光還原催化劑Pt/TiO2富氫條件下CO優(yōu)先氧化反應(yīng)
王 芳 王彩紅 劉國霞
(濱州學(xué)院化學(xué)與化工系,山東濱州256603)
光還原催化劑Pt/TiO2富氫條件下CO優(yōu)先氧化反應(yīng)
王 芳*王彩紅 劉國霞
(濱州學(xué)院化學(xué)與化工系,山東濱州256603)
用光還原法來提高富氫條件下CO優(yōu)先氧化(PROX)催化活性和CO2選擇性,分別對有無氫氣時(shí)CO氧化反應(yīng)參數(shù)進(jìn)行了詳盡研究.X射線光電子能譜(XPS)表征結(jié)果顯示,在光還原催化劑表面產(chǎn)生了部分氧空穴,可為化學(xué)吸附H提供活性中心.針對光還原Pt/TiO2催化劑上CO優(yōu)先氧化反應(yīng)提出了一種可能的雙功能反應(yīng)機(jī)理.
CO優(yōu)先氧化;Pt催化劑;光還原;浸漬
1 Introduction
Currently,the CO oxidation in the absence and presence of H2has attracted extensive attention because of its potential application in indoor or cabin air cleanup and in the purification of hydrogen streams used in proton exchange membrane (PEM)fuel cells.1,2Considerable efforts have been devoted to design the suitable catalysts for the competitive oxidation of CO in the presence of hydrogen.Supported noble metals,such as Au,3,4Pt,5-10Pd,11-13and Rh,14were found applicable for the PROX reaction.Platinum catalysts are by far the most extensively studied catalysts owing to their superior performance in photocatalytic and thermal CO oxidation.Nevertheless,the most commonly used Pt/TiO2catalysts prepared by impregnation method are unsuitable for the reaction of CO preferential oxidation since they require high operation temperature in the range of 150-200°C.In the meantime,significant H2consumption can be observed in the temperature range to work effectively.Furthermore,the impregnated Pt/TiO2catalyst used to be pretreated by oxidation or reduction under an appropriate temperature prior to the catalytic activity tests.15-18This gives an incentive for the development of a highly active and selective catalyst for CO preferential oxidation.
By comparison,the photoreduction method exhibits many advantages,such as simple operation and environment-friendly etc.In this study,we mainly focus on the preparation of Pt/ TiO2catalysts by a photoreduction method.The optimum reaction parameters for CO oxidation in the presence and absence of H2have been investigated in detail.Based on the data of catalytic activity tests and the characterization of catalysts,a possible reaction mechanism for the PROX reaction over the photoreduced Pt/TiO2catalyst has been proposed.
2 Experimental
2.1 Catalyst preparation
Degussa P25 TiO2powder(Degussa,70%-30%anatase) was used as a support.Before deposition,raw TiO2was pretreated at 773 K for 4 h in air to stabilize its surface area and the anatase crystal form.Platinum was directly photodeposited on TiO2in an aqueous solution of chloroplatinic acid(1 mmol· L-1)and methanol(0.1 mol·L-1)under UV illumination(250 W medium-pressure mercury lamp).The samples were dried in air at 120°C for 12 h and had no thermal treatment anymore. The theoretical mass loading amount was 1.5%(w).In order to compare with the photoreduced Pt/TiO2catalysts,the impregnated catalyst was reduced in H2stream at 300°C for 1 h prior to the catalytic activity tests.We denoted the Pt/TiO2catalysts photoreduced for 12,24,and 36 h as Pt/TiO2(PR12),Pt/TiO2(PR24)and Pt/TiO2(PR36),respectively.And the impregnated one was denoted as Pt/TiO2(IM).
2.2 Characterization of catalysts
X-ray diffraction(XRD)patterns of the samples were recorded on a Rigaku D/MAX-RB X-ray diffractometer with a Cu Kαtarget operated at 50 kV and 40 mA with a scanning speed of 0.5(°)·min-1and a scanning angle(2θ)range of 10°-90°. Chemical states of the Pt species on the catalyst surface were investigated by X-ray photoelectron spectroscopy(XPS)on a VG ESCALAB 210 Electron Spectrometer(Mg Kαradiation; hν=1253.6 eV).
2.3 Activity measurement
Catalytic test was carried out at atmospheric pressure in a fixed bed continuous flow quartz reactor(inside diameter is 8mm),consisting of a flow controller unit,a reactor unit,and an analysis unit.A schematic diagram of the experimental system is shown in Fig.1.Typically,the temperature increased from 80 to 300°C at a 5°C increment,and a sample was taken for analysis after stabilizing for 30 min at each investigated temperature.Then 0.1 g catalyst diluted with equal amount of quartz was used in each run.Prior to the experiment,the impregnated catalyst was reduced in situ at 300°C(heating rate: 10°C·min-1)for 3 h with a 50%(volume fraction)H2/N2mixture(flow rate:60 mL·min-1).The feed gas consisted of 2.5% CO and 20%O2in N2balance.In the process of PROX,a gas mixture containing 50%H2,1%CO,and 2%O2in N2was fed at the flow rate of 30 mL·min-1.The gas phase effluents were analyzed on-line chromatographs equipped with thermal conductivity detector(TCD).At the end of the catalytic tests,the catalyst was cooled under an N2stream and stored for characterizations.The catalytic activities were defined in terms of conversion of CO(η),conversion of O2,and selectivity to CO2(S),which were denoted as ηCO,ηO2and SCO2,respectively,and were calculated according to the following equations:
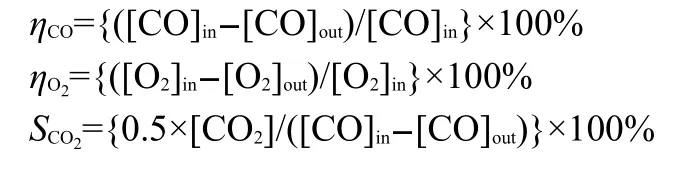
3 Results and discussion
3.1 XRD analysis
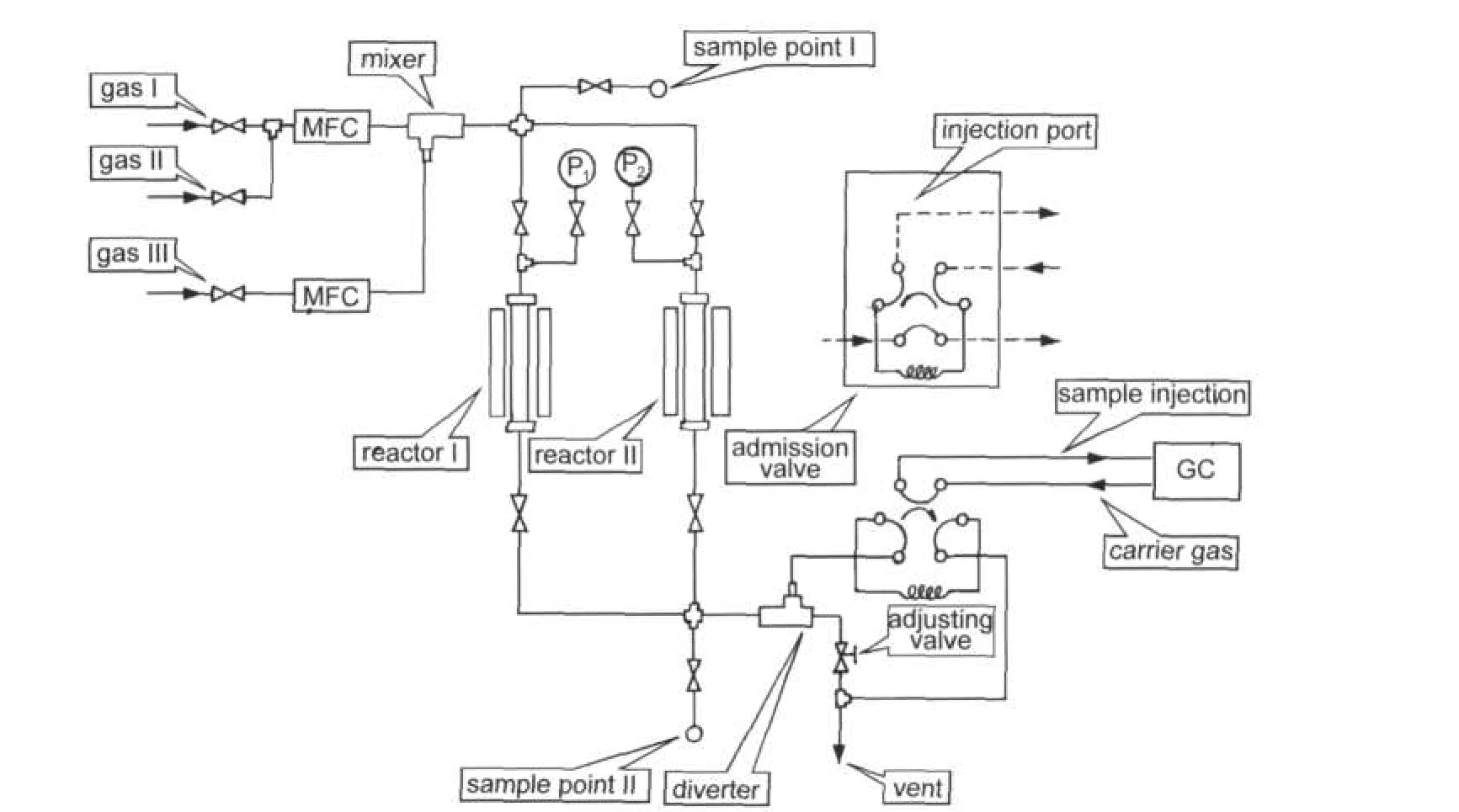
Fig.1 Diagram of apparatus for the test of catalytic activity
XRD patterns of various Pt/TiO2catalysts were shown in Fig.2.No obvious crystallite formation of the Pt species could be found in the XRD patterns of Pt/TiO2(PR12)and Pt/TiO2(PR24)catalysts,which indicated that the low metal content might lead to a high dispersion of Pt and,therefore,that the Pt particles were too small to be detected by XRD analysis.However,only a broader diffraction peak of Pt appeared at 39.68ofor Pt/TiO2(PR36)and Pt/TiO2(IM)catalysts,which could be attributed to Pt(111).10Therefore,the average crystallite sizes of Pt particles could be calculated by applying the Scherrer equation on the Pt(111)diffraction peaks.The calculated average crystallite sizes of Pt particles in Pt/TiO2(PR36)and Pt/ TiO2(IM)samples were 16 and 10 nm,respectively.According to previous report,10the concentrations of chlorine ions could be decreased by photoreduction,resulted in a highly dispersion of active component.These results suggested that the distribution of Pt species on the support surface could be improved by adjusting photoreducation time and the longer time will be detrimental to the enhancement of CO oxidation activity.

Fig.2 XRD patterns of various catalysts(a)Pt/TiO2(PR12),(b)Pt/TiO2(PR24),(c)Pt/TiO2(PR36),(d)Pt/TiO2
3.2 XPS analysis
XPS analyses were carried out to determine the surface concentration and valence state of Pt in Pt/TiO2catalysts.The binding energies of Pt 4f and the derived atomic contents in the different catalysts were summarized in Fig.3 and Table 1.From Fig.3 we found that the line shape and the width of Pt(4f7/2,4f5/2) over the impregnated catalyst matched well with that of the metallic Pt.By comparison,the Pt 4f features obtained from the photoreduced catalysts were quite broad,both zerovalence and cationic Pt could be found.According to previous reports,19,20the Pt 4f bands at 70.9 eV could be related to metallic Pt,while those at 73.2 eV could be assigned to Pt2+,respectively.Therefore,the relative atomic ratios of[Pt]/[Pt2+]decreased with the increase of photoreduction time.These results indicated that the valence state of Pt particles on the catalysts surface could be changed by adjusting photoreduction time.In addition,the atomic content on the support surface in the impregnated catalyst was much higher than those in the photoreduced catalysts, indicating that just partial Pt species could be deposited by photoreduction.

Fig.3 XPS spectra of Pt in various catalysts(A)Pt/TiO2(PR12),(B)Pt/TiO2(PR24),(C)Pt/TiO2(PR36),(D)Pt/TiO2(IM)
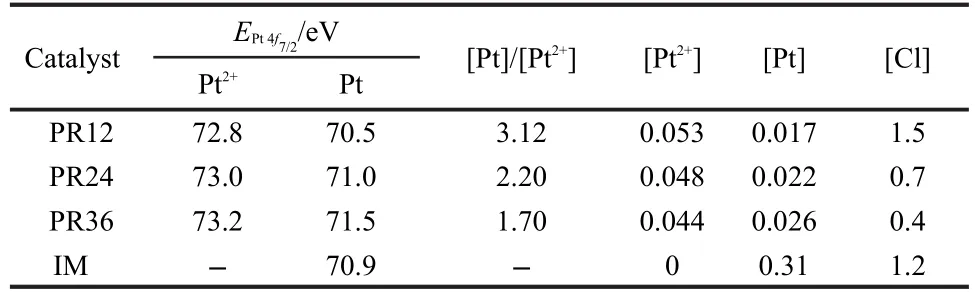
Table 1 XPS data of the catalysts Pt/TiO2(PR12),Pt/TiO2(PR24), Pt/TiO2(PR36),and Pt/TiO2(IM)
The XPS spectra of TiO2were also detected in order to investigate the strong metal-support interaction(SMSI)for precious metal on reducible support.It is widely accepted that such effects may be crucial in many aspects of heterogeneous catalysis.The binding energies of TiO22p in various Pt/TiO2catalysts were shown in Fig.4.From Fig.4(a)we can find that the line shape and the width of TiO2(2p3/2,2p1/2)over the impregnated catalyst matched well with those of the Ti4+,which indicated that TiO2could not be reduced by H2pretreatment.This result also suggested that there was a weak interaction between active center Pt and reducible support TiO2.However,the binding energies of TiO2in photoreducted Pt/TiO2catalysts moved towards higher binding energy,maybe due to the production of part of Ti3+.According to previous report,21the stoichiometric surface exists,in principle,as an oxygen vacancy and two Ti3+.
3.3 Activity tests
CO oxidation in the absence of hydrogen was carried out in a temperature region of 100-260°C with a CO/O2molar ratio of 0.125.For comparison,CO conversions over different catalysts versus reaction temperature were summarized in Fig.5.It can be found that the catalytic activities for CO oxidation are very sensitive to preparation method.Performance of the impregnated catalyst is superior to those of photoreduced ones, which maybe due to the partial deposition of the Pt species resulted from photoreduction.Although both the nature of the active Pt phase and the elucidation of the mechanism for CO oxidation are still debated,herein,we consider that the Pt oxides are major active sites.Comparing the photoreduced catalysts, we found that their catalytic performance could be significantly promoted by adjusting the photoredution time.An optimum photoreduction time was 24 h and the longer time will be detrimental to the enhancement of CO oxidation activity.From Table 1 it can be found that the concentration of chlorine ions can be decreased from 1.5 to 0.4 when we increased photoreduction time from 12 to 36 h.Whereas the excessive photoreduction can result in the aggregation of the Pt species as shown in XRD results above.This result is consistent with previous report.22Therefore,the Pt/TiO2(PR24)catalyst exhibited the best activity for CO oxidation.
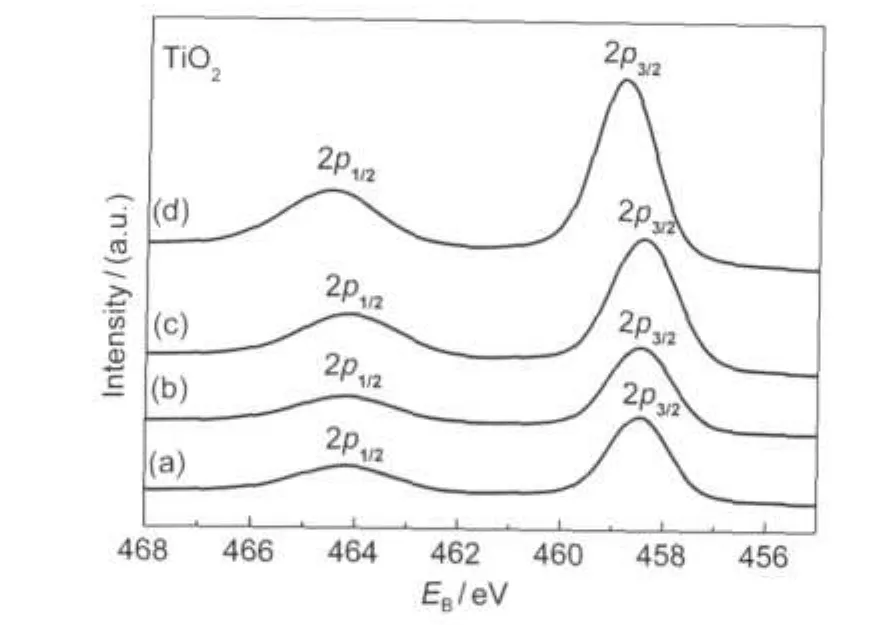
Fig.4 XPS spectra of TiO2for various catalysts(a)Pt/TiO2(IM),(b)Pt/TiO2(PR12),(c)Pt/TiO2(PR24),(d)Pt/TiO2(PR36)
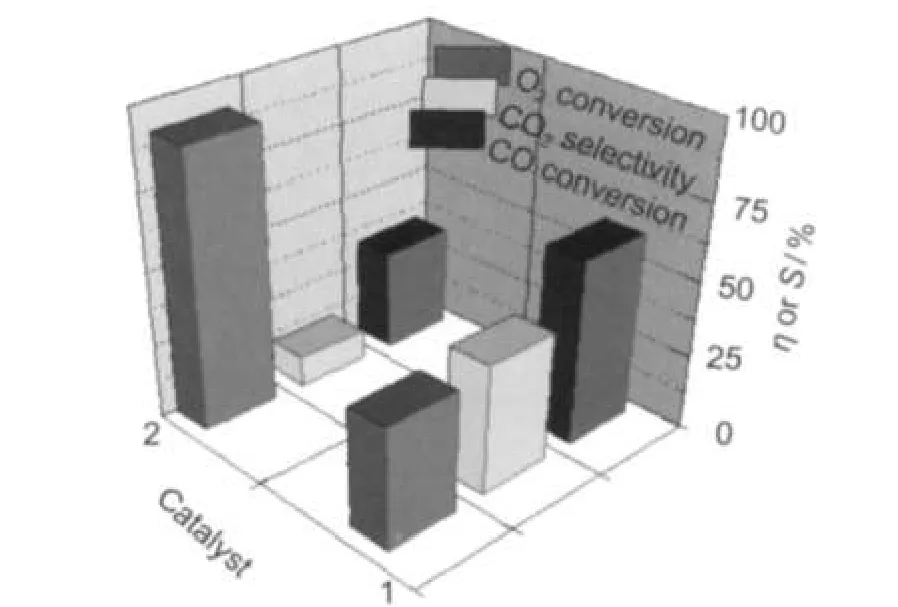
Fig.6 Activity comparison of the CO selective oxidation between Pt/TiO2(PR24)(1)and Pt/TiO2(IM)(2)catalysts
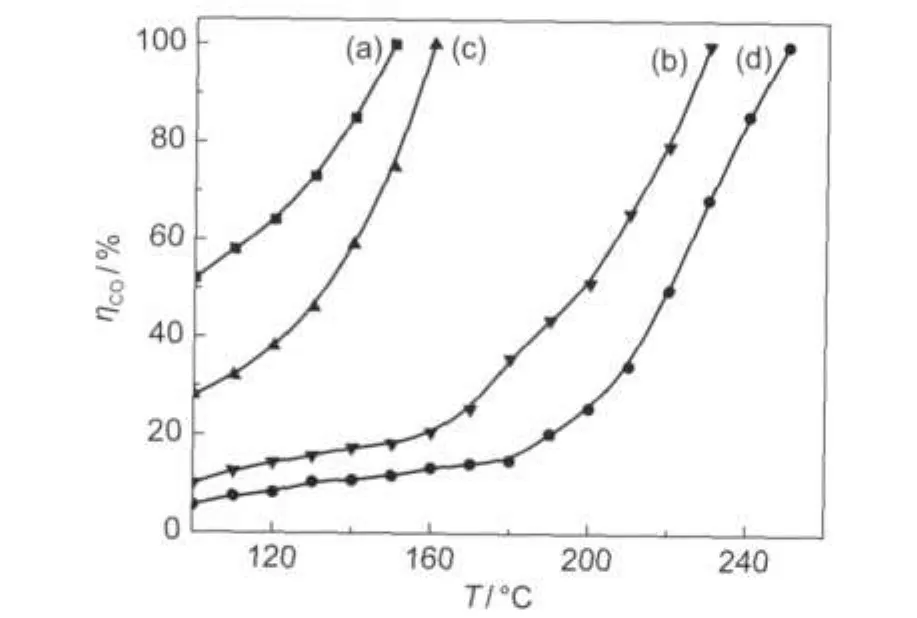
Fig.5 CO conversion vs reaction temperature over various catalysts(a)Pt/TiO2(IM),(b)Pt/TiO2(PR12),(c)Pt/TiO2(PR24),(d)Pt/TiO2(PR36)
Furthermore,in order to investigate the effects of H2,a comparison of the catalytic activities for CO preferential oxidation in the presence of H2between Pt/TiO2(IM)and Pt/TiO2(PR24) was made.The maximum CO conversions,the corresponding O2conversions and selectivities of CO2were shown in Fig.6. Our results showed that the maximum CO conversion of 61.4%on Pt/TiO2(PR24)was achieved at 180°C,the corresponding O2conversion and selectivity of CO2were 40.0%and 43.0%,respectively.However,the maximum CO conversion of 33.7%over the Pt/TiO2(IM)catalyst could not be obtained until the reaction temperature increased to 240°C.Furthermore,the higher O2conversion of 91.4%resulted in a lower selectivity of CO2of 11.2%.
It has been reported that the electron transfer occurs between oxide support and Au nanoparticles or adsorbates,and influences the CO thermocatalytic oxidation.23-25So,it can be proposed that the presence of H2may also play a similar effect on the CO oxidation via electron transfer.These results indicate that the dissociative chemisorption H at surface oxygen vacancy sites of TiO2deduced by photoreduction can act as both the electron-acceptors for the photogeneration electrons and the electron-donors for the chemisorbed O2at TiO2.26We consider that a bi-function reaction mechanism maybe involved in CO preferential oxidation over the photoreduced Pt/TiO2catalysts, where CO adsorbs on the Pt species and H2adsorbs at surface oxygen vacancy sites of TiO2.Therefore,the bi-function reaction mechanism weakens the competitive adsorption between CO and active oxygen on the Pt species,and subsequently enhances the activity for CO preferential oxidation in the presence of H2.
4 Conclusions
In summary,the photoreduction is an effective method to enhance the catalytic activity and selectivity of CO2for the reaction of PROX in H2-rich stream.In photoreduction process,the dissociative chemisorption H at surface oxygen vacancy sites of TiO2can act as the electron-donors for the chemisorbed O2at TiO2,resulted in a bi-function reaction mechanism for CO preferential oxidation in H2-rich stream.In addition,a further research is in progress owing to the numerous influence factors on the photoreduction,such as the irradiate intensity,the pH value,and concentration of solution,etc.
(1)Du,W.P.;Li,Z.;Leng,W.H.;Xu,Y.M.Acta Phys.-Chim.Sin. 2009,25,1530.[杜衛(wèi)平,李 臻,冷文華,許宜銘.物理化學(xué)學(xué)報(bào),2009,25,1530.]
(2) Liu,D.;Xu,Y.M.Acta Phys.-Chim.Sin.2008,24,1584. [劉 鼎,許宜銘.物理化學(xué)學(xué)報(bào),2008,24,1584.]
(3)Wang,F.;Lu,G.X.Catal.Lett.2007,115,46
(4)Wang,F.;Lu,G.X.Catal.Lett.2010,134,72.
(5) Oh,S.H.;Sinkevitch,R.M.J.Catal.1993,142,254.
(6) Kahlich,M.J.;Gasteiger,H.A.;Behm,R.J.J.Catal.1997, 171,93.
(7) Zkara,S.?.;Aksoylu,A.E.Appl.Catal.A-Gen.2003,251,75.
(8) Geng,D.S.;Chen,L.;Lu,G.X.J.Mol.Catal.A 2007,265,42.
(9)Tang,Z.C.;Geng,D.S.;Lu,G.X.Thin Solid Films 2006,497, 309.
(10) Wang,F.;Lu,G.X.J.Power Sources 2008,181,120.
(11) Wang,F.;Lu,G.X.Int.J.Hydrog.Energy 2010,35,7253.
(12)Wang,F.;Lu,G.X.J.Phys.Chem.C 2009,113,4161
(13)Wang,F.;Lu,G.X.J.Phys.Chem.C 2009,113,17070.
(14) Wang,F.;Lu,G.X.Chin.J.Catal.2007,28,27.[王 芳,呂功煊.催化學(xué)報(bào),2007,28,27.]
(15) Zhang,M.;Jin,Z.S.;Zhang,Z.J.;Dang,H.X.Appl.Surf.Sci. 2005,250,29.
(16) Zhang,M.;Jin,Z.S.;Zhang,Z.J.;Dang,H.X.J.Mol.Catal. A-Chem.2005,225,59.
(17) Zhang,M.;Feng,C.X.;Jin,Z.S.;Chen,G.;Du,Z.L.Chin.J. Catal.2005,26,508.[張 敏,馮彩霞,金振聲,程 剛,杜祖亮.催化學(xué)報(bào),2005,26,508.]
(18) Nishiyama,N.;Ichioka,K.;Park,D.H.;Egashira,Y.;Ueyama, K.;Gora,L.;Zhu,W.D.;Kapteijn,F.;Moulijn,J.Ind.Eng. Chem.Res.2004,43,1211.
(19)Kim,K.S.;Winorgrad,N.;Davis,R.E.J.Am.Chem.Soc. 1971,93,6296.
(20) Bornsten,L.In Zahlenwerte und Funktionen aus Naturwissenschaft und Technik;Springer:Berlin,1982.
(21) Robert,G.;Peter,M.;Michael,B.Catal.Lett.2004,98,129.
(22)Yang,J.C.;Kim,Y.C.;Shul,Y.G.;Shin,C.H.;Lee,T.K.Appl. Surf.Sci.1997,121,525.
(23) Lopez,N.;Janssens,T.V.W.;Clausen,B.S.;Xu,Y.; Mavrikakis,M.;Bligaard,T.;N?skov,J.K.J.Catal.2004,223, 232.
(24) Giordano,L.;Goniakowski,J.;Pacchioni,G.Phys.Rev.B 2001, 64,075417.
(25) Molina,L.M.;Hammer,B.Phys.Rev.Lett.2003,90,206102.
(26) Dai,W.X.;Chen,X.;Wang,X.X.;Liu,P.;Li,D.Z.;Li,G.S.; Fu,X.Z.Phys.Chem.Chem.Phys.2008,10,3256.
August 11,2011;Revised:November 2,2011;Published on Web:November 24,2011.
Preferential Oxidation of CO over Photoreduced Pt/TiO2Catalysts in H2-Rich Stream
WANG Fang*WANG Cai-Hong LIU Guo-Xia
(Department of Chemistry&Chemical Engineering,BinzhouUniversity,Binzhou 256603,Shandong Province,P.R.China)
The optimum reaction parameters for CO oxidation in the presence and absence of H2have been investigated by photoreduction method to enhance the catalytic activity and selectivity of CO2for CO preferential oxidation(PROX)in H2-rich stream in detail.X-ray photoelectron spectroscoopy(XPS)results showed that part oxygen vacancies produced on the surface of photoreduced catalysts,which maybe the activity site for the chemisorbed H.Therefore,a possible bi-function reaction mechanism for CO preferential oxidation over the photoreduced Pt/TiO2catalyst has been proposed.
CO preferential oxidation;Pt catalyst;Photoreduction;Impregnation
10.3866/PKU.WHXB201111244www.whxb.pku.edu.cn
*Corresponding author.Email:wangfangosso@yahoo.cn;Tel:+86-18763029669.
The project was supported by the Research Fund of Binzhou University,China(2010Y06).
濱州學(xué)院科研基金(2010Y06)資助項(xiàng)目
O643

