Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function
Qijun Li, Changzheng Dong, Wenling Li, Wei Bu, Jiang Wu, Wenqing Zhao,
1 Graduate School, Hebei Medical University, Shijiazhuang, Hebei Province, China
2 Department of Functional Neurosurgery, Hebei General Hospital, Shijiazhuang, Hebei Province, China
3 Department of Neurosurgery, Third Hospital, Hebei Medical University, Shijiazhuang, Hebei Province, China
Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function
Qijun Li1, Changzheng Dong2, Wenling Li2, Wei Bu3, Jiang Wu2, Wenqing Zhao1,2
1 Graduate School, Hebei Medical University, Shijiazhuang, Hebei Province, China
2 Department of Functional Neurosurgery, Hebei General Hospital, Shijiazhuang, Hebei Province, China
3 Department of Neurosurgery, Third Hospital, Hebei Medical University, Shijiazhuang, Hebei Province, China
Neuropeptide Y has been shown to inhibit the immunological activity of reactive microglia in the rat cerebral cortex, to reduce N-methyl-D-aspartate current (INMDA) in cortical neurons, and protect neurons. In this study, after primary cultured microglia from the cerebral cortex of rats were treated with lipopolysaccharide, interleukin-1β and tumor necrosis factor-α levels in the cell culture medium increased, and mRNA expression of these cytokines also increased. After primary cultured cortical neurons were incubated with the lipopolysaccharide-treated microglial conditioned medium, peak INMDAin neurons increased. These effects of lipopolysaccharide were suppressed by neuropeptide Y. After addition of the neuropeptide Y Y1 receptor antagonist BIBP3226, the effects of neuropeptide Y completely disappeared. These results suggest that neuropeptide Y prevents excessive production of interleukin-1β and tumor necrosis factor-α by inhibiting microglial reactivity. This reduces INMDAin rat cortical neurons, preventing excitotoxicity, thereby protecting neurons.
nerve regeneration; microglia; immunological activity; neuropeptide Y; interleukin-1β; tumor necrosis factor-α; INMDA; neural regeneration
Li QJ, Dong CZ, Li WL, Bu W, Wu J, Zhao WQ. Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function. Neural Regen Res. 2014;9(9):959-967.
Introduction
Microglia are a type of macrophage extensively distributed in the central nervous system (Ginhoux et al., 2010), and strongly associated with immunity. Microglia play major roles in nervous system disease and immune responses (Graeber and Streit, 1990; Aloisi, 1999; Wirenfeldt et al., 2011). Moreover, microglia can produce in fl ammatory factors and mediate in fl ammation in the nervous system (Rivest, 2009; Kraft and Harry, 2011). Under normal conditions, microglia are in a resting state and play an immune surveillance role in the central nervous system. When stimulated, microglia are activated, and can release interleukin-1β and tumor necrosis factor-α, as well as a large number of bioactive substances, including reactive oxygen, nitrogen and lipid species (Giulian et al., 1994; Liu et al., 2002; Rivest, 2009; Wu et al., 2009; Mayer et al., 2011). Excessive release of these bioactive substances can induce neuronal injury (Auvin et al., 2010; Friedman and Dingledine, 2011; Vezzani et al., 2011b). Microglial activation is a key step in the in fl ammatory process in the central nervous system (Hanisch, 2002; Kaur et al., 2010; Kraft and Harry, 2011). Animal experiments and clinical studies show that an in fl ammatory reaction occurs in the focal zone after epileptic seizures (Auvin et al., 2010; Vezzani and Friedman, 2011; Vezzani et al., 2013). Moreover, microglia are noticeably activated in the focal zone, and interleukin-1β and tumor necrosis factor-α expression are increased. Simultaneously, neuronal excitability is elevated, and the number of apoptotic neurons increases (Ravizza et al., 2005; Vezzani and Granata, 2005). In addition, inflammatory factors are strongly associated with epilepsy. Thus, reducing the release of inflammatory factors or blocking their effects could lessen the severity of epilepsy (Maroso et al., 2011; Vezzani et al., 2011a, b; Gao et al., 2012).
Previous studies have shown that some medicines protect neurons by inhibiting microglial reactivity (Tikka et al., 2001; Dheen et al., 2007; Park et al., 2007). For example, one study showed that after microglial activation was suppressed by the cyclooxygenase 2 inhibitor celecoxib in the hippocampus of epileptic rats, spontaneous seizures and neuronal injury were signi fi cantly reduced (Zhang et al., 2008), suggesting that inhibiting microglial activation and bioactive substance release is an effective method of treating epilepsy.
It remains poorly understood whether there was a kind of medicine that directly acted on neurons, regulated the immunological activity of microglia and finally protected neurons against injury through nerve-immunity pathway.
Neuropeptide Y is widely expressed in the central and peripheral nervous systems, and is strongly associated with epilepsy, learning and memory (Wettstein et al., 1995; Vezzani et al., 1999). Neuropeptide Y exerts protective effects against neuronal injury and can alleviate epilepsy (Smialowska et al., 2009; Baptista et al., 2012; Goncalves et al., 2012; Gotzsche et al., 2012; Malva et al., 2012). Neuropeptide Y exerts protective effects on neurons mainly through the Y2 receptor (Greber et al., 1994; Woldbye et al., 2010; Decressac et al., 2012; Goncalves et al., 2012). In a recent study, neuropep-tide Y suppressed the activation of the mouse microglial cell line N9 induced by lipopolysaccharide, and it reduced the generation of interleukin-1β and nitric oxide (Ferreira et al., 2010). Neuropeptide Y was also found to suppress microglial migration and phagocytosis (Ferreira et al., 2011, 2012).
It is unclear whether neuropeptide Y can reduce the release of bioactive substances and protect neurons by regulating microglial immunological activity. Cytokines can activate glutamic acid receptors and induce changes in ion currents, thereby contributing to neuronal injury (Yang et al., 2005). Glutamic acid receptors include ionotropic and metabotropic receptors. The N-methyl-D-aspartate (NMDA) receptor, an ionotropic glutamic acid receptor, is extensively distributed in the central nervous system. The NMDA receptor can be controlled by membrane potential, glutamic acid and NMDA. In the resting state, the NMDA receptor binds to Mg2+, which blocks the opening of the ion channel. When bound to excitatory neurotransmitters such as glutamate and NMDA, the receptor is activated, opening the ion channel and allowing cations such as Ca2+, Na+and K+to enter, resulting in the NMDA current (INMDA). Excessive cation fl ux, especially Ca2+in fl ux, results in excitotoxic neuronal injury (Norris et al., 2006; Deshpande et al., 2008; Zhu et al., 2010).
Previous studies suggested that interleukin-1β and tumor necrosis factor-α elevate NMDA receptor expression, and increase NMDA activity (Balosso et al., 2008; Zheng et al., 2010; Zhu et al., 2010). Therefore, neuropeptide Y may diminish the release of interleukin-1β and tumor necrosis factor-α derived from microglia by inhibiting microglial activation, thereby suppressing NMDA receptor expression, decreasing NMDA activity, reducing INMDA, and ultimately protecting neurons.
In the present study, lipopolysaccharide was used to activate primary cultured microglia derived from the rat cerebral cortex. We observed the effects of neuropeptide Y on interleukin-1β and tumor necrosis factor-α in activated microglia. Microglial conditioned medium treated or untreated with neuropeptide Y was used to incubate primary neurons from the rat cerebral cortex. The patch-clamp technique was used to detect changes in INMDAinduced by the different microglial conditioned media.
Materials and methods
Animals
A total of 64 clean Sprague-Dawley rats born within 24 hours were provided by the Experimental Animal Center of Hebei Medical University in China (animal license No. SCXK (Ji) 2013-1-03). The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and the protocol was approved by the Institutional Animal Care Committee of Hebei Medical University.
Neuronal and microglial cultures
Mixed microglial cultures, microglial isolation and puri fi cation were conducted in accordance with a previous method (Nakajima et al., 1992). The brain was obtained aseptically from neonatal rats after craniotomy. After removal of the meninges and blood vessels, a part of the cerebral cortex was cut into pieces and digested in 0.125% trypsin at 37°C for 15 minutes. Samples were agitated with a pipette, centrifuged at 1,000 r/min for 5 minutes, and fi ltered. The supernatant was discarded. The pellet was resuspended with microglia medium (Dulbecco’s modified Eagle’s medium/F12 medium, DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco), 1 U/mL penicillin and 100 μg/mL streptomycin. The cell suspension was incubated in a 250 mL culture fl ask at 37°C, 5% CO2. On the second day, the medium was replaced once with new medium. From then on, half of the medium was replaced every 3 days. At 14 days, when cells were confluent, they were shaken on a rocker at 37°C, 180 r/min for 2 hours. The cell suspension was centrifuged at 1,000 r/min for 5 minutes. After removal of the supernatant, the samples were agitated into a cell suspension using microglia medium. Cells were adjusted to about 1 × 105/mL, and seeded in 6-well plates precoated with polylysine (Sigma, St. Louis, MO, USA), 3 mL in each well, in a constant-temperature incubator (Heraeus, Hanau, Germany) for 30 minutes without stirring. The medium was replaced once by new medium. After removal of oligodendrocytes, the samples were incubated with microglia medium for 3-5 days, resulting in a puri fi ed microglial culture.
Isolation and culture of rat cerebral cortical neurons were conducted in accordance with a previous method (Yang et al., 2006). The brain was obtained aseptically from neonatal rats by craniotomy. Brain tissue was placed in a petri dish with precooled DMEM/F12 medium. The petri dish was placed on ice. A median sagittal incision was made. After removal of the cerebellum and subcortical tissue, meninges and blood vessels were dissociated with a dissecting microscope. The cortex was placed in an additional petri dish with precooled DMEM/F12 medium. The petri dish was placed on ice. The cerebral cortex was cut into about 1 mm3blocks, digested in 0.125% trypsin at 37°C for 5 minutes, and agitated into a cell suspension. Cell aggregates were removed by fi ltration to obtain a single-cell suspension. Cells were adjusted to about 1 × 105/mL using microglia medium. Cell suspensions were seeded in a 6-well plate with a polylysine-treated coverslip, 3 mL in each well, in a 5% CO2incubator at 37°C for co-culture. Twenty-four hours later, the medium was replaced by neuronal medium (Neurobasal medium) (Gibco), supplemented with 2% B27 (Gibco), 100 U/mL penicillin and 100 μg/mL streptomycin. The medium was replaced once every 3 days. After 7-9 days of culture, puri fi ed neurons were obtained.
Identi fi cation of microglia and neurons
Purity of microglia was assessed using immuno fl uorescence staining (Schluesener et al., 1999; Yamasaki et al., 2010; Azemi et al., 2011; Savard et al., 2013; Yao et al., 2013). Isolated microglia were incubated in a culture plate with polylysine-treated slides for 3 days. The samples were washed with PBS, fixed in 4% paraformaldehyde for 30 minutes, treated with 0.3% Triton X-100 for 10 minutes, blocked with 3% goat serum for 30 minutes, incubated with mouseanti-ionized calcium-binding adapter molecule 1 monoclonal antibody (1:200; Sigma) at 4°C overnight, and then with FITC-labeled goat anti-mouse IgG (1:100; Proteintech, Chicago, IL, USA) at 37°C for 1 hour. Except for goat serum blocking, three PBS washes were performed between each step. After mounting, a fl uorescence microscope (DMI3000B and DFC450C, Leica, Wetzlar, Germany) was used to observe the percentage of positive cells at 400 × magni fi cation. Microglial morphology was observed after treatment with lipopolysaccharide (100 ng/mL; Sigma) for 6 hours.
Immuno fl uorescence staining was used to identify whether the cultured cells were neurons (Andreyeva et al., 2012; Soetedjo et al., 2013). The procedures were identical to that for microglia. The antibody used was rabbit anti-microtubule-associated protein-2 polyclonal antibody (1:100; Proteintech) and TRITC-labeled goat anti-rabbit IgG (1:100; Proteintech). Under a fl uorescence microscope, fi ve high-power fi elds (400 ×) were randomly selected. The percentage of positive cells was calculated with an Image-Pro Plus image analysis system (Media Cybernetics, Silver Spring, ML, USA).
Enzyme linked immunosorbent assay (ELISA) for interleukin-1βand tumor necrosis factor-αprotein levels in lipopolysaccharide-treated microglial conditioned medium
Puri fi ed microglia were seeded in a 6-well polylysine-treated plate at 1 × 105/mL, 3 mL in each well. Three days later, microglia were cultured with fresh serum-free DMEM/F12 medium for 12 hours, and then incubated with serum-free DMEM/F12 medium containing lipopolysaccharide, 100 ng/mL (Sigma), for 1, 3, 6, 12 and 24 hours. Cell culture medium was collected from each group and centrifuged. Interleukin-1β and tumor necrosis factor-α levels in the medium were assessed with an ELISA kit (Bio-Swamp; Wuhan, China). Microtiter plates were assigned to the positive control well, blank control well and sample well. Standard preparations at different concentrations were added in the positive control well, 50 μL in each well. Blank control well did not contain any sample, reagent, biotinylated anti-tumor necrosis factor-α antibody or anti-interleukin-1β antibody. In the sample well, 40 μL of sample was fi rst added, and then biotinylated anti-tumor necrosis factor-α antibody or anti-interleukin-1β antibody, 10 μL, was added. The plate was sealed with membrane, and then placed at 37°C for 30 minutes. The plate was washed fi ve times, and patted dry. ELISA reagents, 50 μL, were added in each well, except the blank well. After incubation for 30 minutes, the plate was washed. The samples were visualized with developer at 37°C in the dark for 15 minutes. Reaction was terminated by the addition of stop buffer, 50 μL, in each well. Absorbance valus of each well were measured at 450 nm using a microplate reader (Thermo Labsystems, Helsinki, Finland). Blank control well was adjusted to zero. The measurement was performed within 15 mintues after adding stop buffer. Protein levels were calculated according to a standard curve of known concentrations.
Microglial treatment with neuropeptide Y and lipopolysaccharide
Microglia at 1 × 105/mL were seeded in a 6-well polylysine-treated plate, 3 mL in each well, for 3 days. Microglia were incubated with fresh serum-free DMEM/F12 medium for 12 hours to synchronize cells. Subsequently, cells were divided into control, lipopolysaccharide, neuropeptide Y + lipopolysaccharide, neuropeptide Y and BIBP3226 + neuropeptide Y + lipopolysaccharide groups. In the control group, microglia were incubated with serum-free DMEM/F12 medium for 6 hours. In the lipopolysaccharide group, microglia were incubated with serum-free medium containing 100 ng/mL lipopolysaccharide for 6 hours. In the neuropeptide Y + lipopolysaccharide group, microglia were incubated with serum-free DMEM/F12 medium containing neuropeptide Y ( fi nal concentration: 1 μmol/L; ENZO, New York, NY, USA) for 0.5 hours, and then treated with lipopolysaccharide ( fi nal concentration: 100 ng/mL) for 6 hours. In the neuropeptide Y group, microglia were incubated with serum-free medium containing neuropeptide Y (1 μmol/L) for 6 hours. In the BIBP3226 + neuropeptide Y + lipopolysaccharide group, microglia were first incubated with serum-free DMEM/F12 medium containing the neuropeptide Y1 receptor antagonist BIBP3226 (N-[(1R)]-4-[(Aminoiminomethyl)amino-1-[[[(4-hydroxyphenyl)methyl]amino] carbonyl]butyl-α-phenylbenzeneacetamide tri fl uoroacetate; molecular weight: 587.59; molecular formula: C27H31N5O3?C F3CO2H; purity > 98%; final concentration: 1 μmol/L; Tocris Bioscience, Ellisville, MO, USA) for 0.5 hours, then with neuropeptide Y ( fi nal concentration: 1 μmol/L) for 0.5 hours, and fi nally with lipopolysaccharide ( fi nal concentration: 100 ng/mL) for 6 hours.
Effects of neuropeptide Y on interleukin-1βand tumor necrosis factor-αmRNA levels in lipopolysaccharidetreated microglia, as detected by real-time PCR
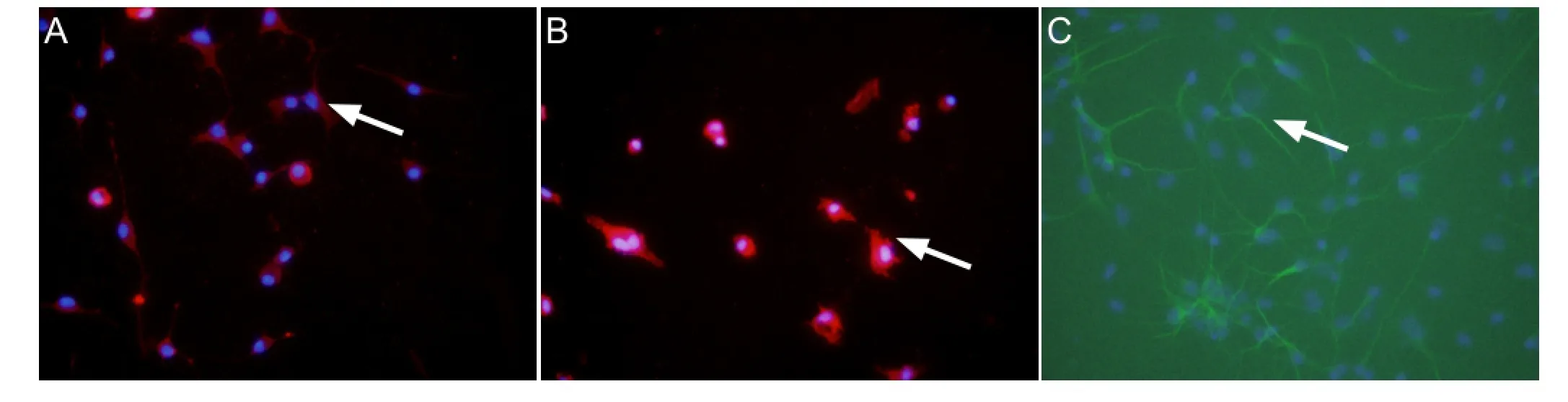
Figure 1 Primary cultured microglia and neurons (immuno fl uorescence, × 400).

Figure 2 Effects of lipopolysaccharide on IL-1β and TNF-α secretion by microglia (enzyme linked immunosorbent assay).
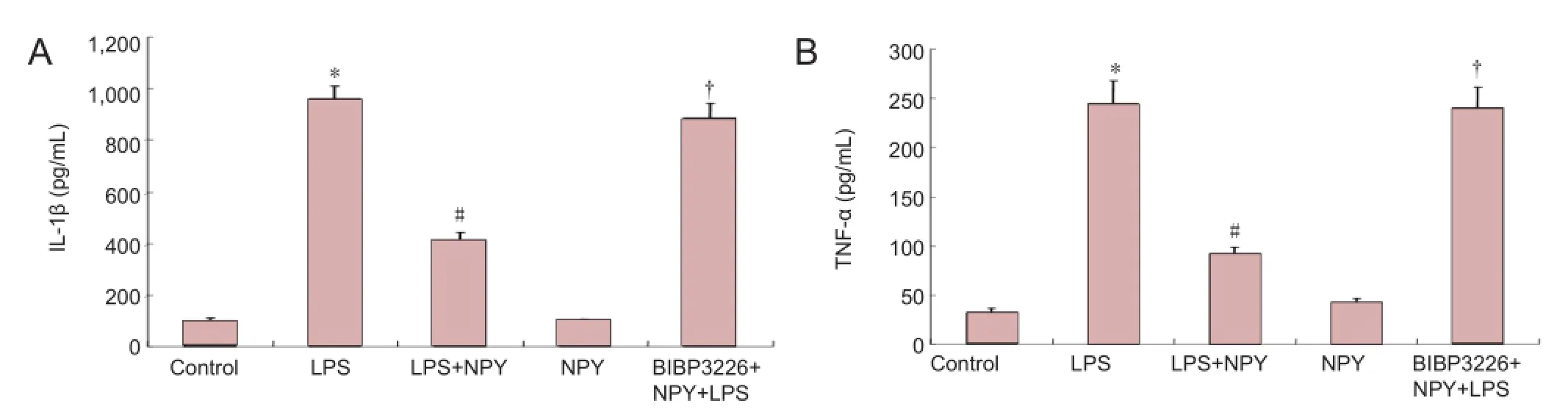
Figure 3 Inhibitory effects of NPY on LPS-induced production of IL-1β and TNF-α by microglia (enzyme linked immunosorbent assay).
Culture medium was discarded and Trizol (1 mL/well) was added in each well. Samples were agitated and placed in an RNAse-free centrifuge tube for 5 minutes. Into each tube, 0.2 mL chloroform was added. After shaking for 15 seconds and incubating for 5 minutes, samples were centrifuged at 12,000 r/min for 15 mintues. The colorless liquid in the upper layer was removed to a new centrifuge tube. An equal volume of isopropanol was added, and mixed by inversion, followed by centrifugation at 12,000 r/min for 10 minutes. A white precipitate was visible at the bottom of the tube. Supernatant was completely discarded. The precipitate, following addition of 1 mL 75% alcohol (in DEPC-treated water), was washed. After centrifugation at 7,500 r/min at 4°C for 5 minutes, the supernatant was discarded. The samples were air-dried for 3-5 minutes. 20-30 μL of DEPC-treated water was added to thoroughly dissolve RNA. To verify RNA integrity and purity, 1 % agarose gel electrophoresis was used. A UV spectrophotometer was used to determine RNAconcentration. Primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China.
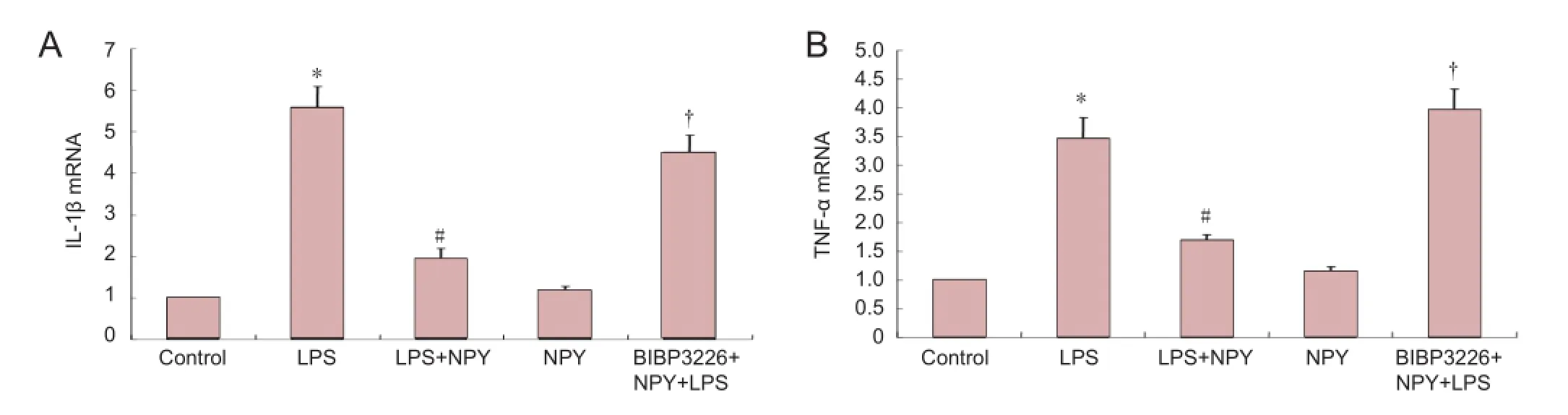
Figure 4 Inhibitory effects of NPY on LPS-induced expression of IL-1β and TNF-α by microglia (real-time PCR).
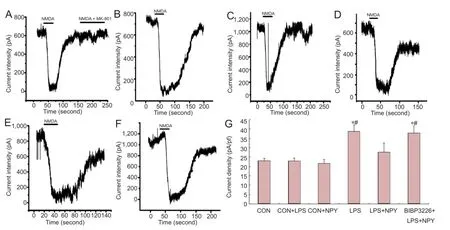
Figure 5 Effects of NPY on INMDAin neurons incubated with LPS-treated microglial conditioned medium.
Primer sequences were shown inTable 1. Reverse transcription reaction: Total RNA 8 μL, random primer 1 μL, 2 × ES reaction mix 10 μL, RT/RI enzyme mix 1 μL; total volume: 20 μL. Amplification system: 2 × UltraSYBR mixture (with ROX) 10 μL, forward primer (10 μmol/L) 1 μL, reverse primer (10 μmol/L) 1 μL, cDNA 8 μL, total volume: 20 μL. RT-PCR parameters: predenaturation at 95°C for 10 minutes; 40 cycles of denaturation at 95°C for 15 seconds, annealing at 58°C for 20 seconds, extension at 72°C for 27 seconds. An ABI 7300 Real-Time PCR System (Applied Biosystems, Foster, CA, USA) was used to detect and calculate the relative quantity of the target gene to the internal reference (GAPDH).
INMDAin neurons as determined by patch-clamp technique
Microglial conditioned media obtained from the variousgroups were individually mixed with neuronal medium at a ratio of 1:1. Control medium after mixing with neuronal medium at 1:1 was divided into three groups: control (CON) group, CON + lipopolysaccharide group and CON + neuropeptide Y group. Lipopolysaccharide ( fi nal concentration of 50 ng/mL) and neuropeptide Y (final concentration of 0.5 μmol/L) were added in the CON + lipopolysaccharide group and CON + neuropeptide Y group, respectively. Puri fi ed neuronal cells (3 × 105/well) in each group were incubated with their corresponding treatment for 6 hours. INMDAin neurons was recorded using the patch-clamp technique (Yang et al., 2005; Zhang et al., 2011). Glass slides with neuronal cells were placed in a 0.3 mL-bath. Cells were continuously perfused with extracellular fl uid at 2 mL/min to ensure that fl uid exchange was fi nished within 2 minutes. An inverted microscope (TE2000-S; Nikon, Tokyo, Japan) was used to observe cells. Neurons with a clear outline, a de fi ned three-dimensional structure and a smooth surface were selected for recording. Using a three-dimensional manipulator (Sutter Instruments Company, Novato, CA), a glass microelectrode at 1-3 MΩ impedance and fi lled with pipette solution (including KCl 10 mmol/L, K-D-gluconate 120 mmol/L, HEPES 10 mmol/L, EGTA 11 mmol/L, NaCl 5 mmol/L, MgCl22 mmol/L, CaCl21 mmol/L, Mg-ATP 2 mmol/L and Li-GTP 1 mmol/L; pH 7.2 adjusted with NaOH) was sealed onto the cell surface. Negative pressure was applied until the patch was formed. Capacitive current and series resistance of the electrode were compensated, and whole-cell recording was commenced. Clamping voltage was set at -60 mV. After whole-cell recording for 5 minutes, the intracellular fluid was thoroughly replaced with electrode solution. At this time, 100 μmol/L NMDA (Sigma) and 10 μmol/L glycine (Sigma) were added extracellularly to induce inward current changes. INMDAwas recorded.
Statistical analysis
The data were analyzed using SPSS 10.0 software (SPSS, Chicago, IL, USA) and expressed as mean ± SD. Test of normality and homogeneity test for variance showed that all data obeyed a normal distribution and had homogeneous variance. Completely random design was employed in analysis of variance. Paired comparison was done using least signi fi cant difference test. A value of P < 0.05 was considered statistically signi fi cant.
Results
Isolated culture of microglia and neurons
Ionized calcium-binding adapter molecule and microtubule-associated protein 2 were used as markers of microglia and neurons, respectively (Izant and McIntosh, 1980; Dehmelt and Halpain, 2005; Nakamura et al., 2013). The purities of microglia and neurons were > 95%, which met the requirements of the experiments (Figure 1).
Lipopolysaccharide promoted the secretion of interleukin-1βand tumor necrosis factor-αby microglia
ELISA results revealed that interleukin-1β and tumor necrosis factor-α levels in the medium of each group were signi fi cantly greater after incubation with lipopolysaccharide compared with the control group (P < 0.01 or P < 0.05). As incubation proceded, interleukin-1β content gradually increased (P < 0.05), peaked at 3 hours, and then gradually decreased (P < 0.05;Figure 2A). Moreover, tumor necrosis factor-α content gradually increased over the incubation period (P < 0.05), peaked at 6 hours, and then gradually diminished (P < 0.05;Figure 2B).
Neuropeptide Y reduced lipopolysaccharide-induced secretion of interleukin-1βand tumor necrosis factor-αby microglia
ELISA results revealed that interleukin-1β and tumor necrosis factor-α levels were signi fi cantly lower in the neuropeptide Y + lipopolysaccharide group than in the lipopolysaccharide group (P < 0.01). Interleukin-1β and tumor necrosis factor-α levels were signi fi cantly increased in the BIBP3226 + neuropeptide Y + lipopolysaccharide group (P < 0.01). Interleukin-1β and tumor necrosis factor-α levels did not signi fi cantly change in the medium after normal cells were treated with neuropeptide Y (P > 0.05;Figure 3).
Neuropeptide Y suppressed interleukin-1βand tumor necrosis factor-αmRNA expression in microglia treated with lipopolysaccharide
At 6 hours after incubation, interleukin-1β and tumor necrosis factor-α mRNA levels were signi fi cantly higher in the lipopolysaccharide group and in the BIBP3226 + neuropeptide Y + lipopolysaccharide group than in the control group (P < 0.01). No significant difference in interleukin-1β or tumor necrosis factor-α mRNA levels was detected between the lipopolysaccharide and BIBP3226 + neuropeptide Y + lipopolysaccharide groups. Interleukin-1β and tumor necrosis factor-α mRNA levels were signi fi cantly lower in the lipopolysaccharide + neuropeptide Y group compared with the lipopolysaccharide group (P < 0.01). Interleukin-1β and tumor necrosis factor-α mRNA levels were signi fi cantly higher in the BIBP3226 + neuropeptide Y + lipopolysaccharide group than in the lipopolysaccharide + neuropeptide Y group (P < 0.01). Interleukin-1β and tumor necrosis factor-α mRNA levels were similar in the neuropeptide Y and control groups (P > 0.05). These results suggest that lipopolysaccharide elevates interleukin-1β and tumor necrosis factor-α mRNA levels in microglia and that neuropeptide Y inhibits the effects of lipopolysaccharide. When BIBP3226 was used to block the neuropeptide Y1 receptor, neuropeptide Y had no effect. Neuropeptide Y did not impact interleukin-1β or tumor necrosis factor-α mRNA levels in microglia (Figure 4).
Neuropeptide Y inhibitsINMDAin neurons after incubation with lipopolysaccharide-treated microglial conditioned medium
Patch-clamp recording showed that, compared with the CON group, INMDAwas higher in the lipopolysaccharide group (P < 0.01). INMDAwas significantly lower in the neuropeptide Y + lipopolysaccharide group compared with the lipopolysaccharide group. INMDAwas significantly higher inthe BIBP3226 + neuropeptide Y + lipopolysaccharide group compared with the neuropeptide Y + lipopolysaccharide group (P < 0.01). INMDAwas not substantially altered in neurons cultured in normal microglia medium (Figure 5).
Discussion
Microglia play an important role in regulating immune function and are strongly associated with the occurrence and development of central nervous system diseases (Harry and Kraft, 2012; Zhao et al., 2013). In central nervous system pathologies, microglia rapidly respond and switch from a resting to an activated state, from a rami fi ed to an amoeboid morphology, with an enlarged body and retracted processes (Harry and Kraft, 2012). Microglia undergo a series of changes, and function as sensors in the central nervous system (Kreutzberg, 1996). Microglias are the macrophages of the central nervous system, and are strongly associated with immune functions in brain tissue. The accumulation of numerous cytokines and bioactive substances after microglial activation is a key event in neuronal injury (Vezzani et al., 2011b). Among these cytokines, interleukin-1β and tumor necrosis factor-α play important roles in physiological and pathological processes in the central nervous system (Lee et al., 2004; Maroso et al., 2011; Savard et al., 2013). The increased concentrations of interleukin-1β and tumor necrosis factor-α in brain tissues exert toxic effects on surrounding neurons (Zheng et al., 2010; Zhu et al., 2010). Lipopolysaccharide-activated microglia produce various factors implicated in pathologies (Lee et al., 2004).
Lipopolysaccharide was used as an activator of microglia in the present study. After 6 hours of intervention with lipopolysaccharide, the immunological activity of microglia increased, and interleukin-1β and tumor necrosis factor-α levels in microglial conditioned medium substantially increased. Neuropeptide Y suppressed the lipopolysaccharide-induced activation of microglia, and it reduced interleukin-1β and tumor necrosis factor-α protein and mRNA levels in these cells. These inhibitory effects of neuropeptide Y were completely suppressed by BIBP3226, indicating that neuropeptide Y diminishes the immunological activity of microglia through the Y1 receptor, thereby reducing interleukin-1β and tumor necrosis factor-α generation, consistent with a previous study (Ferreira et al., 2010). Many studies on the effects of cytokines on neurons suggest that these factors modulate the glutamic acid receptor (Vezzani et al., 2008). For example, interleukin-1β and tumor necrosis factor-α elevate NMDA receptor expression and activity. A previous study demonstrated that interleukin-1β can phosphorylate the NMDA NR2B subunit and activate the receptor (Balosso et al., 2008). Tumor necrosis factor-α increases phosphorylation of the NR1 subunit, and increases its expression as well (Wheeler et al., 2009). In a previous study, after rat hippocampal neurons were incubated with microglial conditioned medium containing tumor necrosis factor-α, NMDARI expression rose in neurons, and this effect was inhibited by addition of an anti-tumor necrosis factor-α antibody (Zhu et al., 2010). Interleukin-1β and tumor necrosis factor-α increase the probability and duration of channel opening, thereby increasing INMDA. The resulting Ca2+in fl ux into neurons causes calcium overload, resulting in cytotoxicity (Viviani et al., 2003). INMDAis a direct index of changes in the NMDA receptor. An increase in INMDAindicates increased activity of the NMDA receptor and an increased number of cations (mainly Ca2+) passing through the ion channel (Malenka and Nicoll, 1999; Gardoni et al., 2001; Yang et al., 2005).
Patch-clamping showed that INMDAwas enhanced after treatment with lipopolysaccharide compared with the control group. Neuropeptide Y suppressed microglial activation and this lipopolysaccharide-mediated increase in INMDA. When BIBP3226 was added to block the neuropeptide Y Y1 receptor, this effect of neuropeptide Y disappeared. INMDAdid not remarkably change after incubation with lipopolysaccharide and neuropeptide Y conditioned medium, suggesting that changes in INMDAin neurons were not caused by lipopolysaccharide or neuropeptide Y, similar to a previous study (Bronstein et al., 1995), but were induced by factors in the conditioned medium of activated microglia.
In summary, neuropeptide Y suppresses microglial reactivity, reduces the release of microglial bioactive factors, diminishes INMDA, and prevents excitotoxicity induced by excessive Ca2+entry into neurons. These effects of neuropeptide Y were fully blocked by the Y1 receptor antagonist BIBP3226. We demonstrated that neuropeptide Y can reduce INMDAin neurons by regulating the immune function of microglia. Thus, neuropeptide Y plays a major role in regulating immune function in the central nervous system. Neuropeptide Y reduces the generation of interleukin-1β and tumor necrosis factor-α, preventing intracellular Ca2+overload induced by NMDA receptor hyperactivation, thereby protecting neurons.
Author contributions:Li QJ wrote the manuscript. All authors participated in study design, implement and evaluation, and approved the final version of the paper.
Con fl icts of interest:None declared.
Aloisi F (1999) The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv Exp Med Biol 468:123-133.
Andreyeva A, Nieweg K, Horstmann K, Klapper S, Muller-Schiffmann A, Korth C, Gottmann K (2012) C-terminal fragment of N-cadherin accelerates synapse destabilization by amyloid-beta. Brain 135:2140-2154.
Auvin S, Mazarati A, Shin D, Sankar R (2010) In fl ammation enhances epileptogenesis in the developing rat brain. Neurobiol Dis 40:303-310.
Azemi E, Lagenaur CF, Cui XT (2011) The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials 32:681-692.
Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, Vezzani A (2008) A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain 131:3256-3265.
Baptista S, Bento AR, Goncalves J, Bernardino L, Summavielle T, Lobo A, Fontes-Ribeiro C, Malva JO, Agasse F, Silva AP (2012) Neuropeptide Y promotes neurogenesis and protection against methamphetamine-induced toxicity in mouse dentate gyrus-derived neurosphere cultures. Neuropharmacology 62:2413-2423.
Bronstein DM, Perez-Otano I, Sun V, Mullis Sawin SB, Chan J, Wu GC, Hudson PM, Kong LY, Hong JS, McMillian MK (1995) Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Res 704:112-116.
Decressac M, Pain S, Chabeauti PY, Frangeul L, Thiriet N, Herzog H, Vergote J, Chalon S, Jaber M, Gaillard A (2012) Neuroprotection by neuropeptide Y in cell and animal models of Parkinson’s disease. Neurobiol Aging 33:2125-2137.
Dehmelt L, Halpain S (2005) The MAP2/Tau family of microtubule-associated proteins. Genome Biol 6:204.
Deshpande LS, Lou JK, Mian A, Blair RE, Sombati S, Attkisson E, De-Lorenzo RJ (2008) Time course and mechanism of hippocampal neuronal death in an in vitro model of status epilepticus: role of NMDA receptor activation and NMDA dependent calcium entry. Eur J Pharmacol 583:73-83.
Dheen ST, Kaur C, Ling EA (2007) Microglial activation and its implications in the brain diseases. Curr Med Chem 14:1189-1197.
Ferreira R, Xapelli S, Santos T, Silva AP, Cristovao A, Cortes L, Malva JO (2010) Neuropeptide Y modulation of interleukin-1{beta} (IL-1{beta})-induced nitric oxide production in microglia. J Biol Chem 285:41921-41934.
Ferreira R, Santos T, Viegas M, Cortes L, Bernardino L, Vieira OV, Malva JO (2011) Neuropeptide Y inhibits interleukin-1beta-induced phagocytosis by microglial cells. J Neuroin fl ammation 8:169.
Ferreira R, Santos T, Cortes L, Cochaud S, Agasse F, Silva AP, Xapelli S, Malva JO (2012) Neuropeptide Y inhibits interleukin-1 beta-induced microglia motility. J Neurochem 120:93-105.
Friedman A, Dingledine R (2011) Molecular cascades that mediate the in fl uence of in fl ammation on epilepsy. Epilepsia 52 Suppl 3:33-39.
Gao F, Liu Y, Li X, Wang Y, Wei D, Jiang W (2012) Fingolimod (FTY720) inhibits neuroin fl ammation and attenuates spontaneous convulsions in lithium-pilocarpine induced status epilepticus in rat model. Pharmacol Biochem Behav 103:187-196.
Gardoni F, Schrama LH, Kamal A, Gispen WH, Cattabeni F, Di Luca M (2001) Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor. J Neurosci 21:1501-1509.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841-845.
Giulian D, Li J, Leara B, Keenen C (1994) Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int 25:227-233.
Goncalves J, Ribeiro CF, Malva JO, Silva AP (2012) Protective role of neuropeptide Y Y(2) receptors in cell death and microglial response following methamphetamine injury. Eur J Neurosci 36:3173-3183.
Gotzsche CR, Nikitidou L, Sorensen AT, Olesen MV, Sorensen G, Christiansen SH, Angehagen M, Woldbye DP, Kokaia M (2012) Combined gene overexpression of neuropeptide Y and its receptor Y5 in the hippocampus suppresses seizures. Neurobiol Dis 45:288-296.
Graeber MB, Streit WJ (1990) Microglia: immune network in the CNS. Brain Pathol 1:2-5.
Greber S, Schwarzer C, Sperk G (1994) Neuropeptide Y inhibits potassium-stimulated glutamate release through Y2 receptors in rat hippocampal slices in vitro. Br J Pharmacol 113:737-740.
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40:140-155.
Harry GJ, Kraft AD (2012) Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology 33:191-206.
Izant JG, McIntosh JR (1980) Microtubule-associated proteins: a monoclonal antibody to MAP2 binds to differentiated neurons. Proc Natl Acad Sci U S A 77:4741-4745.
Kaur G, Han SJ, Yang I, Crane C (2010) Microglia and central nervous system immunity. Neurosurg Clin N Am 21:43-51.
Kraft AD, Harry GJ (2011) Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Res Public Health 8:2980-3018.
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312-318.
Lee SM, Yune TY, Kim SJ, Kim YC, Oh YJ, Markelonis GJ, Oh TH (2004) Minocycline inhibits apoptotic cell death via attenuation of TNF-alpha expression following iNOS/NO induction by lipopolysaccharide in neuron/glia co-cultures. J Neurochem 91:568-578.
Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS (2002) Role of nitric oxide in in fl ammation-mediated neurodegeneration. Ann N Y Acad Sci 962:318-331.
Malenka RC, Nicoll RA (1999) Long-term potentiation--a decade of progress? Science 285:1870-1874.
Malva JO, Xapelli S, Baptista S, Valero J, Agasse F, Ferreira R, Silva AP (2012) Multifaces of neuropeptide Y in the brain--neuroprotection, neurogenesis and neuroin fl ammation. Neuropeptides 46:299-308.
Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, Vezzani A (2011) Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 8:304-315.
Mayer AM, Clifford JA, Aldulescu M, Frenkel JA, Holland MA, Hall ML, Glaser KB, Berry J (2011) Cyanobacterial Microcystis aeruginosa lipopolysaccharide elicits release of superoxide anion, thromboxane B(2), cytokines, chemokines, and matrix metalloproteinase-9 by rat microglia. Toxicol Sci 121:63-72.
Nakajima K, Takemoto N, Kohsaka S (1992) Retinoic acid enhances the secretion of plasminogen from cultured rat microglia. FEBS Lett 314:167-170.
Nakamura R, Nishimura T, Ochiai T, Nakada S, Nagatani M, Ogasawara H (2013) Availability of a microglia and macrophage marker, Iba-1, for differential diagnosis of spontaneous malignant reticuloses from astrocytomas in rats. J Toxicol Pathol 26:55.
Norris CM, Blalock EM, Thibault O, Brewer LD, Clodfelter GV, Porter NM, Land fi eld PW (2006) Electrophysiological mechanisms of delayed excitotoxicity: positive feedback loop between NMDA receptor current and depolarization-mediated glutamate release. J Neurophysiol 96:2488-2500.
Park YK, Chung YS, Kim YS, Kwon OY, Joh TH (2007) Inhibition of gene expression and production of iNOS and TNF-alpha in LPS-stimulated microglia by methanol extract of Phellodendri cortex. Int Immunopharmacol 7:955-962.
Ravizza T, Rizzi M, Perego C, Richichi C, Veliskova J, Moshe SL, De Simoni MG, Vezzani A (2005) In fl ammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia 46 Suppl 5:113-117.
Rivest S (2009) Regulation of innate immune responses in the brain. Nat Rev Immunol 9:429-439.
Savard A, Lavoie K, Brochu ME, Grbic D, Lepage M, Gris D, Sebire G (2013) Involvement of neuronal IL-1beta in acquired brain lesions in a rat model of neonatal encephalopathy. J Neuroinflammation 10:110.
Schluesener HJ, Seid K, Meyermann R (1999) Effects of autoantigen and dexamethasone treatment on expression of endothelial-monocyte activating polypeptide II and allograft-in fl ammatory factor-1 by activated macrophages and microglial cells in lesions of experimental autoimmune encephalomyelitis, neuritis and uveitis. Acta Neuropathol 97:119-126.
Smialowska M, Domin H, Zieba B, Kozniewska E, Michalik R, Piotrowski P, Kajta M (2009) Neuroprotective effects of neuropeptide Y-Y2 and Y5 receptor agonists in vitro and in vivo. Neuropeptides 43:235-249.
Soetedjo L, Glover DA, Jin H (2013) Targeting of vasoactive intestinal peptide receptor 2, VPAC2, a secretin family G-protein coupled receptor, to primary cilia. Biol Open 2:686-694.
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21:2580-2588.
Vezzani A, Granata T (2005) Brain in fl ammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724-1743.
Vezzani A, Friedman A (2011) Brain in fl ammation as a biomarker in epilepsy. Biomark Med 5:607-614.
Vezzani A, Sperk G, Colmers WF (1999) Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci 22:25-30.
Vezzani A, Balosso S, Ravizza T (2008) The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 22:797-803.
Vezzani A, French J, Bartfai T, Baram TZ (2011a) The role of in fl ammation in epilepsy. Nat Rev Neurol 7:31-40.
Vezzani A, Aronica E, Mazarati A, Pittman QJ (2013) Epilepsy and brain in fl ammation. Exp Neurol 244:11-21.
Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T (2011b) IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 25:1281-1289.
Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M (2003) Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 23:8692-8700.
Wettstein JG, Earley B, Junien JL (1995) Central nervous system pharmacology of neuropeptide Y. Pharmacol Ther 65:397-414.
Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ (2009) Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem 109:1237-1249.
Wirenfeldt M, Babcock AA, Vinters HV (2011) Microglia - insights into immune system structure, function, and reactivity in the central nervous system. Histol Histopathol 26:519-530.
Woldbye DP, Angehagen M, Gotzsche CR, Elbrond-Bek H, Sorensen AT, Christiansen SH, Olesen MV, Nikitidou L, Hansen TV, Kanter-Schlifke I, Kokaia M (2010) Adeno-associated viral vector-induced overexpression of neuropeptide Y Y2 receptors in the hippocampus suppresses seizures. Brain 133:2778-2788.
Wu CY, Kaur C, Sivakumar V, Lu J, Ling EA (2009) Kv1.1 expression in microglia regulates production and release of proin fl ammatory cytokines, endothelins and nitric oxide. Neuroscience 158:1500-1508.
Yamasaki R, Tanaka M, Fukunaga M, Tateishi T, Kikuchi H, Motomura K, Matsushita T, Ohyagi Y, Kira J (2010) Restoration of microglial function by granulocyte-colony stimulating factor in ALS model mice. J Neuroimmunol 229:51-62.
Yang S, Zhou W, Zhang Y, Yan C, Zhao Y (2006) Effects of Liuwei Dihuang decoction on ion channels and synaptic transmission in cultured hippocampal neuron of rat. J Ethnopharmacol 106:166-172.
Yang S, Liu ZW, Wen L, Qiao HF, Zhou WX, Zhang YX (2005) Interleukin-1beta enhances NMDA receptor-mediated current but inhibits excitatory synaptic transmission. Brain Res 1034:172-179.
Yao ZG, Zhang L, Huang L, Zhu H, Liu Y, Ma CM, Sheng SL, Qin C (2013) Regional and cell-type speci fi c distribution of HDAC2 in the adult mouse brain. Brain Struct Funct 218:563-573.
Zhang HJ, Sun RP, Lei GF, Yang L, Liu CX (2008) Cyclooxygenase-2 inhibitor inhibits hippocampal synaptic reorganization in pilocarpine-induced status epilepticus rats. J Zhejiang Univ Sci B 9:903-915.
Zhang XJ, Liu LL, Wu Y, Jiang SX, Zhong YM, Yang XL (2011) sigma receptor 1 is preferentially involved in modulation of N-methyl-D-aspartate receptor-mediated light-evoked excitatory postsynaptic currents in rat retinal ganglion cells. Neurosignals 19:110-116.
Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF (2013) Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav Brain Res 236:270-282.
Zheng H, Zhu W, Zhao H, Wang X, Wang W, Li Z (2010) Kainic acid-activated microglia mediate increased excitability of rat hippocampal neurons in vitro and in vivo: crucial role of interleukin-1beta. Neuroimmunomodulation 17:31-38.
Zhu W, Zheng H, Shao X, Wang W, Yao Q, Li Z (2010) Excitotoxicity of TNFalpha derived from KA activated microglia on hippocampal neurons in vitro and in vivo. J Neurochem 114:386-396.
Copyedited by Patel B, Rave W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.133140
Wenqing Zhao, Graduate School, Hebei Medical University, Shijiazhuang 050017, Hebei Province, China; Department of
Functional Neurosurgery, Hebei General Hospital, Shijiazhuang 050051, Hebei
Province, China, 13832121438@163.com.
http://www.nrronline.org/
Accepted: 2014-03-05
- 中國神經(jīng)再生研究(英文版)的其它文章
- Susceptibility-weighted imaging is suitable for evaluating signal strength in different brain regions of a rabbit model of acute hemorrhagic anemia
- Age-related changes of lateral ventricular width and periventricular white matter in the human brain: a diffusion tensor imaging study
- Sequential expression of cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor in rat hippocampal neurons after fl uid percussion injury
- The apparent diffusion coef fi cient does not re fl ect cytotoxic edema on the uninjured side after traumatic brain injury
- Acupuncture and moxibustion reduces neuronal edema in Alzheimer’s disease rats
- Protective effect of alpha-synuclein knockdown on methamphetamine-induced neurotoxicity in dopaminergic neurons

