A novel luminol-based chemiluminescence method for the determination of amikacin sulfate in serum by using trivalent copper-periodate complex
Yu-Fei Hu, Gong-Ke Li, Zhu-Jun Zhang
aSchool of Chemistry and Chemical Engineering, Sun Yat-sen University, 135 Xingang Xi, Guangzhou 510275, China bDepartment of Chemistry, Institute of Analytical Science, Southwest University, Beibei, Chongqing 400715, China
1. Introduction
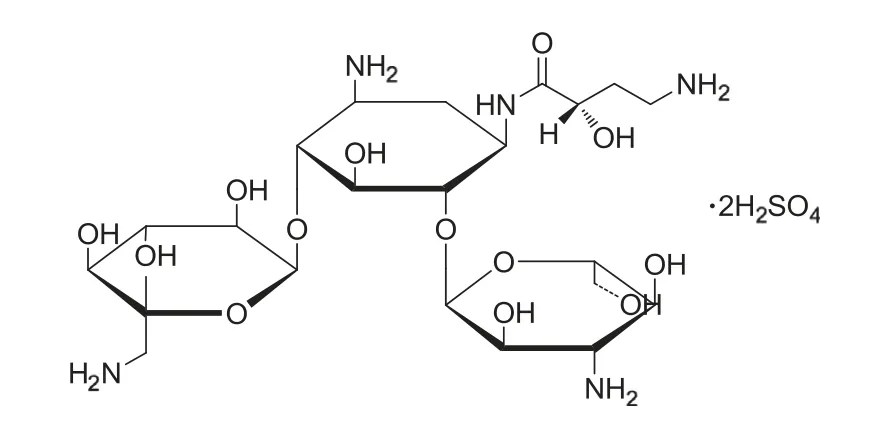
Scheme 1 Structure of amikacin sulfate.
Amikacin(O-3-amino-3-desoxy-α-d-glucopyranosyl-(1-6)-O-[6-amino-6-desoxy-α-d-glucoyra-nosyl-(1-4)]-N1-(4-amino-2-hydroxy-1-oxobutyl)-2-desoxy-d-streptamine, C22H43N5O13) sulfate is one of the most broad-spectrum aminoglycoside antibiotics[1,2].As its structure is shown in Scheme 1,it has the characteristics of being semi-synthetic and water soluble. It can curb drug resistance to gentamicin, kanamycin and tobramycin because of its fewer points susceptible to enzymatic attack than the other amino glycosides. However, inadequate doses in patients can also lead to ototoxicity and nephrotoxicity. Therefore, it is important to monitor the concentration level of amikacin in plasma during the process of therapeutic and toxic control. It is essential to develop a simple, sensitive and specific method for trace analysis of amikacin sulfate (AKS) in plasma. Methods have been reported for the determination of AKS, including atomic absorption spectrometry [3], spectrophotometric method[4], fluorescence method [5,6], chemiluminescence method [7],thin-layer chromatography [8], liquid chromatography [9-11],voltammetry [12] and capillary electrophoresis [13]. However,due to the structural characteristics of AKS, a special pretreatment step (such as derivatization) is needed. Some of the methods mentioned above require special instruments (such as flourescence or HPLC); some are not sensitive enough to determine AKS in real samples. The goal of the present work was to develop a direct and sensitive method for the determination of AKS in serum samples.
Chemiluminescence (CL) is known to be a widely-used analytical method for its higher sensitivity, lower detection limit and wider linear range with a simpler instrument[14].As a highly sensitive detection tool, CL has gained an increasing attention in considerable fields such as clinical, pharmaceutical, environmental and food analyses [15]. The oxidation of luminol (3-aminophthalhydrazide) in alkaline medium is one of the most efficient CL reactions and has been widely used in the quantitative analysis of many inorganic and organic compounds [16]. These luminol-based CL system needs some oxidants such as hydrogen peroxide, oxygen, potassium permanganate, ferricyanide, periodate and oxygen free radicals[17-23].Since then,there has been a continuing interest in the development of novel oxidant reagents for the luminolbased CL reaction, which can widen the application of the luminal-based CL reaction. As valid oxidant reagents, the transition metals in uncommon oxidation state such as Ag(III) and Cu (III) have been reported in recently years. They can be stabilized by the chelation with suitable polydentate ligands [24,25]. The related studies mainly focus on the kinetics and mechanism of oxidant reactions [26,27]. The diperiodatocuprate (III) (K5[Cu(HIO6)2], DPC) [28] is a relatively weak oxidizing agent in alkaline medium with the reduction potential of 0.42 V (Vs SCE) [29]. In our previous work, the H2O2determination method has been proposed for the catalytic effect of Cu (III) complex [30].
We found that the novel CL was based on the oxidizing reaction of luminol and the trivalent copper-periodate complex (K5[Cu(HIO6)2], DPC) in alkaline medium. The CL intensity could be enhanced in the presence of AKS. DPC was obtained by complexation of trivalent copper and periodate in strong alkaline medium. The luminol-based oxidant reaction could occur at a lower concentration of 10-7M,which gave the CL system the characteristic of being selective.The enhanced light emission caused by AKS could be used for the quantitative analysis of AKS concentration in serum samples. A novel, more sensitive and selective chemiluminescence method was developed for trace analysis of AKS in serum by coupling flow injection analysis (FIA) techniques without any pretreatment of samples. Based on the electrochemical reaction and kinetics curve of the CL reaction experiment,the mechanism of the reaction was also discussed.
2. Experimental
2.1. Reagents and chemicals
Luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) was kindly provided by Shaanxi Normal University (Xi'an, China). The standard medicine of AKS was obtained from Chongqing Institute for Drug Control (Chongqing, China). It was dissolved to prepare the stock solution with the concentration of 1×10-4g/mL with distilled water. It was stored at 4°C and diluted to working solution with distilled water. The reagents for DPC preparation were listed as follows:Potassium persulfate(Na2S2O8)was purchased from Shanghai Aijian Chemical Reagent Company(Shanghai, China), cupric sulfate (CuSO4·5H2O) and potassium hydroxide (KOH) were purchased from Chongqing Chemical Reagent Company (Chongqing,China),and potassium periodate(KIO4) was purchased from Shanghai Chemical Reagent Company (Shanghai, China). All of the chemicals were of analytical reagent grade and used without further purification. Doubly distilled water was used throughout the work.The diluted working solutions were prepared and used freshly and daily. A luminol stock solution (0.01 M) was prepared by dissolving 1.772 g luminol in 1 L carbonate buffer (0.1 M), and left to stand for approximately 24 h before use. The luminol solution was stable for at least 1 month when stored in the dark. The DPC stock solution(0.01 M)was prepared by oxidizing Cu(II)in the alkaline medium according to the known method [28]. In briefly, KIO4(0.23 g), CuSO4·5H2O (0.125 g), Na2S2O8(0.14 g) and KOH(0.8 g) were added into 30 mL water. The mixture was heated to boil for about 20 min on a hot plate with constant stirring. After the boiling mixture turned intensely red,the boiling continued for another 20 min for the completion of the synthetic reaction.When the mixture was cooled, it was diluted to 50 mL with distilled water.The stock solution obtained was refrigerated at 4°C,which was found fairly stable for several months, and DPC solutions were freshly prepared before use. The complex was confirmed at 415 nm by UV/Visible spectrum with the molar absorptive (ε) of 6230±100 L3/mol cm.
2.2. Apparatus
The schematic diagram of the used FIA-CL system in this work is shown in Fig.1. All the chemicals were delivered to the system with two peristaltic pumps (HL-2, Shanghai Huxi,China)at a flow rate of 2 mL/min.The polytetrafluorothylene(PTFE) flow tubes (0.8 mm i.d.) were used to connect all the components in the system. A sixteen-port injection valve(Hanzhou, China) was equipped with a loop of 75 μL which was used to process the injection mode. The CL signal was monitored by a BPCL ultra-weak luminescence analyzer(Institute of Biophysics, Chinese Academy of Sciences, Beijing,China) which consists of a flat coil glass flow cell facing the window of the photomultiplier tube(PMT).Data were acquired and processed with BPCL software running under Windows 98.The UV-Vis-2001 spectrophotometer (Hitachi Ltd., Japan) was used for UV-absorbance detection. The CL spectrum was obtained with the LS45/55 fluorospectrophotometer (PerkinElmer Ltd. America). The electrochemical characteristics of copper metal chelate complex were studied using the CHI832 electrochemical workstation (ChenHua Ltd., Shanghai, China).
2.3. Procedure
As shown in Fig.1, the flow lines were inserted into the luminol solution, an analyte (a standard AKS solution or a sample containing AKS,respectively)and DPC solutions.The peristaltic pumps were started to wash the whole system at 2.0 mL/min, and then the in-line and real-time determination models were performed by inserting the flow line ‘‘b'' into the vessel containing the collected samples. When the injection valve was set to the load position,the stream of DPC solution and luminol solution was mixed at ‘‘M'' point, and then the whole system was run until a stable baseline was recorded.When the injection valve was switched to the inject position,the carrier stream DPC solution bypassed the reagent loop(75 μL analyte solution)and ran directly through the flow cell,producing CL emission. The CL signal was then recorded simultaneously. Under the PMT operated at -800 V, the relative CL intensity ΔI, defined as the difference of CL intensity between in the presence and in the absence of analyte respectively, was proportional to the corresponding concentration of AKS solution.
3. Results and discussion
3.1. Kinetics curve of the CL reaction of luminol -DPC-AKS
In the batch mode, with the experimental parameters kept constant, the typical response curve (intensity versus times) of the CL reaction between luminol solution (1×10-7M) and DPC (4×10-4M) in the presence of ASK solution with the concentration of 2×10-7g/mL was recorded to study the kinetic characteristics of the CL reaction. As shown in Fig.2,the CL intensity peak appeared within 1 s after AKS was injected into the mixture solution of luminol and DPC. The CL signals would decrease to the baseline within 10 s. Fig.2 shows that the CL intensity of luminol-DPC could be greatly enhanced by the AKS solution. The CL reaction was obviously quick. The kinetics curve indicated the CL system was rapid and sensitive enough and suitable for the analysis of AKS.
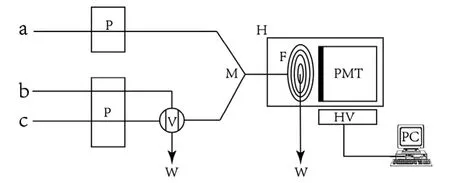
Fig.1 Schematic diagram of the CL-FIA system. P: peristaltic pump; V: injection valve; M: mixtured Position; F: spiral glass flow cell; PMT: photomultiplier tube; W: waste; H: light tight house; PC: personal computer. (a) luminol solution; (b) analyte solution; (c) DPC solution.
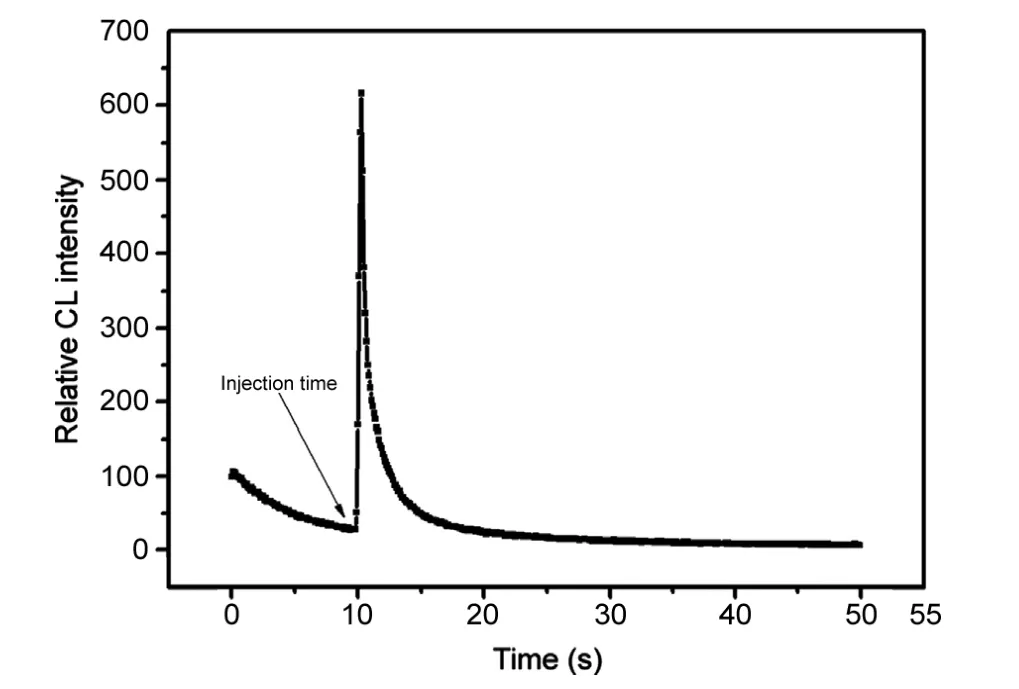
Fig.2 Kinetics curves of luminol-DPC catalyzed by samples.Luminol solution, 1×10-7 M; DPC solution, 4×10-4 M; injected AKS solution, 2×10-7 g/mL.
3.2. Optimization of the experiment procedure
As is well-known, luminol is one of the most popular CL reagents for its oxidation in alkaline medium. These luminolbased CL reactions mainly result from the oxidant reaction of luminol with a range of typical oxidants, such as oxygen,hydrogen peroxide, potassium permanganate, ferricyanide,tetravalent cerium ion, lead dioxide and oxygen free radicals.The oxidant reaction of luminol with these typical oxidants can occur when the concentration of luminol is up to 1×10-5M. The difference of DPC from these typical oxidants enabled the emitted luminal-based CL signal to be clearly observed when luminol was lower than 1.0×10-6M.So the effect of luminol concentration was examined over the range from 1×10-8M to 5×10-7M when the DPC and AKS solutions were fixed at a certain concentration.As shown in Fig.3, the CL intensity increased with an increase of the luminol concentration in the presented range. For avoidance of PMT saturation and minimal reagent consumption were considered, the optimal concentration of luminol was 1×10-7M for the subsequent experiment.The use of luminol with a lower concentration also effectively avoided the interference by other common oxidants.
The kinetics test showed that the luminol-based CL was based on the oxidation reaction with DPC and enhanced by AKS. To test the effect of DPC solution, a range of concentrations of DPC (1×10-5M-1×10-3M) was investigated. As the result in Fig.4 showed, the CL intensity was increased with the increase of the DPC concentration in a lowconcentration range (4×10-4M), and reached the maximum.Above 4×10-4M,the CL intensity decreased probably because a higher concentration of DPC caused self-absorption. Therefore,the optimal concentration was 4×10-4M.
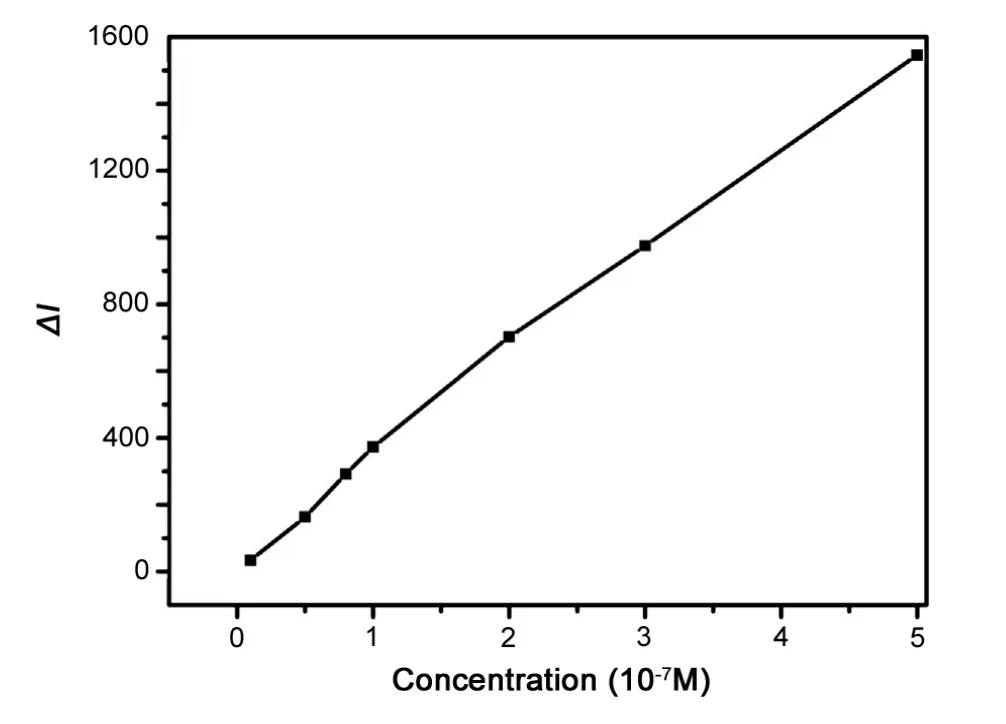
Fig.3 The effect of the luminol concentration. DPC solution,4×10-4 M; AKS solution, 4×10-9 g/mL; flow rate, 2.0 mL/min.
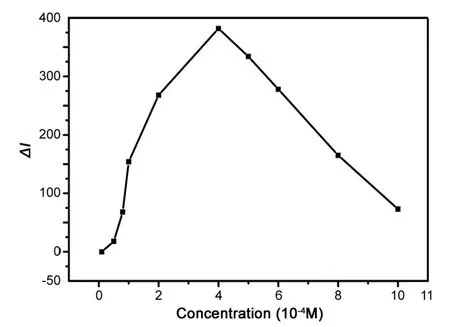
Fig.4 The effect of the DPC concentration. Luminol solution,1×10-7 M; AKS solution, 4×10-9 g/mL; flow rate, 2.0 mL/min.
Generally, the CL oxidation of luminol by oxidants often occurs in basic conditions. Additional DPC was obtained in strong alkaline solution (KOH solution). A solution of KOH(0.1 M) was added to the luminol solutions to offer different pH of CL system. The effects of pH (8-12) medium were investigated. Fig.5 shows that the optimal pH of the reaction was 9.0. For obtaining the highest sensitivity and accuracy, a pH 9.0 of luminol solution(KOH solution)was selected as the optimum.
3.3. The analytical characteristics of the FI-CL method
Under the optimal experimental conditions, the calibration graph of change of CL intensity, ΔI, against AKS concentration, was measured. There was a biphasic linear relationship between the change in the CL intensity and the AKS concentration over the range of 4.0×10-9g/mL-4.0×10-7g/mL and 4.0×10-7g/mL-4.0×10-6g/mL.The regression equation and correlation coefficients are listed in Table 1.The detection limit was 1.2×10-9g/mL (3σ) and the relative standard deviation was 2.3% for 8.0×10-9g/mL AKS (n=9).
3.4. Effects of coexisting foreign species
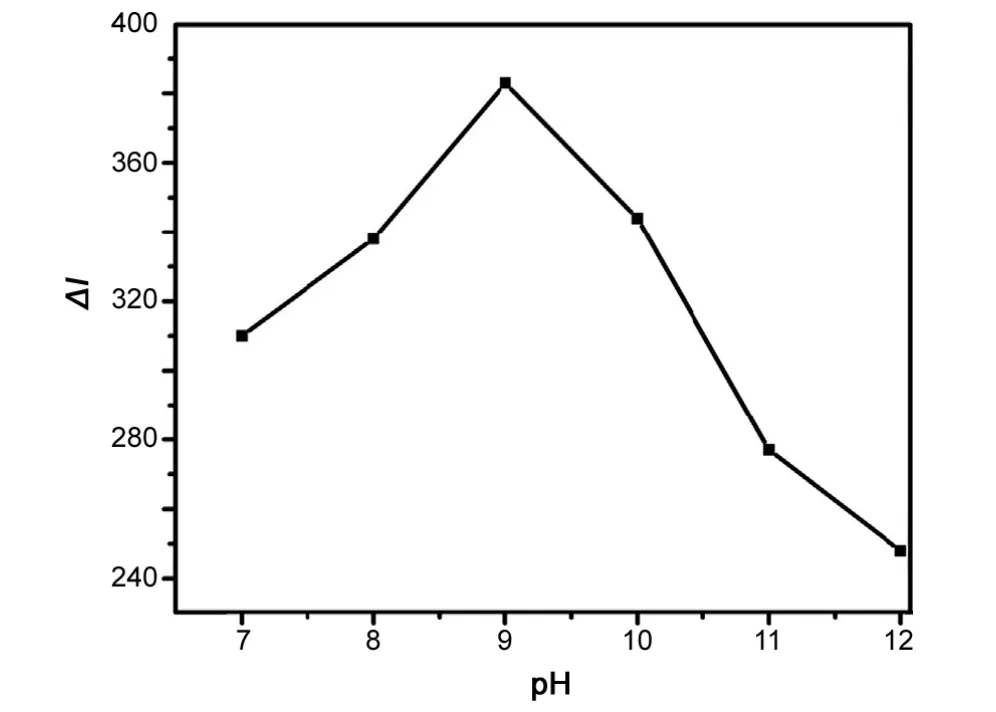
Fig.5 The effect of medium pH of luminol solution. Luminol solution, 1×10-7 M; DPC solution, 4×10-4 M; AKS solution,4×10-9 g/mL; flow rate, 2.0 mL/min.
In order to apply the proposed method to determine AKS in a practical sample, the effects of some possibly coexisting foreign inorganic ions and organic compounds were examined.The effects of foreign substances were tested by analyzing a standard solution of AKS(8×10-9g/mL)to which increasing amounts of interfering substances were added. The tolerable concentration ratios with respect to 8×10-9g/mL AKS at an interference level of 5 % were over 1000 for K+, Na+, Ca2+,Mg2+,Cu2+,Cu+, Al3+,Cd2+,NH, Cl-, Br-, C2O, NO,SO, IO, SeOand F-; 500 for Fe2+and Fe3+; 200 for amylum, urea and lactate; 100 for citrate; 10 for lactose,ascorbic acid and lincomycin; 5 for glucose, Mn2+and uric acid; and 2 for sucrose. The interference of protein in human serum could be ignored by ultra-filtering the sample of human serum.So the present method could be directly applied for the determination of AKS in human serum.
3.5. Analytical applications of the present FIA-CL method
The proposed method was applied to the determination of AKS in human serum. Blank serum sample (2 mL) was collected and transferred to a centrifugal filter unit and then centrifuged at 10,000 rpm for 10 min at 4°C. Then 1 mL filtrate was transferred to a 100 mL volumetric flask and diluted to the mark with doubly distilled water. The blank serum was injected into the CL system, and the blank signal was recorded. The amount of AKS in human serum and the recovery obtained are shown in Table 2.
3.6. Mechanism of the CL reaction
The electrochemical oxidation process is helpful in explaining the oxidant characteristics of DPC. We had studied electrochemical behavior of the copper (II) metal chelate in strong alkaline solution, which contained copper (II) and metaperiodate anion. The detailed description of the electrochemical behavior had been reported in our previous study [31].The obvious oxidation peak was observed at 0.42 V. The results indicated that copper(II)could be oxidated into copper(III), which was stable by chelation with polydentate ligands(IO). Although the oxidative ability of copper (III) metal chelate was weaker than that of other oxidants (such as KMnO4, Ce(IV)), its peculiar oxidation can also make the luminol-based CL reaction occur at a relative lowerconcentration (10-7M). In the oxidant reaction of DPC participation, the cuprous complex was regarded as the final product because the electron transfer process was a single electron process.

Table 1 The analytical characteristics of the FI-CL method.
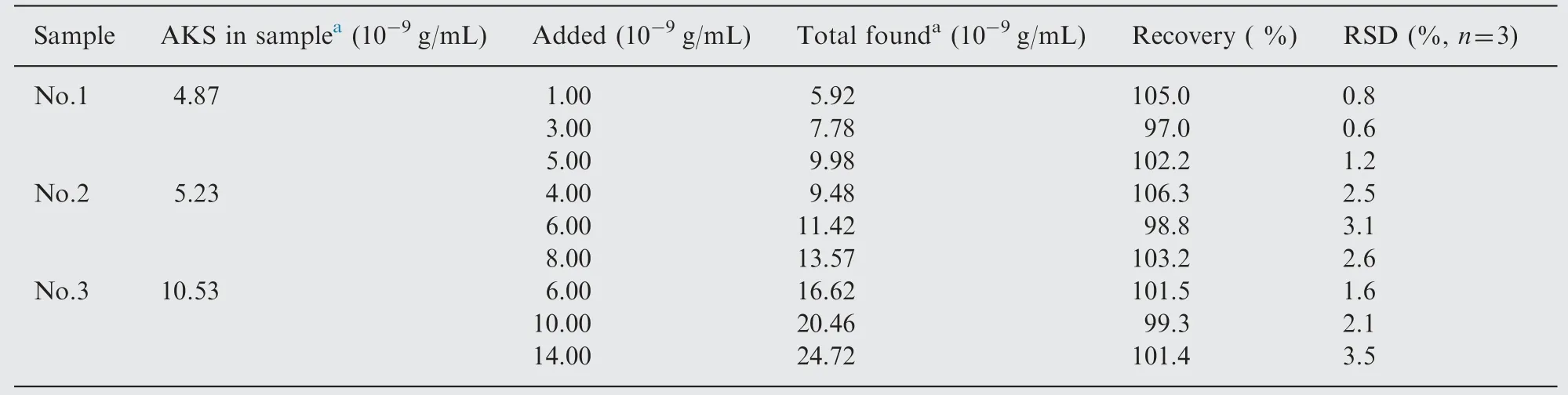
Table 2 Results of recovery tests of AKS in human serum.
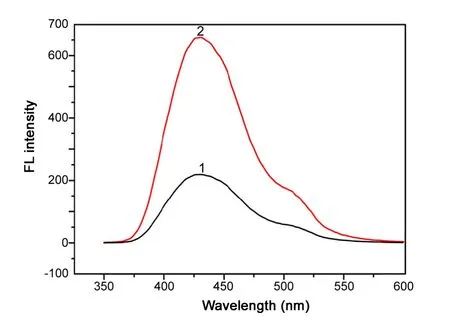
Fig.6 CL spectrum of the luminol-DPC-licomycin system.DPC,1.0 × 10-4 M; luminol, 1× 10-4 M; AKS, 2.0 × 10-4 g/mL; 1,in the absence of AKS; 2, in the presence of AKS.
The CL spectrum of the luminol-DPC system is shown in Fig.6. The fluorescence emission shows an obvious peak at 430 nm when the luminol was mixed with DPC. The obvious intensive peak appeared at the same wavelength when AKS was joined into the luminol-DPC system. It suggested that a reaction between DPC and luminol occurred and the formation of intermediate states (exited Aminophthalate) was with an emission maximum at 425 nm. The CL intensity can be enhanced in the presence of AKS.
Previous studies on oxidation of L-leucine and threonine by Cu(III)[26,28]suggest that formation of[Cu(H3IO6)2(OH)2]3-is the reactive species of water-soluble Cu(III)periodate complex in alkaline medium.From the oxidant reaction of L-leucine[26]and isoniazid [32] by DPC, it is not difficult to speculate the mechanism of luminol-based CL reaction for the similar characteristics of hydrazide in structure. The mechanism could suffer from a free radical intervention. The CL reaction mechanism of DPC-luminol is proposed in Fig.7.Fortunately,the CL intensity of DPC-luminol could be enhanced by AKS.
4. Conclusion
In this work, a novel and sensitive CL method for the direct determination of AKS in serum was developed. It was based on the sensitization of AKS on the oxidant reaction of luminol and DPC. Compared with other oxidants such as H2O2,K3Fe(CN)6, KMnO4and KIO4, DPC has been proved to be a weaker oxidant. However, use of DPC enables the oxidant reaction of luminol to occur at a lower concentration. It makes the CL reaction more selective. Combined with flow injection technique, an attractive FIA-CL method has been developed for its higher sensitivity, lower detection limit and well reproducibility. The detection limit of AKS was at the nanogram level. Recovery tests showed that the DPC-luminol CL method was successfully applied in the determination of AKS in serum without any pretreatment. It indicates that the Cu (III)-based CL method is a promising reagent. Moreover,Cu (III) can broaden the application of CL method in further studies.
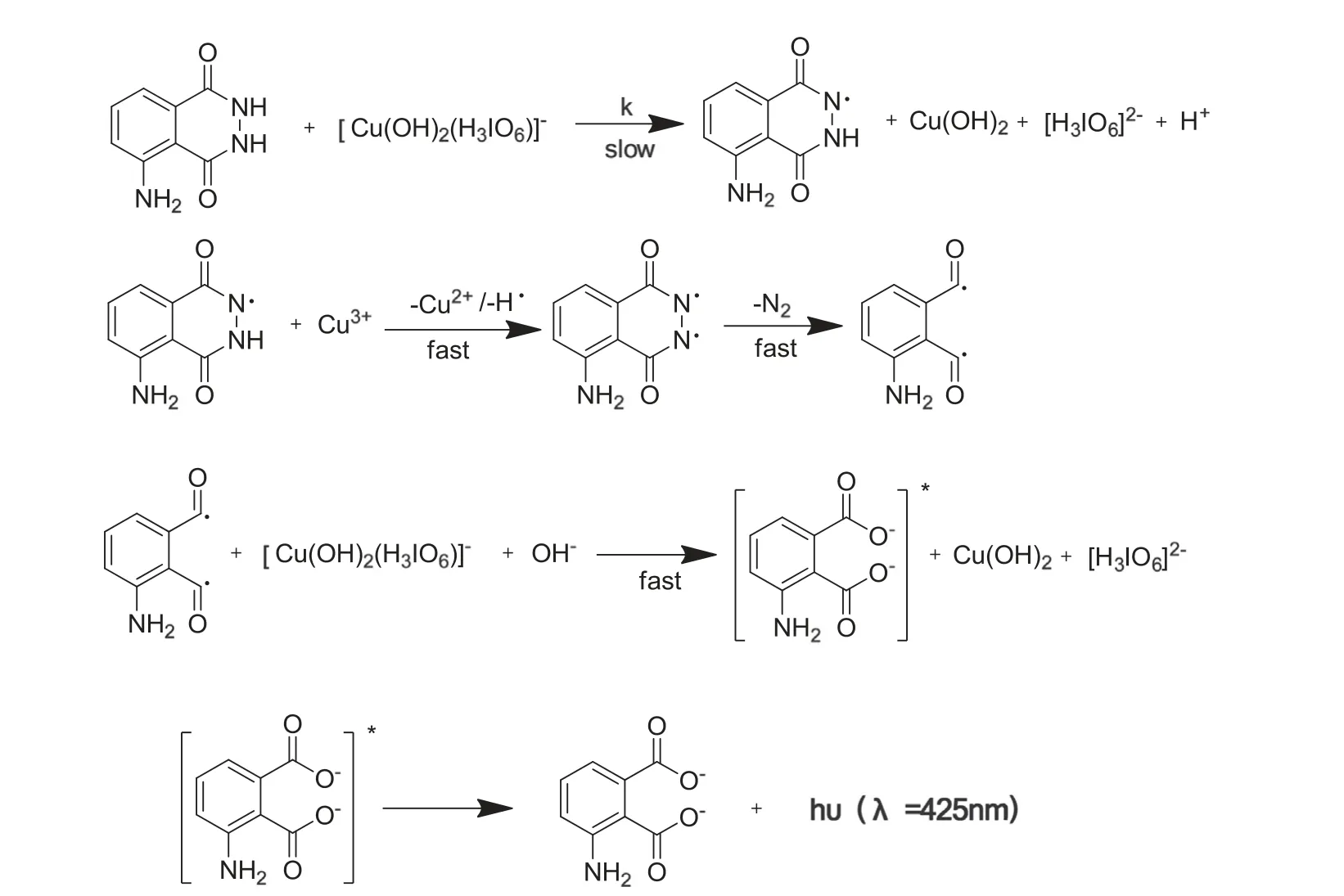
Fig.7 One reaction mechanism suggested for the CL reaction between DPC and luminol.
This work was supported by the National Natural Science Foundation of China (No. 21105133 and No. 21127008) and the Key Program of Guangdong Provincial Natural Science Foundation (No. 9251027501000004).
[1] X.Q. Chen, Y.Y. Jin, G. Tang, New Edition of Pharmacology,People's Medical Publishing House, Beijing, China, 2007, p. 76.
[2] ‘‘Chinese Pharmacopoeia'', Chemical Industry Press, Beijing,China, 2005, p. 286.
[3] S. Thangadurai, L. Giridharan, S.K. Shukla, et al., Analytical utility of atomic absorption spectrometry for the indirect determination of certain aminoglycoside antibiotic drugs,J.Chem.Sci.2 (1) (2004) 62-67.
[4] T.N. Al-Sabha, Spectrophotometric determination of amikacin sulphate via charge transfer complex formation reaction using tetracyanoethylene and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone reagents, Arab, J. Sci. Eng. Section A: Sciences 35 (2A)(2010) 27-40.
[5] T.S. Li, S.P. Liu, Z.F. Liu, et al., Special characteristics of fluorescence and resonance Rayleigh scattering for cadmium telluride nanocrystal aqueous solution and its interactions with aminoglycoside antibiotics, Sci. China, Ser. B 52 (1) (2009) 76-85.
[6] M. Rizk, S.S. Toubar, M.S. Abdel-Mottaleb, et al., A spectrofluorimetric determination of aminoglycoside antibiotics through reaction of their Ruhemann's purple with sodium borohydride,Bulletin of the Faculty of Pharmacy (Cairo University) 41 (2)(2003) 141-148.
[7] J.M. Ramos Fernandez, J.M. Bosque-Sendra, A.M. Garcia-Campana, et al., Chemiluminescence determination of amikacin based on the inhibition of the luminol reaction catalyzed by copper, J. Pharmaceut. Biomed. Anal. 36 (5) (2005) 969-974.
[8] U. Hubicka, J. Krzek, H. Woltynska, et al., Simultaneous identification and quantitative determination of selected aminoglycoside antibiotics by thin-layer chromatography and densitometry, J. AOAC Int. 92 (4) (2009) 1068-1075.
[9] A.A. Al-Majed, A new LC method for determination of some aminoglycoside antibiotics in dosage forms and human plasma using 7-Fluoro-4-nitrobenz-2-oxa-1,3-diazole as a fluorogenic pre-column label, Chromatographia 68 (11-12) (2008) 927-934.
[10] X.J. Chang, J.D. Peng, S.P. Liu, A simple and rapid high performance liquid chromatographic method with fluorescence detection for the estimation of amikacin in plasma—application to preclinical pharmacokinetics, J. Chin. Chem. Soc. (Taipei,Taiwan) 57 (1) (2010) 34-39.
[11] H.K. Trivedi, M.C. Patel, A stability indicating method for the determination of the antioxidant sodium bisulfite in pharmaceutical formulation by RP-HPLC technique, Sci. Pharm. 79 (4)(2011) 909-920.
[12] P. Norouzi, G.R. Bidhendi, M.R. Ganjali, et al., Sub-second accumulation and stripping for pico-level monitoring of amikacin sulfate by fast fourier fransform cyclic voltammetry at a gold microelectrode in flow-injection systems,Microchim.Acta 152(1-2) (2005) 123-129.
[13] S. Oguri, Y. Miki, Determination of amikacin in human plasma by high-performance capillary electrophoresis with fluorescence detection, J. Chromatogr. B 686 (2) (1996) 205-210.
[14] L.J. Wang, Y.H. Tang, Y.H. Liu, Flow injection chemiluminescence determination of loxoprofen and naproxen with the acidic permanganate-sulfite system, J. Pharm. Anal. 1 (1) (2011) 51-56.
[15] L. Gamiz-Gracia, A.M. Garcia-Campana, Huertas-Perez, et al.,Chemiluminescence detection in liquid chromatography:Applications to clinical, pharmaceutical, environmental and food analysis—A review, Anal. Chim. Acta 640 (1-2) (2009) 7-28.
[16] C.A. Marquette, L.J. Blum, Applications of the luminol chemiluminescent reaction in analytical chemistry, Anal. Bioanal.Chem. 385 (3) (2006) 546-554.
[17] J.M.Lin,M.L.Liu,Singlet oxygen generated from the decomposition of peroxymonocarbonate and its observation with chemiluminescence method, Spectrochim. Acta 1 (Part A.72) (2009) 126-132.
[18] J. Tian, Y. Hu, J.J. Zhang, Chemiluminescence detection of permanganate index (CODMn) by a luminol-KMnO4 based reaction, Environ. Sci. 20 (2) (2008) 252-256.
[19] A. Waseem, M. Yaqoob, A. Nabi, Flow-injection determination of cysteine in pharmaceuticals based on luminol-persulfate chemiluminescence detection, Luminescence 23 (3) (2008)144-149.
[20] W.B. Shi, J.D. Yang, Chemiluminescence determination of mezlocillin by the luminol-potassium periodate system, J. Braz.Chem. Soc. 19 (6) (2008) 1180-1185.
[21] X.S. Tang, X.Y. Shi, Y.H. Tang, et al., Chemiluminescence determination of melamine with luminol-K3Fe(CN)6 system,J. Pharm. Anal. 1 (2) (2011) 104-107.
[22] A.A. Ensafi, T. Khayamian, F. Hasanpour, Determination of glutathione in hemolysed erythrocyte by flow injection analysis with chemiluminescence detection, J. Pharm. Biomed. Anal. 48(1) (2008) 140-144.
[23] S. Shleev, J. Wetter, K.E. Magnusson, et al., Simultaneous use of electrochemistry and chemiluminescence to detect reactive oxygen species produced by human neutrophils Cell, Biol. Int.12 (2008) 1486-1496.
[24] B.A. Deganatti, N.P. Shetti, S.T. Nandibewoor, Mechanistic investigations on the oxidation of l-valine by diperiodatocuprate(III) in aqueous alkaline medium: a kinetic model, Transition Met. Chem. 34 (2) (2009) 143-152.
[25] D.S. Munavalli, S.A. Chimatadar, S.T. Nandibewoor, Oxidation of vanillin by a new oxidant diperiodatoargentate(III)in aqueous alkaline medium, Ind. Eng. Chem. Res. 46 (5) (2007) 1459-1464.
[26] K.M. Naik, S.T. Nandibewoor, Kinetics and mechanism of oxidation of L-leucine by alkaline diperiodatocuprate(III) — A free radical intervention, deamination and decarboxylation, J.Chem. Sci. 124 (4) (2012) 809-819.
[27] J.H. Shan, X.Q. Wang, H.X. Shen, Kinetics and mechanism of oxidation of N,N-dimethylethanolamine by diperiodatocuprate(III) complex in alkaline medium, Asian J. Chem. 23 (1)(2011) 180-182.
[28] T.P. Jose, S.M. Tuwar, Oxidation of threonine by the analytical reagent diperiodatocuprate(III) — An autocatalysed reaction, J.Mol. Stru. 827 (1-3) (2007) 137-144.
[29] Z.J. Wu, Z.B. Zhang, L.S. Liu, Electrochemical studies of a Cu(II)—Cu(III) couple: Cyclic voltammetry and chronoamperometry in a strong alkaline medium and in the presence of periodate anions, Electrochim. Acta 42 (17) (1997) 2719-2723.
[30] Y.F.Hu,Z.J.Zhang,C.Y.Yang,The determination of hydrogen peroxide generated from cigarette smoke with an ultrasensitive and highly selective chemiluminescence method, Anal. Chim.Acta 601 (1-2) (2007) 95-100.
[31] Y.F. Hu, Z.J. Zhang, G.K. Li, A novel chemiluminescence method for the determination of ergometrine maleate in serum sample without chemiluminescence reagent, Talanta 81 (1-2)(2009) 499-504.
[32] S.D. Kulkarni, S.T. Nandibewoor, A kinetic and mechanistic study on oxidation of isoniazid drug by alkaline diperiodatocuprate(III) — A free radical intervention, Transition Met. Chem.31 (8) (2006) 1034-1039.
 Journal of Pharmaceutical Analysis2013年5期
Journal of Pharmaceutical Analysis2013年5期
- Journal of Pharmaceutical Analysis的其它文章
- A novel and rapid microbiological assay for ciprofloxacin hydrochloride
- Determination of sodium hyaluronate in pharmaceutical formulations by HPLC-UV
- Copper interactions with DNA of chromatin and its role in neurodegenerative disorders
- A critical quality parameter in quantitative fused-core chromatography: The injection volume
- Application of LC-MS/MS for quantitative analysis of glucocorticoids and stimulants in biological fluids
- In situ modified screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in its formulation
