Influence of Synthesis Parameters with Low Seed Addition on the Crystallinity of ZSM-5
Wang Songlin; Wei Huijuan; Tang Ying; Wang Xiangyu; Li Baojun
(Institute of Industrial Catalysis, College of Chemistry and Molecular Engineering, Zhengzhou Uniνersity, Zhengzhou 450001)
Influence of Synthesis Parameters with Low Seed Addition on the Crystallinity of ZSM-5
Wang Songlin; Wei Huijuan; Tang Ying; Wang Xiangyu; Li Baojun
(Institute of Industrial Catalysis, College of Chemistry and Molecular Engineering, Zhengzhou Uniνersity, Zhengzhou 450001)
ZSM-5 zeolite microparticles (MPs) were synthesized under hydrothermal condition using a low crystal seed addition approach without template. The synthesis parameters such as the seed addition amount, the SiO2/Al2O3ratio, the aluminum source, the feeding addition method, aging, and crystallization were investigated. The structure, morphology and composition of the as-synthesized ZSM-5 zeolite MPs were characterized by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), laser particle size distribution (PSD) measurements, and inductively coupled plasma-atomic emission spectrometry (ICP-AES). The SiO2/Al2O3ratio of ZSM-5 zeolite MPs was in the range of 20—80. The low seed addition was beneficial to improving the crystallinity and shortening the crystallization time, and the suitable amount of seed was 0.25% (SiO2). The ZSM-5 zeolite MPs synthesized with aluminium nitrate nonahydrate used as the aluminum source exhibited a relatively high crystallinity. An appropriate aging time could eliminate the effect of feeding addition method and effectively adjust particle size. The particle size of ZSM-5 zeolite obtained at an aging time of 20 h was around 2.0 μm. Prolonging the aging time appropriately could also shorten the high-temperature crystallization time. The suitable aging time was 24 h, and the relative crystallinity of ZSM-5 zeolite could reach up to 99% after crystallization for 24 h at 180 ℃.
aging; crystal seed; microparticles (MPs); relative crystallinity; ZSM-5 zeolite
1 Introduction
The ZSM-5 zeolite exhibits interesting physical and chemical properties thanks to its superior thermal stability, unique structure, shape selectivity and acidity[1-2]. Nowadays, ZSM-5 materials have been widely used in catalysis, medicine, environmental protection and other fields[3-4]. Olefin aromatization over ZSM-5 zeolite becomes especially effective at atmospheric pressure[5-6]. Specifically, the ZSM-5 zeolite shows an outstanding selectivity up to 99.9% for hydration of olefins such as propene and cyclohexene[7-9].
Since the first successful synthesis of ZSM-5 zeolite by the Mobil Corporation in 1972, novel research activities using rapid and facile techniques have been reported[10]. For effective synthesis of high crystallinity ZSM-5 zeolite, some organic templates, such as tetrapropylamine bromide, n-butyl amine, ethylamine, ethanolamine, and diethylamine[11-14], have been employed. Synthesis using these templates possessed some adverse problems including high production cost and environmental pollution. ZSM-5 zeolite has also been synthesized with cheap ammonia water or alcohol serving as templates[15-16]. Nevertheless, the occurrence of large amount of miscellaneous crystals, low crystallinity and non-uniform particle size are detrimental to the commercial application of catalysts. ZSM-5 zeolite has been synthesized without template by hydrothermal process[17-18]. However, high crystallinity ZSM-5 requires seed addition (>3%)[19-20]. Grose, et al. first reported the synthesis of ZSM-5 zeolite with seeds in the absence of organic cations[21]. Sylvie, et al. assessed the effect of seed addition on the crystallization mechanism[22]. Kumar, et al. studied the influence of synthesis time and stirring mode on the physical and chemical properties of ZSM-5 zeolite[23]. Nowadays, the major concern on ZSM-5 synthesis is related with small particle size in order to achieve high-efficiency utilization of this zeolite in catalytic reactions[24-26]. Unfortunately, for applicationsin industrial processes, small particle size means higher filtration costs for removing the catalyst, once the reaction was finished.
Recently, the issues associated with loss and filtration of small particle size in cyclohexene hydration process need to be tackled. These problems have motivated the development of ZSM-5 microparticles (MPs) from submicroparticle (SMPs) crystal seed. Its excellent catalytic performance and superior sedimentation separation property compared to that of commercial ZSM-5 zeolite have also been reported by our group[27]. Such factors as feeding composition, aging, crystallization, stirring speed, alumina sources and pretreatment can strongly affect the synthesis of ZSM-5 zeolite[28-30]. The effects of these factors on the rapid and facile synthesis of MPs of ZSM-5 zeolite from low seed addition have not been systematically investigated yet.
In this paper, six important factors, including the seed addition amount, the SiO2/Al2O3ratio, the aluminum source, the feeding addition method, aging, and crystallization, were studied. The aim of this work is to provide some useful information on the rapid and facile preparation and potential application of ZSM-5 zeolite in industrial production.
2 Experimental
2.1 Synthesis
The molar composition of gel feeding for ZSM-5 synthesis is listed in Table 1. The typical synthesis process was carried out by dissolving aluminium nitrate nonahydrate in the sodium hydroxide solution, followed by adding the rest of water and colloidal silica solution under vigorous stirring. The quantity of seed added was calculated as follows: (SiO2mass in seed sol)/(total SiO2mass in feeding gel)×100%. The mixture was then stirred at a specified temperature over a constant aging time, and poured into stainless-steel autoclaves. Thereafter, the sealed autoclaves were placed in a preheated oven with the temperature maintained at a value required for crystallization. After a predetermined crystallization time, the autoclaves were taken out from the oven and cooled naturally down to ambient temperature. The products were separated via filtration and washed with distilled water for three times. The as-synthesized samples were dried at 120 ℃ overnight and then heated to 550 ℃ at a heating rate of 10 ℃/min under a flow of air stream and kept for 5 h.
2.2 Characterization
X-ray diffraction (XRD) patterns were recorded on a X’pert PRO diffractometer (PANalytical) using CuKα(λ=0.154 18 nm) radiation (at 40 kV and 40 mA) with a scanning rate of 1.2(°)/min in a range of from 5° to 90°. The relative crystallinity of synthesized samples were calculated by the formula: (the sum of peak intensity of the products at 2θ=7.9°, 8.8°, 14.8°, 23.9° and 24.4°)/ (the sum of peak intensity of the reference sample at 2θ=7.9°, 8.8°, 14.8°, 23.9° and 24.4°)×100%, with the highly crystalline NK (commercial ZSM-5 zeolite) used as the reference sample. Scanning electron microscopy (SEM) measurements were performed by a JSM-6700F instrument operating at a voltage of 20 kV. The average diameter of samples was measured by a Rise-2002 laser particle size distribution meter (PSD). The content of elements in weight percent of samples were determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES) using argon as the support gas.
3 Results and Discussion
3.1 Effect of seed addition
The commercial Na-ZSM-5 zeolite with a SiO2/Al2O3molar ratio of 20.6 and an average particle size of about 0.5 μm serving as the seed was obtained from the Nankai University. Different seed amounts were added to meet the gel feeding composition of 11.5Na2O:100SiO2:3Al2O3:2750H2O. Figure 1 shows the XRD patterns of as-synthesized products, andthe characteristic diffraction peaks of MFI structure could be observed on all the samples[31-32].
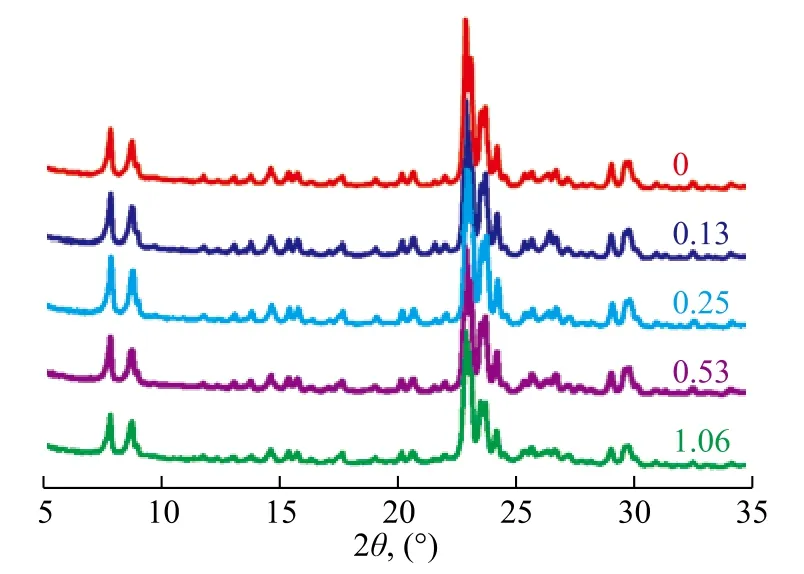
Figure 1 XRD patterns of products formed with different seed amounts
Table 1 demonstrates the effect of seed amount on relative crystallinity, crystal size and particle size of products. The product showed a relatively low crystallinity without seed addition in the course of its preparation. Seed could provide the crystal nucleation or the growth surface for the emergence of crystal products, and therefore a relatively high crystallinity could be obtained with a small amount of seed added. As the seed addition amount increased from 0 to 1.06%, the relative crystallinity increased greatly and then reduced slightly, indicating that the induction nucleation process accelerated with abundant seeds, and the ratio of pure ZSM-5 species increased in the synthesized product. However, the ZSM-5 synthesized solution was not stable, and the gel composition could be changed with excessive seed addition. The formed ZSM-5 nucleation might be dissolved or transformed, and thus the crystallinity decreased. Seeds also could reduce the crystal size of product. Both the crystal size and the particle size decreased with an increasing seed amount. It is worth mentioning that the synthesized products could achieve a 99% crystallinity at about 0.25% of the seed added.
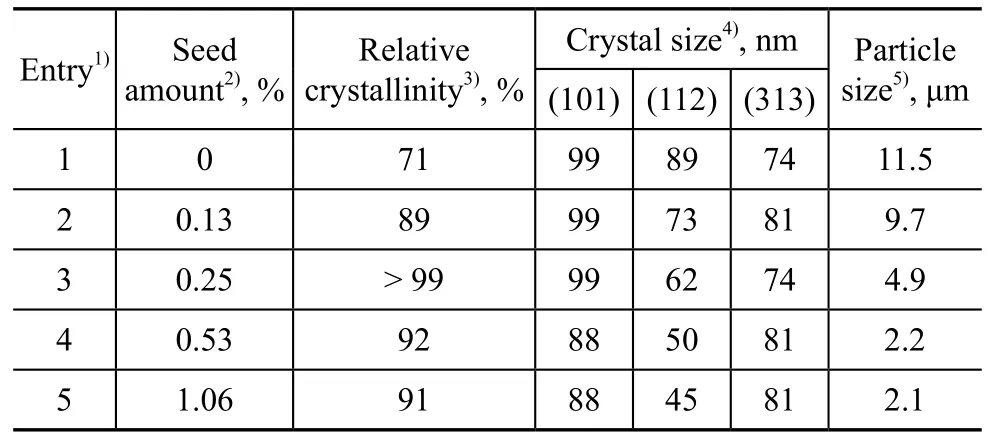
Table 1 Effect of seed amount on the relative crystallinity, crystal size and particle size of products
The morphology of seed and products was observed by SEM (Figure 2). The seed from the commercial sources was slender with an particle size of about 0.5 μm and exhibited the aggregation among crystals (Figure 2a). The product without seed addition was clearly ring-like, and the particle size was well-distributed with an average particle size of about 3.5×9.8 μm, but the crystal surface possessed amorphous debris or small crystals (Figure 2b). The product obtained with seed addition was peanutlike, and the particle size was also well-distributed with an average particle size of about 2.3 μm×4.6 μm (Figure 2c). This distinctive structural feature had verified that the highly crystalline ZSM-5 microparticles (MPs) could be synthesized from a small amount of submicroparticles (SMPs) of crystal seed without the template.

Figure 2 SEM images of (a) seed; (b) product formed without seed addition; and (c) product formed with 0.25% of seed addition
In order to investigate the effect of seed type, the Na-ZSM-5 zeolite was transformed to H-ZSM-5 zeolite by conventional ion-exchange method. The same experiment was repeated, and the results showed that the seed type had little effect on the crystallinity and crystal size of ZSM-5 zeolite. This might be inferred that the H type seed was transformed to the Na type seed again in a strongly alkaline condition (at pH=13—14).
3.2 Effect of SiO2/Al2O3ratio of feeding gels
The SiO2/Al2O3ratio is an important parameter related with the performance of ZSM-5 zeolite. This ratio has a remarkable effect on the properties of ZSM-5 zeolite such as crystallinity, purity, acidity, thermal stability, and hydrothermal stability. ZSM-5 zeolite with a SiO2/Al2O3ratio in the range of 20 to 100 can be easily synthesized. When the SiO2/Al2O3ratio is too high, the α-quartz tends to appear, and when it is lower than 20, the mordenite is likely to form[33]. The SiO2/Al2O3ratio in gel feeding composition was selected in the range of 25—80. Figure 3 presents the characteristic diffraction peaks of XRD patterns for all the samples. Table 2 gives the relative crystallinity and composition of products for different molar ratios of components in feeding gels.
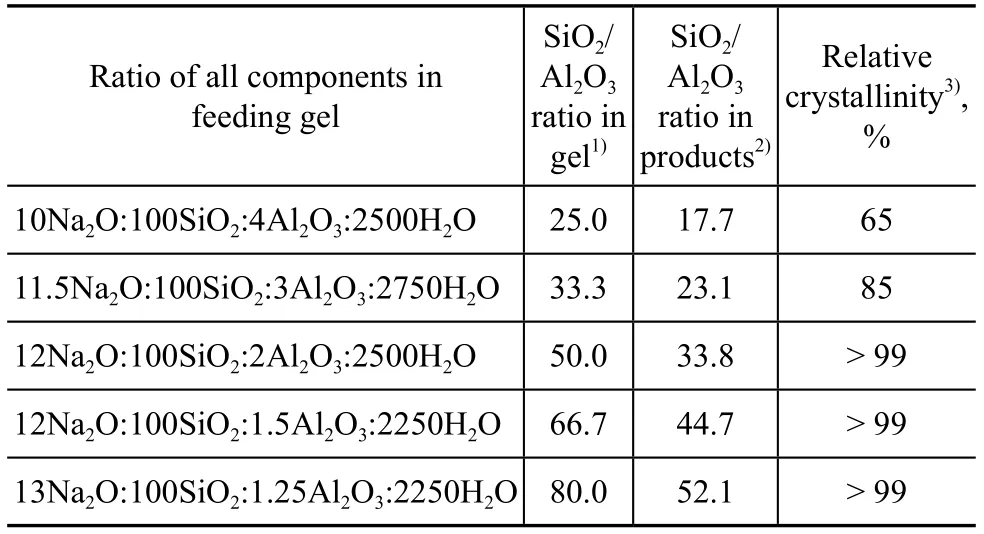
Table 2 Relative crystallinity and composition of products with different SiO2/Al2O3ratios in feeding gels
It can be seen from Table 2 that with an increasing SiO2/ Al2O3ratio in gel feeding composition, the SiO2/Al2O3ratio of crystal products increased. However, the ratio of SiO2/Al2O3of crystal products versus that of gel feeding composition gradually decreased (Figure 4), indicating that it was difficult to insert silicon species into the framework with an increasing SiO2/Al2O3ratio of gel feeding composition. In general, under the same synthesis condition, the crystallinity of products was different in compliance with the difference in SiO2/Al2O3ratio. When the SiO2/Al2O3ratio of gel feeding composition was 25.0, the relative crystallinity of products was low. The miscellaneous crystal phase appeared at the diffraction peaks of 26.6o(Figure 3), which could be attributed to magadiite or α-quartz. As the SiO2/Al2O3ratio of gel feeding composition increased from 25.0 to 80.0, the relative crystallinity of crystal products increased gradually. The reason was that with an increasing SiO2/Al2O3ratio of gel feeding composition, the viscosity of gel feeding composition increased and more vigorous stirring was required. The concentration of various oligomeric silicon species could be increased at the vicinity of aluminum species. It was easier for the polycondensation of aluminum species and silicon species to take place without spatial restriction.

Figure 3 XRD patterns of products with different SiO2/Al2O3ratios
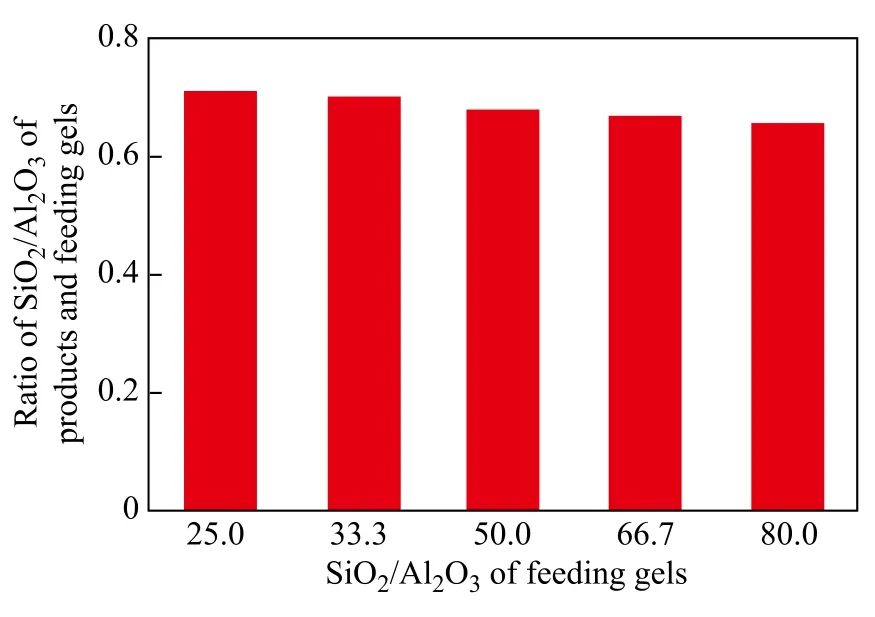
Figure 4 The relationship of SiO2/Al2O3ratio in feeding gels and products
3.3 Effect of aluminum source
The effect of aluminum source on relative crystallinity and composition of crystal products are shown in Table 3. Nitrate ions of aluminum nitrate could enhance the nucleation and crystallization process of ZSM-5 zeolite[34-36]. Thereby a 99% crystallinity was obtained. Aluminum powder could not be dissolved completely in the gel, the available aluminum species decreased, and only a 71% crystallinity was obtained.
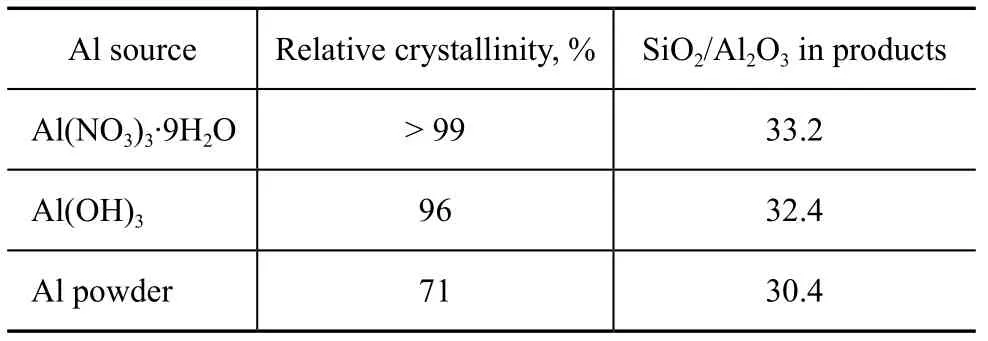
Table 3 Relative crystallinity and composition of products originating from various aluminum sources
3.4 Effect of feeding addition method
The aluminosilicate species formed by the polymerization of acidic silicate ion and acidic aluminum ion determined the nature of ZSM-5 zeolite. The polymerization reaction and distribution state were affected by various factors such as the alkalinity, the cation type, the temperature, the pressure, etc.[37-39]In this work, ZSM-5 zeolite was synthesized in a strongly alkaline condition, the aluminosilicate species existed mainly in the form of monomeric and dimeric state which were beneficial to the synthesis of high-crystallinity ZSM-5 zeolite. In neutral or weakly alkaline conditions, the aluminosilicate species existed in the form of polymeric state.
The alkaline solution upon addition of silicate sol was denoted as the forward addition method (FAM). The silicate sol upon addition of alkaline solution was denoted as the anti-addition method (AAM). Both silicate sol and alkaline solution upon the synchronized addition was denoted as the parallel addition method (PAM). It can be seen from Table 4 that, in a short aging time, different addition feeding methods affected the relative crystallinity distinctly. The silicate sol was added into alkaline solution by FAM, and it was favorable for the nucleation formation because the aluminosilicate species formed in the feed solution could be depolymerized easily into monomeric species under the high alkalinity condition. Thus, the sample synthesized by FAM exhibited a 94% crystallinity after 2 hours of aging time. The precipitates of Al(OH)3and SiO2could be formed by AAM and PAM under the low alkalinity condition, and could not be dissolved rapidly in a short aging time. It was detrimental to the nucleation formation. Therefore, only a 9% crystallinity and a 31% crystallinity were obtained for AAM and PAM, respectively. When the aging time was extended to 24 h, crystallinity of the product was almost not affected by the addition methods, since the precipitates could be dissolved with sufficient time in the alkaline solution. The aluminosilicate species could continue to form under vigorous stirring.
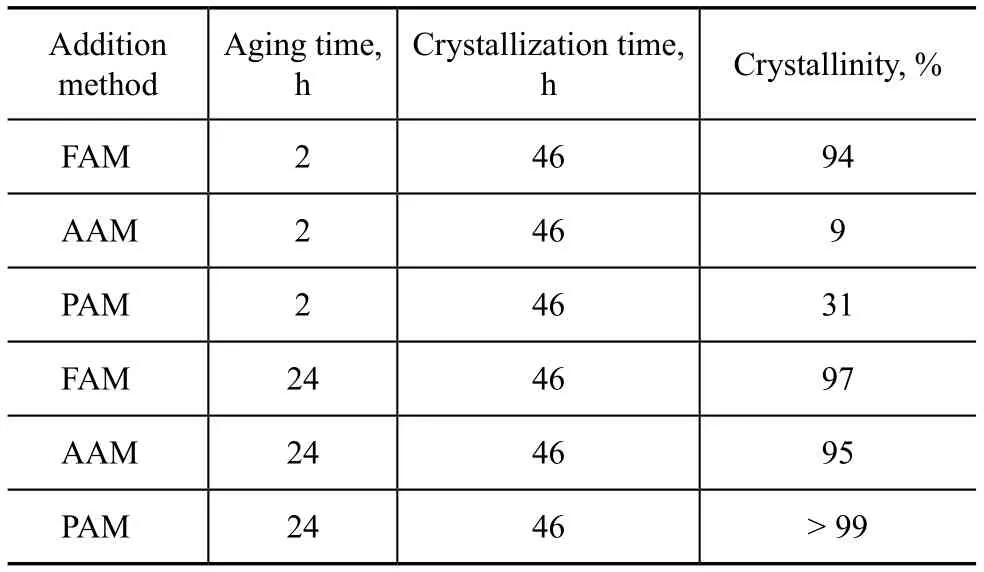
Table 4 Effect of addition feeding method on the crystallinity of products
Figure 5 shows the SEM images of crystal products obtained by three different feeding addition methods. The product of FAM was clearly peanut-like, and the particle size was well-distributed with an average particle size of about 1.5×2.5 μm (Figure 5a and 5b). The product of AAM was olive-like, and the particle size was also well-distributed with an average particle size of about 2.0×4.0 μm (Figure 5c and 5d). The product of PAM was flower-like, spherical and even provided with other visible crystal morphology, with the particle size distributed broadly between 1.5×2.5 μm and 3.0×10.0 μm, respectively (Figure 5e and 5f).
3.5 Effect of crystallization
Table 5 shows the effect of crystallization time. The relative crystallinity was low at a 24-h crystallization time. Moreover, the miscellaneous crystals appeared (Figure 6).The relative crystallinity increased dramatically when the crystallization time was increased. After realization of a complete crystallization process, a trace amount of miscellaneous crystals was dissolved or transformed in the high-temperature alkaline mixture[26,38,40]. Longer crystallization time could also cause the synthesized crystal products to be dissolved in alkaline solution. Therefore, both the crystal size and the particle size decreased.
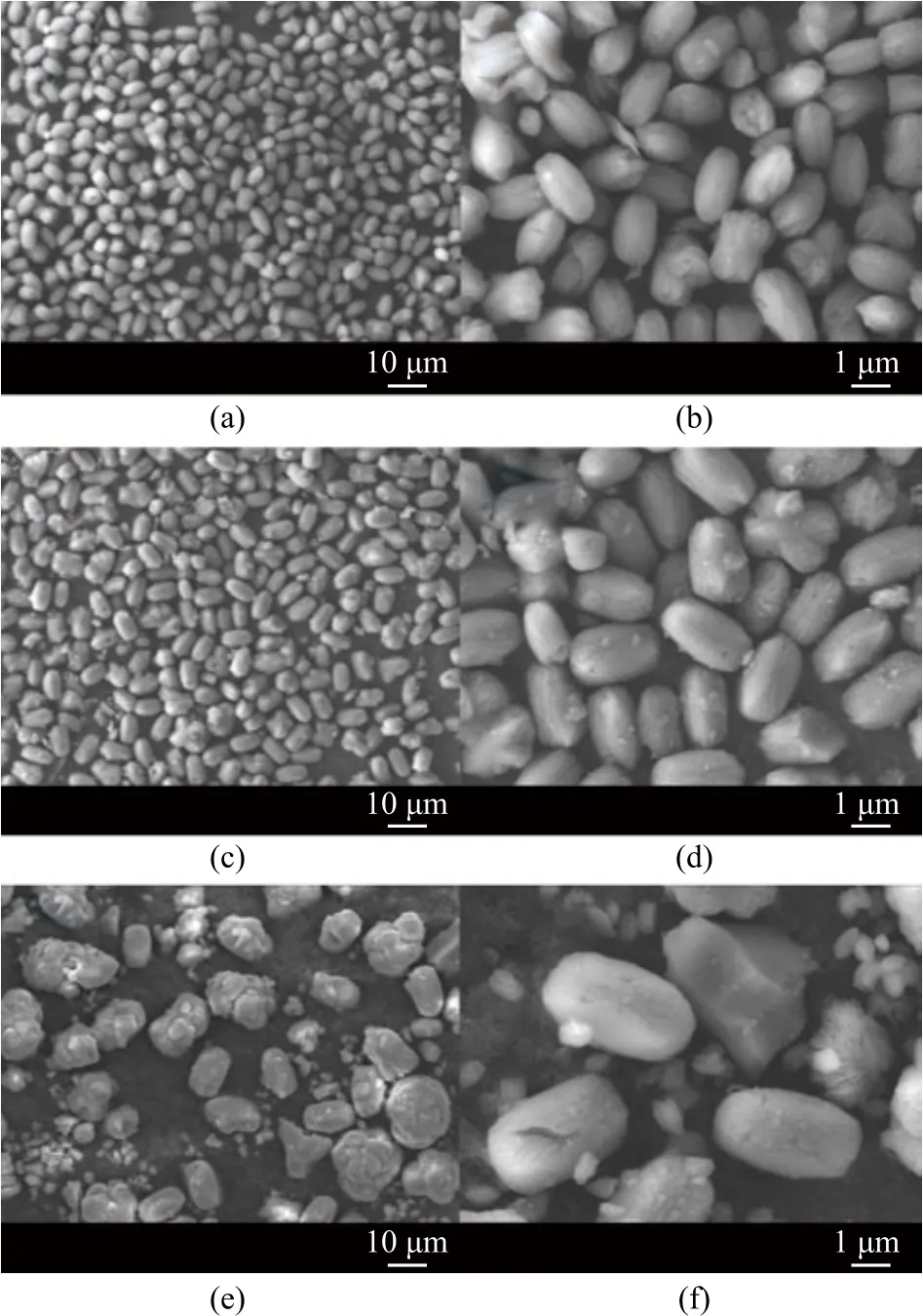
Figure 5 SEM images of products obtained with different feeding addition methods in 24 h of aging at different magnifications of FAM (a, b), AAM (c, d), and PAM (e, f)
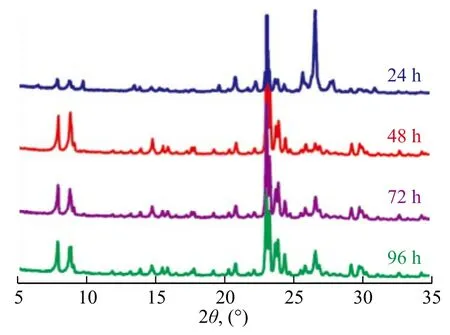
Figure 6 XRD patterns of products obtained at different crystallization time
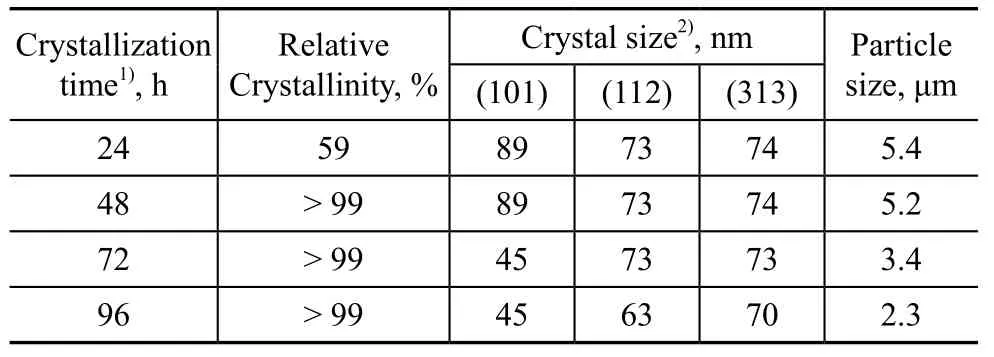
Table 5 Effect of crystallization time on relative crystallinity and particle size of products
In order to study the crystallization kinetics, three series of kinetic runs were carried out by varying crystallization time at 160 ℃, 170 ℃ and 180 ℃, respectively. With an increasing crystallization temperature, the rate of nucleation and crystallization accelerated obviously (Figure 7). The crystallization process was an endothermic reaction. The solubility of aluminosilicate gel could be improved in high-temperature solution. Consequently, the nucleation period was shortened and the rate of nucleation was improved. The rate of crystallization was also improved and the crystallization time was shortened. The formation of nucleation and crystal growth belonged essentially to the condensation reaction. The reaction rate and temperature was almost exponential except for other factors[41-42]. Therefore, increase in crystallization temperature could improve the crystallization rate and shorten the crystallization time. But an excessively high crystallization temperature could lead to the formation of large crystal size and even occurrence of miscellaneous crystals[43-45]. It is worth mentioning that the products synthesized at 180 ℃ required a relatively short crystallization time to bring forthhigh crystallinity and pure crystals.
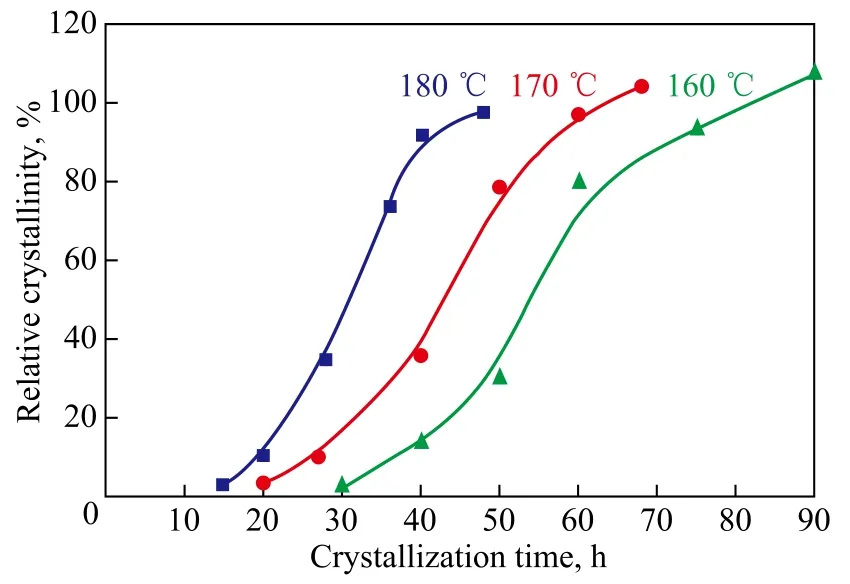
Figure 7 Kinetics curves of crystallization process conducted at different temperature
3.6 Effect of aging time
There are a few reports referring to aging in the course of synthesis of molecular sieves. The aging process was beneficial to the synthesis of ZSM-5 zeolite, and could improve the induction period for nucleation formation of aluminosilicate gel. Moreover, it could also shorten the crystallization time and adjust the crystal size as well as the particle size distribution[46-47].
Table 6 exhibits the effect of aging time. Prolonging aging time to 12 h could improve the crystallinity from 78% to 99% obviously along with decrease in the crystal size. The aging process could change the composition and structure of aluminosilicate gel and then affect the formation of nucleation and crystal growth. Therefore, prolonging aging time was beneficial to the nucleation formation of aluminosilicate ions during the induction period.
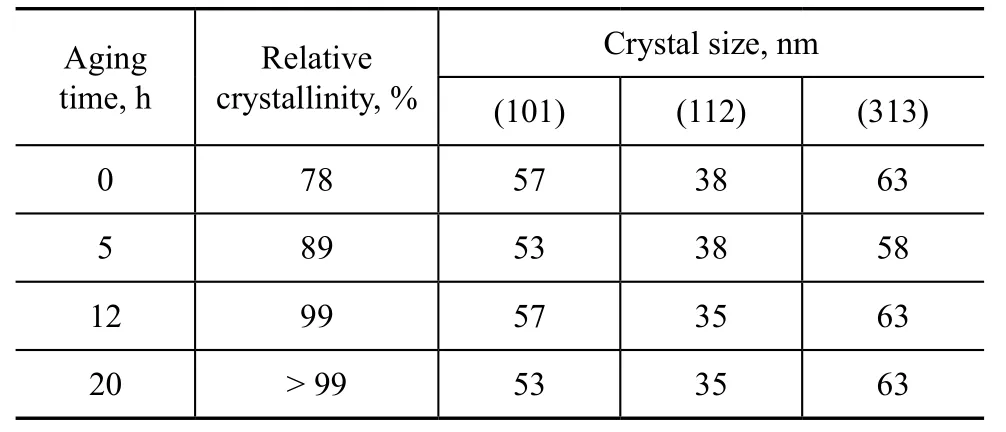
Table 6 Effect of aging time on the crystallinity and crystal size of products
Upon prolonging the aging time, aluminosilicate species were depolymerized in the alkaline environment to form oligomeric species. The size of aluminosilicate polymer became smaller and the nucleates formed thereby became also smaller. Accordingly, the particle size distribution was also reduced and became gradually narrower and well-distributed (Figure 8).
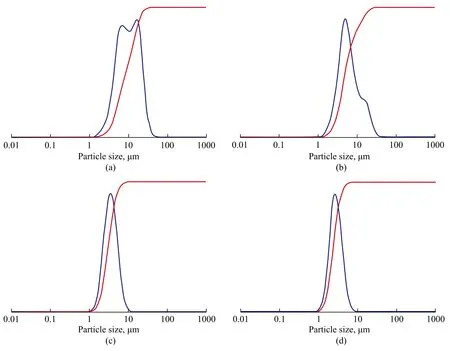
Figure 8 Particle size distribution of products obtained at different aging time
Table 7 exhibits the effect of aging temperature. As the aging temperature increased, the relative crystallinity increased also. Higher temperature could lead to partial water evaporation from the gel, and the relative crystallinity began to drop above 70 ℃.
The effect of extended aging time on shortening thecrystallization time is presented in Table 8. The relative crystallinity of product without aging was only 74% after 48 h of crystallization. When the aging time was extended to 12 h, a 99% crystallinity could be obtained after 30 h of crystallization. This result revealed that appropriate extension of the low-temperature aging time could shorten remarkably the high-temperature crystallization time. Aging could enhance the formation of aluminosilicate during the nucleation induction period to help shorten the high-temperature crystallization time and improve the crystallinity.
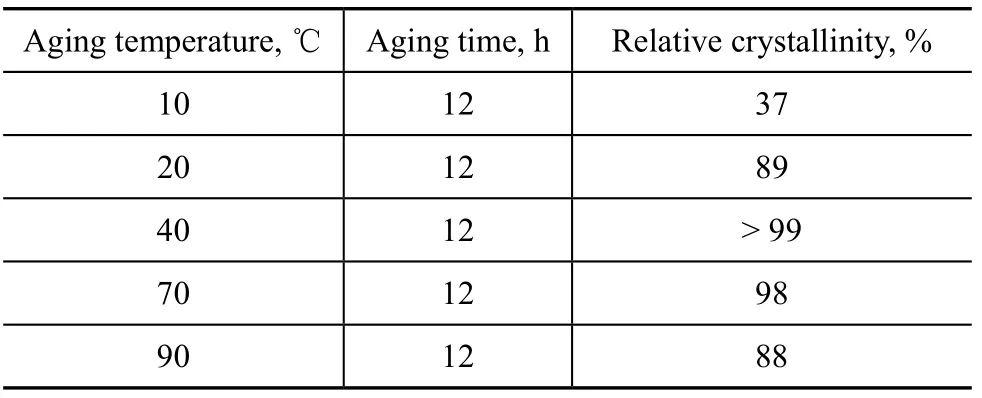
Table 7 Effect of aging temperature on relative crystallinity of products
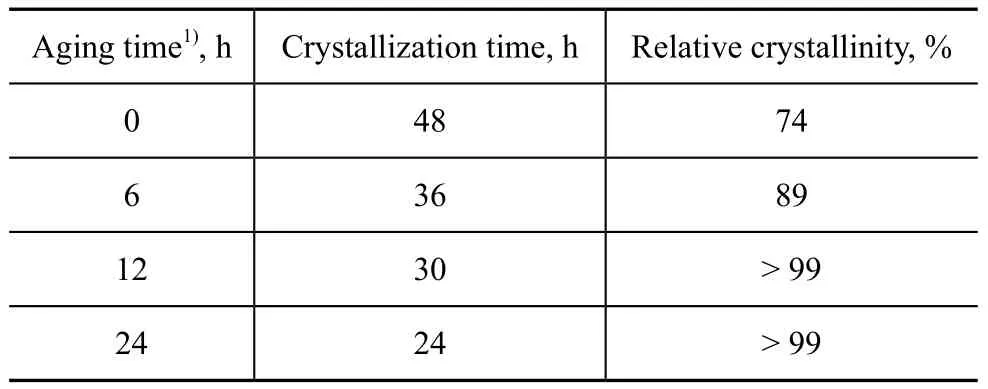
Table 8 Effect of prolonging aging time on shortening crystallization time of products
4 Conclusions
In conclusion, the high crystallinity ZSM-5 microparticles (MPs) with a SiO2/Al2O3ratio in the range of 20—80 have been successfully prepared from submicroparticles (SMPs) of crystal seed. The low seed addition was beneficial to shortening the crystallization time and improving the crystallinity, and the suitable addition amount was 0.25% (SiO2). The crystals exhibited peanut-like shape with good particle size distribution. The relative crystallinity with Al(NO3)3·9H2O serving as the aluminum source could reach 99%. The feeding addition method could affect the crystallinity of ZSM-5 zeolite. Prolonging appropriately the aging time could eliminate the negative effect on the crystallinity, but would affect the morphology and particle size. The particle size could be effectively controlled through adjusting the aging time. The particle size was around 2.0 μm at an aging time of 20 h. Increasing the aging time appropriately could also shorten the crystallization time. The suitable aging time was 24 h, and the relative crystallinity could reach up to 99% upon conducting the crystallization process at 180 ℃ for 24 h.
Acknowledgments:Financial support from the Innovation Fund for Elitists of Henan Province, China (No. 0221001200), the Talent Training Joint Fund of NSFC-Henan (No. U1204203) and the China Postdoctoral Science Foundation (No. 2012M511121) are acknowledged.
[1] Cundy C S, Cox P A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time[J]. Chem Rev, 2003, 103(3): 663-701
[2] Chen L H, Li X Y, Tian G, et al. Highly stable and reusable multimodal zeolite TS-1 based catalysts with hierarchically interconnected three-level micro-meso-macroporous structure[J]. Angew Chem Int Ed, 2011, 50(47): 11156-11161
[3] Davis M E. Ordered porous materials for emerging applications[J]. Nature, 2002, 417(6891): 813-821
[4] Stein A. Advances in microporous and mesoporous solids—highlights of recent process[J]. Adv Mater, 2003, 15(10): 763-775
[5] Mentzel U V, Shunmugavel S, Hruby S L, et al. High yield of liquid range olefins obtained by convertingi-propanol over zeolite H-ZSM-5[J]. J Am Chem Soc, 2009, 131(46): 17009-17013
[6] Byggningsbacka R, Kumar N, Lindfors L E. Comparative study of the catalytic properties of ZSM-22 and ZSM-35 ferrierite zeolites in the skeletal isomerization of 1-butene[J]. J Catal, 1998, 178(2): 611-620
[7] Nuntasri D, Wu P, Tatsumi T. High selectivity of MCM-22 for cyclopentanol formation in liquid-phase cyclopentene hydration[J]. J Catal, 2003, 213(2): 272-280
[8] Huynh L K, Zhang H R, Zhang S W, et al. Kinetics of enol formation from reaction of OH with propene[J]. J Phys Chem A, 2009, 113(13): 317-3185
[9] Svelle S, Joensen F, Nerlov J, et al. Conversion of methanol into hydrocarbons over zeolite H-ZSM-5: Ethene formation is mechanistically separated from the formation of higher alkenes[J]. J Am Chem Soc, 2006, 128(46): 14770-14771
[10] Okuhara T. Water-tolerant solid acid catalysts[J]. Chem Rev, 2002, 102(10): 3641-3666
[11] Yokomori Y, Idaka S. The structure of TPA-ZSM-5 with Si/Al=23[J]. Micropor Mesopor Mater, 1999, 28(3): 405-413
[12] Wright P A, Maple M J, Slawin A M Z, et al. Cation-directed syntheses of novel zeolite-like metalloaluminophosphates STA-6 and STA-7 in the presence of azamacrocycle templates[J]. J Chem Soc Dalton Trans, 2000, (8): 1243-1248
[13] Liu F J, Willhammar T, Wang L, et al. ZSM-5 zeolite single crystals with b-Axis-aligned mesoporous channels as an efficient catalyst for conversion of bulky organic molecules[J]. J Am Chem Soc, 2012, 134(10): 4557-4560
[14] Li B, Sun B, Qian X F, et al. In-situ crystallization route to nanorod-aggregated functional ZSM?5 microspheres[J]. J Am Chem Soc, 2013, 135(4): 1181-1184
[15] Gao X T, Yeh C Y, Angevine P. Mechanistic study of organic template removal from ZSM-5 precursors[J]. Micropor Mesopor Mater, 2004, 70(1/3): 27-35
[16] Kang N Y, Song B S, Lee C W, et al. The effect of Na2SO4salt on the synthesis of ZSM-5 by template free crystallization method[J]. Micropor Mesopor Mater, 2009, 118(1/3): 361-372
[17] Narayanan S, Sultana A, Krishna K. Synthesis of ZSM-5 type zeolites with and without template and evaluation of physicochemical properties and aniline alkylation activity[J]. Catal Lett, 1995, 34(1/2): 129-138
[18] Kim S D, Noh S H, Seong K H, Kim W J. Compositional and kinetic study on the rapid crystallization of ZSM-5 in the absence of organic template under stirring[J]. Micropor Mesopor Mater, 2004, 72(1/3): 185-192
[19] Pan H H, Pan Q X, Zhao Y S, et al. A green and efficient synthesis of ZSM-5 using NaY as seed with mother liquid recycling and in the absence of organic template[J]. Ind Eng Chem Res, 2010, 49(16): 7294-7302
[20] Majano G, Darwiche A, Mintova S, et al. Seed-induced crystallization of nano-sized Na-ZSM-5 crystals[J]. Ind Eng Chem Res, 2009, 48(15): 7084-7091
[21] Grose R W, Flanigen E M. Novel zeolite compositions and processes for preparing and using same: US Patent, US 4257885[P]. 1981
[22] Gonthier S, Thompson R W. Effects of seeding on zeolite crystallisation, and the growth behavior of seeds[J]. Stud Surf Sci Catal, 1994, 85: 43-73
[23] Kumar N, Nieminen V, Demirkan K T, et al. Effect of synthesis time and mode of stirring on physico-chemical and catalytic properties of ZSM-5 zeolite catalysts[J]. Appl Catal A-Gen, 2002, 235(1/2): 113-123
[24] Ren N, Yang Z J, Lv X C, et al. A seed surface crystallization approach for rapid synthesis of submicron ZSM-5 zeolite with controllable crystal size and morphology[J]. Micropor Mesopor Mater, 2010, 131(1/3): 103-114
[25] Petushkov A, Yoon S, Larsen S C. Synthesis of hierarchical nanocrystalline ZSM-5 with controlled particle size and mesoporosity[J]. Micropor Mesopor Mater, 2011, 137(1/3): 92-100
[26] Ren N, Bronic J, Jelic T A, et al. Seed-induced, structure directing agent-free crystallization of sub-micrometer zeolite ZSM-5: A population balance analysis[J]. Cryst Growth Des, 2012, 12(4): 1736-1745
[27] Tang Y, Li B J, Zhang N, et al. Growth of ZSM-5 zeolite microparticles from crystal seeds for catalytic hydration of cyclohexene[J]. Cryst Eng Comm, 2012, 14(11): 3854-3857
[28] Qiu S, Yu J, Zhu G, et al. Strategies for the synthesis of large zeolite single crystals[J]. Micropor Mesopor Mater, 1998, 21(4/6): 245-251
[29] Tosheva L, Valtchev V P. Nanozeolites: synthesis, crystallization mechanism, and applications[J]. Chem Mater, 2005, 17(10): 2494-2513
[30] Kim W J, Lee M C, Hayhurst D T. Synthesis of ZSM-5 at low temperature and atmospheric pressure in a pilot-scale batch reactor[J]. Micropor Mesopor Mater, 1998, 26(1/3): 133-141
[31] Tao Y S, Kanoh H, Abrams L, et al. Mesopore-modified zeolites: Preparation, characterization, and applications[J]. Chem Rev, 2006, 106(3): 896-910
[32] Hardenberg T A J, Mertens L, Mesman P, et al. A catalytic method for the quantitative evaluation of crystallinites of ZSM-5 zeolite preparations[J]. Zeolites, 1992, 12(6): 685-689
[33] Narayanana S, Sultana A, Leb Q T, et al. A comparative and multitechnical approach to the acid character of tem-plated and non-templated ZSM-5 zeolites[J]. Appl Catal A-Gen, 1998, 168(2): 373-384
[34] Kumar R, Bhaumik A, Ahedi R K, et al. Promoter-induced enhancement of the crystallization rate of zeolites and related molecular sieves[J]. Nature, 1996, 381(6580): 298-300
[35] Kumar R, Mukherjee P, Pandey R K, et al. Role of oxyanions as promoter for enhancing nucleation and crystallization in the synthesis of MFI-type microporous materials[J]. Micropor Mesopor Mater, 1998, 22(1/3): 23-31
[36] Bhaumik A, Belhekar A A, Kumar R. A new method for enhancing zeolite crystallization by using oxyacids/salts of group VA and VIIA elements as promoters[J]. Stud Surf Sci Catal, 1997, 105: 141-148
[37] Li P, Liu L P, Xiong G. Effect of zeolite precursor on the formation of MCM-41 molecular sieve containing zeolite Y building units[J]. Phys Chem Chem Phys, 2011, 13(23): 11248-11253
[38] Ren N, Bronic J, Subotic B, et al. Controllable and SDA-free synthesis of sub-micrometer sized zeolite ZSM-5. Part 2: Influence of sodium ions and ageing of the reaction mixture on the chemical composition, crystallinity and particulate properties of the products[J]. Micropor Mesopor Mater, 2012, 147(1): 229-241
[39] Ren N, Subotic B, Bronic J, et al. Unusual pathway of crystallization of zeolite ZSM-5 in a heterogeneous system: Phenomenology and starting considerations[J]. Chem Mater, 2012, 24(10): 1726-1737
[40] Huang X L, Zhang R R, Wang Z B. Controlling crystal transformation between zeolite ZSM-5 and mordenite without organic structure-directing agent[J]. Chin J Catal, 2012, 33(8): 1290-1298
[41] Feoktistov N N, Zhdanov S P. On the kinetics of crystallization of silicalite I [J]. Zeolites, 1989, 9(2): 136-139
[42] Lingappan N, Krishnasamy V. Tetrabutylphosphonium cation as template in the kinetics of crystallisation of ZSM-5[J]. Cryst Res Technol, 1996, 31(3): 275-280
[43] Aguado J, Serrano D P, Escola J M, et al. Low temperature synthesis and properties of ZSM-5 aggregates formed by ultra-small nanocrystals[J]. Micropor Mesopor Mater, 2004, 75(1/2): 41-49
[44] Sang S Y, Chang F X, Liu Z M, et al. Difference of ZSM-5 zeolites synthesized with various templates[J]. Catal Today, 2004, 93/95: 729-734
[45] Kim S D, Noh S H, Park J W, et al. Organic-free synthesis of ZSM-5 with narrow crystal size distribution using twostep temperature process[J]. Micropor Mesopor Mater, 2006(1/3), 92: 181-188
[46] Hsu C Y, Chiang A S T, Selvin R, et al.Rapid synthesis of MFI zeolite nanocrystals[J]. Phys Chem B, 2005, 109(40): 18804-18814
[47] Wu Y J, Ren X Q, Wang J. Effect of microwave-assisted aging on the static hydrothermal synthesis of zeolite MCM-22[J]. Micropor Mesopor Mater, 2008, 116(1/3): 386-393
Recieved date: 2013-08-12; Accepted date: 2013-09-29.
Professor Wang Xiangyu, Telephone: +86-0371-67781064; E-mail: xiangyuwang@zzu.edu.cn.
- 中國煉油與石油化工的其它文章
- Study on Preparation of Waterproofing Agent for Mineral Wool Board from Modified C9Petroleum Resin
- Catalytic Dehydration of 4-Hydroxy-3-hexanone to 4-Hexen-3-one over HZSM-5 Zeolite
- Diffusion and Reaction Model of Catalyst Pellets for Fischer-Tropsch Synthesis
- Simulation of Low-Temperature Coal Tar Hydrocracking in Supercritical Gasoline
- Catalytic Cracking and PSO-RBF Neural Network Model of FCC Cycle Oil
- Effect of Impregnation Sequence on Propane Dehydrogenation Performance of PtSnNa/ZSM-5 Catalyst

