Protective effect of alpha-synuclein knockdown on methamphetamine-induced neurotoxicity in dopaminergic neurons
Yunchun Tai, Ling Chen, Enping Huang, Chao Liu,, Xingyi Yang, Pingming Qiu, Huijun Wang
1 Department of Forensic Medicine, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong Province, China
2 Guangzhou Forensic Science Institute, Guangzhou, Guangdong Province, China
Protective effect of alpha-synuclein knockdown on methamphetamine-induced neurotoxicity in dopaminergic neurons
Yunchun Tai1, Ling Chen1, Enping Huang1, Chao Liu1,2, Xingyi Yang1, Pingming Qiu1, Huijun Wang1
1 Department of Forensic Medicine, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong Province, China
2 Guangzhou Forensic Science Institute, Guangzhou, Guangdong Province, China
Yunchun Tai and Ling Chen
contributed equally to this work.
Pingming Qiu, Ph.D., Department of Forensic Medicine, School of Basic
Medical Sciences, Southern Medical
University, Guangzhou, Guangdong
Province, China, qiupm@163.com.
Huijun Wang, Ph.D., Department of Forensic Medicine, School of Basic
Medical Sciences, Southern Medical
University, Guangzhou, Guangdong
Province, China, hjwangcz@21cn.com.
The over-expression of α-synuclein is a major factor in the death of dopaminergic neurons in a methamphetamine-induced model of Parkinson’s disease. In the present study, α-synuclein knockdown rats were created by injecting α-synuclein-shRNA lentivirus stereotaxically into the right striatum of experimental rats. At 2 weeks post-injection, the rats were injected intraperitoneally with methamphetamine to establish the model of Parkinson’s disease. Expression of α-synuclein mRNA and protein in the right striatum of the injected rats was signi fi cantly downregulated. Food intake and body weight were greater in α-synuclein knockdown rats, and water intake and stereotyped behavior score were lower than in model rats. Striatal dopamine and tyrosine hydroxylase levels were signi fi cantly elevated in α-synuclein knockdown rats. Moreover, superoxide dismutase activity was greater in α-synuclein knockdown rat striatum, but the levels of reactive oxygen species, malondialdehyde, nitric oxide synthase and nitrogen monoxide were lower compared with model rats. We also found that α-synuclein knockdown inhibited methamphetamine-induced neuronal apoptosis. These results suggest that α-synuclein has the capacity to reverse methamphetamine-induced apoptosis of dopaminergic neurons in the rat striatum by inhibiting oxidative stress and improving dopaminergic system function.
nerve regeneration; α-synuclein; Parkinson’s disease; methamphetamine; dopaminergic neurons; neurotoxicity; striatum; oxidative stress; apoptosis; NSFC grant; neural regeneration
Funding: This study was supported by the National Natural Science Foundation of China, No. 81072506.
Tai YC, Chen L, Huang EP, Liu C, Yang XY, Qiu PM, Wang HJ. Protective effect of alpha-synuclein knockdown on methamphetamine-induced neurotoxicity in dopaminergic neurons. Neural Regen Res. 2014;9(9):951-958.
Introduction
Methamphetamine (METH) is an illicit and potent psychostimulant, widely abused by over 35 million people worldwide, which can cause numerous adverse effects on the central nervous system (Tallóczy et al., 2008). With repeated high doses of METH, animals initially experience hyperexcitability, followed by pacing, checking, snif fi ng, exploration, and loss of food intake. Evidence suggests that the behavioral alterations are persistent and correlate with neuron damage (Scott et al., 2007). Redistribution of dopamine from synaptic vesicles to cytoplasmic compartments and the consequent elevation of oxidizable dopamine concentrations may be primarily responsible for dopamine terminal damage by METH (Cubells et al., 1994). The substantial METH-induced release of dopamine from vesicles to cytosol with the resulting formation of toxic reactive oxygen species, such as superoxide and hydroxyl radicals, are well documented in both in vivo (Giovanni et al., 1995) and in vivo (Cubells et al., 1994) studies. Together, this evidence clearly indicates that oxidative stress following administration of METH is the major determinant in METH-induced neuronal impairment. It has also been noted that rats given METH show the same depletion seen in Parkinson’s disease (PD) (Callaghan et al., 2010).
PD is the second most common neurodegenerative disorder, affecting 1-2% of the general population over 60 years of age (Olanow et al., 2009), and is characterized by neuropathology consisting of progressive loss of substantia nigra neurons. Intracellular proteinaceous inclusions, known as Lewy bodies, are also found in PD, and consist largely of α-synuclein, a small acidic protein susceptible to misfolding and aggregation (Leong et al., 2009). Several lines of evidence have shown that α-synuclein accumulation in Lewy bodies, and genomic multiplications and missense mutations of α-synuclein, cause early-onset PD, indicating that α-synuclein has a critical role in the etiology of this disease (Hardy et al., 2009; Devine et al., 2011). Recent investigations emphasize the importance of α-synuclein levels in PD, showing that higher levels are able to cause neuronal dysfunction and disease. An increasing number of studies show that elevated expression of α-synuclein is observed afterMETH administration, in animals (Fornai et al., 2005) and in cells (Ajjimaporn et al., 2005). Oxidative modi fi cations of α-synuclein and generation of stable oligomers contribute to the neurotoxicity of oxidative stress (Norris et al., 2003). In a previous study, we showed that α-synuclein knockdown attenuated METH-induced neurotoxicity in vitro (Chen et al., 2013). The present study investigates the protective mechanism of α-synuclein knockdown in rat striatum against METH intoxication.
Materials and Methods
Preparation of viral vector
The shRNA sequence targeting α-synuclein (Liu et al., 2012) was designed as follows: 5′-CGC GTC CCC GGA AGA TAT GCC TGT GGA TCC TTC AAG AGA GGA TCC ACA GGC ATA TCT TCC TTT TTG GAA AT-3′; its e ffi cacy has been demonstrated previously (Sapru et al., 2006). The shRNA was cloned into a pLVTHM retrovirus vector (Addgene, Cambridge, MA, USA). Retrovirus (titer: 1 × 109TU/mL) was obtained using transfected 293T cells (Liu et al., 2012).
Animal grouping andα-synuclein knockdown in the right striatum of METH intoxicated rats
A total of 60 adult male speci fi c pathogen-free Wistar rats, 8 weeks old, weighing 220-240 g, were purchased from the Experimental Animal Center of Southern Medical University, China (certi fi cate No. SCXK (Yue) 2006-0015). Animals were housed in a standard laboratory environment (25 ± 2°C, 60-70% humidity, 12 hour light/dark cycle) with ad libitum access to food and water. Rats were housed and habituated for 1 week before the experiments started. All procedures complied with the Guidelines for the Care and Use of Experimental Animals and were approved by the Animal Care and Use Committee of Southern Medical University, China.
The rats were randomly and equally divided into four groups: control (saline, saline), model (saline, METH), empty vector (empty vector, METH), and RNAi (shRNA-α-synuclein lentivirus, METH). All rats were housed in solitary cages. Animals were anesthetized with 10% chloral hydrate (350 mg/kg) and placed in a stereotaxic frame (Stoelting Company, Wisconsin, USA) with the tooth bar set at 0.0 mm. Rats in the control and model groups were injected with physiological saline (10 μL/rat). The empty vector group and RNAi group were injected with empty vector (10 μL/rat) and shRNA-α-synuclein lentivirus (10 μL/rat), respectively. Lentivirus or physiological saline was injected stereotaxically into the right striatum (Connor et al., 1999) using a 10-μL Hamilton syringe (Hamilton Company, Reno, NV, USA) with a 30-gauge needle at a rate of 0.5 μL/min. Injection coordinates were 1.6 mm rostral, 3.8 mm lateral and 5.5 mm ventral to bregma (Sapru et al., 2006). The needle was retained in place for 5 minutes before being withdrawn at 1 mm/min.
METH (C6H5-CH2-CH(CH3)-NH-CH3, molecular weight 149, solid, purity > 99.1%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) and dissolved in physiological saline. At 2 weeks after injection of lentivirus or saline, rats in the model group, empty vector group and RNAi group received eight injections of METH (15 mg/kg intraperitoneally, in 1 mL solution) at 12-hour intervals, and control rats received physiological saline (1 mL intraperitoneally) on the same schedule as METH. Body weight, food intake and water intake were measured daily after the fi rst injection. At 24 hours after the fi nal injection, rats were anesthetized and perfused transcardially with 0.9% saline. The brains were removed and the striatum was separated out for neurochemical analyses.
Quantitative real-time PCR analysis of α-synuclein mRNA expression in rat striatum
The right rat striatum was frozen in liquid nitrogen and crushed using a pestle and mortar. Total RNA was extracted using TRIzol reagent according to the manufacturer’s instructions. Reverse transcription reactions were carried out using 1 μg of total RNA and the PrimeScriptTMRT reagent kit (Takara Biotechnology, Dalian, Liaoning Province, China). Quantitative real-time PCR was performed using the SYBR Green quantitative real-time PCR kit (Takara Biotechnology) and the following gene-specific primers: β-actin, forward: 5′-CCC ATC TAT GAG GGT TAC GC-3′, reverse: 5′-TTT AAT GTC ACG CAC GAT TTC-3′; α-synuclein, forward: 5′-CAA AGG CCA AGG AGG GAG TT-3′, reverse: 5′-CCT CCA CTG TCT TCT GAG CG-3′. 2-△△Ctnormalization was used and quantitative real-time PCR was analyzed using ABI 7500 software V2.0.5 (Applied Biosystems, Carlsbad, CA, USA).
Western blot analysis ofα-synuclein expression
Whole right striatum tissue protein extracts were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. The membranes were incubated overnight at 4°C with primary antibody at the following dilutions: rabbit anti-α-synuclein polyclonal antibody (1:1,000; Cell Signaling Technology, Danvers, MA, USA); rabbit anti-β-actin polyclonal antibody (1:1,000; Cell Signaling Technology). Following 1 hour of incubation at room temperature with corresponding horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000; Cell Signaling Technology), the membrane was developed with Chemiluminescence ECL Plus Western Blotting detection reagents (Amersham Biosciences, Piscataway, NJ, USA). Densitometric analysis was carried out to determine the protein content in each band on the membrane, using Image J software (National Institutes of Health, Bethesda, MD, USA). Each western blot reproduced here is typical of at least three separate experiments.
Assessment of stereotyped behavior score
At 2 weeks after METH injection, stereotyped behavior score was assessed according to the standard method reported by Sams-Dodd (Seiden and Vosmer, 1984).
Enzyme-linked immunosorbent assay (ELISA) for dopamine and tyrosine hydroxylase
Dopamine and tyrosine hydroxylase levels were measured in homogenized striatum using ELISA kits (R & D Systems,Boston, MA, USA), according to the manufacturer’s instructions. The color was read in a microtiter plate reader (BioTek Company, Winooski, Vermont, USA) at 450 nm. Sample concentration was calculated using a standard curve of known concentrations of dopamine and tyrosine hydroxylase.
Assay of reactive oxygen species
Homogenized striatum was stained with the oxidant-sensitive dye CM-H2DCFDA, and reactive oxygen species levels were determined using a reactive oxygen species detection kit (Genmed Scientifics Inc., Shanghai, China) according to the manufacturer’s protocol. Fluorescence was measured using a microtiter spectro fl uorometer (Shimadzu Corporation, Kyoto, Japan) at 490 and 530 nm. Data were obtained as relative fl uorescence units after background subtraction. Relative fl uorescence units for untreated controls for each assay were averaged and the values for each treated sample were normalized to this average, followed by statistical analyses.
Determination of levels of nitrogen monoxide and malondialdehyde and activities of nitric oxide synthase and superoxide dismutase
Levels of nitrogen monoxide and malondialdehyde and activities of nitric oxide synthase and superoxide dismutase in rat striatum were measured using kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, China), according to the manufacturer’s instructions. Color was read in a microtiter spectrophotometer at 550, 532, 530, and 550 nm, respectively.
Neuronal apoptosis detection using TUNEL staining
To con fi rm the presence of cell death by an apoptotic-like mechanism following exposure to METH, we used the TUNEL Apoptosis Detection biotin-labeled POD Kit (Genscript Corporation, Piscataway, NJ, USA) according to the manufacturer’s protocol. Images were obtained using a laser confocal microscope (Olympus, Tokyo, Japan); TUNEL-positive neurons appeared green. Cell nuclei were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Apoptotic rate was expressed by normalizing the number of TUNEL-positive cells in six separate but equal visual fi elds to the total cell number in the corresponding areas in each sample.
Statistical analysis
All values are expressed as mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA) and repeated measures ANOVA using SPSS version 13.0 software (SPSS, Chicago, IL, USA). Multiple comparisons were performed using the least significant difference and Student-Newman-Keuls post-hoc tests. A value of P < 0.05 was considered statistically signi fi cant.
Results
α-Synuclein-shRNA downregulatedα-synuclein expression in the striatum of rats with METH intoxication
To inhibit the expression of α-synuclein in the rat striatum, we injected α-synuclein-shRNA lentivirus into the rat striatum. We tested striatal mRNA and protein levels using quantitative real-time PCR and western blot analysis. Compared with the control group, the model group showed signi fi cantly greater α-synuclein mRNA and protein expression after METH treatment (P < 0.05; Figure 1). In contrast, there was no signi fi cant di ff erence in the expression of α-synuclein mRNA or protein between the model group and empty vector group (P > 0.05; Figure 1). α-Synuclein mRNA and protein expression was significantly lower in striatal injected with α-synuclein-shRNA lentivirus (RNAi group) than in striatum from the model group (P < 0.05;Figure 1).
α-Synuclein knockdown improved METH-induced abnormal behavior
We measured body weight and intake of food and water to assess the e ff ect of α-synuclein knockdown on the animals’condition during repeated, high dose METH treatments. Food intake and body weight of the model group rats were significantly lower after METH treatment compared with the control group (P < 0.05). In contrast, no difference was found in either food intake or body weight between the model and empty vector groups (P > 0.05). Food intake and body weight of rats in the RNAi group were markedly greater than those of model group rats (P < 0.05;Figure 2A, C). We also found that water intake of the model group rats was considerably higher than that of control rats (P < 0.05), and rats in the RNAi group drank less water than those in the model group (P < 0.05;Figure 2B). We also measured stereotyped behavior. Compared with control rats, stereotyped behavior in model group rats was noticeably greater after METH treatment (P < 0.05). There was no change in the stereotyped behavior score between the model and empty vector groups (P > 0.05). The stereotyped behavior score of RNAi group rats was markedly lower than that in model group rats (P < 0.05;Figure 2D).
Knockdown ofα-synuclein reversed the reduction in dopamine level and tyrosine hydroxylase activity caused by METH
Dopamine level and tyrosine hydroxylase activity were lower in the striatum of model group rats compared with those in the control group (P < 0.05;Figure 3). There was no signi ficant difference in dopamine level or tyrosine hydroxylase activity between the model and empty vector groups (P > 0.05); however, in α-synuclein knockdown rats, dopamine levels and tyrosine hydroxylase activity were markedly greater than those in the model group (P < 0.05;Figure 3).
Knockdown ofα-synuclein attenuated METH-induced oxidative stress

Figure 1 Effect of α-synuclein-shRNA on α-synuclein mRNA and protein expression in the striatum of rats with methamphetamine intoxication.

Figure 2 Effect of α-synuclein knockdown on methamphetamine-induced abnormal behavior.
To further identify the mechanisms underlying METH-induced oxidative stress and striatal knockdown of α-synuclein, we examined the correlative factors involved in METH-induced neurotoxicity, namely, reactive oxygen species production, nitric oxide synthase activity, nitrogen monoxide level, malondialdehyde expression and superoxide dismutase activity (Figure 4). Upregulation of reactive oxygen species production, nitric oxide synthase activity, nitrogen monoxide level and malondialdehyde expression, and downregulation of superoxide dismutase activity, were detected in striatum of model group rats that had received METH compared with control rats (P < 0.05). In contrast, no difference was found in these indicators between the model and empty vector groups (P > 0.05). We also found that reactive oxygen species production, nitric oxide synthase activity, nitric oxide level and malondialdehyde expression were lower, while superoxide dismutase activity was markedly elevated, in RNAi group rats compared with model group rats (P < 0.05;Figure 4).
α-Synuclein knockdown decreased METH-induced neuronal apoptosis
We investigated the influence of α-synuclein knockdown on METH-induced neuronal apoptosis in rat striatum using TUNEL staining. Apoptotic cells exhibiting inter-nucleosomal DNA fragmentation were sparse in control striatum. A larger number of apoptotic cells were observed in the striata of the model and empty vector group rats when compared with control group (P < 0.05). Knockdown ofα-synuclein markedly reduced the number of apoptotic cells in the striatum compared with the model group (P <0.05;Figure 5).

Figure 3 Effect of α-synuclein knockdown on dopamine (A) and tyrosine hydroxylase (B) levels in the rat striatum.

Figure 4 Effect of α-synuclein knockdown on methamphetamine-induced oxidative stress in rat striatum.
Discussion
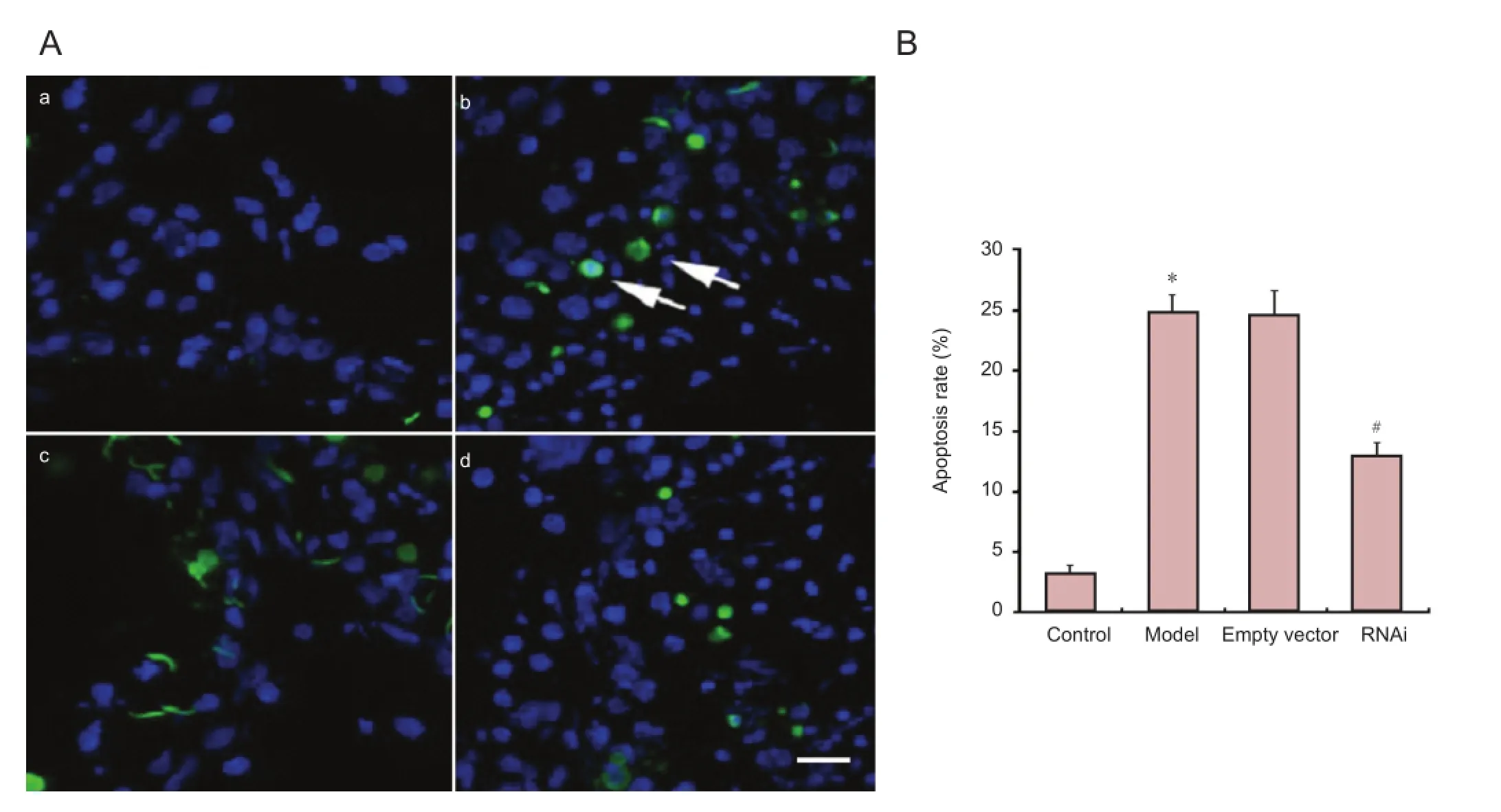
Figure 5 Effect of α-synuclein knockdown on METH-induced neuronal apoptosis in the rat striatum.
Many previous investigations have shown that METH markedly upregulates the expression of α-synuclein in vivo (Fornai et al., 2005) and in vitro (Ajjimaporn et al., 2007). Recently, a role for oxidative stress in the development of METH-induced neurotoxicity has been largely supported by correlative evidence, i.e., increased pro-oxidant production and reduced antioxidant defense are associated with exposure to METH. The present study demonstrates that METH-induced oxidative stress correlates with the cellular level of α-synuclein. Here, we show for the fi rst time that suppression of α-synuclein expression significantly attenuates METH-induced neurotoxicity in the rat striatum by inhibiting oxidative stress and restoring the dopaminergic system.
METH is potentially toxic to the central nervous system. It was found to induce signi fi cant nerve terminal damage in animal models (Kita et al., 2003), create persistent interference in the dopaminergic system of the brain (Volkow et al., 2001a, b), and generate structural abnormalities in the brain (Thompson et al., 2004). α-Synuclein is an unstructured soluble protein that can assemble into amyloid aggregates forming intracellular inclusion bodies called Lewy bodies, which are present in PD. Several studies indicate that overexpression of α-synuclein is found in METH-intoxicated animals, leading to the proposition of METH-induced dopaminergic toxicity as a model for PD. Thus, knockdown of α-synuclein expression has the capacity to protect against neurotoxicity in this METH-induced PD model.
To investigate the effects of α-synuclein knockdown in METH intoxicated rats, we first established α-synuclein reduction in rats using α-synuclein-shRNA lentivirus. Rats in this group (RNAi) showed markedly lower α-synuclein mRNA and protein expression in the striatum compared with model group rats. In contrast, there was no signi fi cant di ff erence in α-synuclein mRNA and protein expression between model group and empty vector group rats. Thus, these data confirmed the effectiveness of the α-synuclein knockdown in the striatum.
METH abuse has many negative consequences in humans. Acute toxicity, altered behavioral and cognitive functions and neurological injury are observed after METH administration (Murray, 1998; Albertson et al., 1999; Barr et al., 2006; Scott et al., 2007). In the present study, we measured food and water intake, body weight and stereotyped behavior score to estimate the effect of α-synuclein knockdown on animals’ survival condition during repeated high dose METH administration. Compared with the control group, the body weight and food intake of rats in the model group was markedly lower, whereas water intake and stereotyped behavior was greater. We found that RNAi group rats presented opposite tendencies. Together, these data indicate that α-synuclein knockdown alleviates METH-induced abnormal behavior in rats.
The chemical structure of METH is similar to dopamine, allowing it to enter dopamine axons, to be released with dopamine from synaptic vesicles into the cytoplasm and to undergo reverse transport into the synaptic cleTh. Indeed, several investigations have a ffi rmed that dopamine plays a critical role in the mechanisms of METH neurotoxicity. Death of neuronal cell bodies occurs in METH-treated rat striatum (Jayanthi et al., 2005). In the present study, dopamine levels in striatum were notably lower in the model group rats than in controls, consistent with previous reports (Fukumura etal., 1998; Cappon et al., 2000; Chapman et al., 2001; Truong et al., 2005; Yue et al., 2012). Tyrosine hydroxylase activity, as a rate-limiting factor, can regulate dopamine synthesis. Decreased tyrosine hydroxylase activity is observed in the METH-induced PD rat model (Hotchkiss et al., 1979; Hotchkiss and Gibb, 1980; Morgan and Gibb, 1980). α-Synuclein co-localizes with tyrosine hydroxylase (Perez et al., 2002), suggesting that α-synuclein plays a critical role in the adjustment of dopamine biosynthesis by reducing tyrosine hydroxylase activity. Previous results also show that overexpression of α-synuclein causes cellular oxidative stress and generates a 30-80% reduction of nigral dopaminergic neurons and a 40-50% depletion of striatal dopamine (Kirik et al., 2002). In the present study, striatal dopamine levels were signi fi cantly elevated in RNAi group rats after METH treatment. To reveal the mechanism of α-synuclein knockdown on dopamine levels, we measured tyrosine hydroxylase activity, and found signi fi cantly greater striatal tyrosine hydroxylase activity in RNAi group rats compared with model group rats. These data indicate that knockdown of α-synuclein protects against METH-induced deficits in striatal dopamine and tyrosine hydroxylase activity.
Treatment with multiple high doses of METH can induce oxidative damage, including formation of dopamine-mediated reactive oxygen species, which may lead to the neurotoxic damage of monoamine neurons and long-lasting deficit of dopamine in the striatum. In the present study, abundant production of reactive oxygen species was observed after METH treatment. It was previously shown that nitrogen monoxide is involved in METH-induced neurotoxicity because the nitric oxide synthase inhibitors Nw-nitro-L-arginine and monomethyl-L-arginine attenuated METH-induced cell death (Sheng et al., 1996). Studies also have shown that the selective neuronal nitric oxide synthase inhibitor 7-nitroindazole was able to protect against METH-induced dopamine and 5-HT de fi cit in the striatum (Di Monte et al., 1996; Itzhak and Ali, 1996; Ali and Itzhak, 1998). Previous reports also indicate larger METH-induced elevation in neuronal nitric oxide synthase activity (Imam et al., 2005). In the present study, we found that the nitrogen monoxide levels and nitric oxide synthase activity in the striatum were notably elevated after METH administration. Nitrogen monoxide has the ability to react with superoxide radicals to generate the powerful oxidant and primary neurotoxin, peroxynitrite (Pacher et al., 2007). Nitric oxide synthase-related e ff ects on α-synuclein may play an important role in PD. For example, inducible nitric oxide synthase and neuronal nitric oxide synthase associated with PD can increase the aggregation polymorphisms of α-synuclein. In the present study, α-synuclein knockdown in the striatum attenuated the elevated expression of reactive oxygen species induced by METH. In addition, both nitrogen monoxide level and nitric oxide synthase activation in the striatum were signi fi cantly lower in RNAi group rats than in model group rats after METH treatment.
Several investigations have revealed that METH is able to activate oxidative stress by changing the balance between reactive oxygen species products and the ability of antioxidant enzyme systems to clear reactive oxygen species (Kobeissy et al., 2008; Li et al., 2008). Dysfunction of the antioxidative stress system could cause reduced elimination of free radicals, leading to lipid peroxidation and severe cell and tissue damage (Kessler et al., 2003). Malondialdehyde, a product of lipid peroxidation by reactive oxygen species, is commonly used as a marker of oxidative damage (Kessler et al., 2003), and treatment with multiple high doses of METH increases malondialdehyde reactivity in the striatum of humans and experimental animals. In the present study, a remarkably elevated level of malondialdehyde was observed in the striatum of model group rats compared with control rats. Superoxide dismutase is an essential enzyme, clearing superoxide free radicals from aerobic organisms and protecting against the toxic e ff ect of oxygen free radicals. In our experiment, superoxide dismutase activity was markedly lower in the striatum of model group rats compared with that of control group rats. Generation of stable oligomers and oxidative modifications of α-synuclein are involved in the neurotoxicity of oxidative stress (Norris et al., 2003). Here, we found that malondialdehyde expression in the striatum was signi fi cantly lower and superoxide dismutase activity was much greater in RNAi group rats compared with model group rats. Together, these data suggest that suppression of α-synuclein expression can rescue the balance between reactive oxygen/nitrogen species and antioxidant enzyme systems, which is damaged in the rat striatum treated with METH. Cell apoptosis or death is the ultimate consequence after METH treatment. Here, the ratio of neuronal apoptosis was greater in the striatum of model group rats compared with the control group rats, whereas it was markedly lower in RNAi group rats compared with model group rats.
In summary, we have shown that knockdown of α-synuclein expression markedly attenuates METH-induced oxidative stress, improves dopaminergic system de fi cits and increases neuronal cell viability in the rat striatum. Based on the above data, we suggest that the development of clinical α-synuclein knockdown may represent a potential approach for the treatment of PD.
Author contributions:Tai YC, Qiu PM and Wang HJ conceived and designed the experiments. Tai YC and Huang EP performed the experiments. Tai YC, Huang EP, Liu C and Chen L analyzed the data. Tai YC, Huang EP, Liu C, Chen L and Yang XY provided reagents/materials/analysis tools. Tai YC wrote the paper. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Ajjimaporn A, Swinscoe J, Shavali S, Govitrapong P, Ebadi M (2005) Metallothionein provides zinc-mediated protective e ff ects against methamphetamine toxicity in SK-N-SH cells. Brain Res Bull 67:466-475.
Ajjimaporn A, Phansuwan-Pujito P, Ebadi M, Govitrapong P (2007) Zinc protects SK-N-SH cells from methamphetamine-induced α-synuclein expression. Neurosci Lett 419:59-63.
Albertson TE, Derlet RW, Van Hoozen BE (1999) Methamphetamine and the expanding complications of amphetamines. West J Med 170:214-219.
Ali SF, Itzhak Y (1998) E ff ects of 7-nitroindazole, an NOS inhibitor on methamphetamine-induced dopaminergic and serotonergic neurotoxicity in mice. Ann N Y Acad Sci 844:122-130.
Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T (2006) The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci 31:301-313.
Callaghan RC, Cunningham JK, Sajeev G, Kish SJ (2010) Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov Disord 25:2333-2339.
Cappon GD, Pu C, Vorhees CV (2000) Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res 863:106-111.
Chapman DE, Hanson GR, Kesner RP, Keefe KA (2001) Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther 296:520-527.
Chen L, Huang E, Wang H, Qiu P, Liu C (2013) RNA interference targeting α-synuclein attenuates methamphetamine-induced neurotoxicity in SH-SY5Y cells. Brain Res 1521:59-67.
Connor B, Kozlowski DA, Schallert T, Tillerson JL, Davidson BL, Bohn MC (1999) Di ff erential e ff ects of glial cell line-derived neurotrophic factor (GDNF) in the striatum and substantia nigra of the aged Parkinsonian rat. Gene Ther 6:1936-1951.
Cubells JF, Rayport S, Rajendran G, Sulzer D (1994) Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci 14:2260-2271.
Devine MJ, Gwinn K, Singleton A (2011) Parkinson’s disease and alpha-synuclein expression. Mov Disord 26:2160-2168.
Di Monte DA, Royland JE, Jakowec MW, Langston JW (1996) Role of nitric oxide in methamphetamine neurotoxicity: protection by 7-nitroindazole, an inhibitor of neuronal nitric oxide synthase. J Neurochem 67:2443-2450.
Fornai F, Lenzi P, Ferrucci M, Lazzeri G, di Poggio AB, Natale G, Busceti CL, Biagioni F, Giusiani M, Ruggieri S, Paparelli A (2005) Occurrence of neuronal inclusions combined with increased nigral expression of alpha-synuclein within dopaminergic neurons following treatment with amphetamine derivatives in mice. Brain Res Bull 65:405-413.
Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV (1998) A single dose model of methamphetamine-induced neurotoxicity in rats: e ff ects on neostriatal monoamines and glial fi brillary acidic protein. Brain Res 806:1-7.
Giovanni A, Liang LP, Hastings TG, Zigmond MJ (1995) Estimating hydroxyl radical content in rat brain using systemic and intraventricular salicylate: impact of methamphetamine. J Neurochem 64:1819-1825.
Hardy J, Lewis P, Revesz T, Lees A, Paisan-Ruiz C (2009) The genetics of Parkinson’s syndromes: a critical review. Curr Opin Genet Dev 19:254-265.
Hotchkiss AJ, Morgan ME, Gibb JW (1979) The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci 25:1373-1378.
Hotchkiss AJ, Gibb JW (1980) Long-term e ff ects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther 214:257-262.
Imam SZ, Jankovic J, Ali SF, Skinner JT, Xie W, Conneely OM, Le WD (2005) Nitric oxide mediates increasedsusceptibility to dopaminergic damage in Nurr1 heterozygous mice. Faseb J 19:1441-1450.
Itzhak Y, Ali SF (1996) The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J Neurochem 67:1770-1773.
Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL (2005) Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A 102:868-873.
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095-3105.
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bj?rklund A (2002) Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci 22: 2780-2791.
Kita T, Wagner GC, Nakashima T (2003) Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci 92:178-195.
Kobeissy FH, Warren MW, Ottens AK, Sadasivan S, Zhang Z, Gold MS, Wang KK (2008) Psychoproteomic analysis of rat cortex following acute methamphetamine exposure. J Proteome Res 7:1971-1983.
Leong SL, Cappai R, Barnham KJ, Pham CL (2009) Modulation of alpha-synuclein aggregation by dopamine: a review. Neurochem Res 34:1838-1846.
Li X, Wang H, Qiu P, Luo H (2008) Proteomic pro fi ling of proteins associated with methamphetamine-induced neurotoxicity in di ff erent regions of rat brain. Neurochem Int 52:256-264.
Liu Y, He P, Zhang M, Wu D (2012) Lentiviral vector-mediated RNA interference targeted against prohibitin inhibits apoptosis of the retinoic acid-resistant acute promyelocytic leukemia cell line NB4-R1. Mol Med Rep 6:1288-1292.
Morgan ME, Gibb JW (1980) Short-term and long-term effects of methamphetamine on biogenic amine metabolism in extra-striatal dopaminergic nuclei. Neuropharmacology 19:989-995.
Murray JB (1998) Psychophysiological aspects of amphetamine-methamphetamine abuse. J Psychol 132:227-237.
Norris EH, Giasson BI, Ischiropoulos H, Lee VM (2003) Effects of oxidative and nitrative challenges on alpha-synuclein fi brillogenesis involve distinct mechanisms of protein modification. J Biol Chem 278:27230-27240.
Olanow CW, Stern MD, Sethi K (2009) The scienti fi c and clinical basis for the treatment of Parkinson disease. Neurology 72:S1-136.
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315-424.
Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ (2002) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22:3090-3099.
Sapru MK, Yates JW, Hogan S, Jiang L, Halter J, Bohn MC (2006) Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol 198:382-390.
Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I (2007) Neurocognitive e ff ects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17:275-297.
Seiden LS, Vosmer G (1984) Formation of 6-hydroxydopamine in caudate nucleus of the rat brain after a single large dose of methylamphetamine. Pharmacol Biochem Behav 21:29-31.
Sheng P, Cerruti C, Ali S, Cadet JL (1996) Nitric oxide is a mediator of methamphetamine (METH)-induced neurotoxicity. In vitro evidence from primary cultures of mesencephalic cells. Ann NY Acad Sci 801:174-186.
Tallóczy Z, Martinez J, Joset D, Ray Y, Gácser A, Toussi S, Mizushima N, Nosanchuk JD, Goldstein H, Loike J, Sulzer D, Santambrogio L (2008) Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog 4:e28.
Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED (2004) Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci 24:6028-6036.
Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, Hanson GR, FleckensteinAE (2005) Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: implications for neurotoxicity. J Pharmacol Exp Ther 314:1087-1092.
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J (2001a) Loss of dopamine transporters in methamphetami ne abusers recovers with protracted abstinence. J Neurosci 21:9414-9418.
Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN (2001b) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377-382
Yue X, Qiao D, Wang A, Tan X, Li Y, Liu C, Wang H (2012) CD200 Attenuates methamphetamine-induced microglial activation and dopamine depletion. J Huazhong Univ Sci Technolog Med Sci 32:415-421.
Copyedited by Murphy JS, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.133146
http://www.nrronline.org/
Accepted: 2014-03-24
 中國(guó)神經(jīng)再生研究(英文版)2014年9期
中國(guó)神經(jīng)再生研究(英文版)2014年9期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Susceptibility-weighted imaging is suitable for evaluating signal strength in different brain regions of a rabbit model of acute hemorrhagic anemia
- Age-related changes of lateral ventricular width and periventricular white matter in the human brain: a diffusion tensor imaging study
- Sequential expression of cyclooxygenase-2, glutamate receptor-2, and platelet activating factor receptor in rat hippocampal neurons after fl uid percussion injury
- The apparent diffusion coef fi cient does not re fl ect cytotoxic edema on the uninjured side after traumatic brain injury
- Acupuncture and moxibustion reduces neuronal edema in Alzheimer’s disease rats
- Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function
