Difference between resistant and susceptible maize to systematic colonization as revealed by DsRed-labeled Fusarium verticillioides
Lei Wu,Xioming Wng,Rongqi Xu,Hongjie Li,*
aThe National Key Facility for Crop Gene Resources and Genetic Improvement(NFCRI)/Institute of Crop Science,Chinese Academy of Agricultural Sciences,Beijing 100081,China
bCollege of Life Science and Technology,Hebei Normal University of Science&Technology,Changli,Hebei 066600,China
cBiotechnology Research Institute,Chinese Academy of Agricultural Sciences,Beijing 100081,China
Difference between resistant and susceptible maize to systematic colonization as revealed by DsRed-labeled Fusarium verticillioides
Lei Wua,b,Xiaoming Wanga,Rongqi Xuc,Hongjie Lia,*
aThe National Key Facility for Crop Gene Resources and Genetic Improvement(NFCRI)/Institute of Crop Science,Chinese Academy of Agricultural Sciences,Beijing 100081,China
bCollege of Life Science and Technology,Hebei Normal University of Science&Technology,Changli,Hebei 066600,China
cBiotechnology Research Institute,Chinese Academy of Agricultural Sciences,Beijing 100081,China
A R T I C L E I N F O
Article history:
Received 25 February 2013
Received in revised form 15 May 2013
Accepted 28 May 2013
Available online 10 July 2013
DsRed
Zea mays
Infection
Fumonisin
Colony forming unit
CFU
Fusarium verticillioides was labeled with DsRed via Agrobacterium tumefaciens-mediated transformation to examine differences in colonization and reactions of resistant and susceptible inbred lines of maize(Zea mays L.).The extent of systemic colonization of F.verticillioides in roots from maize lines either resistant or susceptible to the fungus was studied by visualizing the red fluorescence produced by the fungus expressing DsRed.The difference in quantities of colony forming units(CFU)in roots and basal stems,production of fumonisin B1,and pH of root were determined.Although F.verticillioides colonized both resistant and susceptible lines,differences were observed in the pattern and extent of fungal colonization in the two types of maize lines.The fungus colonized the susceptible lines producing mosaic patterns by filling the individual root cells with hyphae.Such a pattern of colonization was rarely observed in resistant lines,which were less colonized by the fungus than the susceptible lines in terms of CFUs.The production of mycotoxin fumonisin B1 in roots from different lines was closely correlated with the amount of F.verticillioides colonization,rather than the pH or amylopectin concentrations in the root.The findings from this study contribute to a better understanding of the defense mechanism in resistant maize lines to F.verticillioides.
?2013,Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.All rights reserved.
1.Introduction
Fusarium verticillioides(Sacc.)Nirenberg(teleomorph Gibberella moniliformis Wineland,syn.Fusarium moniliforme J.Sheld.)is widely distributed in most maize(Zea mays L.)growing regions throughout the world.This fungus not only damages all parts of the plant with obvious symptoms during the entire growing period[1],but also behaves as an endophyte with invisiblesymptoms[2].In addition to maize,this filamentous fungus invades numerous plant species of economic importance, including food,vegetable and horticultural crops,as well as trees.
A pathogen is regarded as a“root pathogen”or“foliar pathogen”primarily based on its ability to incite symptoms on roots or leaves rather than where infection occurs,and its ability to colonize these tissues[3].However,some pathogens, such as Magnaporthe grisea(T.T.Hebert)M.E.Barr,Cercospora beticola Sacc.,and Colletotrichum graminicola(Ces.)G.W.Wils, are able to infect through both above-and below-ground tissues of plants[3–5].F.verticillioides shares similar features as it causes symptoms on both the above-and below-ground parts of plants.Although it can survive in crop residues,such as senescent roots and leaves in the soil,to initiate subsequent infection,infected seeds also serve as a source of inoculum[6]. The maize lateral roots are assumed to be the major areas that are initially infected by F.verticillioides[7].Because the pathogen is not able to produce penetration structures that break the epidermis directly,it tends to attack the primary maize tissues, e.g.,silks and lateral roots[8,9].Most studies on the movement and development of F.verticillioides in maize were conducted with susceptible maize lines;consequently,difference in systemic infection of maize roots with different reactions to F.verticillioides is not well understood.
F.verticillioides produces a number of mycotoxins and other secondary metabolites.Fumonisin B1(FB1)is the major mycotoxin[10].Boddu et al.[11]demonstrated that the amount of deoxynivalenol(DON)increased when Fusarium graminearum Schwabe attacked the roots of barley(Hordeum vulgare L.). Although trichothecenes are not virulence factors during infection of the seed coat,they facilitate the penetration of F.graminearum into the thick cell walls of wheat rachis nodes [12].It is important to understand the importance of mycotoxin accumulation(in particular FB1)produced by F.verticillioides during the host–fungus interaction.
The biosynthesis of FB1 is not only regulated by genetic factors,but also influenced by environmental factors,such as pH,temperature,and composition of maize tissues,as well as the soil in which the fungus resides[13–16].The accumulation of FB1 induces the programmed cell death(PCD)in leaves of Arabidopsis thaliana and maize[17,18].The structure of FB1 is similar to that of ceramide synthase,which increased the free sphingoid bases in plants[15,19].
Fluorescent reporter genes,e.g.,gfp,are useful because they can be used to visualize complex interactions between pathogenic fungi and their hosts without destruction of the target tissues.GFP-labeled pathogens have been used to study the systematic colonization and infection of Fusarium spp.in maize [20,21].Red fluorescent protein(DsRed),discovered in radiating mushroom coral(Discosoma striata),has an emission spectrum in the far-red zone[22]and permits dual or multi-color labeling of many fungal species.The DsRed protein has been used effectively to label a number of filamentous fungi,such as Aspergillus,Trichoderma,and Oculimacular spp.[23–25].
In a previous study,we generated F.verticillioides strains expressing red fluorescence by introducing the gene DsRed via Agrobacterium tumefaciens-mediated transformation(ATMT) [26].Using a DsRed-labeled fungal strain,this study was initiated to investigate the differences in colonization and reaction of resistant and susceptible maize lines challenged with F.verticillioides.
2.Materials and methods
2.1.Fungal and plant materials
Wild type strain Fv-1 of F.verticillioides was isolated from Yayuan County,Jilin Province,China.Its identity was confirmed by morphological and interval transcribed spacer(ITS)sequence analyses.Susceptible maize inbred lines B73,P138 and Lu 9804, and the resistant lines Qi 319,Dan 340 and Zhongzi 01,were used in the study.
2.2.Generation of F.verticillioides strains expressing DsRed protein
The plasmid pCAMDsRed[27],which contains the gene DsRed driven by the promoter PgpdA,as well the selectable gene hpg for resistance to the antibiotic hygromycin,was used in ATMT of F.verticillioides as described previously[26,28].Analyses of mitotic stability of DsRed protein expression,growth rates of colonies,and metabolism ofextracellular enzymes(i.e.,protease, cellulase,amylase,and pectase)in the transformants were performed to characterize the DsRed-labeled strain of F.verticillioides [26].
2.3.Inoculation of F.verticillioides on maize roots
Seeds of the maize inbred lines were washed with running water,surface sterilized in 75%alcohol for 5 min and in 0.4% sodium hypochlorite for 15–20 min,and then rinsed with distilled water.The surface-sterilized seeds were sown in pots (10 L)filled with vermiculite in a greenhouse set at 25–30°C for 16 h of light and at 16°C for 8 h of darkness.When the second seedling leaves were unfolded,the top 12 cm of vermiculite was removed from pots,mixed with the suspensions of the DsRed-labeled fungus(108conidia mL?1)at a rate of 1:5(V/W), and returned to the pots.In the untreated checks,soil similarly treated with distilled water was used as mock inoculation.
2.4.Microscopic observation of root tissues infected with DsRed-labeled F.verticillioides
Root cross sections were prepared using a Microtome(MTH-I, Tokyo,Japan)without fixation to ensure living root cells and real-time observation.Systemic colonization by F.verticillioides in root tissues was determined by observing the red fluorescence emitted by the DsRed-labeled fungus with an epifluorescent microscope(BX60,Olympus,Tokyo,Japan)under emission wavelengths of 515/560 nm.Light microscopy was performed with the same microscope without a filter.
To determine the infectionand colonization by F.verticillioides in maize root hairs,seeds of B73 were germinated and grown on buffered nodulation medium(BNM)agar medium as described previously[34,35].For inoculating the fungus expressing DsRed,the maize roots 1 cmin length were immersed in a suspension of spores(105conidia mL?1)for 5 min before transfer to 1 mL of BNM medium on a slide.The growth andcolonization of F.verticillioides were subjected to epifluorescent microscopy.
2.5.Analysis of histochemical staining
Following infection with F.verticillioides,the dyes of neutral red(0.01%,W/V)and Evans blue(0.2%,W/V)(Sigma,St.Louis, MO,USA)were used to stain the cross and longitudinal sections of the roots for 5 min each,rinsed with water,and then observed under a microscope.Dead cells were stained blue with Evans blue,whereas living cells were stained red with neutral red.
Certain characteristic indicators,e.g.,accumulation of peroxide,can be detected when PCD occurs.To investigate whether infection of F.verticillioides induced PCD in maize leaves,peroxide staining using 3,3-diaminobenzidine(DAB) as the substrate was performed to detect the accumulation of H2O2following infection of F.verticillioides as described previously[33].At the two-leaf-stage,the leaves of maize plants inoculated with the F.verticillioides strain expressing DsRed were excised and incubated in a 1 mg mL?1solution of DAB(pH 3.8)for 2 h under light at 25°C and then boiled in ethanol(96%)for 10 min.After cooling,the leaves were extracted with fresh ethanol at room temperature.The degree of dark brown polymerization indicated the amount of H2O2accumulated in the treated leaves.
2.6.Analysis of root colony forming units in roots and basal stems
Selective Fusarium agar(SFA)[29,30]medium was used to analyze the colonization of F.verticillioides on/in maize roots. DsRed-labeled fungus-infected and mock-inoculated roots and basal stems of maize were sampled at different times after the inoculation from two replicated greenhouse trials to determine the numbers of colony forming units(CFU)as previously described[31].A randomized complete block design with four replicates consisting of two plants each was used to arrange the inoculated maize plants.The roots were removed from the vermiculite,washed thoroughly with tap water,and surface sterilized for 3 min in 0.5%(V/V)NaOCl solution.After rinsing with sterile deionized water several times,roots were wiped with sterile filter paper.Roots and basal stems from the two plants in each replicate were weighted,ground,and mixed into 10 ml of sterile deionized water with a Fast-Prep-24 Instrument(MP Biomedicals,Solon, OH,USA)at high speed for 1 min.Homogenized suspensions of root and basal stem samples were filtered through four layers of cheesecloth to remove plant debris and diluted 20-fold with sterile deionized water.The diluted samples were separately spread with a sterile glass rod onto the SFAplates.Each inoculated sample consisted of five replicated plates with 50 μL of diluted tissue suspension.All the plates were incubated at 25°C in the dark.The F.verticillioides colonies on each plate were counted to determine the number of CFUs per gram of root and/or stem.
2.7.Determination of FB1 accumulation in maize roots
DsRed-labeled fungus-and mock-inoculated roots were sampled at 24,48,72,96 and 144 h after inoculation(HAI), ground using a mortar and a pestle,and then mixed in 10 ml of acetonitrile/water(1:1,V/V)containing 5%formic acid.The mixture was shaken vigorously on a rocker shaker(220 r min?1) for 3 h[32]to disrupt the fungal colonies prior to incubation. The extracts were diluted 1000-fold with acetonitrile/water (3:7,V/V)containing 1%formic acid and diluted samples were subjected to competitive ELISA using a Beacon FB1 plate kit (Portland,OR,USA).The absorbance of samples was read at 450 nm with a fluoremeter RT-6000(Rayto Life and Analytical Sciences Co.,Ltd.,Shenzhen,China).
2.8.Determination of pH and amylopectin in maize roots
F.verticillioides is sensitive to the pH of maize roots which can affect its growth and metabolism.To determine the pH of maize roots inoculated with the DsRed-labeled fungus,roots (0.5 g)were sampled from plants at 144 h after inoculation, ground,and suspended in 5 ml of deionized water.A standard pH electrode(VWR Scientific,West Chester,PA,USA)was used to determine the pH of each sample.Analysis of total starch in root was performed by the amyloglucosidase/ α-amylase method(AOAC method 996.11)with the total starch assay kit from Megazyme(Wicklow,Ireland).Three samples were analyzed for each maize line as replicates.
2.9.Statistical analysis
The experiments for parameter determination were carried out twice.Analysis of variance(ANOVA)was performed using PROC GLM procedure in SAS statistical package(version 9.1, SAS Institute,Cary,NC,USA).Treatment means were compared by Duncan's multiple-range test(P<0.05).
3.Results
3.1.Transformation with DsRed via ATMT and characterization of the transformants
Wild type of F.verticillioides was co-cultured with A.tumefaciens cells containing the target gene DsRed to generate DsRedlabeled fungal strains.After three rounds of selection on hygromycin B-containing PDA,hygromycin B-resistant transformants were subjected to epifluorescent microscopic observation.Using the gene-specific primers,amplification of DNA confirmed the integration of DsRed gene in the genome of the wild type.Based on analyses of mitotic stability of DsRed protein expression,growth rates of colonies,and metabolism of extracelluar enzymes,i.e.,protease,cellulase,amylase and pectase,strain FVR-12 was used in the study because it resembled the wild type for most of the characteristics examined(data not shown).
3.2.Infection of F.verticillioides on maize lines
The susceptible maize line B73 and resistant line Qi 319 were infected with F.verticillioides strain expressing DsRed through root inoculation.The conidia germinated on the root surfaces of both lines at 24 HAI.On line B73 the fungus formed runner hyphae(Fig.1-a).The quantity of hyphae colonized on B73was greater than that on Qi 319(Fig.1-b and c).The lateral roots seemed to be the most vulnerable to invasion by the fungus regardless of whether the genotype was resistant or a susceptible to F.verticillioides.

Fig.1–Systemic infection and colonization of DsRed-tagged Fusarium verticillioides strain FvR-12 on the roots of susceptible maize line B73 and resistant line Qi 319.a:Fungal colonization of on the susceptible line B73 tended to form runner hyphae (arrow);b:colonization of F.verticillioides in the lateral root of B73(arrow);c:colonization by F.verticillioides of the lateral root of Qi 319(arrow);d:net-like structure formed on the root surface of B73;e:colonization by F.verticillioides of the root surface of Qi 319;f and g:comparison of root surfaces between B73(f)and Qi 319(g)in bright field;h and i:mosaic patterns formed on the root of B73;j and k:mosaic patterns observed in bright field;l and m:colonization of F.verticillioides in the root of Qi 319;n and o:colonization of F.verticillioides inside of B73 shown as cross(n)and longitudinal root sections(o);p and q:colonization of F.verticillioides inside of Qi 319 in bright field(p)and fluorescence(q)views.
At seven days(144 h)after inoculation(DAI),the hyphae had formed a network on the root surface of B73(Fig.1-d),but only a few hyphae were detected on the roots of Qi 319 (Fig.1-e).The architecture of the root surface of B73 differedfrom that of Qi 319 for the number of root hairs(Fig.1-f and g). Some cells of roots were filled with hyphae showing a mosaic pattern of colonization on B73(Fig.1-h and i).These patterns were observed on the root hairs(Fig.1-j and k).Such a pattern of hyphal colonization was rarely observed in the roots of Qi 319 in which the spread of hyphae in Qi 319 appeared to be limited(Fig.1-l).Fewer root hairs were observed on root surface of this line(Fig.1-m).
Atthe early stages of infection,smalland round lesions were present on the roots of susceptible line B73,and conidiospores were able to germinate on the surface of roots.In cross sections of the root,the hyphae extended directly through or across the root cells(Fig.1-n);the hyphae also grew longitudinally along the roots(Fig.1-o).The root cells of resistant line Qi 319 tended to become necrotic(Fig.1-p).The senescent areas displayed auto-fluorescence,but fungal hyphae were rarely observed in such areas(Fig.1-q).
3.3.Colonization of F.verticillioides on maize root hairs
DsRed-labeled F.verticillioides tended to colonize the base of root hairs of B73(Fig.2-a and b).The mosaic patterns of colonization formed by the hyphae were observed near or at the base of hair roots(Fig.2-c and d).When stained with neutral red and Evans blue,the areas showing mosaic patterns of colonization did not exhibit blue color(Fig.2-e and f),indicating that these cells may be viable.The hair roots colonized by hyphae were stained red with neutral red(Fig.2-g and h).The hyphae were able to grow inside the hair roots(Fig.2-i).When the hyphae reached the ends of the hair roots,they formed“ball-like structure”(Fig.2-j),or broke through the ends and continued to grow from the“ball-like structure”(Fig.2-k).The hyphae in maize hair roots always grew longitudinally(Fig.2-l and m),and sometimes were folded on themselves(Fig.2-n).
3.4.Cell death caused by F.verticillioidesThe roots of susceptible line B73 inoculated with FVR-12 were strongly stained with Evans blue(Fig.2-o).Only a few cells displayed blue color due to staining by Evans blue in the roots of resistant line Qi 319(Fig.2-p).However,roots of both types showed similar patterns of fluorescence(Fig.2-q and r).
Maize leaves were treated with DAB at different times after inoculation with strain FVR-12.Susceptible lines B73,P138 and Lu 9801 displayed a brown color as early as 24 HAI,whereas resistant lines Qi 319,Dan 340 and Zhongzi 01 showed no DAB staining until 144 HAI(data not shown).The mock-inoculated leaves showed no staining at any time.
3.5.Quantification of F.verticillioides colonization in maize roots and basal stems
At the early stages of infection and even after 144 HAI,the symptoms in both susceptible(B73,Lu 9801 and P138)and resistant(Qi 319,Dan 340 and Zhongzi 01)lines were not obvious,but the color of inoculated roots was darker than those of mock-inoculated roots,especially the root hairs.In 14 DAI samples,all inoculated roots had typical symptoms.In the root samples from resistant lines,the CFU values ranged from 260.8±22.8 to 527.8,and the CFUs of the basal stems ranged from 125.0±9.1 to 573.3±28.3.The CFU values for root samples of susceptible lines ranged from 1146.4±13.7 to 3826.9±455.6 and from 1158.3±24.7 to 6134.2±646.4,respectively,and were significantly greater than those for the resistant lines(Fig.3).
3.6.Accumulation of FB1 in maize roots
The toxin FB1 was not detected in any of the mock-inoculated roots at any time.Accumulation of FB1 in DsRed-labeled fungus-inoculated root samples was not detected until 48 HAI. No statistically significant difference was observed in the titers of FB1 until 96 HAI(data not shown).At 144 HAI,accumulations of FB1 in the susceptible lines ranged from 11.5±0.3 to 38.4± 1.1 ng mL?1(Fig.4).The titer of FB1 in line P138 was significantly greater than the other lines.In the resistant lines,the concentrations of FB1 remained atlow levels with a range of1.74±0.08 to 5.0±0.46 ng mL?1,significantly lower than those of the susceptible lines(P<0.05).To determine the relationship between the production of FB1 and amount of F.verticillioides colonization, CFUs of maize roots were measured at 144 HAI.All CFU values in the susceptible lines were higher than those of the resistant lines (Fig.5).Correlation analysis indicated thatthe accumulation of FB1 was associated with CFU value(R2=0.8095,P<0.0001).
3.7.Influence of maize root secondary metabolites to fungal toxin production
To determine if biosynthesis of FB1 might be influenced by pH or amylopectin content,root samples were collected from susceptible and resistant genotypes at 144 HAI,ground and suspended in distilled water.The pH of roots ranged from 6.0 to 6.3 with no significant difference between susceptible and resistant lines,despite variation among individual lines(Fig.6). The amounts of amylopectin in roots were also measured,but the amounts in allthe samples were below the limitofdetection (data not shown).
4.Discussion
Infection and systemic colonization of maize by F.verticillioides can occur in different parts of the plants,such as roots, crowns,stalks,and ears,and have been studied using different methods[7,9,36,37].However,F.verticillioides,as well as the other kernel rot pathogens,do not form penetration structures,such as appressoria[8,38].There might be a mechanism for the passive movement of conidia along the surface of tissues,allowing the pathogen to get access to an infection court[9].In the present study,infection and colonization by DsRed-labeled F.verticillioides in maize were examined on maize lines with different reactions to the fungus.The roots of resistant lines showed limited surface growth of F.verticillioides compared to susceptible lines.In the initial stages of infection,F.verticillioides developed similar lesions on the roots regardless of the response of the host. Most likely,the quantity of fungal inoculum in the soil would be far less concentrated than the artificial suspensions used to inoculate the roots in the present study.When the maize roots were treated with a moderate level of F.verticillioidesinoculum,the resistant lines supported less fungal growth than the susceptible ones.Typical lesions and runner hyphae in mosaic patterns of colonization were readily observed on the roots of susceptible lines,whereas the cells in the roots of resistant lines tended to become necrotic,apparently limiting hyphal extension within the root tissues.
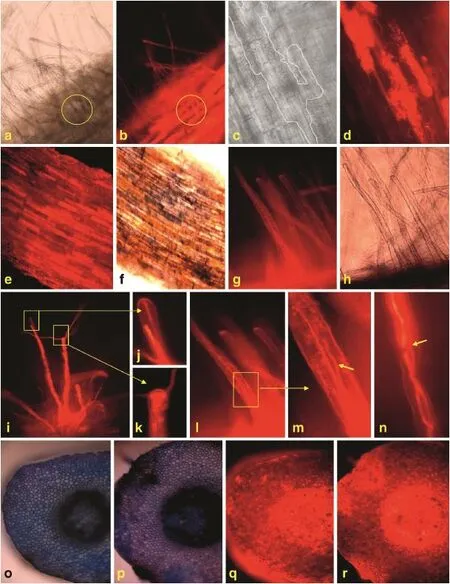
Fig.2–Systemic infection and colonization of DsRed-tagged Fusarium verticillioides strain FVR-12 on the root hairs of maize.a and b:Root hairs observed in bright field(a)and fluorescence(b).Circles indicate colonization of F.verticillioides in the root hairs;c and d:mosaic patterns observed in bright(c)and fluorescence(d)field.The region of heavily colonized root cells showing bright fluorescence in the fluorescent image(d)are outlined in the corresponding regions in the black and white image(c);e and f:surface of maize roots observed in fluorescence(e)and bright(f)fields after staining with Evans blue;g and h: root hairs colonized with FVR-12 observed under fluorescence(g)and in bright(h)fields after staining with neutral red;i,j,k,l, m,and n:colonization of FVR-12 in maize root hairs;arrow in Fig.2n indicates folding of hyphae;o and p:cross sections of roots from B73(o)and Qi 319(p)stained with Evans blue in bright field;q and r:the cross section of a root from B73(q)and Qi 319(r)stained with Evans blue and observed in fluorescence.
The cellular junctions that form between the lateral roots and root hairs are considered to be the entry points forpenetration into the root tissues[39].Verticillium longisporum (C.Stark)Karapapa,Bainbr.&Heale 1997,Fusarium oxysporum Schlecht.emend.Snyder&Hansen,and Klebsiella oxytosa Klebsiella oxytoca(Schroeter 1886)Trevisan 1887 initially enter roots by following the root hairs[40–42].There might exist a common mode of infection used by vascular pathogens to enter root hair zones where they first attach and then penetrate directly into the epidermal cells,due to a stronger chemical attraction of the fungus to the root hairs than the root surface[7,41].A similar observation that the root hairs are entry points of F.verticillioides into the inner and upper parts of maize was made in the present study.The roots of resistant maize lines(i.e.,Qi 319,Dan 340 and Zhongzi 01)had fewer root hairs than susceptible lines(i.e.,B73,Lu 9801 and P138),and were less heavily colonized by the pathogen. Analysis of CFU at the same time-points showed that the quantities of F.verticillioides in the roots of susceptible maize lines were higher than in those of resistant lines.
Several factors influenced the accumulation of toxin when F.graminearum attacked root system of barley[11].Factors such as ambient pH,amylopectin concentration,nitrogen limitation, and carbon nutrient specificity also affected FB1 production in F.verticillioides infections of maize[14,43].Although acidic conditions are reported to be favorable for the production of FB1,no significant difference in pHofthe roots ofsusceptible and resistant maize lines was observed in the present study.The amount of amylopectin in maize roots was below the limit of detection.The titers of FB1 that accumulated in susceptible maize roots were greater than those in the resistant roots. The CFU values at 144 HAI were significantly associated with the production of FB1.This suggests that the quantity of F.verticillioides seems to be a main factor determining the production of FB1 at the early stages of the plant–fungus interaction.
FB1 toxin was shown to induce PCD in Arabidopsis thaliana leaves and in protoplasts of maize leaves[17,18].The formation of hypersensitive reaction and PCD restricts the spread of the fungus,because biotrophic fungi require living host tissues for nutrients[44].DAB staining revealed that cell death in maize leaves was induced by the root infection of F.verticillioides. However,the susceptible maize lines were sensitive as early as 24 HAI,whereas the resistant maize lines did not show any visible color staining until 144 HAI.These results suggest that the accumulation of FB1 and the amount of fungal growth may play a key role in inducing PCD in maize roots when attacked by F.verticillioides,and rapid cell death following infection seems to be a major factor in constraining the spread of F.verticillioides on the roots of resistant plants.
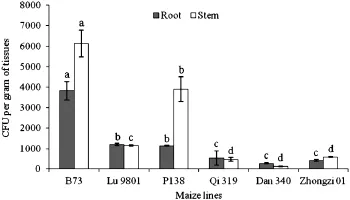
Fig.3–Comparison of CFU values produced by maize roots and basal stems at 14 DAI inoculated with DsRed-tagged Fusarium verticillioides strain FVR-12.
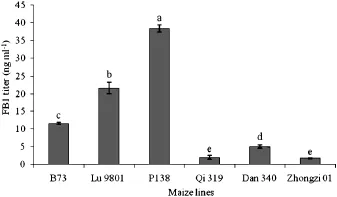
Fig.4–Production of fumonisin B1 in maize roots inoculated with DsRed-tagged Fusarium verticillioides strain FVR-12 at 144 HAI.
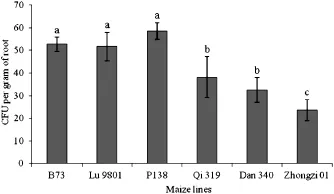
Fig.5–CFU values in maize roots inoculated with DsRed-tagged Fusarium verticillioides strain FVR-12 at 144 HAI.
5.Conclusions
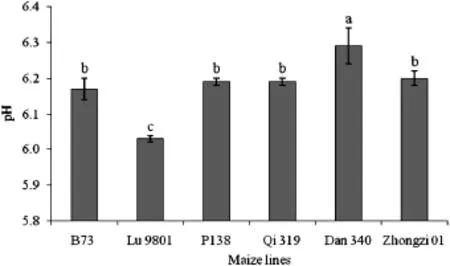
Fig.6–pH of maize roots inoculated with DsRed-tagged Fusarium verticillioides strain FVR-12.
F.verticillioides attacked maize roots by the initial infection of the root hairs,and then colonizing without killing them.Insusceptible lines,F.verticillioides tended to formmosaic patterns of infection by filling individual cells with hyphae.Resistant maize lines were less colonized by the fungus and apparently used cell necrosis to limit the spread of the pathogen.The production of FB1 at early stages of infection was associated with the amount of F.verticillioides in the colonized roots. The pH and amylopectin concentration of the roots were not associated with accumulation of FB1.The use of a DsRedlabeled F.verticillioides strain allows direct visualization of colonization by the fungus in maize roots.
Acknowledgments
The authors are grateful to Marina Franceschetti,John Innes Centre,UK,for providing the plasmid pCAMDsRed.Financial support from the National Natural Science Foundation (31170080)and China Agricultural Research Service(CARS-02) was greatly appreciated.
R E F E R E N C E S
[1]In:D.G.White(Ed.),Compendium of Corn Disease,3rd edn, APS Press,St.Paul,MN,USA,1999.
[2]C.W.Bacon,D.M.Hinton,Symptomless endophytic colonization ofmaize by Fusarium moniliforme,Can.J.Bot.74(1996)1195–1202.
[3]S.A.Sukno,V.M.García,B.D.Shaw,M.R.Thon,Root infection and systemic colonization of maize by Colletotrichum graminicola,Appl.Environ.Microbiol.74(2008)823–832.
[4]M.Dufresne,A.E.Osbourn,Definition of tissue-specific and general requirements for plant infection in a phytopathogenic fungus,Mol.Plant Microbe Interact.14(2001)300–307.
[5]J.Vereijssen,H.J.H.M.Schneider,A.J.Termorshuizen,Possible root infection of Cercospora beticola in sugar beet,Eur.J.Plant Pathol.110(2004)103–106.
[6]G.P.Munkvold,W.M.Carlton,Influence of inoculation method on systemic Fusarium moniliforme infection of maize plants grown from infected seeds,Plant Dis.81(1997) 211–216.
[7]L.Oren,S.Ezrati,D.Cohen,A.Sharon,Early events in the Fusarium verticillioides-maize interaction characterized by using a green fluorescent protein-expressing transgenic isolate,Appl.Environ.Microbiol.81(2003)556–565.
[8]R.J.Howard,Breaching the outer barriers–cuticle and cell wall penetration,in:G.Carroll,P.Tudzynski(Eds.),Plant Relationships,vol.5A,Springer-Verlag,New York,NY,1997, pp.43–60.
[9]K.E.Duncan,R.J.Howard,Biology of maize kernel infection by Fusarium verticillioides,Mol.Plant Microbe Interact.23(2010)6–16.
[10]C.W.Bacon,A.E.Glenn,I.E.Yates,Fusarium verticillioides: managing the endophytic association with maize for reduced fumonisins accumulation,Toxin Rev.27(2008)441–446.
[11]J.Boddu,S.Cho,W.M.Kruger,G.J.Muehlbauer, Transcriptome analysis of the barley–Fusarium graminearum interaction,Mol.Plant Microbe Interact.19(2006)407–417.
[12]C.Jansen,D.Wettstein,W.Sch?fer,K.H.Kogel,A.Felk,M.F. Maier,Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum,Proc.Natl.Acad.Sci.U.S.A. 102(2005)16892–16897.
[13]W.B.Shim,J.E.Flaherty,C.P.Woloshuk,Comparison of fumonisin B1 biosynthesis in maize germ and degermed kernels by Fusarium verticillioides,J.Food Prot.66(2003) 2116–2122.
[14]B.H.Bluhm,C.P.Woloshuk,Amylopectin induces fumonisin B1 production by Fusarium verticillioides during colonization of maize kernels,Mol.Plant Microbe Interact.18(2005) 1333–1339.
[15]L.D.Williams,A.E.Glenn,C.W.Bacon,M.A.Smith,R.T.Riley, Fumonisin production and bioavailability to maize seedlings grown from seeds inoculated with Fusarium verticillioides and grown in natural soils,J.Agric.Food Chem.54(2006)5694–5700.
[16]A.L.Wilke,C.R.Bronson,A.Tomas,G.P.Munkvold,Seed transmission of Fusarium verticillioides in maize plants grown under three different temperature regimes,Plant Dis.91 (2007)1109–1115.
[17]J.M.Stone,J.E.Heard,T.Asai,F.M.Ausubel,Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant(fbr)Arabidopsis mutants,Plant Cell 12 (2000)1811–1822.
[18]T.Asai,J.M.Stone,J.E.Heard,Y.Kovtun,P.Yorgey,J.Sheen, F.M.Ausubel,Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-,ethylene-,and salicylate-dependent signaling pathways,Plant Cell 12(2000) 1823–1835.
[19]R.T.Riley,E.Wang,A.H.J.Merrill,Liquid chromatography of sphinganine and sphingosine:use of the sphinganine–tosphingosine ratio as a biomarker for consumption of fumonisins,J.Assoc.Off.Anal.Chem.77 (1994)533–540.
[20]J.M.Lorang,R.P.Tuori,J.P.Martinez,T.L.Sawyer,R.S. Redman,J.A.Rollins,T.J.Wolpert,K.B.Johnson,R.J. Rodriguez,M.B.Dickman,L.M.Ciuffetti,Green fluorescent protein is lighting up fungal biology,Appl.Environ.Microbiol. 67(2001)1987–1994.
[21]E.Larrainzar,F.O'Gara,J.P.Morrissey,Application of autofluorescent proteins for in situ studies in microbial ecology,Annu.Rev.Microbiol.59(2005)257–277.
[22]M.V.Matz,A.F.Fradkov,Y.A.Labas,A.P.Savitsky, A.G.Zaraisky,M.L.Markelov,S.A.Lukyanov,Fluorescent proteins from nonbioluminescent Anthozoa species,Nat. Biotechnol.17(1999)969–973.
[23]L.Mikkelsen,S.Sarrocco,M.Lübeck,D.F.Jensen,Expression of the red fluorescent protein DsRed-express in filamentous Ascomycete fungi,FEMS Microbiol.Lett.223(2003)135–139.
[24]M.W.Toews,J.Warmbold,S.Konzack,P.Rischitor,D.Veith, K.Vienken,C.Vinuesa,H.Wei,R.Fischer,Establishment of mRFP1 as a fluorescent marker in Aspergillus nidulans and construction of expression vectors for high-throughput protein tagging using recombination in vitro(GATEWAY), Curr.Genet.45(2004)383–389.
[25]M.Eckert,K.Maguire,M.Urban,S.Foster,B.Fitt,J.Lucas, K.Hammond-Kosack,Agrobacterium tumefaciens-mediated transformation of Leptosphaeria spp.and Oculimacula spp. with the reef coral gene DsRed and the jellyfish gene gfp,FEMS Microbiol.Lett.253(2005)67–74.
[26]L.Wu,X.M.Wang,R.Q.Xu,H.J.Li,Root infection and systematic colonization of DsRed-labeled Fusarium verticillioides in maize,Acta Agron.Sin.37(2011)793–802.
[27]A.Sesma,A.E.Osbourn,The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi,Nature 431(2004)582–586.
[28]E.D.Mullins,X.Chen,P.Romaine,R.Raina,D.M.Geiser, S.Kang,Agrobacterium-mediated transformation of Fusarium oxysporum:An efficient tool for insertional mutagenesis and gene transfer,Phytopathology 91(2000)173–180.
[29]S.M.Nash,W.C.Snyder,Quantitative estimations by plate counts of propagules of the bean root rot fusarium in field soils,Phytopathology 52(1962)567–572.
[30]L.W.Burgess,C.M.Liddell,Laboratory Manual for Fusarium Research,University of Sydney,Australia,1983.162.
[31]S.Li,A.Lygin,O.Zernova,V.Lozovaya,G.L.Hartman, J.Widholm,Genotype response of soybean(Glycine max)whole plants and hairy roots to Fusarium solani f.sp.glycines infection,Soybean Sci.27(2008)275–282.
[32]A.E.Glenn,N.C.Zitomer,A.M.Zimeri,L.D.Williams, R.T.Riley,R.H.Proctor,Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings,Mol.Plant Microbe Interact.21(2008)81–97.
[33]M.L.Orozco-Cárdenas,C.Ryan,Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway,Proc.Natl.Acad.Sci. U.S.A.96(1999)6553–6557.
[34]H.Imaizumi-Anraku,N.Takeda,M.Charpentier,J.Perry, H.Miwa,Y.Umehara,H.Kouchi,Y.Murakami,L.Mulder, K.Vickers,J.Pike,J.A.Downie,T.Wang,S.Sato,E.Asamizu, S.Tabata,M.Yoshikawa,Y.Murooka,G.J.Wu,M.Kawaguchi, S.Kawasaki,M.Parniske,M.Hayashi,Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots, Nature 433(2005)527–531.
[35]Y.Murakami,H.Miwa,H.Imaizumi-Anraku,H.Kouchi, A.Downie,M.Kawaguchi,S.Kawasaki,Positional cloning identifies Lotus japonicus NSP2 a putative transcription factor of the GRAS family,required for NIN and ENOD40 gene expression in nodule initiation,DNA Res.13(2006)255–265.
[36]S.Danielsen,D.Jensen,Relationships between seed germination,fumonisin content,and Fusarium verticillioides infection in selected maize samples from different regions of Costa Rica,Plant Pathol.47(1998)609–614.
[37]D.J.Jardine,J.F.Leslie,Aggressiveness to mature maize plants of Fusarium strains differing in ability to produce fumonisin, Plant Dis.83(1999)690–693.
[38]Y.P.Zhang,Y.E.Choi,X.X.Zou,J.R.Xu,The FvMK1 mitogen-activated protein kinase gene regulates conidiation, pathogenesis,and fumonisin production in Fusarium verticillioides,Fungal Genet.Biol.48(2011)71–79.
[39]G.Grunewaldt-St?cker,N.Riediger,C.Dietrich,Suitability of GFP-transformed isolates of the fungal root endophyte Acremonium strictum W.Gams for studies on induced Fusarium-wilt resistance in flax,Plant Roots 1(2007)46–56.
[40]Q.L.An,X.J.Yang,Y.M.Ding,L.J.Feng,B.J.Kuang,J.D.Li,Using confocal scanning microscope to visualize the infection of rice roots by GFP-labeled Klebsiella oxytoca SA2,an endophytic diazotroph,Acta Bot.Sin.6(2001)23–27.
[41]A.L.Lagopodi,A.F.J.Ram,G.E.M.Lamers,P.J.Punt,Novel aspects of tomato root colonization and infection by Fusarium oxysporum f.sp.radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker,Mol.Plant Microbe Interact.15(2001) 172–179.
[42]C.Eynck,B.Koopmann,G.Grunewaldt-Stoecker, P.Karlovsky,P.von Tiedemann,Differential interactions of Verticillium longisporum and V.dahliae with Brassica napus detected with molecular and histological techniques,Eur.J. Plant Pathol.118(2007)259–274.
[43]W.B.Shim,C.P.Woloshuk,Nitrogen repression of fumonisin B1 biosynthesis in Gibberella fujikuroi,FEMS Microbiol.Lett. 177(1999)109–116.
[44]J.Keon,J.Antoniw,R.Carzaniga,S.Deller,J.L.Ward, J.M.Baker,M.H.Beale,K.Hammond-Kosack,J.J.Rudd, Transcriptional adaptation of Mycosphaerella graminicola to programmed cell death(PCD)of its susceptible wheat host, Mol.Plant Microbe Interact.20(2007)178–193.
*Corresponding author.
E-mail address:lihongjie@caas.cn(H.Li).
Peer review under the responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
- The Crop Journal的其它文章
- Expression of an(E)-β-farnesene synthase gene from Asian peppermint in tobacco affected aphid infestation
- Anatomical and chemical characteristics associated with lodging resistance in wheat
- Zea mays(L.)P1 locus for cob glume color identified as a post-domestication selection target with an effect on temperate maize genomes
- Genome-wide association of 10 horticultural traits with expressed sequence tag-derived SNP markers in a collection of lettuce lines
- Dissection of two quantitative trait loci for grain weight linked in repulsion on the long arm of chromosome 1 of rice(Oryza sativa L.)
- Variation of high-molecular-weight glutenin subunits and glutenin macropolymer particle distribution in wheat grains produced under different water regimes

