Atrial fibrillation
Thomas M. MungeLi-Qun Wu, Win K. Shen
aHeart Rhythm Services, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN 55905, USA;
bDepartment of Cardiology, Rui Jin Hospital, Shanghai Jiao Tong University of Medicine, Shanghai 200025, China;
cDivision of Cardiovascular Diseases, Mayo Clinic, Phoenix, AZ 85054, USA.
Atrial fibrillation
aHeart Rhythm Services, Division of Cardiovascular Diseases, Mayo Clinic, Rochester, MN 55905, USA;
bDepartment of Cardiology, Rui Jin Hospital, Shanghai Jiao Tong University of Medicine, Shanghai 200025, China;
cDivision of Cardiovascular Diseases, Mayo Clinic, Phoenix, AZ 85054, USA.
Atrial fibrillation is the most common arrhythmia affecting patients today. Disease prevalence is increasing at an alarming rate worldwide, and is associated with often catastrophic and costly consequences, including heart failure, syncope, dementia, and stroke. Therapies including anticoagulants, anti-arrhythmic medications, devices, and non-pharmacologic procedures in the last 30 years have improved patients' functionality with the disease. Nonetheless, it remains imperative that further research into AF epidemiology, genetics, detection, and treatments continues to push forward rapidly as the worldwide population ages dramatically over the next 20 years.
atrial fibrillation, arrhythmias, cardiac, stroke, dementia, heart failure
INTRODUCTION
More than three centuries ago, William Harvey is credited with being the first to describe unusual chaotic movements of the right atrium in experimental animals who were dying[1]. The earliest descriptions of human patients who had grossly irregular heart pulsations were published in 1749 by John Baptist Senac, and an Irish physician, Robert Adams, in 1827[1]. In 1909, using the newly invented electrocardiogram, Sir Thomas Lewis[2]concluded that the usual cause for the arrhythmia MacKenzie[3]and Cushny[4]had described clinically in the prior decade, was atrial fibrillation. It was indeed a common clinical condition, and correlated with the first AF ECG that Einthoven had published 3 years earlier. As a clinical arrhythmia, it has rapidly become the most prevalent rhythm disorder for which electrophysiologists are consulted and especially among the rapidly expanding global elderly population.
This contemporary review of atrial fibrillation will be divided into two halves. A review on the epidemiology, mechanisms, clinical manifestations of atrial fibrillation, and risk stratification and prevention of stroke will be discussed in the first half of the review. In the second half, we will focus on the topics of therapy for rate control and rhythm control, from drugs to intervention.
EPIDEMIOLOGY
From the Framingham Trial[5], the two-year incidence of transition into chronic atrial fibrillation was approximately 30-50 percent higher in males at all ages examined. The risk of AF development is enhanced by the presence of rheumatic heart disease and cardiac failure, particularly in women. The cumulativeincidence of new AF at 22-year follow-up in Framingham was 21.5 per 1,000 men and 17.1 per 1,000 women[6]. Advancing age is the predominant risk factor for AF and contributes to the increased population prevalence in Western countries; this has been known for nearly 50 years[7].
The prevalence of AF at various decades of life has been characterized from 3 earlier studies: ATRIA, Framingham, and Olmsted County[8-10]. AF incidence doubles in each decade for patients who are of age beyond 50 years. The incidence at each age is higher in men than women. The number of patients afflicted with AF in the United States is expected to more than double over the next 35 years. Data from several recent studies in Europe and the United States suggest that the prevalence of AF is also increasing and it is becoming a global epidemic[11-14]; nonetheless, baseline prevalence data recently collected in a Chinese population from the Mainland is more than 50% lower than equivalent Western populations[15]. Additionally, the incidence of AF in African-American men is 25% lower (45% when age/risk factor-adjusted) as compared to American Caucasians[16].
Below (Fig. 1) are the common demographic factors associated with AF incidence. Most of these factors either increase intra-atrial pressure or alter the autonomic nerve balance of the heart (sympathetic or vagal). Additionally, genetic variations in younger families with AF have been identified that are associated with chiefly potassium channel kinetics[17]. Particularly in younger men, long-distance endurance type sports increase the incidence of AF[18]. The effect of alcohol as a promoter of AF appears to also have a dosing-threshold effect[19]. Despite now a plethora of clinical epidemiologic reports, about half of all cases of AF are still not attributable to the common associated risks in Fig. 1[20].
MECHANISMS
In 1962, Gordon Moe published his initial paper on the “Multiple Wavelet Hypothesis” for the mechanism of perpetuated atrial fibrillation[21], which served as a mechanistic template for the design of the surgical MAZE procedure nearly 30 years later[22,23]. Moe noted that there had been conflicting theories on the mechanisms for the etiology of atrial fibrillation over the preceding 70 years. These included: 1) Ectopic focus theory[24,25]; 2) Ion flux theory (electrical stimulation coupled with potassium depletion and acetylcholine or vagal stimuli)[26]; 3) Circus-movement theory. We now know that all 3 postulates play active mechanistic roles in the initiation and perpetuation of AF.

Fig. 1 Demographic, exogenous, and underlying disease associations with atrial fibrillation.
Moe noted that a shortened atrial refractory period (ARP) determined the frequency of repetitive ectopy and thus would play a role in AF initiation and maintenance particularly when coupled with the inhomo-geneous features of premature activation and recovery in sheets of cardiac tissue. From this, he concluded wavelets and subsequent “daughter wavelets” were a frequent consequence of any of the triggering mechanisms. He concluded the self-sustaining feature of AF was related to a minimally sufficient cardiac mass (adult or large animals were able to maintain AF while young or small animals were NOT able to maintain AF). A critical mass of tissue being required for AF maintenance had been reinforced by studies showing that cutting fibrillating tissue in half would terminate AF. Moe concluded a critical number of “daughter wavelets” after an appropriate trigger would perpetuate AF over time.
Currently (Fig. 2), we know that individuals have differing propensities to develop AF over time. The initial description of a familial link to AF was reported in 1943[27]. In the last 15 years, multiple different genes have been identified as being linked with AF in families and include mutations for the sodium and potassium channels as well as gap junction proteins[17].
Elevation of the atrial pressure is well known as a promoter of AF. Stretch-induced vulnerability has been demonstrated to be dose-dependent in rabbits[28].
Associations with a cadre of “pressure” diseases, viz.: hypertension, mitral valve regurgitation and stenosis, obstructive sleep apnea, hypertrophic cardiomyopathy, and congestive heart failure support this observation. Diastolic heart failure in particular is associated with the development of non-valvular atrial fibrillation in the elderly[29]. So called heart failure with preserved ejection fraction (HEpEF) has a high prevalence in heart failure populations (50%), is increasing as the disease is associated with aging and female sex, and is a systemic disease associated with progressive vascular stiffening, renal dysfunction, and anemia[30].
Inflammation has long been known to play a role in the pathogenesis of atrial fibrillation. Patients with myocardial infarction, acute myo-pericarditis, chronic rheumatic heart disease, or following cardiac surgery have augmented rates of AF. In fact, for the patients following coronary bypass grafting, the link has been noted since the report of the first 100 cases was published in 1969[31]; the initial incidence reported in that paper was 12% but has since been demonstrated to be 17-33% (and higher in valvular or hybrid procedures[32]. Multiple inflammatory markers have been linked with a propensity towards AF, including several of the interleukins (IL-2, IL-6, and IL-8), as well as C-reactive protein (CRP), tissue necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1)[33]. Leukocyte activation enhances thromboembolism[34]via the thrombosis cascade, as well as possibly enhancing the chronicity of AF[35].
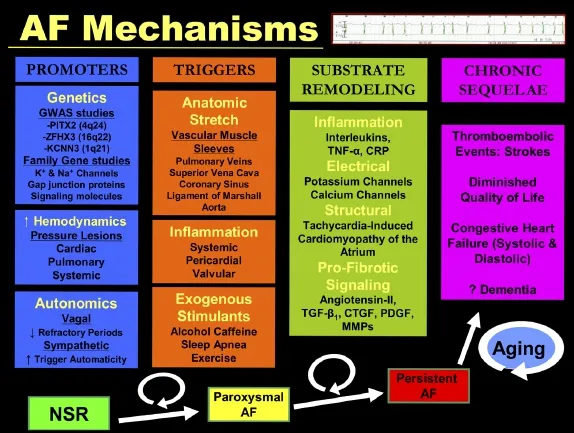
Fig. 2 Genetic, autonomic, hemodynamic, and inflammatory mechanisms underlying atrial fibrillation pathogenesis followed by clinical sequelae.
In 1998, the Bordeaux group published that the anatomic sources for focal initiation of AF were chiefly sleeves of left atrial muscle tissue that extend onto the epicardial surface of the four pulmonary veins (PVs) which drain oxygenated blood back into the LA from the lungs[36]. The PV muscle sleeves, described initially by Nathan and Eliakim in 1966[37], extend typically into each PV 1-3 cm, are susceptible to stretch-induced firing, have been the major targets for catheterbased therapies of AF in the last 15 years, and are richly innervated from adjacent ganglionic plexi (GP). Small amounts of IK1 activity[38], as well as susceptibility to enhanced calcium loading[39]with stretch or manipulation, help explain the PV muscle's tendency for triggered activity, short refractoriness and rapid firing. Augmented atrial pressures appear to centralize the LA-PV junction areas as a source for dominant reentrant rotors[40]. The PV osteums have also been demonstrated to have the highest dominant frequency sites of activation in PAF patients using power spectral wave analysis[41]. However, in longstanding persistent patients, the PV-LA junction does not appear to contain the highest dominant frequency sites in AF, suggesting a more prominent role for extra-pulmonary vein triggers in a more chronic patient[42].
Cardiac autonomic inputs originate from the central nervous system via pre-ganglionic fibers of the vagus and sympathetic chains as well as the intrinsic cardiac autonomic nerves. Within the latter, there are five major left atrial GPs that are located within epicardial fat pads (adjacent to the four PVs) and the Ligament of Marshall. The GPs contain post-ganglionic efferent parasympathetic and sympathetic axons as well as interconnecting neurons amongst the GPs. Firing of the GPs produces both parasympathetic and sympathetic outputs, which facilitate firing of the PV muscle sleeves[43]. Stimulation of the vagal and sympathetic trunks inhibits GP firing and PV automaticity[44]. GP location appears to be correlated with the presence of complex fractionated atrial electrograms (CFAEs) as assessed during endocardial catheter mapping[45]. CFAE-type electrograms (EGMs) can be produced via injection of acetylcholine (Ach) into GPs[46]; additionally, GP localization can be performed with 20 Hz high frequency pacing that produces AV block[47].
While the initiation of AF is a focal event modulated by genetics, autonomics, hemodynamics, endocrine & exogenous factors, and inflammatory mechanisms, the persistent nature of AF due to ongoing reentry (as Moe suggested) is an iterative process: an axiom attributed to Allessie and colleagues better known as“Atrial fibrillation begets atrial fibrillation”[48]. Over the first several weeks to months the atrium persistently fibrillates, electrical remodeling occurs characterized by: a shortening of the atrial action potential duration (APD) (via a decrease in the inward calcium current and outward potassium currents in Phase III), a slowing of conduction (due to a decrease in inward sodium current, tissue fibrosis, and impaired connexin function and gap-junction conductance), and then finally with structural atrial remodeling. The latter is a form of atrial tachycardia-induced cardiomyopathy initially due to hypocontractility because of abnormal calcium handling (transient) and later characterized by more permanent inter-cellular fibrosis and scar. With the new substrates of shortened refractoriness, slowed conduction and atrial enlargement with associated fibrosis, the conditions for a critical number of reentrant wavelets are met and AF can become selfsustaining[49].
As the atrial rate suddenly increases after AF occurs, severe intracellular Ca++loading occurs which is modulated by the L-type Ca++-channel reducing influx of the ion, preventing overload, but also shortening the APD; with persistence, the L-type calcium channels are down-regulated[50]. Potassium currents, including IK1[51]and IKAch[52]are increased during this remodeling phase as well, hyperpolarizing the atrial myocyte. Expression of connexin-40 is diminished contributing to lower conduction velocities[53]. Provided that the AF is short-term, electrical remodeling and the associated atrial hypocontractility and dilatation can all reverseremodel as well with a predictable time course[54].
Following many months of persistent AF associated with electrical and structural remodeling, more permanent pro-fibrotic changes to the interstitial atrial substrate begin to occur. This is a critical component for AF to become chronic, with enhanced difficulty of maintaining sinus rhythm despite medical and interventional therapies[55]. Ongoing inflammation, worsening hemodynamics, ongoing concurrent medical illnesses (hypertension, OSA, heart failure, as well as aging) continue to further adversely affect the atrial substrate during this time. Mediators of fibroblast activation and subsequent collagen synthesis and fibrosis include: angiotensin II (increased in response to tachycardia mediated heart failure), transforming growth factor beta-1 (TGF-β1), and platelet-derived growth factor (PDGF), and connective tissue growth factor (CTGF)[49,56]. It is during this time that unless sinus rhythm is restored, AF becomes chronic for a lifetime.
LATE CLINICAL OUTCOMES
As atrial fibrillation becomes more permanent, the most devastating complication increases in frequency,that being embolic stroke. Systemic embolization becomes more frequent with aging and several risk factors have been identified in patients with paroxysmal and permanent forms of AF; these have been analyzed within the CHADS2and CHA2DS2-VASc scoring systems (Fig. 3)[57,58]and are useful for counseling Western patients in regards to initiation of anti-platelet therapy with aspirin versus oral anticoagulant (OAC) therapies with vitamin-K antagonists (VKA) like warfarin or newer novel agents. A CHA2DS2-VASc score of zero truly predicts a group of patients at low risk for events akin to the group of Mayo Clinic lone-AF patients under age 60 years, Kopecky and colleagues identified over a quarter-century ago[59]. More recent studies from Asian populations have suggested the risk of stroke to be lower as compared to Western populations, even when applying the CHADS-type risk stratification systems[60-62]; hypertension may play a more prominent role in Asian populations, which is not accounted for as strongly in the CHADS-type systems[62].
Transthoracic echocardiogram (TTE), left atrial size above 44 mm, as well as transesophageal echocardiogram (TEE) parameters like: sluggish left atrial appendage (LAA) velocities, large LAA dimensions, and spontaneous echo contrast have been shown to correlate with higher stroke event rates[63-65].
Most episodes of AF are actually asymptomatic[66,67]. Recently, it has been demonstrated that sub-clinical AF as detected by implanted pacemaker or ICD, predicts a 2.5-fold increase for ischemic stroke or systemic embolization over a 2.5 year follow-up (4.2 % versus 1.7 %)[68]. The study included 2,580 patients who were 65 years of age or older with hypertension and no prior history of atrial fibrillation. The patients were initially monitored for 3 months during which time 10.1% of the cohort had sub-clinical atrial arrhythmias detected of greater than 6 minutes duration.
AF has been described in association with tachycardia-induced cardiomyopathy (TICM) as a causative factor since the early 20th century[69-71]. In this disease, patients do not feel symptoms from the AF and thus only present clinically with systolic heart failure due to fallen ejection fractions from uncontrolled rapid rates that often occur for weeks or months. Many patients mistakenly attribute their symptoms to pneumonia or an upper respiratory infection. Fortunately, this represents one of the few reversible causes of congestive heart failure (CHF), once the rates are controlled medically, with electrical cardioversion, or with ablative therapy. AF also exacerbates CHF symptoms in patients where the arrhythmia occurs secondary to other diseases like dilated cardiomyopathies (DCMs), rheumatic valvular heart disease, congenital heart disease, and end-stage coronary disease. Multiple (but not all) studies have demonstrated that AF has an adverse effect on overall CHF mortality (1.5-2X increase), particularly new-onset AF[71].
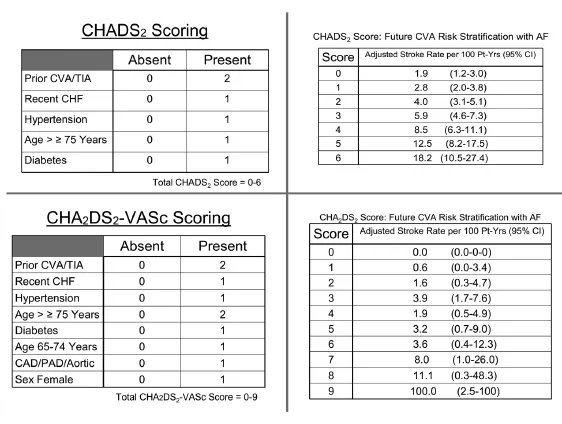
Fig. 3 CHADS and CHADS-Vasc: Scoring systems for assessment of subsequent annual stroke risk in the setting of nonrheumatic atrial fibrillation based on underlying disease processes and demographics. CVA: cerebrovascular accident; TIA: transient ischemic attack; CHF: congestive heart failure; CAD: coronary artery disease; PAD: peripheral artery disease.
Syncope and falls are quite common in elderly patients and can be precipitated by the “tachy-brady syndrome” in this disease, also called “sick sinus syndrome (SSS)”, patients develop AF with rapid response that co-exists with severe sinus node dysfunction which is unmasked when the patient transitions from AF into sinus rhythm. Subsequently, hemodynamically significant pauses develop during the restitution of sinus rhythm, prompting loss of consciousness or at a minimum, presyncope and a fall. As the population ages, this problem increasingly grows common. In a retrospective study of syncope in 711 very old institutionalized patients (mean age = 87 years), the 1-year incidence was 7% and the 10-year prevalence was 23%[72]; nearly a quarter of these syncope patients had a cardiac etiology: aortic stenosis or bradyarrhythmias. Diagnosis can be facilitated with longer-term telemetry monitoring such as 48-hour Holter, 10-30 day event recording, or implantable loop recorder (ILR) devices that can monitor well over a year. Pacemakers are effective in preventing further syncope in patients with SSS. Fewer patients develop persistent AF and experience less CHF if paced dual chamber (atrially) as compared in the ventricle alone[73].
A cross-sectional examination of the Rotterdam Study from 1997 suggested a relationship between dementia (of the Alzheimer's type) and the occurrence of AF in the elderly, particularly young elderly women (2X increase)[74]. Six years later, a subsequent sub-study correlated “silent” brain infarcts with the risk of dementia and decline in cognitive function in older patients[75]. A recent review of the existing literature suggests an association between AF and decline in cognitive function over time at 2-3 fold[76]. The reviewers cautioned, however, that a direct independent effect of AF causing dementia is yet not present. Nonetheless, they noted a higher incidence of silent strokes and more severe cognitive impairment in patients with persistent AF than those with paroxysmal AF, and both groups were more advanced than normal without AF.
THROMBOEMBOLIC PROPHYLAXIS
Peri-cardioversion
For over 3 decades, non-rheumatic AF has been a known independent risk for ischemic stroke, particularly in the elderly[63,77]. Since prior to the 1950 s, pharmacologic and electrical cardioversions have been known to enhance stroke risk. Following the introduction of warfarin in the 1950s, stroke rates following pharmacologic or electrical conversions to sinus rhythm were reduced. A prospective cohort study from 40 years ago documented the incidence of embolic events to be at 5.3 percent in patients not receiving, and 0.8 percent in those receiving warfarin[78,79]. Other studies from the 1960s[80,81]documented similar patterns. Conversion with antiarrhythmic drugs also can pose risks, as a retrospective study using quinidine suggested a comparable risk of embolization (1.5%)[78,82]. Anticoagulation prior to conversion thus is mandated in patients with atrial fibrillation of more than 48 hours or when duration is uncertain[83,84]. Indeed, for patients with structural heart disease, a cutoff of 24-36 hours may be more appropriate. In 1997, Weigner and coworkers examined the risk for thromboembolism associated with active conversion of atrial fibrillation to sinus rhythm in patients with AF for less than 48 hours[85]. Of 357 patients, 107 patients converted spontaneously without an event; 250 underwent pharmacologic or electrical conversion. Thromboembolic events occurred in 3 individuals (1%). While this rate is low, it was not negligible, and suggested that, for higher risk patients, a 24-36 hour cutoff may be more reasonable.
For patients who are to undergo elective cardioversion, it is recommended that a minimum of 3 weeks of therapeutic oral anticoagulant (OAC) be given prior to the conversion either with a warfarin or the NOAC (novel oral anticoagulant) dabigatran[86,87]. A minimum of 4 weeks of OAC is prescribed following cardioversion, based on the assumption that it takes approximately four weeks for a thrombus to organize and adhere to the atrial wall once it has developed, provided that anticoagulation therapy has been prescribed. Atrial contractility does not return after cardioversion for up to four weeks[88,89].
Transesophageal echocardiography (TEE) can be used as an alternative to the requisite 3 weeks of OAC prior to cardioversion[90,91]. In patients whose atrial fibrillation is of longer than 24-48 hours duration, TEE has documented LAA thrombi in approximately 15 percent of individuals with low blood velocity by Doppler seen in approximately 40 percent[92]. A prospective study on the utility of TEE in AF patients undergoing cardioversion demonstrated 6 of 40 clots in the right atrium, while 34 were localized to the left atrial appendage[93]. Thrombus size ranged from 2 to 20 mm. Factors associated with LAA thrombus included recent stroke or transient ischemic attack (TIA), decreased ejection fraction, spontaneous left atrial contrast (smoke), and rheumatic heart disease. Ninetyfive percent of atrial thrombi visualized by TEE were not visualized by accompanying TTE.
A negative TEE does not, however, guarantee cardioversion of AF without embolization[94]. Im-proved sensitivity for identification of LAA thrombus by TEE has been achieved utilizing echo-contrast agents[95]. If an LAA thrombus is identified by TEE, it remains unclear how long to anticoagulate the patient prior to cardioversion. In one study, repeat TEE evaluations were performed in 21 patients with LAA clot; only 43% of LAA clots identified on TEE resolved within 5-17 weeks and an additional 28% were rendered immobile[96].
Long-term antithrombotic management in AF
The European Society of Cardiology in their 2012 update guideline statement reviewed long-term recommendations for OAC therapy for patients with AF[86]. OAC therapy should be given to AF patients with rheumatic or prosthetic valvular heart disease, hypertrophic cardiomyopathy, thyrotoxicosis[83], and to patients at high risk based on CHA2DS2-VASc scores of 2 or more (the 1 point for female sex is only included if patient is > 65 years). These recommendations are irrespective of whether the patient is paroxysmal or persistent.
Multiple trials in the 1980s and 1990s demonstrated the superiority of warfarin over aspirin in AF patients. Aspirin typically would only reduce stroke rates by 20% while warfarin would by 70%. Hylek, et al. demonstrated the occurrence of thromboembolic events in warfarin treated patients was inversely related to the intensity of anticoagulation[97]. Whereas stroke risk was very low in patients with an INR maintained between 2 and 4, the event rate rose sharply with international normalized ratio (INR) values below 1.8. These investigators also identified INR values above 4 as associated with intracerebral hemorrhage complications[98]. More recently, Asian investigators have proposed an optimal level for OAC therapy in Chinese patients at 1.8-2.4 rather than the 2-3 suggested in Western populations and is consistent with clinical observations of more bleeding in Chinese populations treated with higher dose warfarin[99].
Warfarin is effective, but despite its discovery over 70 years ago[100], it has remained a challenging drug for clinicians and patients, due to its narrow therapeutic index, multiple drug and dietary interactions, variable metabolism (that only recently has been addressed with pharmacogenomics [VKORC1, CYP2C9, CYP4F2 enzyme pathways][101]), long half-life, and constant need for INR monitoring, either at a physician office or home monitoring[102,103]. Patients remain outside of therapeutic INR values on a regular basis, 55% of the time[104]. Unfortunately, three recent trials have shed doubt on the value of pharmacogenomics for initiation of OAC therapy compared to clinical algorithms[105-107].
Over the last decade, a multitude of new approaches for management of the LAA space with NOACS (dabigatran[108], rivaroxaban[109], apixaban[110], and edoxaban[111](Fig. 4)) and mechanical procedures (resection/amputation either surgically[112-114]or via video assisted thoracic surgery (VATS)[115], surgical clipping (instead of suturing or stapling)[112], percutaneous endocardial mechanical plugs (WATCHMAN[117], AMPLATZER[118], PLAATO[119]), or percutaneous epicardial suture closure (LARIAT)[120]) have been utilized and in many cases have compared equally or favorably to warfarin[86].
It should be noted that the dabigatran dose in Figure 4 from RE-LY was 150 mg twice daily. Dabigatran is the most dependent on renal excretion of the 4 NOACs while apixaban is the least. These two agents are dosed twice a day while rivaroxaban and edoxaban are once a day dosing. The relative efficacy of these agents is currently unknown in head-to-head comparisons as are their risks and benefits when coupled with dual anti-platelet therapies in patients who have received coronary stents. Recommendations concerning the use of warfarin therapy with dual antiplatelet therapies has been addressed in recent ESC guidelines risk stratifying these patients based on HAS-BLED scores 0-2 vs. 3 or greater[86].
The maximum HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/ Alcohol Concomitantly) score is 9 with annualized bleeding rates while on warfarin for scores of 0-5 as follows: 0.9%, 3.4%, 4.1%, 5.8%, 8.9%, and 9.1%[121]. Major bleeding (usually gastrointestinal or intracerebral) as well as repeated falls remain concerns amongst clinicians attempting to reduce stroke rates with OACs. Nevertheless, older patients with multiple CHADS risk factors stand greatly to benefit from such therapies.
As an alternative to OAC, mechanical LAA occlusion or isolation has also received attention. Surgical resection of the LAA as a means to prevent recurrent arterial emboli in patients with rheumatic heart disease was reported in 1949[112]. Localization to the LAA of thrombi is seen in 91% of patients with non-valvular AF as compared to 57% of patients with rheumatic valvular related AF[113]. Using a combination of the Cox-Maze to achieve sinus rhythm and surgical LAA removal/isolation in the 1980s-1990s, Cox achieved an annual incidence of stroke in a surgical series of over 300 patients (of whom 19% had had prior stroke or TIA) of < 0.5% per year[114].
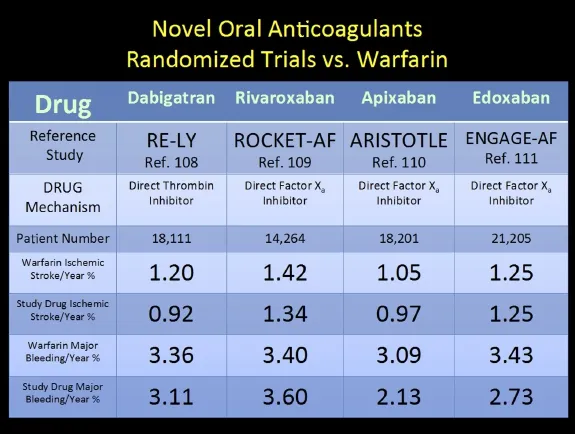
Fig. 4 Novel oral anticoagulants (NOACs) and representative prospective randomized trials against conventional warfarin therapy. Comparison of NOAC versus warfarin with respect to annualized risk of stroke and bleeding.
However, the optimal technique has not been established; LAA complete occlusion rates as determined by post-op TEE with suture or stapling closure in one study were reported at only 45% and 72%, respectively[122]. While patent LAA mouth connections to the LA might be associated with higher risks of stroke off OAC[123], similar rates have been found in surgical[124]and device[125]trials thus far. Additionally, the safety profiles of the current percutaneous devices are just being clarified[126]and are the subject of ongoing study. At present, there is no role for LAA isolation/occlusion as a substitute for OAC[86]. Patients to be considered for LAA isolation or occlusion will have multiple risk factors for stroke and either contraindications, major complications, or prior failure of OACs.
Understanding the variability of LAA anatomy[127](20% single lobe, 54% double lobed, 23% triple lobed, 3% four lobed in a US group of normal hearts) and how that influences optimal technique and device selection will be an area of future investigation. A recent Chinese paper suggested single lobes (60%) were actually more common than double (27%) or higher lobe number (13%) in AF patients as compared to ASD patients[128]. These investigators described 8 morphologies of the LAA: tube, claw, sphere-like, tadpole, willow-leaf, sword, duckbill, and irregular with the tube morphology being the most common in AF patients.
In this first half of review, the incidence and prevalence of atrial fibrillation are increasing in the Western countries. The impact on public health, particularly in the most rapidly growing elderly population is alarming. Mechanisms of atrial fibrillation are multiple and complex, encompassing focal triggers to diffuse substrates with complex interactions from structural changes, electrophysiological modulation, inflammatory reactions, autonomic balance to genetic/molecular predisposition and modification. One of the most devastating complications associated with atrial fibrillation is stroke. Risk stratification schemes of stroke have been developed and will continue to evolve. Risks of bleeding while taking OAC have been stratified into scoring systems. A number of new OACs have been approved in clinical use in the last few years. It is anticipated that future studies will provide additional information guiding clinicians to the most appropriate use of a given new OAC for a particular population to achieve the most cost effective outcomes.
RATE CONTROL
Medical therapy
Most patients, who are in persistent AF and have adequate rate control, feel remarkably well, particularly the elderly who are not as physically active asyounger individuals and typically have slower AV nodal conduction. Until the 1990s, it had been surmised that there was a mortality benefit in being in sinus rhythm and great effort was made to insure that even in asymptomatic individuals. Several studies including the AFFIRM[129,130]trial proved this wrong; the trial included over 4,000 patients who were randomized to either rhythm or rate control following cardioversion. It demonstrated there was neither survival nor stroke benefit imparted by restoration of sinus rhythm over a 5-year follow-up. Therefore, the goals of rate control would include relief of symptom, which could include fatigue and mental dullness, prevention of tachycardia-induced cardiomyopathy, appropriate OAC prophylaxis, and prevention of medication side effects. The RACE II Investigators demonstrated that a rate control strategy that used resting heart rate < 110 beats per minute as a more strict value of < 80 beats per minute was as effective in regards to death, CHF hospitalization, stroke and embolism, bleeding, and life-threatening arrhythmic events was at 2 years follow-up[131]. The authors found similar incidence of “symptoms” in both groups although severity of symptoms was not quantified. These investigators previously noted that quality of life is impaired in AF patients compared to normal age-matched controls and may be improved if sinus rhythm can be maintained[132].
Rate control to allow cardiac resynchronization therapy (CRT) to maximize pacing benefits to patients with systolic CHF with rapid AF is also a desired endpoint from rate control[133]. Occasionally, urgent cardioversion is necessary to stabilize a patient's rate if there is no response to conventional IV forms of diltiazem or beta-blockers. This is not infrequent in patients with severe underlying structural heart disease (severe coronary artery disease, aortic stenosis, hypertrophic cardiomyopathy); additionally, patients who are at risk for sudden death in the setting of Wolff-Parkinson-White syndrome[134]and do not respond to conversion with intravenous antiarrhythmic drugs or ibutilide should be considered for urgent cardioversion for rate control.
Digoxin is the oldest available of the AV nodal blocking drugs, dating to the 18th century when Withering reported on its use from the foxglove plant in heart failure in a series of 163 patients[135,136]. As a single agent, it is inferior to the calcium and beta-adrenergic antagonists for ventricular rate control. It has not been shown to facilitate conversion to sinus rhythm. It should be considered a second-tier rate drug unless the patient has severe left ventricular dysfunction and heart failure. Beta-blockers such as metoprolol, atenolol, propranolol, and carvedilol should be considered first tier therapy for rate control, particularly for patients with structural heart disease. Calcium channel blockers like diltiazem and verapamil are also frequently prescribed, particularly in the emergency room setting for acute medical rate control. There is an additive effect of these agents favorably influencing resting and exercise heart rates[137]. Clonidine, a central alphaantagonist, has also been shown to have a favorable effect on AV nodal conduction during AF[138].
Catheter ablation of the AV node
Atrioventricular node (AVN) catheter ablation using direct current was first reported in 1982 as a method to slow down medically unresponsive supraventricular arrhythmias, including atrial fibrillation[139,140]. Complete disruption of the conduction system implied insertion of an electronic pacing system. In the late 1980s, the technique has utilized alcohol coronary injection, and most commonly radiofrequency heating to cause the lesions. In the absence of structural heart disease, the survival of patients with AF undergoing AVN catheter ablation is excellent and is similar to the age-matched general population[141]. In a recent meta-analysis of 5 historical trials back to 1997 of AVN ablation vs. pharmacologic therapy[142], there was no difference in mortality between the 2 groups, and procedure-related mortality was low at 0.27%; at a 27-month follow-up, there was a 2.1 % incidence of sudden cardiac death. Quality of life and the symptoms of palpitations and dyspnea were definitely improved in the procedural group as was the ejection fractions in the majority of patients who had preexisting left ventricular dysfunction. In a more recent randomized trial examining the use of CRT versus conventional RV pacing in patients with pre-existing systolic heart failure and AF undergoing ablation, it was found that the composite endpoint of death from CHF + hospitalization or worsening CHF occurred in 11% of the CRT group and 26% of the RV group[143].
RHYTHM CONTROL
Acute conversion with antiarrhythmic drugs
In general, younger patients who are more active are the ones who cannot be adequately managed with rate control and seek sinus rhythm maintenance. Fortunately, these symptomatic patients are those most likely to remain in sinus rhythm once it has been restored, either spontaneously, medically, or with electrical cardioversion.
For most new-onset AF patients, spontaneous conversion does occur. In one retrospective study of 356patients with AF < 72 hours duration before presentation, the spontaneous conversion rate was noted to be 68%[144]; of that group, 66% converted within 24 hours, another 17% in 24-48 hours, and another 17% in 48-72 hours. Spontaneous conversion was only predicted by an AF presentation that had been < 24 hours. In Europe, intravenous forms of the Class I-C drugs flecainide and propafenone have been available; in the United States, IV amiodarone is available which offers rate control but no real enhanced conversion rates until patients have been on the drug for 24 hours[145,146]. The Class I-A procainamide is also available IV in the US and can enhance early conversion at 2-4 hours[147]. Oral flecainide (200-300 mg) or propafenone (450-600 mg) can be given on an outpatient basis “pill-in-pocket approach” or in the emergency setting to facilitate early conversions in the 3-8 hour time frame[148]. Intravenous ibutilide, an IKr blocker that enhances late sodium currents, can also be given. Ibutilide, released in 1996, can facilitate AF conversion rates of 50% in 0.5-2 hours and higher with atrial flutter[149]. However, patients require monitoring for 6-8 hours after dosing for excessive prolongation of QT intervals and Torsade's de Pointes (1.7%).
Vernakalant (RSD1235) is a novel “atrial repolarization-delaying agent” with its main target the ion Kv1.5 channel that carries the IKur current which is chiefly in the atriums and not the ventricles[150]. Multiple trials have been completed in Europe where the drug has been approved since 2010 for intravenous conversion of AF. A recently completed study examining conversion rates at 90 minutes of recent onset (< 48 hours) AF in 254 patients demonstrated a 52% conversion rate for IV vernakalant versus 5% for IV amiodarone[151,152]. Another trial comparing vernakalant to flecainide demonstrated a mean time to AF conversion of 10 minutes versus 2.7 hours and a reduction of hospital stay of about half (P < 0.0001)[153]. There were no cases of ventricular arrhythmias, making this a very promising drug for acute conversion.
Long-term sinus rhythm maintenance with antiarrhythmic drugs
The antiarrhythmic drugs and so-called “upstream”drugs for aiding in sinus rhythm maintenance are shown in Fig. 5 and are patterned after the AHA/ACC 2011 AF guideline update[154].
While digoxin, beta-blockers, and calcium blockers help control the rate of AF, they do not have any effect on the incidence of recurrences unless linked with another rhythm like PSVT or medical conditions like hyperthyroidism, hypertension, or heart failure. A list of the currently available oral membrane-active antiarrhythmic drugs in the United States is shown in Fig. 5.
All of the oral membrane active antiarrhythmic medications except the Vaughn-Williams Class I-B drugs (mexiletine and phenytoin) have activity in the atria. Quinidine is the oldest of the medications; the Inca Indians of Peru used the bark of the cinchona tree in the 15th century to treat fevers (likely malarial)[155]. Quinidine, a related compound to quinine (both alkaloids from the tree's bark) was described by Van Heymingen in 1848 and named by Pasteur in 1853 when he used the drug as an alternative to quinine as an antimalarial[156]. In 1914, one of Wenckebach's patients pointed out to him that the quinine he had prescribed for malaria had made the patient's irregular heart beat (AF) become regular once again; in 1918, Frey established that quinidine was more effective than quinine as an antiarrhythmic[157]. While the drug is not first line antiarrhythmic therapy for atrial fibrillation any more, it has made a resurgence as a desired agent for several contemporary arrhythmia syndromes: Brugada syndrome, short QT syndrome, and J-wave syndrome[156].
In fact, quinidine's clinical history over the last century really mirrors concerns about all the other AF antiarrhythmic agents on the list in Fig. 5: the increased risk of stroke with medical conversion to sinus rhythm and ventricular proarrhythmia with syncope or sudden death, particularly in patients with underlying structural heart disease. A systematic review at the Harvard and Yale hospitals of quinidine use was reported in 1923 and suggested that two-thirds of persistent AF patients could be restored, at least temporarily to sinus rhythm with CHF adversely effecting longer term maintenance of AF; reports of embolization and sudden death in association with sinus rhythm restoration were noted[158].Thirty-five years later, after the advent of OAC therapy and continuous ECG monitoring, it became clear that quinidine could cause sudden death not related to embolization in up to 4% of patients receiving treatment[159]. Selzer and Wray coined the term quinidine syncope in 1964 to describe the symptoms due to polymorphic VT that was characteristic of drug induced excessive QT prolongation and the associated early afterdepolarization (EAD) activity[160]. Other drugs have replaced quinidine as first line therapy for long-term sinus rhythm maintenance, but still require vigilance in the safety of their use[161-163].
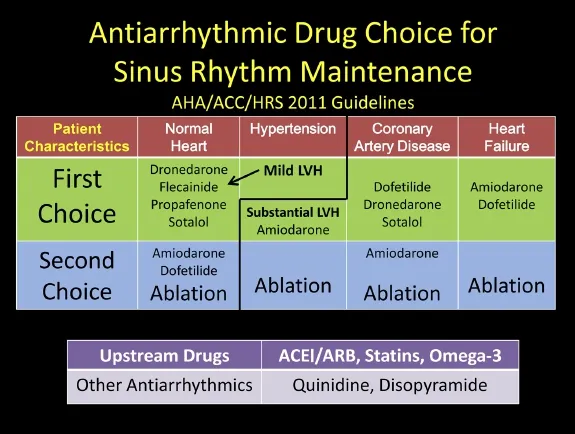
Fig. 5 Antiarrhythmic drug choice for maintenance of sinus rhythm based on underlying structural heart diseases. Nonantiarrhythmic drugs (upstream) demonstrated to be of benefit in patient groups with AF for primary or secondary prevention.
As seen in Fig. 5, patients with structurally normal hearts are advised to take sodium channel blocking agents like the class I-C drugs flecainide or propafenone or potassium blocking class III agents like dronedarone or sotalol. These four medications can be initiated in the outpatient setting safely during sinus rhythm for the younger patient without structural or conduction system disease[83]. These medications are all available as twice daily dosing that aids in patient treatment compliance. Patients initiated on a Class IC drug should have a follow-up ECG after 5 drug half-lives to insure that the QRS duration (QRSD) has increased by 10%-20%, indicating the appropriate conducting slowing effect of a sodium channel blocker. Patients begun on Class III agents should also be monitored with ECGs to be assured that there is some QTc prolongation, not exceeding 500 msec, which indicates a threshold for significant increase in ventricular proarrhythmia. For amiodarone patients, the threshold for concern is higher, in the 550-msec. range. For paroxysmal patients, both drugs are very well tolerated, although flecainide slightly more so than propafenone[164-166]; both medications are equally effective[167].
In fact, most of the antiarrhythmic drugs have similar rates of effectiveness and are definitely superior to placebo (Fig. 7)[168-173]. In 2009, a large meta-analysis (Fig. 8) involving 44 randomized trials and over 11,000 patients was compiled[174]. The study included drug vs. placebo and drug vs. drug trials with at least 6 months of follow-up. The study suggests overall response rates for maintenance of sinus rhythm at follow-up for AF patients as follows: no antiarrhythmic drug (AAD) therapy—10%-35%, Class I & III agents (except amiodarone)—25%-60%, Amiodarone—55%-70%. Ranges in these studies occur due to variable patient demographics (especially age), time to follow-up reporting, mix of persistent versus paroxysmal patients, time period historically the study was conducted, and the degree of surveillance monitoring[175]that was done to assure a patient is in sinus rhythm. Of all the agents, amiodarone is the singly most effective for preserving sinus rhythm long-term, although it has never received an FDA labeling for that indication[176-179]. The various toxicities of amiodarone should be sought in the patient on the agent long-term, and include but are not exclusive of: pulmonary, optic neuritis, liver, thyroid (either hyperthyroidism or hypothyroidism), skin discoloration and photosensitivity[180]. Important drug interactions with amiodarone include those of warfarin and statins.
The more recently released Class III AADs dofetilide[181](which is very similar to sotalol without the beta-blocking activity)and dronedarone[182](related to amiodarone without the iodine components) also have similar effectiveness profiles compared to the older drugs and are well tolerated. Dronedarone does not share the propensity to cause toxicity to the lungs,skin, eyes, and thyroid as compared to amiodarone although it can affect liver function tests and aggravate heart failure[184]; it is also less effective[185]. The drug has a low ventricular proarrhythmia profile and does not need to be started in the hospital. There is a significant drug interaction with the calcium channel blocker diltiazem.
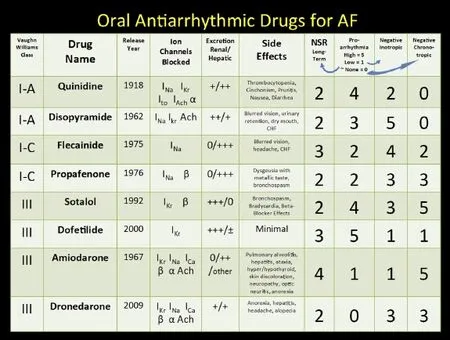
Fig. 6 Antiarrhythmic drug choice for maintenance of sinus rhythm based on underlying structural heart diseases. Nonantiarrhythmic drugs (upstream) demonstrated to be of benefit in patient groups with AF for primary or secondary prevention.
Dofetilide is excreted by the kidneys and like sotalol should be dose-adjusted in patients with impaired renal function. The drug has been mandated by the US Food & Drug Administration (FDA) to be initiated in the hospital setting and has several drug interactions including verapamil, hydrochlorothiazide, cimetidine, ketoconazole, trimethoprim, prochlorperazine, and megestrol since these agents can lead to increased levels of dofetilide. Similar to amiodarone, it does not adversely affect mortality in congestive heart failure patients and thus can be used safely in patients with severely impaired left ventricular dysfunction[185-188].
Since the AADs all have a similar efficacy (except amiodarone), the physician chooses the drug for the patient deemed to be a candidate for rhythm management on several considerations, including:
1. Baseline left ventricular (LV) function (avoid drugs that significantly depress LV function like disopyramide, flecainide, and sotalol).
2. Presence of coronary artery disease (avoid Class IC drugs: flecainide in particular implicated in ventricular pro-arrhythmia in the CAST trial[189]).
3. “Vagally-induced” AF in younger patients (disopyramide might be preferred).
4. Patient compliance (Drugs dosed once or twice a day like amiodarone, sotalol, flecainide, dronedarone, dofetilide, propafenone SR, disopyramide SR would be favored over 3-4 x per day scheduling like with short acting quinidine or disopyramide).
5. Patient cost (Short acting quinidine and disopyramide would be the cheapest, while the newer agents dronedarone and dofetilide would be the most expensive).
6. Nuisance side effects.
7. Post-MI or CHF would favor amiodarone or dofetilide.
8. Coincident medical illnesses (avoid quinidine in patients with myasthenia gravis; avoid sotalol in patients with asthma or renal insufficiency; avoid amiodarone in a patient with severe emphysema). (See Fig. 6).
Upstream drugs for primary and secondary prevention of AF
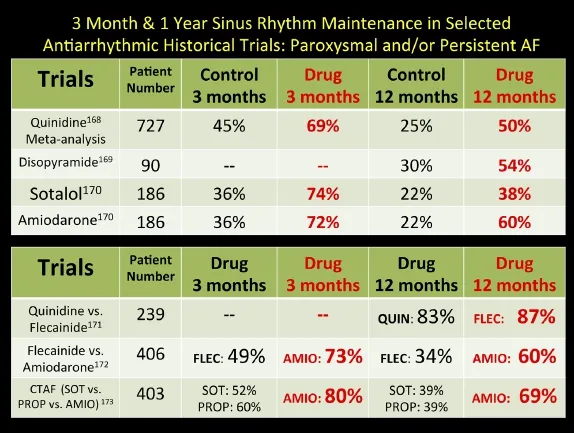
Fig. 7 Relative effectiveness of oral membrane-active antiarrhythmic medications in the long-term maintenance of sinus rhythm in patients with atrial fibrillation based on selected randomized controlled trials.
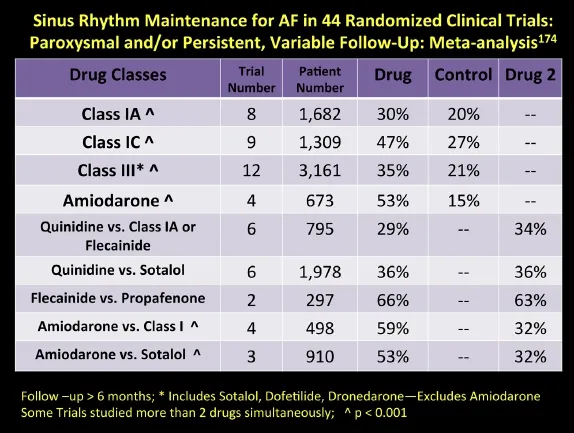
Fig. 8 Relative effectiveness of oral membrane-active antiarrhythmic medications in the long-term maintenance of sinus rhythm in patients with atrial fibrillation based on various meta-analyses of 44 randomized controlled trials.
These drugs are non-antiarrhythmic drugs that have been shown to have a favorable effect on the subsequent incidence of AF. Angiotensin Converting Enzyme inhibitors (ACEis) and Angiotensin Receptor Blockers (ARBs) are classes of drugs that are vasodilators. Captopril, an ACEi, was first tested in heart failure patients in the late 1970s[190]. These classes ofdrugs were shown to have multiple favorable pharmacologic effects in diabetic nephropathy[191-193], post-MI with left ventricular dysfunction[194], and for the prevention of AF[195-200]. Meta-analyses confirmed the effect in hypertension and heart failure trials, indicating prospectively a 50% reduction in future AF burden in patients treated with these agents as compared to beta-blockers[201,202]. Atrial fibrosis is a prominent feature in advanced heart failure and can be favorably altered by ARBs and statins[203,204]; besides a beneficial consequence on atrial hemodynamics in hypertensive patients, these drugs also display “direct” electrophysiological effects in vitro. In canine pulmonary vein muscle sleeves, the drugs losartan and enalapril reduced or eliminated delayed after-depolarization (DAD) triggered firing[205].
Statins were released in the 1980s for management of hyperlipidemia, a risk factor for coronary artery disease. They also have an anti-inflammatory component that can be used favorably in patients with atrial fibrillation[206]. Collating multiple studies show a 40-60% reduction rate in AF incidence, most prominent in secondary prevention situations and less so in longterm primary prevention[207-209]. Omega-3 fatty acids, as found in fish oil, have also been proposed as agents for prevention of AF after a small post-surgical study of 160 randomized patients demonstrated a 54% reduction in post-operative atrial fibrillation (POAF)[210]. However, several meta-analyses have been completed in recent years and have shown no clear benefit for secondary prevention or in prevention for POAF patients[211-214].
Post operative atrial fibrillation (POAF)
The incidence of POAF remains high approaching 40%, and adds significant hospitalization time and costs to a patient convalescence from cardiac surgery[215-217]. Meta-analyses and large prospective randomized clinical trials have demonstrated the advantageous effects (reductions of over 50%) of multiple pharmacologic strategies including betablockers (carvedilol favored over metoprolol), sotalol, amiodarone, and statins[218-225]. Several studies have also revealed the value of perioperative atrial epicardial pacing[226]as well as intravenous magnesium administration[227]. ACEi and ARBs do not seem to have similar benefits in POAF patients as compared to primary prevention patients with hypertension or heart failure[228].
Ablation targets & strategies
In 1964, Moe and coworkers used a digital computer to examine a mathematical model of conduction through a nonuniform two-dimensional space[229]. The model was similar to Moe's concept of AF, not the result of fixed focus generators or circuits, but rather as was described: “irregular drifting eddies which varied in position, number, and size.” By lengthening the refractory period the computer-generated arrhythmia could be terminated. By reducing the area of the model a similar phenomenon was observed.
In the next decade, with these observations in mind, Cox performed animal experiments and developed a surgical operation he termed the Maze, that effectively reduced the area allowed for reentrant circuits to wander around the atrium, thus promoting termination[230]. Included in the Maze lesion sets was also isolation of the PVs, as well as the maintenance of sinus node conduction to the AV node. The lesion sets of the Cox-Maze-III cut and sew procedure still serve as the gold standard for a non-pharmacologic therapy to maintain sinus rhythm.
In the early 1990s, clinical experiences from both the Cleveland Clinic[231]and Mayo Clinic[232]demonstrated 1 and 3 year follow-up sinus rhythm maintenance rates of 90% with associated surgical mortality rates of 1% and need for pacemakers of approximately 5%. A more recent review from Mayo demonstrated 10-year follow-up sinus rhythm maintenance rates of 62-64% for both paroxysmal and persistent patients[233].
Last year, an international task force from Asia, Australia, Europe, and North America representing 7 professional societies published a consensus statement about the current state-of-the-art in regards to surgical and catheter ablation of atrial fibrillation[234]. Radiofrequency ablation began for SVT type rhythms in the late 1980s. Early in the 1990s, Swartz, et al. demonstrated linear portions of the Cox-Maze procedure could be replicated with endovascular telescoping sheaths and catheters[235]. Three cases of pulmonary vein ablation for treatment of AF came from Bordeaux in 1994[236].
During the latter half of the 1990s, various RA and LA linear lesions sets were studied, but it soon became apparent that the muscle sleeves[37]in the pulmonary veins were the triggers for atrial fibrillation in up to 80-90% of patients, particularly paroxysmal patients. Spontaneous PV firing was mediated by late phase 3 EAD, as well as DAD triggered activity[205,237-239]. Initially, ablation was carried out in the candidate vein based on spontaneous PV activity and imaging using PV angiography. Over time, PV isolation has remained the cornerstone of ablation paradigms[240-242].
In the late 1990s, intracardiac ultrasound, multipolar electrode circle catheters, and 4-D mapping sys-tems were introduced and aided in patient monitoring, detailed PV mapping, and the assessment of lesion continuity. Pappone found increased efficacy of the procedure using electroanatomic mapping combined with wide area circular lesion sets around all four veins[243]. Enhancing effectiveness during this time was the introduction of the 8 mm catheter electrode and the irrigated-tip catheter electrode, which were capable of creating larger, deeper lesions[244,245].
Chen and colleagues categorized non-pulmonary vein focuses including: LA posterior free wall, the superior vena cava (SVC), the crista terminalis, the ligament of Marshall, the coronary sinus ostium, and the inter-atrial septum[246]. More recently, it has been appreciated that muscle sleeves in the non-coronary cusp of the aorta can also be an AF generator site[247]. Nonetheless at re-do procedures, approximately 80% of patients have recurrent PV conduction present that requires re-treatment[241]. Several studies were conducted in the early-mid 2000s assessing optimal lesion sets[248-254]; while PV isolation (PVI) was satisfactory for paroxysmal patients, PVI alone for more persistent patients was unsatisfactory. Thus, linear lesion sets involving the LA roof and mitral isthmus were added, akin to the Maze operation[255].
Most of the 8 randomized clinical trials of ablation have compared it against antiarrhythmic drugs and involved mostly PAF patients with 1-year followups[234]. Freedom from AF has been in the 66-89% range in the ablation groups and 9-58% in the drug arms. In surveys from approximately 265 centers, redo rates were quoted at 27-33% with major complications at 6% and freedom from AF at 10 months without drug therapy at 70%[234]. A single center report from the Mayo Clinic demonstrated that 24% of patients were on antiarrhythmic drugs at 2 years, 15% had been redone in the first 10 months and the univariate predictors of recurrence were: age, hypertension, diabetes, persistent AF, a family history of AF, and LA size larger than 45 mm[256].
By the mid 2000s, other substrate targets were being sought. The ganglionated plexi of autonomic nerves as characterized by complex fractionated atrial electrograms (CFAEs) were included in the ablation lesion sets[257-263]. Meta-analyses of studies where randomization was made to include CFAE-directed ablation found enhanced success in persistent patients (as with linear) although no incremental benefit over PVI alone in paroxysmal patients[264,265].
Complications of ablation
A worldwide survey recently documented the most frequent serious complications associated with catheter ablation as tamponade (the most common cause of death), esophageal-atrial fistula, and embolic stroke[266]. Cardiac tamponade can occur in 0.2-6.0% of series and if unrecognized can lead to death[234,267]. Delayed tamponade has been also reported and can be associated with Dressler's syndrome and later constrictive pericarditis; early recurrence of AF is more common in these patients who have elevated inflammatory markers[268-271]. Treating patients with corticosteroids can prevent early AF after radiofrequency catheter ablation[272]; what remains unanswered is whether there is an unfavorable effect on scar formation late following the ablation that could lead to more recurrence[273].
Stroke and TIA rates have been reduced (0-7%) with transseptal puncture following heparin anticoagulation and the use of intracardiac ultrasound[274,275]. Silent emboli as detected by MRI have been reported in 7-38% of cases[234]. The long-term significance of these findings has yet to be characterized fully. Fifteen years ago the risk of pulmonary vein stenosis was at least 5% and has fortunately fallen to < 1 %, but still occurs. It may be asymptomatic or present similar to a pulmonary embolism with dyspnea, chest pain, and hemoptysis, usually in the 2-6 month time frame after the procedure[276]. Patients can receive balloon venoplasty and stenting if appropriate for relief[277].
Thermal injury to the esophagus has occurred in surgery, with endocardial RF ablation and with cryoablation. If severe enough, this forms a fistula between the LA and the esophagus causing endocarditis, sepsis, seizures, strokes, and/or gastrointestinal bleeding at 2-5 weeks following the procedure[278-282]. No reliable way of avoiding this complication, which occurs in 0.1% of cases, has been found. Low powers applied to the posterior wall (20 Watts), esophageal temperature monitoring, avoidance, moving the esophagus, and post-procedural proton pump inhibitors have all been advocated, but unproven[234].
Right phrenic nerve injury is also a known complication related to ablation at the posterolateral SVC or the right pulmonary veins, particularly the superior[283]. In the early European experience with the Cryoballoon device, the incidence was 8-10% with freezing of the RSPV, which is now avoided using a larger balloon and by simultaneous diaphragmatic pacing during freeze application[284]. About half of all patients have symptoms of dyspnea with diaphragm paralysis, which is reversible, but can last up to 12-18 months. Other less common complications include acute occlusion of the left circumflex coronary artery during mitral isthmus line ablation[285]and entrapment of circular mapping catheters in the mitral valve appara-tus[286]. Radiation exposure to patients and operators is increased in obese patients[287]and can be decreased by the use of robotic-driven catheter systems[288].
Ablation in 2014
Over the last decade, catheter ablation has been demonstrated to be cost effective[289]. For several subgroups of patients, including those who are obese[290], with hypertrophic cardiomyopathy[291]or heart failure[292,293], with diastolic dysfunction[294], and the very elderly[295,296], results and safety profiles are consistent with other studied groups. Yet, no randomized clinical trials exist in large numbers of women, ethnic minorities, patients with longstanding persistent AF, or those over 75 years and will need to be completed.
Newer energy sources have just begun to be utilized[297-299]and small studies of the Cryoballoon versus RF ablation have been reported[300,301]. Robotics driven mapping and RF ablation have been performed and also await rigorous prospective study[302,303]. Assessment of cell death acutely has been challenging and has been based on PV exit and entrance block. Lately, adenosine has been used as a pharmacologic tool by which to assess the difference between acute cell death versus cell “stunning”[304,305]. The role of new integrated imaging techniques is also being explored[306,307].
In the surgical arena, minimally invasive techniques, utilizing microwave, radiofrequency, and cryoablation energy sources have grown with results similar to catheter ablation series[308]. Hybrid procedures involving both the electrophysiologist and cardiac surgeon involving thoracoscopic video-assisted (VATS) PVI and ganglionated plexus ablation are being performed[309].
Atrial fibrosis remains a large unsolved problem. Age remains the most important factor that drives the prevalence of AF and the failure to restore sinus rhythm long-term[310]. Atrial fibrosis burden has been assessed by MRI by the University of Utah group and may prove to be an important tool for judging the efficacy of medical or invasive techniques for long-term sinus rhythm maintenance[311]. It appears it is a marker for stroke risk and sinus node dysfunction[312,313]. The ability of the atrium to remodel after restoration of sinus rhythm is also likely a marker of the atrium's ability to maintain sinus rhythm long-term and perhaps of atrial fibrosis[314]. The ability to ablate chronic AF may not portend well for atrial transport function if the muscle carries with it a significant fibrosis burden[315,316].
The atrium that has minimal fibrosis may not require extensive ablation if rotors prove to be a viable target[317]. Narayan and coworkers recently reported on the use of computer-identified rotor wavefronts from the atrial endocardium that when ablated, terminate AF[318]. This work is exciting and hopefully can be reproduced.
CONCLUSION
In the future, we need a nationwide or worldwide registry of atrial fibrillation treatment; for ablation, in the United States, the SAFARI registry has been proposed[319], but is not yet up for enrollment. The appropriate strategies for utilization of invasive rate control vs. rhythm control strategies are needed for both the elderly[320]and heart failure patients[321]. New tactics for inexpensive and centralized monitoring may have a dramatic effect on stroke incidence[322-324]. As worldwide AF dramatically increases in the next 20 years, a significant burden on health care systems in multiple countries will occur. It remains imperative that further research into the epidemiology, genetics, detection, and treatments of AF pushes forward rapidly.
[1] McMichael J. History of atrial fibrillation 16281819, HarVvey, de Senac, Laennec. Br Heart J 1982; 48:193-7.
[2] Lewis T. Auricular fibrillation: a common clinical condition. Br Med J 1909; 2:1528.
[3] Mackenzie J. The interpretation of the pulsations in the jugular veins. Am J Med Sci 1907; 134:12-34.
[4] Cushny AR, Edmunds CW.Paroxysmal irregularity of the heart and auricular fibrillation. In Bulloch W (ed). Studies in Pathology. Aberdeen, Scotland: University of Aberdeen, 1906, 95-110.
[5] Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997; 96: 2455-61.
[6] Kannel WB, AbbottRD, Savage DD, McNamara PM.Epidemiologic features of chronic atrial fibrillation: theFramingham Study. N Engl J Med 1982; 306: 1018-22.
[7] Wood P. Paul Wood's Diseases of the Heart and Circulation. 3rded. Philadelphia: JB Lippincott, 1968, 278.
[8] Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults (ATRIA). JAMA 2001; 285: 2370-5.
[9] Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in Incidence off atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006; 114: 119-25.
[10] LloydJones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110: 1042-6.
[11] Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace 2013; 15: 486-93.
[12] Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104: 1534-9.
[13] Reardon G, Nelson WW, Patel AA, Philpot T, Neidecker M. Prevalence of atrial fibrillation in US nursing homes: results from the national nursing home survey, 19852004. JAMDA 2012; 13: 529-34.
[14] Ball J, Carrington MJ, McMurray JJV, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21stcentury. Int J Cardiol 2013; 167: 1807-24.
[15] Zhou Z, Hu D. An epidemiologic study on the prevalence of atrial fibrillation in the Chinese population of Mainland China. J Epidemiology 2008; 18: 209-16.
[16] Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: The cardiovascular health study. J Am Geriatric Soc 2013; 61: 276-80.
[17] Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, et al. Atrial Fibrillation: Current knowledge and future directions in epidemiology and genomics. Circulation 2011; 124: 1982-93.
[18] Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, et al. Longdistance endurance sport practice increases the incidence of lone atrial fibrillation in men. Europace 2008; 10: 618-23.
[19] Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, et al. Alcohol consumption and risk of atrial fibrillation: a metaanalysis. J Am Coll Cardiol 2011; 57: 427-36.
[20] Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a populationbased cohort: the Framingham Heart Study. JAMA 1994; 271: 840-4.
[21] Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn 1962; CXL: 183-8.
[22] Cox JL, Schuessler RB, D'Agostino HJ Jr., Stone CM, Chang BC, Cain ME, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991; 101: 569-83.
[23] DiMarco JP. Surgical therapy for atrial fibrillation: a first step on what may be a long road. J Am Coll Cardiol 1991; 17: 976-7.
[24] Engelmann TW. Ueber den einfluss der systole auf der motorische leitung in der herzkammer, mit bemerkungen zur theorie allorhythmischer herzstorungen. Archivs Physiologie 1896; 62: 543-66.
[25] Scherf D. Studies on auricular tachycardia caused by aconitine administration. Proc Soc Exper Biol Med 1947; 64: 233-9.
[26] Holland WC, Burn JH. Production of fibrillation in isolated atria of rabbit heart. Brit Med J 1957; 1: 1031-3.
[27] Wolff L. Familial auricular fibrillation. N Engl J Med1943; 229: 396-8.
[28] Bode F, Katchman A, Woosley RL, Franz MR. Gadolinium decreases stretchinduced vulnerability to atrial fibrillation. Circulation 2000; 101: 2200-5.
[29] Tsang TSM, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 2002; 40: 1636-44.
[30] Yamamoto K, Sakata Y, Ohtani T, Takeda Y, Mano T. Heart failure with preserved ejection fraction: what is known and unknown? Circ J 2009; 73: 404-10.
[31] Favaloro RG, Effler DB, Groves LK, Sheldon WC, Riahi M. Direct myocardial revascularization with saphenous vein autograft: Clinical experience in 100 cases. Dis Chest 1969; 56: 279-83.
[32] Hakala T, Hedman A. Predicting the risk of atrial fibrillation after coronary artery bypass surgery. Scand Cardiovasc J 2003; 37: 309-15.
[33] Guo Y, Lip GYH, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol 2012; 60: 2263-70.
[34] Akar JG, Jeske W, Wilber DJ. Acute onset atrial fibrillation is associated with local cardiac platelet activation and endothelial dysfunction. J Am Coll Cardiol 2008; 51: 1790-3.
[35] Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, et al. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J 2010; 31: 1730-6.
[36] Ha?ssaguerre M, Ja?s P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998; 339: 659-66.
[37] Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation 1966; 34: 412-22.
[38] Ehrlich JR, Cha TJ, Zhang L, Chartier D, Melnyk P, Hohnloser SH, et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol 2003; 551: 801-13.
[39] Honjo H, Boyett MR, Niwa R, Inada S, Yamamoto M, Mitsui K, et al. Pacinginduced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation 2003; 107: 1937-43.
[40] Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, et al. Intraatrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation 2003; 108: 668-71.
[41] Sanders P, Berenfeld O, Hocini M, Ja?s P, Vaidyanathan R, Hsu LF, et al. Spectral analysis identifies sites of highfrequency activity maintaining atrial fibrillation in humans. Circulation 2005; 112: 789-97.
References of 42-325 are available online as a supplementary file.
Received 04 November 2013, Accepted 04 December 2013, Epub 28 December 2013
The authors reported no conflict of interests.
10.7555/JBR.28.20130191
 THE JOURNAL OF BIOMEDICAL RESEARCH2014年1期
THE JOURNAL OF BIOMEDICAL RESEARCH2014年1期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- A new free-hand pedicle screw placement technique with reference to the supraspinal ligament
- Pediatric restrictive cardiomyopathy due to a heterozygous mutation of the TNNI3 gene
- Genetic variants at 10q23.33 are associated with plasma lipid levels in a Chinese population
- Mechanisms simultaneously regulate smooth muscle proliferation and differentiation
- Extracellular matrix synthesis in vascular disease: hypertension, and atherosclerosis
- Renal denervation as an option for the management of hypertension
