Vapor-Liquid Equilibrium Data for Carbon Dioxide+Dimethyl Carbonate Binary System
CHEN Li ZHU Rong-Jiao FANG Yuan YUAN Ping-Fang CAO Li-Qian TIAN Yi-Ling
(Department of Chemistry,School of Science,Tianjin University,Tianjin 300072,P.R.China)
1 lntroduction
The importance of carbon dioxide as a C1feedstock is obvious:CO2is environmentally benign,nontoxic,nonflammable,and cheap.1,2In particular,the reactivity of supercritical(SC)CO2is dramatically increased.One of the most interesting synthetic targets starting from SCCO2is dimethyl carbonate(DMC).3-5Dimethyl carbonate is popularly used as a methylation or a carbonation agent,6and even as a promising oxygenative agent for fuel.7Phase equilibrium data are helpful to design the optimal synthesis technology and efficient separation process.But there are only few vapor-liquid equilibrium data for the CO2+DMC system.8-10Camyet al.8reported the equilibrium data for this system at 322.65,348.15,and 373.55 K in the pressure range of 1.33 to 12.75 MPa.Imet al.9reported the data at 310.27,320.36,330.30,and 340.27 K in the pressure range of 0.827 to 6.136 MPa.Luet al.10reported the data at 273,283,and 293 K in the pressure range of 0.66 to 4.19 MPa.So the phase equilibrium study for such an integrated reactionseparation scheme is obviously necessary.
In our previous study,10-15series of phase equilibrium data have been reported for many binary systems including the vaporliquid equilibrium data for carbon dioxide+DMC system at lower temperatures(273,283,and 293 K).In this paper,the phase equilibria at higher temperatures were studied using a variable-volume autoclave.The experimental pressure range was from 3.98 to 13.75 MPa and temperature range from 333.0 to 393.0 K.At the same time,the experimental data were correlated with the Peng-Robison(PR)cubic equation of state and van der Waals-1 mixing rule.
2 Experimental
2.1 Chemicals
CO2with a purity of 99.99%(mass fraction)was supplied by Jing-Jin Gas Limited Corporation.The gas was dried with anhydrous CaCl2powder.The DMC used was obtained from Lanes,Heyshan,Research Chemicals Ltd.with a purity of more than 99.8%(mass fraction)as verified by Chromatographic analysis.
2.2 Experimental apparatus and procedure
The variable-volume view cell similar to that in literature16,17was used in this work which is shown in Fig.1.The main part of the apparatus is a high-pressure view cell of 100 cm3.The pressure was generated with a manually operated screw driven pump and was measured with a pressure sensor.The contents inside the autoclave were stirred with a magnetic stirrer.The circulated water(T≤353.15 K)or a heating jacket(T>353.15 K)was used to adjust the temperature.The temperature was measured with a calibrated thermocouple inside the cell.The temperature and pressure can be controlled within±0.1 K and±0.01 MPa,respectively.
The experimental procedure and analysis method have been described in our previous paper.11,13,18Firstly,DMC and carbon dioxide were charged into the vacuum cell.The phase equilibrium was achieved when the pressure and temperature of the system kept invariant for 2 h after stirring.Then the liquid phase and vapor phase of the sample were taken from the lower and higher valve into the small steel vessels which had been evacuated and weighted previously.The mole fraction was determined by the desorption method.The density was obtained by the appropriate mass divided by the volume of the sample taken in each phase.The uncertainty of the mole fraction is below 0.1%.The uncertainty of the density is within 0.5%.
3 Correlating
To describe multi-component mixtures,a suitable equation of state(EOS)model which considers the interaction of the components in the mixture along with the proper mixing rulesmust be used.The cubic equation of state-PR equation is not only suitable for the non-polar compounds,but also applicable for slightly polar compounds.In this work,The experimental data were correlated using the PR EOS with van der Waals I mixing rule.19,20

Table 1 Physical properties of pure substances CO2+DMC
As pointed out by Wichterle,21the choice of the objective functionFfor the evaluation of the binary parameter is determined in the performance of a model.Generally this criterion corresponds to the relative difference between calculated and experimental equilibrium pressure or gas composition.

where the subscripts exp and cal represent the experimental and the calculated values,respectively.prepresents the pressure.yrepresents the mole fraction of the vapor phase.
The use of this model requires the data of pure component parametersTc,pc,andω.Tc,pc,andωrepresent critical temperature,critical pressure,and acentric factor,respectively.For each component,these parameters were taken from the literature22and they are listed in Table 1.
4 Results and discussion
4.1 VLE data for the binary system(SCCO2+DMC)
Isothermal vapor-liquid phase equilibrium(VLE)data were measured at 333.0,353.0,373.0,and 393.0 K over a pressure ranging from 3.98 to 13.75 MPa.The experimental results are compiled in Table 2,wherex1andy1are the mole fractions of CO2in the liquid and vapor phase,respectively.The molar volumes of liquid and vapor mixtures at different temperatures(T)and pressures(p)were obtained from densities and mole fractions.The equilibrium ratios(Kfactors)are given by:


Fig.1 Schematic diagram of the high-pressure apparatus
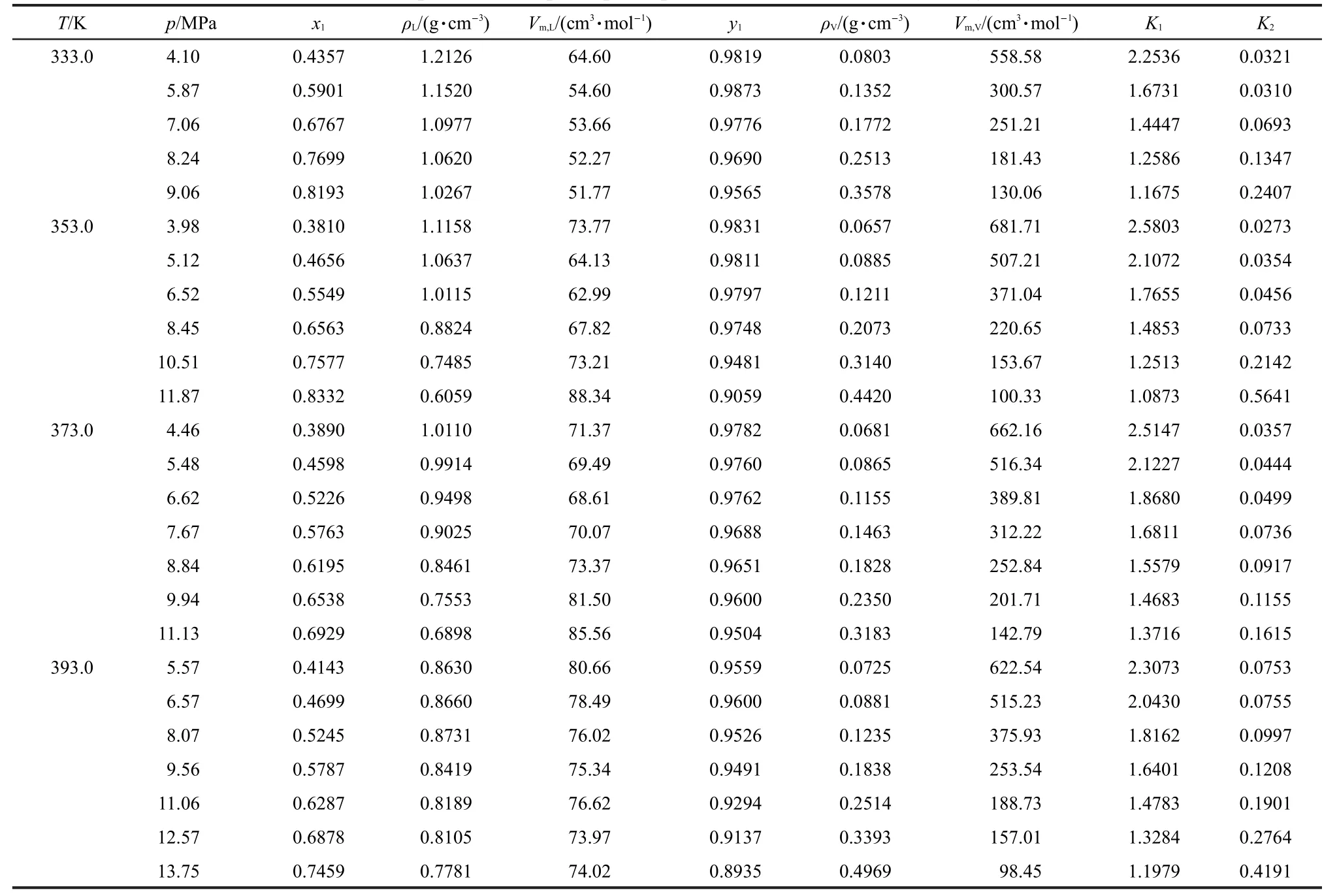
Table 2 Experimental vapor-liquid equilibrium data of SCCO2+DMC system
wherex2andy2are the mole fractions of DMC in the liquid and vapor phase,respectively.They were also calculated and listed in Table 2.
From Table 2,it was shown that the solubility of SCCO2in DMC was influenced obviously by pressure,while the solubility of DMC in SCCO2changed little from low pressure to high pressure which can be observed in SCCO2-methanol binary system.23So it should pay more attention to liquid phase when using SCCO2and methanol to produce DMC,and the reaction should be operated at higher pressure to guarantee the higher solubility of SCCO2in DMC.

Fig.2 Temperature dependence of the optimum binary parameters kij/kjiof PR equation for CO2+DMC system
4.2 Correlation of the experimental data

Fig.4 p-ρ plots of CO2(1)+DMC(2)system at four temperatures
The experimental data were correlated with the PR cubic equation of state and van der Waals-1 mixing rule.The binary interaction parameterskij(kji)were obtained when correlating.The values were-0.011,-0.035,-0.063,and-0.074 at temperatures from 333.0 K to 393.0 K.Because of the wide range temperatures investigated,binary interaction parameters were found to be dependent on temperature.The dependence of the binary interaction parameter on temperature is shown in Fig.2.From Fig.2,it can be obtained that the relationship betweenkij/kjiandTis linear andkij/kjidecreases obviously with the increase of the temperature.
Fig.3 presented the comparisons of the experimental data and the literature data inp-x1(y1)curves.Fig.4 to Fig.6 presented thep-ρ,Vm-x1(y1),andK-pcurves,respectively.In the figures,the points represent the experimental data except the stars,the stars represent the estimated critical points,and the lines represent the correlated results.From the figures,it was shown that the trend of our experimental results was consistent with the literature data.It can be also obtained that both the solubilities of CO2in DMC rise when the pressure increases,which results in the decrease of densities in the liquid phase and increase of densities in the vapor phase.The molar volume of the vapor phase decreases sharply with increasing pressure,while there are no remarkable changes for the liquid phase.Kvalues of CO2are much larger than those of DMC.And all ofKvalues of CO2are greater than 1,while all ofKvalues of DMC are less than 1.The estimated critical points are listed in Table 3.From Table 3,it can be obtained that increasing the critical temperature,critical pressure and critical density increase while the critical composition decreases for CO2+DMC system in our research ranges.

Fig.5 Vm-x1(y1)plots of CO2(1)+DMC(2)system at four temperatures

Fig.6 K-p polts of CO2(1)+DMC(2)system at four temperatures

Table 3 Estimated critical pressure pc,critical density ρc,and critical composition xcof CO2(1)+DMC(2)system at critical temperatures Tc

Fig.7 Percentage deviations in the pressure calculated with the PR equation for CO2+DMC system at different temperatures

Fig.8 Absolute deviations in vapor phase composition of CO2for CO2+DMC system at different temperatures
Fig.7 and Fig.8 show the percentage deviations in pressure and absolute deviations in vapor phase composition of CO2between calculated and experimental values as a function of liquid phase concentration.From the figures,it shows that the calculated results are in good with the experimental data.The largest relative error in pressure was 3.14%.The largest absolute error in vapor phase composition of CO2was 0.018.From another point of view,this work has shown the ability of PR model to predict phase equilibrium data of CO2+DMC system is good under high pressure.
5 Conclusions
(1)The phase equilibrium data of CO2+DMC system were measured at four temperatures from 333.0 to 393.0 K and pressures ranging from 3.98 to 13.75 MPa.
(2)The experimental data were calculated with PR equation of state.The agreement between experimental and correlated phase equilibrium data was satisfactory.
(3)The largest percentage deviation in pressure was 3.14%.The largest absolute deviation in vapor phase composition of CO2was 0.018.
(4)The binary interaction parameters were obtained and it corrected the experimental data.
(1) Song,J.L.;Zhang,B.B.;Zhang,P.;Ma,J.;Liu,J.L.;Fan,H.L.;Jiang,T.;Han,B.X.Catalysis Today2012,183,130.doi:10.1016/j.cattod.2011.08.042
(2)Watile,R.A.;Deshmukh,K.M.;Dhake,K.P.;Bhanage,B.M.Catalysis Science&Technology2012,2,1051.doi:10.1039/c2cy00458e
(3)Cui,H.Y.;Wang,T.;Wang,F.J.;Gu,C.R.;Wang,P.L.;Dai,Y.Y.Industrial&Engineering Chemistry Resesarch2003,42,3865.doi:10.1021/ie021014b
(4)Zhong,S.H.;Li,H.S.;Wang,J.W.;Xiao,X.F.Acta Phys.-Chim.Sin.2000,16,226.[鐘順和,黎漢生,王建偉,肖秀芬.物理化學(xué)學(xué)報(bào),2000,16,226.]doi:10.3866/PKU.WHXB20000307
(5) Ning,Y.N.;Tang,H.;Mao,G.D.Journal of Chemical Industry&Engineering2011,32,35.[寧英男,唐 浩,毛國梁.化學(xué)工業(yè)與工程技術(shù),2011,32,35.]
(6) Ono,Y.Catalysis Today1997,35,15.doi:10.1016/S0920-5861(96)00130-7
(7) Pacheco,M.A.;Marshall,C.L.Energy Fuels1997,11,2.doi:10.1021/ef9600974
(8) Camy,S.;Pic,J.S.;Badens,E.;Condoret,J.S.Journal of Supercritical Fluids2003,25,19.doi:10.1016/S0896-8446(02)00087-6
(9) Im,J.;Kim,M.;Lee,J.;Kim,H.J.Chem.Eng.Data2004,49,243.doi:10.1021/je034089a
(10)Lu,C.M.;Tian,Y.L.;Xu,W.;Li,D.;Zhu,R.J.J.Chem.Thermodyn.2008,40,321.doi:10.1016/j.jct.2007.05.017
(11) Li,H.L.;Zhu,R.J.;Xu,W.;Li,Y.F.;Su,Y.J.;Tian,Y.L.J.Chem.Eng.Data2011,56,1148.doi:10.1021/je101086r
(12) Su,Y.J.;Li,Y.F.;Zhou,J.G.;Zhu,R.J.;Tian,Y.L.Fluid Phase Equilibria2011,307,72.doi:10.1016/j.fluid.2011.05.005
(13) Zhu,R.J.;Zhou,J.G.;Liu,S.C.;Ji,J.;Tian,Y.L.Fluid Phase Equilibria2010,291,1.doi:10.1016/j.fluid.2009.12.011
(14) Zhu,R.J.;Liu,S.C.;Ji,J.;Tian,Y.L.Chem.J.Chin.Univ.2009,30,2263.[朱榮嬌,劉淑參,季 甲,田宜靈.高等學(xué)?;瘜W(xué)學(xué)報(bào),2009,30,2263.]
(15)Tian,Y.L.;Han,M.;Chen,L.;Feng,J.J.;Qin,Y.Acta Phys.-Chim.Sin.2001,17,155.[田宜靈,韓 銘,陳 麗,馮季軍,秦 穎.物理化學(xué)學(xué)報(bào),2001,17,155.]doi:10.3866/PKU.WHXB20010213
(16)Han,F.;Xue,Y.;Tian,Y.L.J.Chem.Eng.Data2005,50,36.doi:10.1021/je049887v
(17) Lentz,H.;Weber,M.Fluid Phase Equilibria1994,93,363.doi:10.1016/0378-3812(94)87019-5
(18)Tian,Y.L.;Zhu,H.G.;Xue,Y.;Liu,Z.Z.;Yin,L.J.Chem.Eng.Data2004,49,1554.doi:10.1021/je034224j
(19)Valderrama,J.Ind.Eng.Chem.Res.2003,42,1603.doi:10.1021/ie020447b
(20) Soave,G.Chem.Eng.Sci.1972,27,1197.doi:10.1016/0009-2509(72)80096-4
(21)Wichterle,I.Collect.Czech.Chem.Commun.1979,44,3515.doi:10.1135/cccc19793515
(22) Poling,B.;Prausnitz,J.;O'Connell,J.The Properties of Gases and Liquids,5th ed.;McGraw-Hill:New York,2001.
(23)Joung,S.N.;Yoo,C.W.;Shin,H.Y.;Kim,S.Y.;Yoo,K.P.;Lee,C.S.;Huh,W.S.Fluid Phase Equilibria2001,185,219.doi:10.1016/S0378-3812(01)00472-1

