Heterogeneity and renal mass biopsy: a review of its role and reliability
Jeffrey J. Tomaszewski, Robert G. Uzzo, Marc C. Smaldone
1Division of Urology, Department of Surgery, MD Anderson Cancer Center at Cooper, Rowan University School of Medicine, Camden, NJ, 08103, USA;
2Division of Urologic Oncology, Department of Surgical Oncology, Fox Chase Cancer Center-Temple University Health System, Philadelphia, PA, 19111, USA
Heterogeneity and renal mass biopsy: a review of its role and reliability
Jeffrey J. Tomaszewski1, Robert G. Uzzo2, Marc C. Smaldone2
1Division of Urology, Department of Surgery, MD Anderson Cancer Center at Cooper, Rowan University School of Medicine, Camden, NJ, 08103, USA;
2Division of Urologic Oncology, Department of Surgical Oncology, Fox Chase Cancer Center-Temple University Health System, Philadelphia, PA, 19111, USA
Increased abdominal imaging has led to an increase in the detection of the incidental small renal mass (SRM). With increasing recognition that the malignant potential of SRMs is heterogeneous, ranging from benign (15%-20%) to aggressive (20%), enthusiasm for more conservative management strategies in the elderly and infirmed, such as active surveillance (AS), have grown considerably. As the management of the SRM evolves to incorporate ablative techniques and AS for low risk disease, the role of renal mass biopsy (RMB) to help guide individualized therapy is evolving. Historically, the role of RMB was limited to the evaluation of suspected metastatic disease, renal abscess, or lymphoma. However, in the contemporary era, the role of biopsy has grown, most notably to identify patients who harbor benign lesions and for whom treatment, particularly the elderly or frail, may be avoided. When performing a RMB to guide initial clinical decision making for small, localized tumors, the most relevant questions are oen relegated to proof of malignancy and documentation (if possible) of grade. However, signi fi cant intratumoral heterogeneity has been identi fi ed in clear cell renal cell carcinoma (ccRCC) that may lead to an underestimation of the genetic complexity of a tumor when single-biopsy procedures are used. Heterogeneous genomic landscapes and branched parallel evolution of ccRCCs with spatially separated subclones creates an illusion of clonal dominance when assessed by single biopsies and raises important questions regarding how tumors can be optimally sampled and whether future evolutionary tumor branches might be predictable and ultimately targetable.is work raises profound questions concerning the genetic landscape of cancer and how tumor heterogeneity may a ff ect, and possibly confound, targeted diagnostic and therapeutic interventions. In this review, we discuss the current role of RMB, the implications of tumor heterogeneity on diagnostic accuracy, and highlight promising future directions.
Renal cell carcinoma (RCC); renal mass biopsy (RMB); tumor heterogeneity
Introduction
The incidence of renal cell carcinoma (RCC) has been steadily increasing over the past decade1, in large part due to the increased incidental detection of small renal masses (SRMs) on crosssectional abdominal imaging2. Nephron sparing surgery (NSS) is the standard of care for clinically localized T1a SRMs, howeveralternative minimally invasive and conservative treatment options are possible in select comorbid or elderly patients3-5.e optimal treatment modality is based on clinical assessment of patient comorbidities and tumor characteristics, but SRMs represent a heterogeneous group of benign and malignant histologic entities, with a range of clinical and biologic behaviors unpredictable by conventional imaging6.
Traditionally, all localized solid renal masses have been considered potentially malignant and treated with surgical excision in an effort to minimize the risk of metastatic dissemination7. Renal mass biopsy (RMB) has had a limited role in SRM management given concerns regarding accuracy, inconclusiveness, and complications, and its use was largely reserved for evaluationof suspected metastases or extrarenal malignancies8. However, given expanded treatment options including active surveillance (AS) and ablative therapies, RMB may help de fi ne tumor subtype and stratify aggressive potential, allowing for a more rational treatment protocol7. Indeed RMB is emerging as a safe and useful tool for the preoperative identi fi cation of benign lesions to avoid the potential morbidity of extirpative or ablative treatment, particularly in the older population9. However, RCC is now recognized as a heterogeneous disease process, with a number of distinct histopathological subtypes, having substantial variance in biological aggressiveness7.e recent identi fi cation of signi fi cant intratumoral heterogeneity in clear cell renal cell carcinoma (ccRCC) further complicates the role of RMB, as it may lead to an underestimation of the genetic complexity of a tumor when single-biopsy procedures are used5,10-12. Herein we review the current role of RMB, evolving indications, the implications of tumor heterogeneity on diagnostic accuracy, and highlight future directions including the promising role of RMB combined with biomarkers and molecular profiling to stratify tumor aggressiveness.
Established indications for RMB
While extirpative therapy is the preferred management modality for clinically localized RCC3,4, there is emerging consensus that a significant proportion of patients with incidentally detected tumors may be over treated. Traditionally, RMB has been utilized in specific clinical scenarios in which a tissue diagnosis would obviate surgery, including lymphoma, metastatic carcinoma, infection/abscess, or concurrent with ablative therapies7,13. Eight percent to 13% of SRMs represent metastatic disease14, with lung, colon, melanoma, and liver cancer most frequently metastasizing to the kidney15. Among patients with a known extrarenal primary cancer, RMB has a sensitivity of 90% for malignancy detection, but over half of such lesions will prove to be new primary renal tumors14. Among 100 patients with non-renal malignancies diagnosed with renal masses at presentation or follow-up, progression of the non-renal malignancy and lack of enhancement of the renal mass were predictive of a metastasis to the kidney12.
Renal lymphoma presents as a solitary renal mass in 10% to 25% of patients, and can frequently mimic RCC on imaging16. Use of RMB to evaluate suspected lymphoma can establish the correct pathological diagnosis and ensure appropriate treatment with chemotherapy. A renal mass detected in the clinical seing of febrile urinary tract infection should increase suspicion for renal abscess or focal pyelonephritis and prompt percutaneous aspiration with drain placement at the time of biopsy to expedite recovery17. In patients with unresectable or metastatic RCC, or those who are poor operative candidates, the precise histological classi fi cation obtained from RMB can guide targeted molecular therapy18,19.
Rationale for expanded RMB indications
Beyond the aforementioned established indications for RMB, concerns regarding RMB safety, diagnostic yield, accuracy, and the limited ability of RMB to in fl uence treatment decisions based on the perception that all solid SRMs have malignant potential and should be removed with surgery upfront have limited the widespread use of RMB. However, increasing detection of incidental SRMs, development of treatment alternatives in select patients, and the discovery of several e ff ective biologically targeted drugs for metastatic disease have raised the awareness that pretreatment tumor histology can be useful and necessary to individualize treatment decisions6. Increased expertise in biopsy performance and pathological interpretation of RMB, utilization of modern biopsy techniques, and increasing confidence of urologists in using biopsy results to support treatment decisions have helped to overcome the traditional limitations of RMB and fuel the renewed interest in RMB as a diagnostic tool6-8.
The role of RMB has expanded to include the evaluation of complex cystic lesions, SRMs <4 cm, and determination of tumor subtype (Table 1)7,13,20-23. Renal mass size is an important predictor of malignant histology, and since the odds of benign pathology significantly increase with decreasing tumor size, SRMs are benign in a significant proportion of cases20,24,25. In review of 2,770 solid renal mass resections over a thirty-year period, 30% of renal lesions <4 cm were benign23. As clinicians cannot rely on imaging alone to differentiate benign from malignant renal masses26, RMB can help stratify oncological risk in patients with SRM. The largest increase in incidentally detected SRMs has occurred among patients 70-89 years of age, in whom comorbidities are more frequent and the risk of competing-cause mortality is higher27. Competing cause mortality increases with increasing patient age, regardless of tumor size28, and increased comorbidity (as measured by Charlson comorbidity index) is associated with worse overall survival after surgical treatment29-32. The perception that active treatment for SRM may not significantly influence OS in patients with a short life expectancy has led to the development of conservative and minimally invasive treatment options for select elderly and surgically high-risk patients with a SRM33. For patients who are candidates for a wide range of treatment options ranging from AS to surgery, RMB can be useful in the management of all solid, contrast-enhancing SRMs when histologic diagnosis has the potential for supporting treatment, decisions7. Among young and healthy patients, RMB is not routinely recommended because long-term oncologic outcomes of non-surgical therapies are not available, and there may be a risk of histologic transformation when a renal tumor is observed for a prolonged period of time6.

Table 1 Current indications and contraindications for renal mass biopsy
An exciting and expanding indication for RMB is assessment of renal primary lesions in patients with metastatic RCC. Over the past decade, the treatment landscape in metastatic RCC has changed dramatically31, and identification of histologic subtype and relevant molecular pathways may allow for more precise targeted systemic treatment32,34-36. Identification of sarcomatoid differentiation, for example, represents a poor prognosis with limited response to systemic treatment and may represent a contraindication to cytoreductive nephrectomy to avoid morbidity6,37. Clinical responses to sunitinib and sorafenib are low in papillary RCC35, but efficacy of the mTOR inhibitor temsirolimus appears more pronounced in non-clear cell and papillary type RCC36. RMB of the primary renal tumor allows ideal targeted therapy selection, and is recommended when a cytoreductive nephrectomy is not indicated or when neoadjuvant systemic therapy is planned4. In the metastatic RCC setting, interest in precision beyond “cancer” versus “benign” can be challenging, re fl ecting increased tumor heterogeneity, occasional divergent pathologies, and predominance of tumor necrosis7,14,38.
Clinical nomograms to predict malignant potential
Efforts have also been focused on development of clinical nomograms to predict malignant potential prior to surgery and safely substitute for RMB. Early efforts to predict malignant pathology and tumor grade using tumor size and other clinical variables (such as age, gender, smoking history and presence of symptoms) were highly inaccurate, which limited their clinical utility39-41. Combining individual descriptors of the nephrometry score with patient characteristics (age, gender), Kutikov et al.41developed a nomogram that could predict malignant RCC histology and high-grade features. Recently externally validated42, these models represent the most accurate preoperative predictors of malignant potential of localized renal tumors to date, and their accuracy for predicting tumor grade matches that of percutaneous core biopsy40. Although early efforts have been encouraging, the role of statistical modeling, including nomogram development, for risk prediction during AS is likely to evolve and expand in the future43.
Safety
RMB is a relatively safe procedure with minimal morbidity. Contemporary series reveal overall complications rates ranging from 1.4%7,44-51to 4.7%13,14,52-57, with major complications reported in 0.46%7,13,44,45,47,48,58,59. Potential complications of RMB include bleeding, tumor seeding, infection, pneumothorax, and arteriovenous fi stula6,7,56. Most RMB related complications are minor and related to bleeding, but clinically significant bleeding is unusual and almost always self-limiting. While small pneumothoraces can occur, especially following biopsy of posterior upper pole tumors, they are rare and usually managed conservatively48. The most feared and controversial potential complication of RMB is tumor seeding of the biopsy tract.e overall estimated risk of tract seeding is < 0.01%6,11,60,61with only a handful of case reports documenting its occurrence and one reported case since 199462, tumor seeding should be considered anecdotal21. Among 1,377 patients undergoing RMB in contemporary series using coaxial techniques with guides or cannulas, no cases of biopsy tract tumor seeding were reported6.While the risk of RMB related complications is small but not zero, risks should be weighed against managing a patient with suboptimal information.
Diagnostic accuracy for malignancy
The ultimate goal of RMB is to appropriately match tumor treatment with tumor biology, so its utility becomes dependent upon the clinical scenario encountered and the accuracy of RMB for determination of malignancy and tumor grade.e diagnostic accuracy of RMB is currently estimated at >95%7, but care must be taken when determining accuracy because the definitions used vary and can result in artifactually high estimates. Most series de fi ne accuracy as the percentage of informative biopsies for which the pathological diagnosis appeared to be correct; that is not a false-positive or a false-negative, based on final surgical pathology or radiographic and clinical surveillance7.is de fi nition is inherently limited, however, as it assumes that radiographic surveillance is a reliable surrogate for pathologic diagnosis, and ignores non-informative biopsies.
Historically, many non-informative biopsies were inappropriately considered “false-negative”, representing a major criticism of RMB, in that missed malignancies would potentially remain untreated7. Among recent series, diagnostic yield of RMB ranges from 78% to 100%, while sensitivity and specificity for the diagnosis of malignancy are 86%-100% and 100%, respectively (Table 2)9,44,45,48,49,51,54,59,61. Among review of 2,474 recent RMB results, PPV and NPV for the diagnosis of malignancy were 97.5% and 82%, respectively, with an overall sensitivity of 92.1% and specificity of 89.7%13. While the rate of non-diagnostic biopsy remains in the range of 10%-20%, in patients with an initially non-diagnostic biopsy, the diagnostic rate on re-biopsy ranges from 75% to 100%44,47,49-52,54,58,59,65-68. Although tumor size, location, and character can certainly play a role9, the similarity between re-biopsy and initial biopsy rates suggests there is nothing intrinsic to tumors themselves that results in a nondiagnostic biopsy and that repeat biopsy is both feasible and can be expected to identify tumors and cancers9. It is important to note that a nondiagnostic biopsy is not a surrogate for a benign diagnosis and should not be considered a reassurance that the patient has a benign lesion9.
Inaccurate RMB
Inaccurate RMB, including true false-negative and falsepositive results, represents the most concerning outcome for clinicians. Concern is justified, as false-negative results could lead to surveillance of a malignancy with metastatic potential. Fortunately, the rate of false-negative RMB (excluding noninformative RMB) among modern series ranges from 0% to 3.8%7,13,48,51,69,70. Sampling error, tumor necrosis, and tumor heterogeneity are responsible for most false-negative biopsy results14. Smaller tumors can be more difficult to visualize and target56, but larger tumors are also prone to sampling error given the greater incidence of necrosis14,65. In a series of 115 core RMBs, the false-negative rate was lowest for tumors 4 to 6 cm in diameter (2.3%), compared to small (1 to 3 cm; 13%) and large (>6 cm; 12%) tumors14. In a larger series of 345 RMBs, the odds ratio for a diagnostic result was 2.3 (95% CI, 1.5-6.3) for each 1-cm increase in tumor size9.
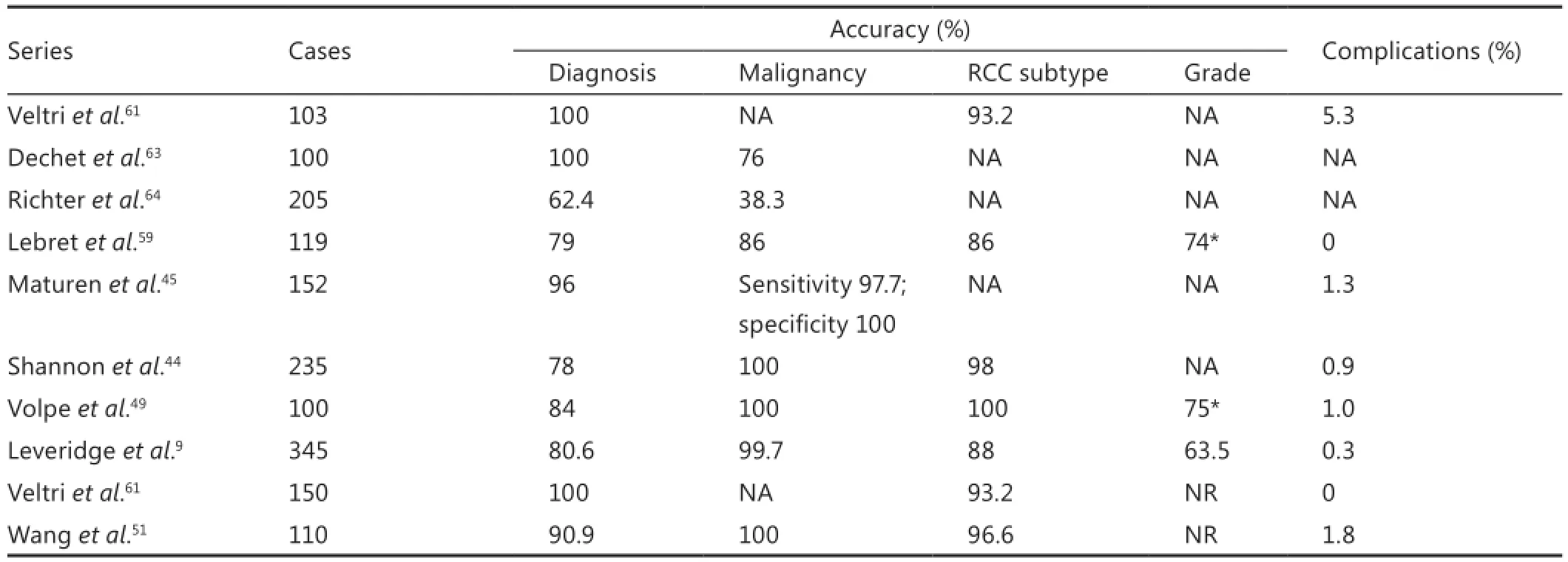
Table 2 Contemporary outcomes from renal mass biopsy series
Concern for a coexisting malignancy in otherwise benign tumors is also a signi fi cant barrier to routine RMB, undermining the validity of RMB and likely deterring its routine use13,67. While hybrid histology has been largely described in patients with multifocal tumors and known genetic syndromes, concern persists despite relatively sparse data on sporadic solitary tumors68. In the largest series to date to examine the rates of coexisting malignant and high grade pathology, 1,829 patients with benign solid solitary renal tumors underwent tumor excision, and 147 masses were found to contain a benign component [oncocytoma (5.2%), angiomyolipoma (2.4%), or another solid benign pathology (0.38%)]68. Only four patients (2.7%) had hybrid malignant pathology, all of which were chromophobe in the seing of oncocytoma, and importantly, no benign tumor coexisted with high grade malignancy68. Hybrid tumor rates as high as 27.1% have been reported67, but this discrepancy stems from the pathological criteria used to classify the malignant component, and hybrid tumors are generally believed to be nonaggressive67,68,71,72. Collectively, these data suggest that uncertainty regarding hybrid malignant pathology coexisting with benign pathological components should not deter renal biopsy in efforts to minimize over-treatment of the renal mass, especially in the frail and comorbid populations68.
Accuracy of tumor grading and histological subtype
Histological subtype and grade are known prognostic factors in renal carcinoma and potentially important in the staging and management of small renal cancers26. The accuracy of grading renal cell cancers with percutaneous biopsy is controversial and largely unreliable, with reported accuracy for grading ranging from 43% to 75%26,49,54,59,70,71. Even the most recent series reveal only moderate concordance between biopsy and surgery grade (Kappa score 0.52)26. By compressing the Fuhrman nuclear classifications, accuracy of differentiation between “l(fā)ow ” and“high” grade tumors can improve to as high as 93%26. The reproducibility of these fi ndings will remain in question however until verified by additional larger reports. Fuhrman grade intratumor heterogeneity further complicates accurate grade determination via RMB, and grade heterogeneity in a single tumor has been observed in up to 25% of cases (Figure 1)72. While not as powerful a prognostic factor in RCC nomograms and predictive models73, diagnostic accuracy for histologic subtyping has also been examined. When core RMBs are compared to nephrectomy specimens, accuracy for subtyping is high, ranging from 86% to 100%26,44,51,56,59. Given patients with clear cell RCC have a poor prognosis compared to those with papillary Type 1 and chromophobe RCC74, accurate subtype determination may impact clinical management.

Figure 1 Photomicrograph from nephrectomy specimen illustrates intratumor grade heterogeneity. Low grade tumor cells with Fuhrman grades 1 and 2 nuclei (upper half) are sharply demarcated from high grade tumor cells with Fuhrman 3 and 4 nuclei (lower half). In addition, many high grade tumor cells had rhabdoid features, characterized by densely eosinophilic cytoplasmic aggregates of intermediate fi laments (reproduced with permission from Elsevier)56.
Increasing the accuracy of conventional RMB
Utilization of molecular characteristics and refined RMB techniques has the potential to improve the accuracy of conventional RMB. Use of larger (18 gauge) needles is safe and allows acquisition of su ffi cient tissue for accurate diagnosis and may increase the diagnostic accuracy of RMB56. The presence of sarcomatoid dedifferentiation or necrosis correlates with decreased recurrence-free survival75-78, and expression of carbonic anhydrase IX, a ccRCC marker, is an independent predictor of survival79. Adding fl uorescence in situ hybridization to evaluate chromosomal abnormalities increased the diagnostic accuracy of ex vivo RMB from 87% with histopathology alone to 94%77, and addition of real-time polymerase chain reaction data on the expression of 4 select genes showed similar promise78. In evaluation of RMB tissue from 60 tumors, use of molecular diagnostics increased the accuracy of histological subtyping form 90% to 95%78. Microarray technology has demonstrated some ability to differentiate tumors by gene expression profiling80, and the molecular fingerprinting of histological subtype could someday be used to predict likelihood of recurrence13. Gene expression microarrays have been used to classify aggressive variants of RCC81, and these profiles are concordant with final surgical pathology80. Combining molecular pro fi ling with patientfactors, tumor size, and radiographic parameters may provide refined risk-stratification of patients with renal tumors for counseling and management.
Intratumoral heterogeneity
A potential limitation in the imagined future of oncology is its underestimation of tumor heterogeneity—not just heterogeneity between tumors, which is a central feature of the new image of personalized medicine, but heterogeneity within an individual tumor (Figure 1)82. Gerlinger et al.11performed unbiased whole-exome sequencing of multiple primary and metastatic renal-cell carcinoma tumor sites in several different patients to map genetic heterogeneity within a single tumor. A majority of somatic genetic mutations were not present ubiquitously within a tumor, and branched evolutionary tumor development was evident11. Approximately two thirds of the mutations (including mutations, allelic imbalance, and ploidy) that were found in single biopsies were not uniformly detectable throughout all the sampled regions of the same patient’s tumor11. “Favorable”and “unfavorable” prognostic gene profiles were expressed in different regions of the same tumor. Unlike previous studies utilizing next-generation sequencing of a single index lesion per patient and targeted sequencing of the mutated genes in other sites, the author’s independently sequenced and validated mutant gene expression and altered function throughout primary and metastatic sites.
Further, there were widespread alterations in the total number of tumor cell chromosomes (aneuploidy) and detection of many allelic imbalances at the chromosomal level, in which one allele of a gene pair is lost11,12,82,83. These imbalances can be due to chromosome loss or gene imprinting and may alter gene expression82. Convergent evolution was also evident, with different tumor regions containing different mutations within the same genes. This underscores the importance of changing particular tumor-cell functions as the tumor expands and evolves11,12,82,83. Tumor heterogeneity presents a considerable therapeutic challenge because treatment choices based on a biomarker present in a single biopsy specimen may not be relevant72, and genomics analyses from single tumorbiopsy specimens may underestimate the mutational burden of heterogeneous tumors11.us, a single tumor biopsy, the standard of tumor diagnosis and the cornerstone of personalized-medicine decisions, cannot be considered representative of the landscape of genomic abnormalities in a tumor. Given that selective gene activation and inactivation occurs to guarantee tumor survival, the genes that are affected by convergent evolution may be suitable targets for functional inhibition or restoration. However, the concept of directing therapy on the basis of genetic tumor markers is probably too simple. Reconstructing tumor clonal architectures and the identi fi cation of common mutations located in the trunk of the phylogenetic tree may contribute to more robust biomarkers and therapeutic approaches11.
Risk strati fi ed RMB and utility in complex scenarios
Incorporation of RMB results could allow clinicians to reduce the treatment burden for patients without compromising disease specific survival, and incorporation of RMB into risk stratified management algorithms has been proposed as the next refinement in RMB72. Using management based on final pathology as the reference standard for patients with SRM ≤4 cm, RMB had a 100% treatment PPV and 69% surveillance NPV for correctly determining management72. Revision of the histological risk-grouping to account for undergrading of grade 1 ccRCC on biopsy increased sensitivity to 96% and improved the negative (surveillance) predictive value to 86%, while the positive (treatment) predictive value remained at 100%72. Using risk stratified histology groups and maximum mass diameter on imaging accurately defines patients as surveillance or treatment candidates, and incorporation of additional factors could improve the results. Although silent discrepancies may exist between initial biopsy and final pathology due to the heterogeneous nature of some tumors, this variability becomes clinically irrelevant when using a well-defined management protocol72. A “biopsy for all” strategy might avoid unnecessary surgeries, even among young patients with an incidentaloma (Figure 2).
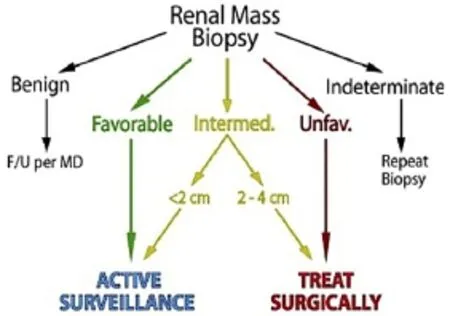
Figure 2 Simplified biopsy directed management algorithm designating active surveillance vs. treatment based on mass size and histological risk category (reproduced with permission from Elsevier)72.
Patients with bilateral synchronous (BSRT) or unilateralmultifocal renal tumors pose multiple prognostic and therapeutic challenges, similar to those of patients with hereditary RCC84-87. Numerous issues need to be addressed in patients with BSRT, including the need for complex nephron-sparing surgery, staged procedures, prognosis, decline in renal function, the prospect of progressive renal insufficiency, hyperfiltration injury, and the need for future renal replacement therapy85. Malignant and nuclear grade concordance rates are high for contralateral disease, ranging from 84%-95% and 79%-85%, respectively, while benign concordance rates are much lower (39%-67%)85. The data suggest that RCC of any type present on one side indicates RCC will be present on the contralateral side, but when benign disease is present, there is a lower risk of only benign disease on the contralateral side85. In patients with BSRT and confirmed benign disease on one side, consideration of contralateral RMB is reasonable.
Between 5% and 25% of patients who undergo surgery for a presumed single renal mass are found to have multifocal disease83, and diagnosis of unilateral synchronous multifocal renal masses at presentation impacts the intensity of evaluation, use of RMB, and treatment planning. Although the pathological concordance of sporadic bilateral masses is relatively high, rates of pathological concordance from unilateral multifocal cases of RCC are poorly de fi ned. In the largest series to date, 97 patients with unilateral synchronous multifocal renal masses underwent partial nephrectomy84. Malignant, benign, histological concordance rates were 77.2%, 48.6%, and 58.8%, respectively84.e low concordance rates indicate that single renal mass biopsy in patients with multifocal disease may be insufficient patient counseling and treatment planning.
In patients presenting with a renal tumor and clinical evidence of metastatic disease, RMB of the primary tumor and/ or metastatic lesion can be done to obtain a tissue diagnosis. Indications for primary tumor RMB include inability to make a tissue diagnosis from a metastatic site, atypical appearance of the primary tumor on preoperative imaging, suspicion of multiple primary neoplasms and/or the need to make a histological diagnosis to guide treatment86. In the largest series evaluating percutaneous RMB findings compared to nephrectomy specimens in patient with metastatic RCC86, tumor grade was accurately assessed in only 33% of cases and discordance by two or more grades was reported in 17%86. Sarcomatoid dedifferentiation was found in 20.5% of final pathological specimens and yet preoperative biopsy failed to identify this in almost 90%. Biopsy failed to specify the primary histological subtype in 41% of cases and many biopsies were non-diagnostic for RCC86. Physicians should use caution when using biopsy data assigning grade or sarcomatoid elements to enroll patients with metastatic RCC in neoadjuvant clinical trials or to make complicated treatment decisions. Future development of better imaging techniques, new molecular markers and/ or improved immunohistochemical techniques may help improve the predictive accuracy of percutaneous biopsy of large heterogeneous primary tumors in patients with metastatic RCC86.
Future directions
Cytogenetic alterations in clear cell RCC, such as loss of 9p, are known to correlate with a signi fi cantly worse 5-year cancerspecific survival89, and have been examined among a cohort of 282 patients90. Combining loss of 9p (as measured by FISH) with clinical stage and Fuhrman grade into a nomogram yielded high prognostic accuracy to predict 3-year cancer-speci fi c survival90. DNA or RNA expression microarrays can also be performed using core biopsy specimens, although adequate tissue is needed to effectively extract DNA and RNA for analysis. One recently identified gene array was able to distinguish two groups of clear cell RCC’s with significantly different 5-year recurrencefree survival rates of 68% and 42%, respectively64,80,84,91,92. While results of early genetic and molecular tissue markers are highly promising, prospective studies are needed to validate the fi ndings and expand applicability to non-clear cell histotypes.
Limitations
While the current renaissance in RMB is exciting, several notable limitations deserve mention. A definite assessment of RMB accuracy remains difficult given most series are small, single institutional, and use varying de fi nitions for biopsy success6.e accuracy of RMB is limited by factors intrinsic to the procedure(inconsistent tumor sampling), to the histology of renal tumors (difficult differential diagnosis of tumor histotypes, evaluation of Fuhrman grade, presence of intratumoral heterogeneity), and to the interpretation of biopsy specimens (interobserver variability in pathologic assessment)6. Sampling error and tumor heterogeneity contribute to inaccuracy of RMB, and differentiation among conventional RCC with granular cytoplasm, oncocytic papillary RCC, the eosinophilic variant of chromophobe RCC and oncocytoma can also be particularly problematic13. Further practical concerns include the risk of renal hemorrhage given renal vascularity, and technical failure leading to indeterminate or inaccurate pathological findings. Finally, assessment of Fuhrman grade on RMB is challenging with suboptimal accuracy9,15,48,49,51,52,59,66, moderate interobserver concordance for grade assessment, and intratumoral grade heterogeneity in 5%-25% of renal tumors48.
Conclusion
Advances in the understanding of the limited biological potential of many SMRs, expanding treatment and surveillance options for RCC, improved biopsy techniques, and the integration of molecular factors into prognostic and therapeutic algorithms have renewed interest in RMB. Current indications for RMB include diagnostic work-up of renal tumors that are indeterminate on imaging, assessment of the primary renal tumor prior to initiation of systemic therapy for metastatic RCC, and of incidentally detected radiologically suspicious SRMs in patients at high surgical risk to support treatment decisions and avoid unnecessary surgery. Intratumoral heterogeneity, sampling error, and inconsistent classi fi cation of RMB failures in published studies make a precise determination of RMB accuracy di ffi cult. Uniform reporting of RMB safety and efficacy in the literature as well as further studies addressing tumor heterogeneity and sampling error are needed. Differentiation of oncocytoma from oncocytic neoplasms poses a diagnostic dilemma, but incorporation of more sophisticated molecular analyses into enhanced RMB has promising potential.
Despite these limitations, RMB has a de fi nite and expanding role in the evaluation and treatment of renal masses, but remains significantly underutilized. While the ability to differentiate between high and low grade malignancies remains the chief limitation of RMB, we anticipate the further integration of percutaneous biopsy into clinical algorithms will guide patient counseling and inform personalized decision making. Future studies will focus on the role of repeat biopsy and the use of biomarkers and molecular fi ngerprinting in order to facilitate a more rational approach to the management of renal masses.
Con fl ict of interest statement
No potential con fl icts of interest are disclosed.
1. Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al.e epidemiology of renal cell carcinoma. Eur Urol 2011;60:615-621.
2. Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer 2008;113:78-83.
3. Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271-1279.
4. Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010;58:398-406.
5. Gill IS, Aron M, Gervais DA, JeweMA. Clinical practice. Small renal mass. N Engl J Med 2010;362:624-634.
6. Volpe A, Finelli A, Gill IS, JeweMA, Martignoni G, Polascik TJ, et al. Rationale for percutaneous biopsy and histologic characterisation of renal tumours. Eur Urol 2012;62:491-504.
7. Samplaski MK, Zhou M, Lane BR, Herts B, Campbell SC. Renal mass sampling: an enlightened perspective. Int J Urol 2011;18:5-19.
9. Leveridge MJ, Finelli A, Kachura JR, Evans A, Chung H, Shi ff DA, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 2011;60:578-584.
10. Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, et al. Genomic architecture and evolution of clear cell renal cell carcinomas de fi ned by multiregion sequencing. Nat Genet 2014;46:225-233.
11. Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-892.
12. Sánchez-Ortiz RF, Madsen LT, Bermejo CE, Wen S, Shen Y, Swanson DA, et al. A renal mass in the seing of a nonrenal malignancy: When is a renal tumor biopsy appropriate? Cancer 2004;101:2195-2201.
13. Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy--a renaissance? J Urol 2008;179:20-27.
14. Rybicki FJ, Shu KM, Cibas ES, Fielding JR, vanSonnenberg E, Silverman SG. Percutaneous biopsy of renal masses: sensitivity andnegative predictive value strati fi ed by clinical seing and size of masses. AJR Am J Roentgenol 2003;180:1281-1287.
16. Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: paerns of disease with pathologic correlation. Radiographics 2006;26:1151-1168.
17. Lang EK, Macchia RJ, Gayle B, Richter F, Watson,omas R, et al. CT-guided biopsy of indeterminate renal cystic masses (Bosniak 3 and 2F): accuracy and impact on clinical management. Eur Radiol 2002;12:2518-2524.
18. Atkins M, Regan M, McDermoD, Mier J, Stanbridge E, Youmans A, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res 2005;11:3714-3721.
19. Lane BR, Rini BI, Novick AC, Campbell SC. Targeted molecular therapy for renal cell carcinoma. Urology 2007;69:3-10.
20. Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging 2009;9:44-55.
21. Silverman SG, Gan YU, Mortele KJ, Tuncali K, Cibas ES. Renal masses in the adult patient: the role of percutaneous biopsy. Radiology 2006;240:6-22.
22. Tsivian M, Mouraviev V, Albala DM, Caso JR, Robertson CN, Madden JF, et al. Clinical predictors of renal mass pathological features. BJU Int 2011;107:735-740.
23. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol 2003;170:2217-2220.
25. Sahni VA, Ly A, Silverman SG. Usefulness of percutaneous biopsy in diagnosing benign renal masses that mimic malignancy. Abdom Imaging 2011;36:91-101.
26. Millet I, Doyon FC, Hoa D,uret R, Merigeaud S, Serre I, et al. Characterization of small solid renal lesions: can benign and malignant tumors be di ff erentiated with CT? AJR Am J Roentgenol 2011;197:887-896.
27. Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA 1999;281:1628-1631.
28. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival aer surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer 2007;109:1763-1768.
29. Santos Arrontes D, Fernández Ace?ero MJ, García González JI, Martín Mu?oz M, Paniagua Andrés P. Survival analysis of clear cell renal carcinoma according to the Charlson comorbidity index. J Urol 2008;179:857-861.
30. Lane BR, Abouassaly R, Gao T, Weight CJ, Hernandez AV, Larson BT, et al. Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer 2010;116:3119-3126.
31. Allory Y, Culine S, de la Taille A. Kidney cancer pathology in the new context of targeted therapy. Pathobiology 2011;78:90-98.
32. Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol. 2009;27:3225-3234.
33. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment e ff ect. J Natl Cancer Inst 2006;98:1331-1334.
35. Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, et al. E ffi cacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 2008;26:127-131.
36. Dutcher JP, de Souza P, McDermoD, Figlin, Berkenblit A,iele A, et al. E ff ect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of di ff erent tumor histologies. Med Oncol 2009;26:202-209.
37. Shuch B, Bratslavsky G, Shih J, Vourganti S, Finley D, Castor B, et al. Impact of pathological tumour characteristics in patients with sarcomatoid renal cell carcinoma. BJU Int 2012;109:1600-1606.
38. Niceforo J, Coughlin BF. Diagnosis of renal cell carcinoma: value of fi ne-needle aspiration cytology in patients with metastases or contraindications to nephrectomy. AJR Am J Roentgenol 1993;161:1303-1305.
39. Jeldres C, Sun M, Liberman D, Lughezzani G, de la Taille A, Tostain J, et al. Can renal mass biopsy assessment of tumor grade be safely substituted for by a predictive model? J Urol 2009;182:2585-2589.
41. Kutikov A, Smaldone MC, Egleston BL, Manley BJ, Canter DJ, Simhan J, et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol 2011;60:241-248.
42. Wang HK, Zhu Y, Yao XD, Zhang SL, Dai B, Zhang HL, et al. External validation of a nomogram using RENAL nephrometry score to predict high grade renal cell carcinoma. J Urol 2012;187:1555-1560.
43. Smaldone MC, Corcoran AT, Uzzo RG. Active surveillance of small renal masses. Nat Rev Urol 2013;10:266-274.
44. Shannon BA, Cohen RJ, de Bruto H, Davies RJ.e value of preoperative needle core biopsy for diagnosing benign lesions among small, incidentally detected renal masses. J Urol 2008;180:1257-1261; discussion 1261.
45. Maturen KE, Nghiem HV, Caoili EM, Higgins EG, Wolf JS Jr, Wood DP Jr. Renal mass core biopsy: accuracy and impact on clinical management. AJR Am J Roentgenol 2007;188:563-570.
46. Reichelt O, Gajda M, Chyhrai A, Wunderlich H, Junker K, Schubert J. Ultrasound-guided biopsy of homogenous solid renal masses. Eur Urol 2007;52:1421-1426.
48. Schmidbauer J, Remzi M, Memarsadeghi M, Haitel A, Klingler HC, Katzenbeisser D, et al. Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol 2008;53:1003-1011.
50. Masoom S, Venkataraman G, Jensen J, Flanigan RC, Wojcik EM. Renal FNA-based typing of renal masses remains a useful adjunctive modality: evaluation of 31 renal masses with correlative histology. Cytopathology 2009;20:50-55.
51. Wang R, Wolf JS Jr, Wood DP Jr, Higgins EJ, Hafez KS. Accuracy of percutaneous core biopsy in management of small renal masses. Urology 2009;73:586-590; discussion 590-591.
52. Johnson PT, Nazarian LN, Feld RI, Needleman L, Lev-Toa ff AS, Segal SR, et al. Sonographically guided renal mass biopsy: indications and e ffi cacy. J Ultrasound Med 2001;20:749-753; quiz 755.
53. Eshed I, Elias S, Sidi AA. Diagnostic value of CT-guided biopsy of indeterminate renal masses. Clin Radiol 2004;59:262-267.
54. Neuzillet Y, Lechevallier E, Andre M, Daniel L, Coulange C. Accuracy and clinical role of fi ne needle percutaneous biopsy with computerized tomography guidance of small (less than 4.0 cm) renal masses. J Urol 2004;171:1802-1805.
55. Hara I, Miyake H, Hara S, Arakawa S, Hanioka K, Kamidono S. Role of percutaneous image-guided biopsy in the evaluation of renal masses. Urol Int 2001;67:199-202.
56. Volpe A, Kachura JR, Geddie WR, Evans AJ, Gharajeh A, Saravanan A, et al. Techniques, safety and accuracy of sampling of renal tumors by fi ne needle aspiration and core biopsy. J Urol 2007;178:379-386.
57. Caoili EM, Bude RO, Higgins EJ, Ho ff DL, Nghiem HV. Evaluation of sonographically guided percutaneous core biopsy of renal masses. AJR Am J Roentgenol 2002;179:373-378.
58. Vasudevan A, Davies RJ, Shannon BA, Cohen RJ. Incidental renal tumours: the frequency of benign lesions and the role of preoperative core biopsy. BJU Int 2006;97:946-949.
59. Lebret T, Poulain JE, Molinie V, Herve JM, Denoux Y, Guth A, et al. Percutaneous core biopsy for renal masses: indications, accuracy and results. J Urol 2007;178:1184-1188; discussion 1188.
60. Herts BR, Baker ME. The current role of percutaneous biopsy in the evaluation of renal masses. Semin Urol Oncol 1995;13:254-261.
62. Mullins JK, Rodriguez R. Renal cell carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J 2013;7:E176-E179.
63. Dechet CB, Zincke H, Sebo TJ, King BF, LeRoy AJ, Farrow GM, et al. Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol 2003;169:71-74.
64. Richter F, Kasabian NG, Irwin RJ Jr, Watson, Lang EK. Accuracy of diagnosis by guided biopsy of renal mass lesions classi fi ed indeterminate by imaging studies. Urology 2000;55:348-352.
65. Lechevallier E, André M, Barriol D, Daniel L, Eghazarian C, De Fromont M, et al. Fine-needle percutaneous biopsy of renal masses with helical CT guidance. Radiology 2000;216:506-510.
66. Wood BJ, Khan MA, McGovern F, Harisinghani M, Hahn PF, Mueller PR. Imaging guided biopsy of renal masses: indications, accuracy and impact on clinical management. J Urol 1999;161:1470-1474.
68. Ginzburg S, Uzzo R, Al-Saleem T, Dulaimi E, Walton J, Corcoran A, et al. Coexisting hybrid malignancy in a solitary sporadic solid benign renal mass: implications for treating patients following renal biopsy. J Urol 2014;191:296-300.
69. Beland MD, Mayo-Smith WW, Dupuy DE, Cronan JJ, DeLellis. Diagnostic yield of 58 consecutive imaging-guided biopsies of solid renal masses: should we biopsy all that are indeterminate? AJR Am J Roentgenol 2007;188:792-797.
70. Blumenfeld AJ, Guru K, Fuchs GJ, Kim HL. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology 2010;76:610-613.
71. Ficarra V, Brunelli M, Novara G, D’Elia C, Segala D, Gardiman M, et al. Accuracy of on-bench biopsies in the evaluation of the histological subtype, grade, and necrosis of renal tumours.Pathology 2011;43:149-155.
72. Halverson SJ, Kunju LP, Bhalla R, Gadzinski AJ, Alderman M, Miller DC, et al. Accuracy of determining small renal mass management with risk strati fi ed biopsies: con fi rmation by fi nal pathology. J Urol 2013;189:441-446.
73. Novara G, Martignoni G, Artibani W, Ficarra V. Grading systems in renal cell carcinoma. J Urol 2007;177:430-436.
74. Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 2003;27:612-624.
75. Cheville JC, Lohse CM, Zincke H, Weaver AL, Leibovich BC, Frank I, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol 2004;28:435-441.
76. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002;168:2395-2400.
77. Barocas DA, Mathew S, DelPizzo JJ, Vaughan ED Jr, Sosa RE, Fine RG, et al. Renal cell carcinoma sub-typing by histopathology and fl uorescence in situ hybridization on a needle-biopsy specimen. BJU Int 2007;99:290-295.
78. Barocas DA, Rohan SM, Kao J, Gurevich RD, Del Pizzo JJ, Vaughan ED Jr, et al. Diagnosis of renal tumors on needle biopsy specimens by histological and molecular analysis. J Urol 2006;176:1957-1962.
79. Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 2003;9:802-811.
80. Yang XJ, Sugimura J, Schafernak KT, Tretiakova MS, Han M, Vogelzang NJ, et al. Classi fi cation of renal neoplasms based on molecular signatures. J Urol 2006;175:2302-2306.
81. Vasselli JR, Shih JH, Iyengar SR, Maranchie J, Riss J, Worrell R, et al. Predicting survival in patients with metastatic kidney cancer by gene-expression pro fi ling in the primary tumor. Proc Natl Acad Sci U S A 2003;100:6958-6963.
82. Longo DL. Tumor heterogeneity and personalized medicine. N Engl J Med 2012;366:956-957.
83. Li QL, Guan HW, Zhang QP, Zhang LZ, Wang FP, Liu YJ. Optimal margin in nephron-sparing surgery for renal cell carcinoma 4 cm or less. Eur Urol 2003;44:448-451.
84. Simhan J, Canter DJ, Sterious SN, Smaldone MC, Tsai KJ, Li T, et al. Pathological concordance and surgical outcomes of sporadic synchronous unilateral multifocal renal masses treated with partial nephrectomy. J Urol 2013;189:43-47.
85. Rothman J, Crispen PL, Wong YN, Al-Saleem T, Fox E, Uzzo RG. Pathologic concordance of sporadic synchronous bilateral renal masses. Urology 2008;72:138-142.
86. Abel EJ, Culp SH, Matin SF, Tamboli P, Wallace MJ, Jonasch E, et al. Percutaneous biopsy of primary tumor in metastatic renal cell carcinoma to predict high risk pathological features: comparison with nephrectomy assessment. J Urol 2010;184:1877-1881.
87. Eichelberg C, Junker K, Ljungberg B, Moch H. Diagnostic and prognostic molecular markers for renal cell carcinoma: a critical appraisal of the current state of research and clinical applicability. Eur Urol 2009;55:851-863.
89. Brunelli M, Eccher A, Gobbo S, Ficarra V, Novara G, Cossu-Rocca P, et al. Loss of chromosome 9p is an independent prognostic factor in patients with clear cell renal cell carcinoma. Mod Pathol 2008;21:1-6.
91. Lane BR, Li J, Zhou M, Babineau D, Faber P, Novick AC, et al. Di ff erential expression in clear cell renal cell carcinoma identi fi ed by gene expression pro fi ling. J Urol 2009;181:849-860.
92. Rogers CG, Ditlev JA, Tan MH, Sugimura J, Qian CN, Cooper J, et al. Microarray gene expression pro fi ling using core biopsies of renal neoplasia. Am J Transl Res 2009;1:55-61.
Cite this article as:Tomaszewski JJ, Uzzo RG, Smaldone MC. Heterogeneity and renal mass biopsy: a review of its role and reliability. Cancer Biol Med 2014;11:162-172. doi: 10.7497/j.issn.2095-3941.2014.03.002
Jeffrey J. Tomaszewski
E-mail: tomaszewski.jeffrey@gmail.com
Received May 29, 2014; accepted June 25, 2014. Available at www.cancerbiomed.org
Copyright ? 2014 by Cancer Biology & Medicine
 Cancer Biology & Medicine2014年3期
Cancer Biology & Medicine2014年3期
- Cancer Biology & Medicine的其它文章
- Breast metastasis from lung cancer: a report of two cases and literature review
- The gene expression patterns of BMPR2, EP300, TGFβ2, and TNFAIP3 in B-Lymphoma cells
- Neoadjuvant chemoradiotherapy for resectable esophageal cancer: an in-depth study of randomized controlled trials and literature review
- Hepatitis B virus X protein accelerates the development of hepatoma
- Systemic treatment in EGFR-ALK NSCLC patients: second line therapy and beyond
- Restricting carbohydrates to fi ght head and neck cancer—is this realistic?
