycoside distribution and morphological characteristics, Levin et al. (1985, 1986) described the taxonomic relationship between the North Pacific representatives of family Stichopodidae and order Aspidochirotida. However, this chemical approach is not effective for deeply processed sea cucumber, because it requires the purification of species-specificchemical components, which is complicate and difficult when sea cucumber is processed.
In order to avoid the difficulty of species identification on morphological characteristics and species-specific chemical constitution, diverse methods of identifying marine species through DNA analysis have been reported. These methods included PCR and sequencing, forensically informative nucleotide sequencing (FINS), rapid analysis of polymorphic DNA (RAPD), single-stranded conformation polymorphism and restriction fragment length polymorphism (RFLP) (Lockley and Bardsley, 2000; Comi et al., 2005; Gil, 2007; Aranceta et al., 2011; Espi?eira et al., 2009; Botti and Giuffra, 2010). Of these methods, direct sequencing of PCR products and FINs require expensive equipments and agents. RAPD is complex in bands profile and poor in reproducibility (Mochizuki et al., 1997; Kac, 2000). RFLP is rapid and simple, which has been extensively used to identifying diverse fish species (Di Finizio et al., 2007; Hsieh et al., 2007; Rea et al., 2009; Chuang et al., 2012). The sequence divergence of mitochondrial cytochrome oxidase I gene (COI) can be used to identifing closely related species of most animals (Hebert et al., 2003). Actualy, it has been used to identifying sea cucumber species (Arndt et al., 1996; Zuo et al., 2012). Therefore, the aim of this study was to identify and distinguish sea cucumber species through PCR-RFLP analysis of COI.
2 Materials and Methods
2.1 Materials
Fresh sea cucumber individuals of Apostichopus japonicus, Cucumaria frondosa, Thelenota ananas, Parastichopus californicus and Actinopyga lecanora were purchased from a retail market in Qingdao, China. Species identification was initially performed according to their taxonomic characteristics of dermal ossicles (Liao, 1997; Massin, 1999), and subsequently confirmed by DNA sequence analysis of COI by referring to the deposited in GenBank. After identification, the muscle tissue of these sea cucumber species each was sampled and preserved in ethanol. These sea cucumber species are commonly consumed and easily available in market. Dried and frozen sea cucumber samples were collected from local supermarket and retail market located in the same area, and treated as were done for the fresh sea cucumber individuals.
2.2 DNA Isolation
The genomic DNA was isolated from 100 mg of muscle with modified CTAB method (Grewe et al., 1993). The DNA concentration was measured on a UV-2550 spectrophotometer (Shimadzu, Japan). DNA was stored at -20℃.
2.3 COI Gene Amplification
To amplify the 692 bp fragment of COI, a pair of primers, COIef, 5’-ATA ATG ATA GGA GGR TTT GG-3’ and COIer, 5’-GCT CGT GTR TCT ACR TCC AT-3’ (Arndt et al., 1996) was used. On a MJ Mini Personal Thermal Cycler (BIO-RAD, USA), PCR was performed in a volume of 50 μL containing 100 ng DNA, 5 μL of 10× PCR buffer, 1 μL of dNTP (10 mmol L-1), 3 μL of MgCl2(25 mmol L-1), 1 μL of each primer (10 μmol L-1), and 1 μL of 5 U μL-1of Taq DNA polymerase (TaKaRa, Japan). The reaction was thermocycled by denaturing at 94℃ 5 min, followed by 30 cycles at denaturing at 94℃ for 50 s, annealing at 46℃ for 1 min, and extending at 72℃ for 1 min, and a extra extension at 72℃ for 10 min. The PCR product was analyzed through electrophoresis in 1% agarose gel, and purified with an AxyGenTMDNA gel extraction kit (Beijing, China). The purified DNA was cloned into a pUCm-T vector (BBI, Sangon, China) following manufacturer’s procedure, and sequenced by Sangon Biotech Co., Ltd. (Shanghai, China).
2.4 RFLP Analysis
The 692 bp fragment of five sea cucumbers was analyzed using DNAStar (version 6.1; DNASTAR Inc., Madison, WI) and DNAMAN (version 6.0; Lynnon Biosoft, Quebec, Canada) software to detect the restriction sites suitable for the characterization of these species.
Five restriction endonucleases, BamHI, KpnI, PstI, XbaI and Eco31I (Fermentas, MBI, USA), were chosen for RFLP analysis. A 30 μL reaction mixture containing 10 μL of PCR product, 1 FDU of each enzyme and 2 μL of 10× Fast Digest Universal Buffer (Fermentas, MBI, USA) was incubated at 37℃ for 15 min. The restriction fragments were separated in 10% native-polyacrylamide gel electrophoresis (PAGE) at 100 V for 2 h with BIO-RAD PowerPac Universal (USA). The length of fragments was determined by referring to a DL2000 marker (TaKaRa, Japan). The gel was visualized under UV light and photographed using Tanon GIS-2008 (Shanghai, China). The analysis was performed at least 3 times for each species.
To validate species-specific PCR-RFLP assay, two species were discretionarily chosen from the five reference sea cucumber species to prepare ten mixtures of the same amount of DNA. Then, PCR-RFLP was analyzed in order to distinguish each of them.
3 Results and Discussion
The mitochondrial DNA (mtDNA) inherits maternally, which acts independently of nuclear DNA (nDNA) (Taanman, 1999). Compared with nDNA, mtDNA is devoid of introns, pseudogenes, repetitive sequences, and recombination sites that are generally associated with sexual processes (Avise et al., 1987; Linacre and Tobe, 2011). The sequence of mtDNA is more conservative than that of nDNA (Rokas et al., 2003). The base substitution rate in mtDNA is higher than that in nDNA; thus, mutations can arise in a population more rapidly (Brown et al., 1979; Cawthorn et al., 2012). Accordingly, several mtDNA genes have been commonly used as genetic markers for species identification, which included COI,16S rRNA, 18S rRNA, and Cytb (Joshi et al., 2004; Chen et al., 2005; Naderi et al., 2007). With BamHI, KpnI, PstI, XbaI and Eco31I, the 692 bp fragment of mitochondrial COI was digested, yielding RFLP among sea cucumber species. Five sea cucumber species, A. japonicus, C. frondosa, T. ananas, P. californicus, and A. lecanora, were easily identifiable with the RFLP yielded.
3.1 Use of Mitochondrial COI for Species Identification of Sea Cucumbers
In this study, COI of five sea cucumber species was amplified, yielding a 690 bp fragment as expected (Fig.1). The 690 bp COI from five sea cucumber species fully matched that deposited in GenBank with high similarities ranging from 98% to 100%.
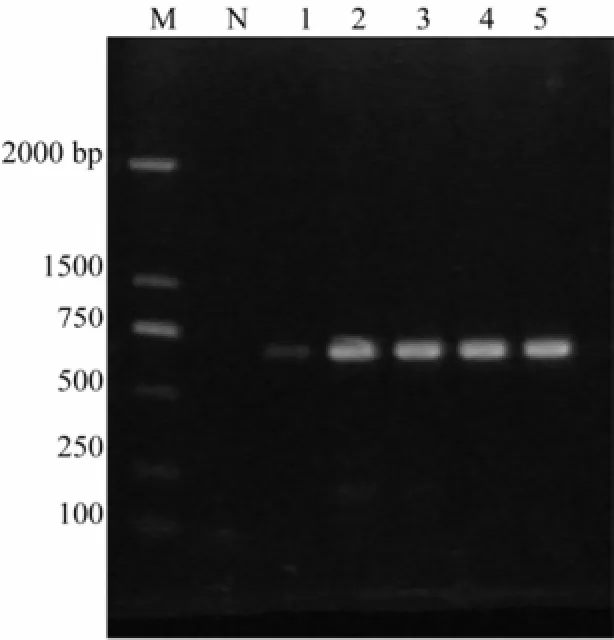
Fig.1 PCR product of COI of five sea cucumber species. 1% agarose gel; M, DL2000 Marker (Takala); N, negative control; 1, Apostichopus japonicas; 2, Cucumaria frondosa; 3, Thelenota ananas; 4, Parastichopus californicus; 5, Actinopyga lecanora.
3.2 Species Identification by PCR-RFLP of COI
PCR-RFLP analysis is a well-established technique for rapid identification of species in food science (Lin and Hwang, 2007). After amplification and sequencing, the sequences were analyzed using DNAMAN 6.0 software. According to the restriction map of the sequences, BamHI, KpnI, PstI, XbaI and Eco31I were selected to differentiate each sea cucumber species. The restriction sites and the sizes of the fragments cleaved by each restriction enzyme were listed in Table 1.
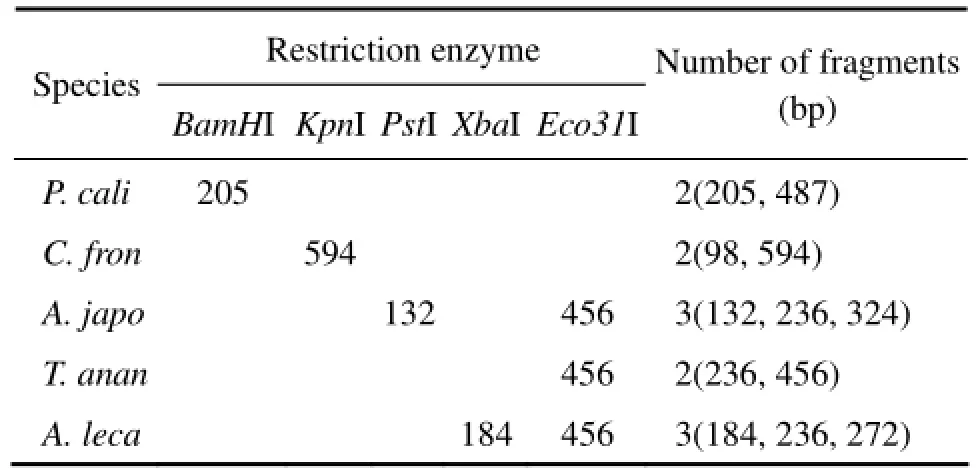
Table 1 Positions of digestion sites of selected endonucleases on the amplified 692 bp fragment of cytochrome oxidase subunit I gene and restriction fragment length in tested sea cucumbers
The length of these restriction fragments was different from each other and the minimum length difference between them was more than 30 bp which can be easily resolved in PAGE. However, DNA fragments less than 50 bp were not applicable to the identification because these short fragments might be primer dimmers and is difficult to visualize. Thus, to avoid the generation of ambiguous fragments, an endonuclease was selected if its restriction fragments were all more than 100 bp in length.
RFLP analysis revealed that five sea cucumber species examined in this study could be distinguished using the restriction enzymes selected (Fig.2). Digesting with BamHI generated two specific restriction fragments for P. californicus (205 and 487 bp). Digesting with KpnI generated two specific fragments for C. frondosa (98 and 594 bp). Digesting with PstI and Eco31I generated three specific fragments for A. japonicus (132, 236, and 324 bp). Digesting with Eco31I generated two specific fragments for T. ananas (236 and 456 bp). A common restriction pattern (184, 236, and 272 bp) was observed in A. lecanora when its DNA was digested with XbaI and Eco31I.
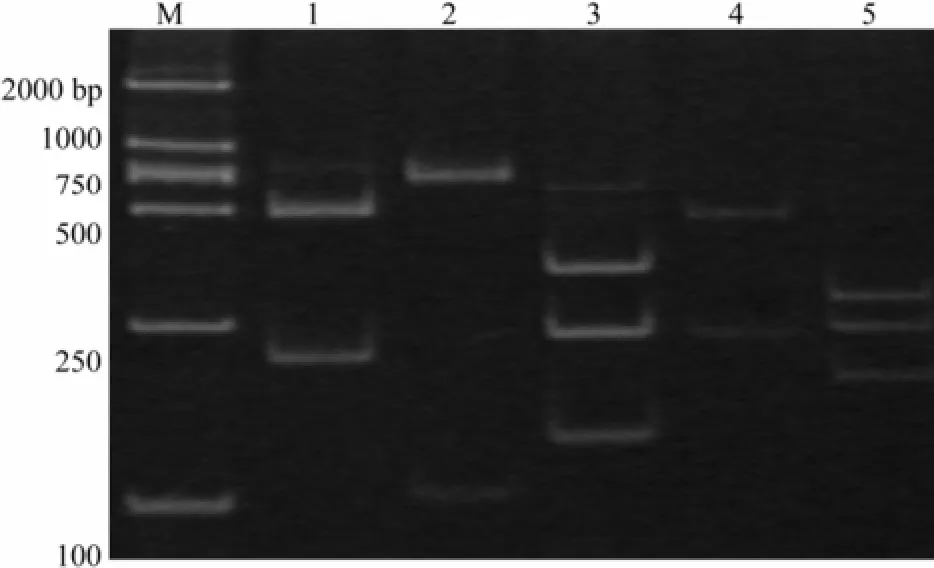
Fig.2 Restriction fragments generated through BamHI, KpnI, PstI, XbaI and Eco31I digestion and 10% native-PAGE. M, DL2000 Marker (Takala); 1, Parastichopus californicus; 2, Cucumaria frondosa; 3, Apostichopus japonicas; 4, Thelenota ananas; 5, Actinopyga lecanora.
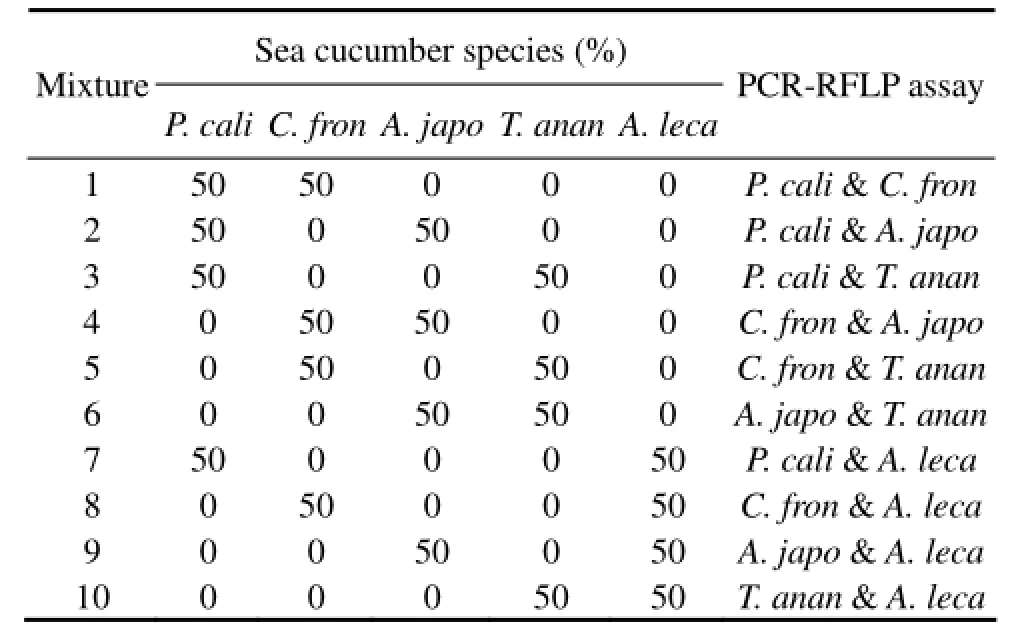
Table 2 Species identification of the artificial mixtures prepared with reference sea cucumbers through PCR-RFLP assay
PCR-RFLP analysis has the advantage of clearly de-tecting and identifying the target species in mixed products. To assess the capability of the PCR-RFLP assay in simultaneously detecting various species in one sample, artificially generated mixtures containing the same amounts of COI gene from two sea cucumber species discretionarily chosen from the five reference sea cucumbers were analyzed. Our results showed that the two sea cucumber species were simultaneously detected in the ten mixtures (Fig.3 and Table 2), indicating that PCRRFLP analysis can effectively identify at least two sea cucumber species in one sample.
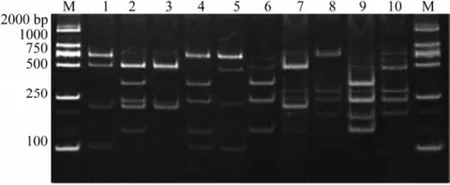
Fig.3 Species identification through PCR-RFLP analysis. M, DL2000 Marker (Takala); 1, P. cali & C. fron; 2, P. cali & A. japo; 3, P. cali & T. anan; 4, C. fron & A. japo; 5, C. fron & T. anan; 6, A. japo & T. anan; 7, P. cali & A. leca; 8, C. fron & A. leca; 9, A. japo & A. leca; 10, T. anan & A. leca. P. cali, Parastichopus californicus; C. fron, Cucumaria frondosa; A. japo, Apostichopus japonicus; T. anan, Thelenota ananas; A. leca, Actinopyga lecanora.
In conclusion, PCR-RFLP analysis developed in this study is a reliable, simple and rapid method for the unambiguous identification of sea cucumber species. With appropriate restriction enzymes, this method is applicable to identifying a wider range of sea cucumber species. It will serve as a useful tool for quality control and tracking sea cucumber products, preventing commercial fraud.
Acknowledgements
This research was supported by National Natural Science Foundation of China (Nos. 31101281 and 31071525) and National Marine Public Welfare Scientific Research Project of China (No. 201105029).
Aranceta-G, F., Perez-E, R., and Cruz, P., 2011. PCR-SSCP method for genetic differentiation of canned abalone and commercial gastropods in the Mexican retail market. Food Control, 22: 1015-1020.
Arndt, A., Marquez, C., Lambert, P., and Smith, M. J., 1996. Molecular phylogeny of eastern Pacific sea cucumbers (Echinodermata: Holothuroidea) based on mitochondrial DNA sequence. Molecular Phylogenetics and Evolution, 6 (3): 425-437.
Avise, J. C., Arnold, J., Ball, R. M., Bermingham, E., Lamb, T., Neigel, J. E., Reeb, C. A., and Saunders, N. C., 1987. The mitochondrial DNA bridge between population genetics and systematics. Annual Review of Ecology and Systematics, 18: 489-522.
Botti, S., and Giuffra, E., 2010. Oligonucleotide indexing of DNA barcode: Identification of tuna and other scombrid species in food products. BMC Biotechnology, 10: 60.
Brown, M. W., Georage, M., and Wilson, A. C., 1979. Rapid evolution of animal mitochondrial DNA. Proceedings of the National Academy of Sciences of the United States of America, 79: 3246-3250.
Cawthorn, D., Steinman, H., and Witthuhn, R., 2012. Evaluation of the 16S and 12S rRNA genes as universal markers for the identification of commercial fish species in South Africa. Gene, 491 (1): 40-48.
Chen, J. X., 2003. Overview of sea cucumber farming and sea ranching practices in China. Beche-de-mer Information Bulletin, 18: 18-23.
Chen, S. Y., Su, Y. H., Wu, S. F., Sha, T., and Zhang, Y. P., 2005. Mitochondrial diversity and phylogeographic structure of Chinese domestic goats. Molecular Phylogenetics and Evolution, 37 (3): 804-814.
Chuang, P., Chen M., and Shiao, J., 2012. Identification of tuna species by a real-time polymerase chain reaction technique. Food Chemistry, 133: 1055-1061.
Comi, G., Iacumin, L., Rantsiou, K., Cantoni, C., and Cocolin, L., 2005. Molecular methods for the differentiation of species used in production of codfish can detect commercial frauds. Food Control, 16 (1): 37-42.
Di Finizio, A., Guerriero, G., Russo, G. L., and Ciarcia, G., 2007. Identification of gadoid species (Pisces, Gadidae) by sequencing and PCR-RFLP analysis of mitochondrial 12S and 16S rRNA gene fragments. European Food Research and Technology, 225: 337-344.
Espi?eira, M., González-Lavín, N., Vieites, J. M., and Santaclara, F. J., 2009. Development of a method for the genetic identification of commercial bivalve species based on mitochondrial 18S rRNA sequences. Journal of Agricultural and Food Chemistry, 57: 495-502.
FAO, 2008. Sea cucumbers: A global review of fisheries and trade. In: Fisheries and Aquaculture Technical Paper, 516. FAO, Rome, 317pp.
Gil, L. A., 2007. PCR-based methods for fish and fishery products authentication. Trends in Food Science and Technology, 18 (11): 558-566.
Grewe, P. M., Krueger, C. C., Aquadro, C. F., Bermingham, E., Kincaid, H. L., and May, B., 1993. Mitochondrial DNA variation among lake trout (Salvenilus namaycush) strains stocked into Lake Ontario. Canadian Journal of Fisheries and Aquatic Sciences, 50: 2397-2403.
Gudimova, E. N., 1991. Methods of quantitative analysis of the sclerite shapes of the sea cucumbers belonging to the genus Cucumaria. Biologia Morya, 6: 80-87.
Hebert, P. D. N., Ratnasingham, S., and deWaard, J. R., 2003. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society of London Series B: Biological Sciences, 270: 96-99.
Hirata, T., Zaima, N., Yamashita, K., Ryoko, N., Xue, C. H., and Sugawara, T., 2005. Recent advances in researches on physiologically active substances in holothurians. Journal of Ocean University of China, 4 (3): 193-197.
Hsieh, H. S., Chai, T., and Hwang, D. F., 2007. Using the PCR-RFLP method to identify the species of different processed products of billfish meats. Food Control, 18 (4): 369-374.
Joshi, M. B., Rout, P. K., Mandal, A. K., Tyler-Smith, C., Singh, L., and Thangaraj, K., 2004. Phylogeography and origin ofIndian domestic goats. Molecular Biology and Evolution, 21 (3): 454-462.
Kac, G., 2000. Molecular approaches to the study of dermatophytes. Medical Mycology, 38 (5): 329-336.
Kalinin, V. I., Levin, V. S., and Stonik, V. A., 1994. The Chemical Morphology: Triterpene Glycosides of Sea Cucumbers (Holothurioidea, Echinodermata). Dalnauka, Vladivostok, 284pp (in Russian).
Levin, V. S., Kalinin, V. I., Maltsev, I. I., and Stonik, V. A., 1985. Structure of triterpene glycosides and taxonomy of aspidochirotian sea cucumbers. Biologiya Morya, 2: 3-11.
Levin, V. S., Kalinin, V. I., Fedorov, S. N., and Smiley, S., 1986. Structure of triterpene glycosides and taxonomical position of two species of the family Stichopodidae. Biologiya Morya, 4: 72-77.
Levin, V. S., and Gudimova, E. N., 1997. On taxonomical relationships of the sea cucumbers Cucumaria frondosa and C. japonica (Dendrochirotida, Cucumariidae). Zoologichesky Zhurnal, 76: 575-584.
Liao, Y. L., 1997. Fauna Sincia: Phylum Echinodermata, Class Holothuroidea. Science Press, Beijing, 334pp.
Lin, W. F., and Hwang, D. F., 2007. Application of PCR-RFLP analysis on species identification of canned tuna. Food Control, 18 (9): 1050-1057.
Linacre, A., and Tobe, S., 2011. An overview to the investigative approach to species testing in wildlife forensic science. Investigative Genetics, 2 (1): 2.
Lockley, A. K., and Bardsley, R. G., 2000. DNA based-methods for food authentication. Trends in Food Science and Technology, 11: 67-77.
Massin, C., 1999. Reef-dwelling Holothuroidea (Echinodermata) of the Spermonde Archipelago (Southwest Sulawesi, Indonesia). Zoologische Verhandelingen, 329: 3-144.
Mochizuki, T., Sugie, N., and Uehara, M., 1997. Random amplification of polymorphic DNA is useful for the identification of several anthropophilic dermaophytes. Mycoses, 40: 405-409.
Naderi, S., Rezaei, H. R., Taberlet, P., Zundel, S., Rafat, S. A., Naghash, H. R., el-Barody, M. A., Ertugrul, O., and Pompanon, F., 2007. Large-scale mitochondrial DNA analysis of the domestic goat reveals six haplogroups with high diversity. PLoS One, 2 (10), e1012.
Rea, S., Storani, G., Mascaro, N., Stocchi, R., and Loschi, A. R., 2009. Species identification in anchovy pastes from the market by PCR-RFLP technique. Food Control, 20 (5): 515-520.
Rokas, A., Ladoukakis, E., and Zouros, E., 2003. Animal mitochondrial DNA recombination revisited. Trends in Ecology and Evolution, 18: 411-417.
Smiley, S., 1994. Holothuroidea. In: Microscopic Anatomy of Invertebrates, Echinodermata, 14. Harrison, F. W., and Chia, F. S., eds., Wiley-Liss, New York, 401-471.
Taanman, J. W., 1999. The mitochondrial genome: Structure, transcription, translation and replication. Biochimica et Biophysica Acta, 1410: 103-123.
Toral-Granda, M., 2005. The use of calcareous spicules for the identification of the Galápagos sea cucumber Isostichopus fuscus on the international market. SPC Beche-de-mer Information Bulletin, 22: 3-5.
Zuo, T., Li, Z., Lv, Y., Duan, G., Wang, C., Tang, Q., Xue, Y., and Xue, C., 2012. Rapid identification of sea cucumber species with multiplex-PCR. Food Control, 26 (1): 58-62.
(Edited by Qiu Yantao)
(Received July 7, 2013; revised November 4, 2013; accepted May 28, 2014)
? Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2014
* Corresponding author. Tel: 0086-532-82032597
E-mail: tangqingjuan@ouc.edu.cn
# These two authors contributed equally to the present work.
 Journal of Ocean University of China2014年5期
Journal of Ocean University of China2014年5期
- Journal of Ocean University of China的其它文章
- Identification of Fucans from Four Species of Sea Cucumber by High Temperature1H NMR
- The Appearance of Ulva laetevirens (Ulvophyceae, Chlorophyta) in the Northeast Coast of the United States of America
- Metabolic and Phylogenetic Profile of Bacterial Community in Guishan Coastal Water (Pearl River Estuary),South China Sea
- Distribution and Source of Main Contaminants in Surface Sediments of Tidal Flats in the Northern Shandong Province
- Analgesis and Wound Healing Effect of Chitosan and Carboxymethyl Chitosan on Scalded Rats
- Histological Observation of Germ Cell Development and Discovery of Spermatophores in Ovoviviparous Black Rockfish (Sebastes schlegeli Hilgendorf) in Reproductive Season
