Mechanisms simultaneously regulate smooth muscle proliferation and differentiation
Ning Shi, Shi-You Chen
Department of Physiology & Pharmacology, University of Georgia, Athens, GA 30602, USA.
Mechanisms simultaneously regulate smooth muscle proliferation and differentiation
Department of Physiology & Pharmacology, University of Georgia, Athens, GA 30602, USA.
Vascular smooth muscle cell (VSMC) differentiation and proliferation are two important physiological processes during vascular development. The phenotypic alteration from differentiated to proliferative VSMC contributes to the development of several major cardiovascular diseases including atherosclerosis, hypertension, restenosis after angioplasty or bypass, diabetic vascular complications, and transplantation arteriopathy. Since the VSMC phenotype in these pathological conditions resembles that of developing VSMC during embryonic development, understanding of the molecular mechanisms that control VSMC differentiation will provide fundamental insights into the pathological processes of these cardiovascular diseases. Although VSMC differentiation is usually accompanied by an irreversible cell cycle exit, VSMC proliferation and differentiation occur concurrently during embryonic development. The molecular mechanisms simultaneously regulating these two processes, however, remain largely unknown. Our recent study demonstrates that cell division cycle 7, a key regulator of cell cycle, promotes both VSMC differentiation and proliferation through different mechanisms during the initial phase of VSMC differentiation. Conversely, Krüppel-like factor 4 appears to be a repressor for both VSMC differentiation and proliferation. This review attempts to highlight the novel role of cell division cycle 7 in TGF-β-induced VSMC differentiation and proliferation. The role of Krüppel-like factor 4 in suppressing these two processes will also be discussed.
vascular smooth muscle, differentiation, proliferation, cell division cycle 7, Krüppel-like factor 4
SMOOTH MUSCLE CELL PROLIFERATION AND DIFFERENTIATION
Vascular smooth muscle cells (VSMCs) are one of the major cellular components of blood vessel wall where they exist in a differentiated contractile phenotype to maintain vascular tone[1]. VSMCs within adult blood vessels proliferate at an extremely low rate, display very low synthetic activity, and express a unique set of contractile proteins such as smooth muscle myosin heavy chain (SMMHC), smooth muscle α-actin (α-SMA), SM22α, and calponin, etc.[2,3]. During embryonic development, VSMCs can be differentiated from various sources of progenitor cells[4-6]. For example, VSMCs of large arteries near the heart originate from the neural crest cells of the ectoderm, whereas other VSMCs are believed to be derived from mesoderm-derived mesenchymal cells. Among themesoderm-derived VSMCs, coronary VSMCs are reported to come from the proepicardial organ[7]; VSMCs of the root of the pulmonary artery and the lung artery stem from the second heart field[8]. A detailed description about SMC diversity and origins can be found in an elegant review published a few years ago[5].
Unlike either skeletal or cardiac myocytes that are terminally differentiated, VSMCs preserve remarkable plasticity and may undergo reversible alterations in phenotype in response to changes in local environmental cues[3]. When contractile VSMCs collected from human or animal artery are cultured in vitro, they immediately transform into proliferative or dedifferentiated VSMCs and begin to proliferate under various conditions. These transformed cells show the same characteristics as the proliferative VSMCs observed in vivo, including the inability to contract and secretion of extracellular matrix (ECMs) such as collagen, elastin and proteoglycans[9]. Similarly, upon injury due to angioplasty, stent implantation, or bypass surgery, VSMCs dedifferentiate and re-enter the cell cycle. They exhibit an increased rate of proliferation, migration, and synthesis of ECMs, and at the same time, display a decrease in the expression of VSMC contractile proteins[10]. This dedifferentiated, proliferative phenotype plays a major pathophysiologic role in the development of atherosclerosis, hypertension, restenosis after angioplasty or bypass, diabetic vascular complications, and transplantation arteriopathy[11].
A large number of environmental cues including growth factors, inflammatory mediators, matrix component, and cell-cell interactions have been shown to regulate VSMC proliferation and differentiation[3]. Transforming growth factor-β (TGF-β) is among the most potent soluble growth factors that promote VSMC differentiation[12-18]. The effects of TGF-β on VSMC proliferation remain controversial. It has been reported that TGF-β exerted a growth-inhibitory effect on VSMCs by inducing cell cycle arrest at G1 phase[19,20]. However, Stouffer et al. have shown that TGF-β promotes proliferation of cultured VSMC[21], which is consistent with recent findings that TGF-β increases VSMC proliferation through the Smad3 and ERK MAPK pathways[22,23]. In addition to TGF-β, other growth factors including platelet-derived growth factor (PDGF), fibroblast growth factor-2 (FGF-2) and epidermal growth factor (EGF) have also been shown to stimulate VSMC proliferation or help maintain a dedifferentiated SMC phenotype[24,25].
VSMC proliferation and differentiation are generally considered as opposite processes. Cell differentiation is usually accompanied by an irreversible cell cycle exit[26,27]. However, it is now believed that VSMC differentiation and proliferation are not necessarily mutually exclusive, and cell cycle exit may not necessarily lead to VSMC differentiation. A great example is that heparin, a powerful inhibitor of VSMC growth both in vitro and in vivo, has no effect on differentiation of human VSMC[28-30]. These results show that cessation of proliferation alone is not sufficient to promote SMC differentiation. Moreover, during late embryogenesis and postnatal development, VSMCs exhibit an extremely high rate of proliferation, yet they undergo a rapid rate of induction of multiple VSMC differentiation marker genes[3,31]. Consistent with this, Lee et al. have reported that proliferation and differentiation of VSMC precursors occurs simultaneously during the development of the vessel wall[32].
CELL DIVISION CYCLE 7 (CDC7) IN CELL PROLIFERATION
Cdc7 has been shown to play a critical role in cell growth or proliferation. Knockout of Cdc7 in mice causes early embryonic lethality between day 3.5 and 6.5[33]. Inactivation of the Cdc7 gene in mouse ES cells is also lethal[33]. These cells cease DNA synthesis, accumulate DNA damages, and eventually undergo cell death in a p53-dependent manner. In cancer cells, depletion of Cdc7 has been demonstrated to cause an abortive S phase, leading to p53-independent apoptotic cell death or aberrant mitosis. In contrast, in untransformed cells, Cdc7 depletion results in a reversible arrest in G1, and cells remain in a viable non-proliferative state[34,35]. On the other hand, overexpression of Cdc7 is associated with neoplastic transformation for certain tumors or transformed cell lines[36].
Cdc7 is a serine-threonine kinase and was first identified in budding yeast as a mutant defective in the initiation of DNA replication, conserved from yeast to humans[37,38]. Cdc7 is activated by binding to a regulatory protein called either dumbbell former 4 (Dbf4) or activator of S phase kinase (ASK)[39]. Cdc7 plays a very important role in cell cycle progression through the G1/S transition and is critical for the initiation, but not the elongation, of DNA replication in the mitotic cell cycle[40]. Initiation of DNA replication in eukaryotes requires an assembly of various replication proteins at the origin of the replication in order to form a pre-replicative complex (preRC)[41]. These proteins include origin recognition complex (ORC), Cdc6, Cdc45, minichromosomal maintenance (MCM) proteins, DNA polymerase, and single-stranded DNA binding protein, etc. However, preRC cannot induce replication unless an additional cue is provided from upstream cell cycle events. This cue is protein phosphorylation mediated by Cdc7-Dbf4 and cyclin-dependent kinase (Cdk)[42,43].
Cdc7 functionally links to Cdks. Cdk is essential for G1/S transition although its target for S phase initiation has not been elucidated. It is also unclear if Cdc7 and Cdk act in parallel or in the same pathway for S phase initiation. Recent evidences show that Cdk including Cdk2-Cyclin E can phosphorylate human Cdc7 and ASK proteins in vitro. A potential phosphorylation site mutation (T376A) in Cdc7 significantly affects its kinase activity. This critical threonine 376 residue appears to be phosphorylated by Cdk2-cyclin E, Cdk2-cyclin A, and Cdc2-cyclin B in vitro, providing a possible functional link between Cdk and Cdc7[44]. Moreover, one-hybrid assays in yeast indicate that Dbf4 protein is tethered at the origins of DNA replication[45], strongly suggesting that the Cdc7 kinase complex is present on chromatin and is in association with replication complexes at the origin. The Cdc7 kinase complex along with Cdk is known to be the ultimate trigger of chromosomal replication in yeast[46].
Cdc7 activation of MCM proteins requires prior phosphorylation by Cdk. Activation of MCM2-7 unwinds the origin of DNA replication and allows the priming of leading and lagging strands by DNA polymerase α. The MCM2-7 complex is an important target of Cdc7 kinase[47-49]. Genetic studies also demonstrate that MCM2 is a physiologically important substrate of Cdc7[50]. Dbf4 recruits Cdc7 to MCM2 because Dbf4, but not Cdc7, directly binds to MCM2[51]. Interestingly, de-phosphorylation of MCM2 causes a loss of phosphorylation by Cdc7 in yeast[52], suggesting that prior phosphorylation of the MCM substrate is a prerequisite for Cdc7 target site recognition. This is also true for mammalian MCM2 proteins[44]. The efficacy of MCM2 phosphorylation by Cdc7 is significantly increased when the substrate is pre-phosphorylated by Cdk2. These results suggest that Cdk and Cdc7 work together to achieve efficient phosphorylation of MCM2 in the complex for initiation of DNA replication. Phosphorylation of MCM2 by Cdc7-Dbf4 and Cdk is likely to create direct binding sites for other factors such as Cdc45, or cause a structural change in the MCM2-7 complex that is important for activation of the helicase[39,53,54].
Initiation of DNA replication is a crucial decision point during cell proliferation and lies at the convergence point of complex networks of signaling molecules that have evolved to specify when and where cells divide in an organism[55]. Cdc7 not only lies at an integration point for mitogenic signaling pathways, but also plays a key role in maintaining genomic stability through intra-S-phase checkpoint pathways in response to DNA damage and delayed replication fork progression[56].
CDC7 IN VSMC DIFFERENTIATION
VSMC differentiation is a very complex process that involves multi-level regulations. The molecular mechanisms underlying VSMC differentiation have been extensively studied including transcriptional, post-transcriptional, and posttranslational regulations. Although it is well established that SMC differentiation and proliferation can be regulated simultaneously, the molecular mechanisms governing these two somewhat uncompromised processes, however, are less well studied. Most growth factors block VSMC differentiation while inducing VSMC proliferation, or vice versa, but thrombin has been shown to induce VSMC proliferation and increase the expression of VSMC marker genes even though the detailed mechanism remains to be determined[57]. We found that TGF-β can also simultaneously induce differentiation and proliferation in the initial phase of VSMC differentiation. It appears that cell cycle regulator Cdc7 mediates both TGF-β-induced VSMC differentiation and proliferation[58].
Cdc7 expression is significantly upregulated by TGF-β although Cdc7 level is normally constant throughout the cell cycle[59,60]. Blockade of Cdc7 activity inhibits TGF-β-mediated proliferation of cells undergoing VSMC differentiation. More importantly, inactivation of Cdc7 also strongly inhibits TGF-βinduced VSMC differentiation. Conversely, overexpression of Cdc7 promotes expression of early VSMC marker genes such asα-SMA, SM22α, and calponin[58], demonstrating the dual role of Cdc7 in VSMC differentiation and proliferation. Cdc7 regulates VSMC differentiation through modulating VSMC marker gene transcription. Cdc7 is required for TGF-β-mediated activation of α-SMA and SM22α promoters though Cdc7 does not directly bind to promoter DNA. Cdc7 function in VSMC marker gene transcription appears to be dependent on Smad3, a well-known TGF-β signaling intermediate and transcription factor important for VSMC differentiation. In fact, Cdc7 promotes Smad3 binding to Smad-binding element in VSMC gene promoters by physically interacting with Smad3, leading to activation of the marker gene transcription. In addition to the direct effect on marker gene transcription, Cdc7 also indirectly regulates marker gene transcription by enhancing Smad3 accumulation in the nuclei, which is achieved by enhancing the expression or stability of Smad nuclear retention factor TAZ.
In addition to SMC marker gene, Cdc7 has been shown to regulate the transcription of a meiosisspecific transcriptional activator, NTD80 in yeast[61].Cdc7-Dbf4 promotes NDT80 transcription by relieving repression mediated by a complex of Sum1, Rfm1, and a histone deacetylase Hst1[62]. The other roles of Cdc7 in meiosis include facilitating the premeiotic DNA replication and the initiation of recombination[62].
Cdc7 regulates VSMC differentiation and proliferation through different mechanisms. Although Dbf4, the regulatory subunit of Cdc7, is required for VSMC proliferation during differentiation, Dbf4 is not involved in VSMC differentiation because knockdown of Dbf4 by its specific shRNA has no effect on VSMC marker gene expression. Because the differentiation and proliferation occur simultaneously via different mechanisms (Fig. 1), VSMC marker gene activation and cell cycle progression may take place concurrently in the same cells, which requires further careful investigation. Nevertheless, it is reasonable to believe that cells use cell cycle regulators to control another important cellular process to accomplish the high rates of VSMC differentiation and proliferation during late embryogenesis and postnatal development.
KLF4, A NEGATIVE REGULATOR OF BOTH VSMC DIFFERENTIATION AND PROLIFERATION
In contrast to Cdc7, KLF4 negatively regulates both VSMC differentiation and proliferation. KLF4 is a member of the Kruppel family of transcription factors and one of the four induced-pluripotential stem cell pluripotency factors[63,64]. The combined retroviralmediated overexpression of KLF4 and Sox2, Oct4 and c-Myc in differentiated cells can convert the cells into embryonic stem cell-like cells. Although KLF4 is not normally expressed in healthy adult SMCs, it is rapidly induced during SMC phenotypic modulation following vascular injury[65], or in cultured VSMC while treated with PDGF-BB or oxPLs[66,67]. KLF4 binds to G/C repressor elements in VSMC marker genes, and is critical in mediating the down-regulation of these genes during VSMC phenotypic modulation in vivo and in vitro[68]. KLF4 suppresses VSMC marker gene expression through multiple mechanisms (Fig. 1), including reducing serum response factor binding to CArG box, decreasing myocardin expression, and recruiting histone deacetylases to silence gene transcription, etc.[65,69,70]. SMC-specific knockout of KLF4 causes a transient delay in injury-induced repression of VSMC differentiation markers[69], which is likely due to the absence of KLF4 binding to G/C repressor. The transient but not long-term repression of VSMC genes may be due to a compensatory effect of other KLFs such as KLF5, KLF13 and/or KLF1571.
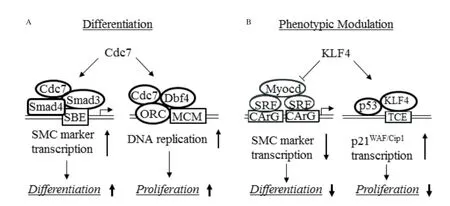
Fig. 1 Positive and negative regulation of cell differentiation and proliferation during vascular smooth muscle cell (VSMC) differentiation and phenotypic modulation. A: Cdc7 stimulates VSMC differentiation and proliferation. Cdc7 interacts with Smad3 and enhances Smad3 binding to SBE in VSMC marker gene promoter, resulting in activation of SMC gene transcription and VSMC differentiation. Cdc7 binds to Dbf4, and Cdc7/Dbf4 phosphorylates MCM located on ORC, leading to initiation of DNA replication and VSMC proliferation. B: KLF4 inhibits VSMC differentiation and proliferation. KLF4 blocks SRF binding to CArG box, decreases myocardin expression, and recruits histone deacetylases to silence VSMC marker gene transcription and thus suppresses VSMC differentiation. KLF4 binds to p21WAF/Cip1 promoter and recruits p53 to induce p21WAF/Cip1 expression and thus inhibits VSMC proliferation.
Interestingly, although KLF4 suppresses VSMC gene expression, KLF4 SMC-specific knockout in mice enhances neointimal formation in response to vascular injury, which is caused by increased VSMC proliferation in artery media[69], suggesting that KLF4 inhibits VSMC proliferation. Indeed, overexpressionof KLF4 in cultured VSMC reduces cell proliferation. The underlying mechanism is that KLF4 binds to KLF4 binding site and recruits p53 to bind to p53 binding site in the promoter/enhancer of the cell cycle inhibitor p21WAF1/Cip1and thus induces p21WAF1/Cip1expression[69], leading to inhibition of VSMC proliferation (Fig. 1).
PERSPECTIVE
VSMC differentiation and proliferation are separate but concurrent processes that may be regulated independently and simultaneously with a high possibility of crosstalk between their controls. Cdc7 simultaneously stimulates VSMC differentiation and cell proliferation, indicating that cell cycle regulators may play an important role in synchronizing these two seemingly paradoxical processes. Therefore, it is highly likely that other cell cycle regulators are also involved in regulating both VSMC differentiation and proliferation during VSMC development. Although KLF4 suppresses both VSMC differentiation marker and cell proliferation, it would be interesting to determine if KLF4 exerts these effects simultaneously during VSMC phenotypic modulation or differentiation from progenitors. In addition, it is important to define how cells balance the activities of Cdc7 and KLF4 in VSMC differentiation and proliferation. The answers to these questions or identification of other novel mechanisms shall increase our understanding of VSMC biology during late embryonic development and vascular remodeling in pathological conditions.
[1] Ammit AJ, Panettieri RA, Jr. The circle of life: Cell cycle regulation in airway smooth muscle. J Appl Physiol 2001; 91: 1431-7.
[2] Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 1995; 75: 487-517.
[3] Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84: 767-801.
[4] Hirschi KK, Majesky MW. Smooth muscle stem cells. Anat Rec A Discov Mol Cell Evol Biol 2004;276:22-33.
[5] Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 2007; 27: 1248-58.
[6] Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res 2005; 96: 280-91.
[7] Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 1996; 174: 221-32.
[8] Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol 2005; 281: 78-90.
[9] Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev 1979; 59: 1-61.
[10] Sobue K, Hayashi K, Nishida W. Expressional regulation of smooth muscle cell-specific genes in association with phenotypic modulation. Mol Cell Biochem 1999; 190: 105-18.
[11] Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg 2007; 45 Suppl A: A25-32.
[12] Hirschi KK, Rohovsky SA, D'Amore PA. Pdgf, tgf-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10t1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 1998; 141: 805-14.
[13] Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, et al. Central role for rho in tgf-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol 2003; 284: F911-924.
[14] Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol 2003; 29: 397-404.
[15] Chen S, Lechleider RJ. Transforming growth factorbeta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res 2004; 94: 1195-202.
[16] Sinha S, Hoofnagle MH, Kingston PA, McCanna ME, Owens GK. Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol 2004; 287: C1560-8.
[17] Deaton RA, Su C, Valencia TG, Grant SR. Transforming growth factor-beta1-induced expression of smooth muscle marker genes involves activation of pkn and p38 mapk. J Biol Chem 2005; 280: 31172-81.
[18] Tang Y, Urs S, Boucher J, Bernaiche T, Venkatesh D, Spicer DB, et al. Notch and transforming growth factorbeta (tgfbeta) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J Biol Chem 2010; 285: 17556-63.
[19] Reddy KB, Howe PH. Transforming growth factor beta 1-mediated inhibition of smooth muscle cell proliferation is associated with a late g1 cell cycle arrest. J Cell Physiol 1993; 156: 48-55.
[20] Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther 2005; 315: 1005-12.
[21] Stouffer GA, Owens GK. Tgf-beta promotes proliferation of cultured smc via both pdgf-aa-dependent and pdgf-aa-independent mechanisms. J Clin Invest 1994;93: 2048-55.
[22] Suwanabol PA, Seedial SM, Shi X, Zhang F, Yamanouchi D, Roenneburg D, et al. Transforming growth factor-beta increases vascular smooth muscle cell proliferation through the smad3 and extracellular signal-regulated kinase mitogen-activated protein kinases pathways. J Vasc Surg 2012; 56: 446-54.
[23] Tsai S, Hollenbeck ST, Ryer EJ, Edlin R, Yamanouchi D, Kundi R, et al. Tgf-beta through smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am J Physiol Heart Circ Physiol 2009; 297: H540-549.
[24] Hayashi K, Saga H, Chimori Y, Kimura K, Yamanaka Y, Sobue K. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J Biol Chem 1998; 273: 28860-7.
[25] Dempsey EC, McMurtry IF, O'Brien RF. Protein kinase c activation allows pulmonary artery smooth muscle cells to proliferate to hypoxia. Am J Physiol 1991; 260: L136-145.
[26] Maione R, Amati P. Interdependence between muscle differentiation and cell-cycle control. Biochim Biophys Acta 1997; 1332: M19-30.
[27] Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev 1997; 7: 597-602.
[28] Au YP, Kenagy RD, Clowes MM, Clowes AW. Mechanisms of inhibition by heparin of vascular smooth muscle cell proliferation and migration. Haemostasis 1993; 23 (S1): 177-182.
[29] Karnovsky MJ, Wright TC, Jr., Castellot JJ, Jr., Choay J, Lormeau JC, Petitou M. Heparin, heparan sulfate, smooth muscle cells, and atherosclerosis. Ann N Y Acad Sci 1989; 556: 268-81.
[30] Orlandi A, Ropraz P, Gabbiani G. Proliferative activity and alpha-smooth muscle actin expression in cultured rat aortic smooth muscle cells are differently modulated by transforming growth factor-beta 1 and heparin. Exp Cell Res 1994; 214: 528-36.
[31] Cook CL, Weiser MC, Schwartz PE, Jones CL, Majack RA. Developmentally timed expression of an embryonic growth phenotype in vascular smooth muscle cells. Circ Res 1994; 74: 189-96.
[32] Lee SH, Hungerford JE, Little CD, Iruela-Arispe ML. Proliferation and differentiation of smooth muscle cell precursors occurs simultaneously during the development of the vessel wall. Dev Dyn 1997; 209: 342-52.
[33] Kim JM, Nakao K, Nakamura K, Saito I, Katsuki M, Arai K, et al. Inactivation of cdc7 kinase in mouse es cells results in s-phase arrest and p53-dependent cell death. EMBO J 2002; 21: 2168-79.
[34] Kulkarni AA, Kingsbury SR, Tudzarova S, Hong HK, Loddo M, Rashid M, et al. Cdc7 kinase is a predictor of survival and a novel therapeutic target in epithelial ovarian carcinoma. Clin Cancer Res 2009; 15: 2417-25.
[35] Montagnoli A, Tenca P, Sola F, Carpani D, Brotherton D, Albanese C, et al. Cdc7 inhibition reveals a p53-dependent replication checkpoint that is defective in cancer cells. Cancer Res 2004; 64: 7110-6.
[36] Hess GF, Drong RF, Weiland KL, Slightom JL, Sclafani RA, Hollingsworth RE. A human homolog of the yeast cdc7 gene is overexpressed in some tumors and transformed cell lines. Gene 1998; 211: 133-40.
[37] Masai H, Sato N, Takeda T, Arai K. Cdc7 kinase complex as a molecular switch for DNA replication. Front Biosci 1999; 4: D834-840.
[38] Hartwell LH, Culotti J, Pringle JR, Reid BJ. Genetic control of the cell division cycle in yeast. Science 1974; 183: 46-51.
[39] Jackson AL, Pahl PM, Harrison K, Rosamond J, Sclafani RA. Cell cycle regulation of the yeast cdc7 protein kinase by association with the dbf4 protein. Mol Cell Biol 1993; 13: 2899-908.
[40] Hartwell LH, Mortimer RK, Culotti J, Culotti M. Genetic control of the cell division cycle in yeast: V. Genetic analysis of cdc mutants. Genetics 1973; 74: 267-86.
[41] Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol 1989; 5: 197-245.
[42] Buck V, White A, Rosamond J. Cdc7 protein kinase activity is required for mitosis and meiosis in saccharomyces cerevisiae. Mol Gen Genet 1991 ;227: 452-7.
[43] Ohtoshi A, Miyake T, Arai K, Masai H. Analyses of saccharomyces cerevisiae cdc7 kinase point mutants: Dominant-negative inhibition of DNA replication on overexpression of kinase-negative cdc7 proteins. Mol Gen Genet 1997; 254: 562-70.
[44] Masai H, Matsui E, You Z, Ishimi Y, Tamai K, Arai K. Human cdc7-related kinase complex. In vitro phosphorylation of mcm by concerted actions of cdks and cdc7 and that of a criticial threonine residue of cdc7 by cdks. J Biol Chem 2000; 275: 29042-52.
[45] Dowell SJ, Romanowski P, Diffley JF. Interaction of dbf4, the cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo. Science 1994; 265: 1243-6.
[46] Stillman B. Cell cycle control of DNA replication. Science 1996; 274: 1659-64.
[47] Sato N, Arai K, Masai H. Human and xenopus cdnas encoding budding yeast cdc7-related kinases: In vitro phosphorylation of mcm subunits by a putative human homologue of cdc7. EMBO J 1997; 16: 4340-51.
[48] Brown GW, Kelly TJ. Purification of hsk1, a minichromosome maintenance protein kinase from fission yeast. J Biol Chem 1998; 273: 22083-90.
[49] Takeda T, Ogino K, Matsui E, Cho MK, Kumagai H, Miyake T, et al. A fission yeast gene, him1(+)/dfp1(+), encoding a regulatory subunit for hsk1 kinase, plays essential roles in s-phase initiation as well as in s-phase checkpoint control and recovery from DNA damage. Mol Cell Biol 1999; 19: 5535-47.
[50] Lei M, Kawasaki Y, Young MR, Kihara M, Sugino A, Tye BK. Mcm2 is a target of regulation by cdc7-dbf4 during the initiation of DNA synthesis. Genes Dev 1997; 11: 3365-74.
[51] Bruck I, Kaplan D. Dbf4-cdc7 phosphorylation of mcm2 is required for cell growth. J Biol Chem 2009; 284: 28823-31.
[52] Kihara M, Nakai W, Asano S, Suzuki A, Kitada K, Kawasaki Y, et al. Characterization of the yeast cdc7p/ dbf4p complex purified from insect cells. Its protein kinase activity is regulated by rad53p. J Biol Chem 2000; 275: 35051-62.
[53] Hardy CF, Dryga O, Seematter S, Pahl PM, Sclafani RA. Mcm5/cdc46-bob1 bypasses the requirement for the s phase activator cdc7p. Proc Natl Acad Sci U S A 1997; 94: 3151-5.
[54] Fletcher RJ, Bishop BE, Leon RP, Sclafani RA, Ogata CM, Chen XS. The structure and function of mcm from archaeal m. Thermoautotrophicum. Nat Struct Biol 2003; 10: 160-7.
[55] Stoeber K, Tlsty TD, Happerfield L, Thomas GA, Romanov S, Bobrow L, et al. DNA replication licensing and human cell proliferation. J Cell Sci 2001; 114: 2027-41.
[56] Costanzo V, Gautier J. Single-strand DNA gaps trigger an atr- and cdc7-dependent checkpoint. Cell Cycle 2003; 2: 17.
[57] McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, 2nd, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest 1993; 91: 94-8.
[58] Shi N, Xie WB, Chen SY. Cell division cycle 7 is a novel regulator of transforming growth factor-betainduced smooth muscle cell differentiation. J Biol Chem 2012; 287: 6860-7.
[59] Yoon HJ, Campbell JL. The cdc7 protein of saccharomyces cerevisiae is a phosphoprotein that contains protein kinase activity. Proc Natl Acad Sci U S A 1991; 88: 3574-8.
[60] Yoon HJ, Loo S, Campbell JL. Regulation of saccharomyces cerevisiae cdc7 function during the cell cycle. Mol Biol Cell 1993; 4: 195-208.
[61] Lo HC, Wan L, Rosebrock A, Futcher B, Hollingsworth NM. Cdc7-dbf4 regulates ndt80 transcription as well as reductional segregation during budding yeast meiosis. Mol Biol Cell 2008; 19: 4956-67.
[62] Lo HC, Kunz RC, Chen X, Marullo A, Gygi SP, Hollingsworth NM. Cdc7-dbf4 is a gene-specific regulator of meiotic transcription in yeast. Mol Cell Biol 2012; 32: 541-57.
[63] Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663-76.
[64] Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008; 451: 141-6.
[65] Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem 2005; 280: 9719-27.
[66] Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, et al. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res 2007; 101: 792-801.
[67] Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of klf4 is required for pdgf-bb-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol 2009; 296: H1027-37.
[68] Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, Mc-Donald OG, Owens GK. A g/c element mediates repression of the sm22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res 2004; 95: 981-8.
[69] Yoshida T, Kaestner KH, Owens GK. Conditional deletion of kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res 2008; 102: 1548-57.
[70] McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of srf binding to carg box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest 2006; 116: 36-48.
[71] Autieri MV. Kruppel-like factor 4: Transcriptional regulator of proliferation, or inflammation, or differentiation, or all three? Circ Res 2008; 102: 1455-7.
Received 31 August 2013, Accepted 13 November 2013, Epub 28 December 2013
This work was supported by grants from National Institutes of Health (HL093429 and HL107526 to S.-Y.C.).
The authors reported no conflict of interests.
10.7555/JBR.28.20130130
 THE JOURNAL OF BIOMEDICAL RESEARCH2014年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2014年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- A new free-hand pedicle screw placement technique with reference to the supraspinal ligament
- Pediatric restrictive cardiomyopathy due to a heterozygous mutation of the TNNI3 gene
- Genetic variants at 10q23.33 are associated with plasma lipid levels in a Chinese population
- Extracellular matrix synthesis in vascular disease: hypertension, and atherosclerosis
- Renal denervation as an option for the management of hypertension
- Atrial fibrillation
