凝膠燃燒法合成Li1.07Mn1.93O4納米片及其高倍率放電和循環(huán)穩(wěn)定性
毛 景 代克化 翟玉春
(東北大學(xué)材料與冶金學(xué)院,沈陽110004)
凝膠燃燒法合成Li1.07Mn1.93O4納米片及其高倍率放電和循環(huán)穩(wěn)定性
毛 景 代克化*翟玉春*
(東北大學(xué)材料與冶金學(xué)院,沈陽110004)
利用聚乙烯吡咯烷酮(PVP)作為聚合物配位劑和燃料,通過凝膠-燃燒法合成了Li1.07Mn1.93O4納米片.采用熱重/差熱分析(TG/DTA)研究了凝膠的燃燒過程.采用X射線多晶衍射(XRD)分析了材料的結(jié)構(gòu),結(jié)果表明合成的Li1.07Mn1.93O4結(jié)晶完整,無雜質(zhì)相.掃描電鏡(SEM)結(jié)果顯示材料的二次形貌為厚度約100 nm的片狀,由大小約100 nm的一次顆粒構(gòu)成.充放電測(cè)試表明Li1.07Mn1.93O4納米片具備極佳的倍率放電性能和優(yōu)秀的循環(huán)性能.0.5C(1C=120 mA·g-1)倍率的初始放電容量為115.4 mAh·g-1,即使倍率增大到40C,放電容量仍有105.3 mAh·g-1.在10C倍率的放電條件下,循環(huán)850次容量保持率為81%.電化學(xué)阻抗譜(EIS)測(cè)試表明Li1.07Mn1.93O4納米片的界面電荷轉(zhuǎn)移電阻(Rct)遠(yuǎn)小于同類商業(yè)材料.
鋰離子電池;錳酸鋰;燃燒合成;倍率性能;循環(huán)性能
1 Introduction
The rapid development of electric vehicles requires advanced lithium ion batteries with higher power density and longer cycling life.Spinel LiMn2O4is at present a very prospective candidate for the cathode material due to its low cost, good safety,environmental friendliness,and relatively high voltage.1Enhancing the rate capability and cycling stability of LiMn2O4has recently become one of the most attractive topics of both scientific and industrial interests.2-12
Nanosized particles provide short diffusion pathways for both Li-ions and electrons,resulting in an improvement in Li-ion intercalation kinetics,which should allow for a higher charge-discharge rate and minimize the structural distortion at the surface of the cathode grains.13-15Recently,nanostructured LiMn2O4with various morphologies has been extensively prepared trying to improve the rate capability.A variety of synthetic routes have been chosen such as ball milling,16,17room-temperature solid-state coordination process,18,19sol-gel,20,21flame spray pyrolysis,22,23hard-template route,24-26electrochemical precipitation,27self-assembly process,28biomimetic synthetic process,29and hydrothermal method.3,8,12,30-32However,most of these methods involve several steps and some are quite complicated or expensive.
Combustion method has been known as a simple,fast,and energetically economic method that yields high purity products.33Urea,34triethanolamine(TEA)-starch,35starch,36hexamethylenetetramine(HMTA),33polyacrylic acid(PAA),37polyvinylalcohol(PVA),38citric acid/glycol,39and glycine40have been chosen as fuels to synthesize LiMn2O4with high capacity, but the rate capability was not reported or was poor.Rojo et al.41,42reported a sucrose-aided combustion method and synthesized doubly doped LiMn1.99-yLiyM0.01O4(M=Al3+,Ni2+,Cr3+,Co3+; y=0.01,0.06)spinels.Among them the LiMn1.93Li0.06Co0.01O4can deliver 105 and 101 mAh·g-1at 0.2C and 5C rates between 3.1 and 4.4 V,respectively.
The choice of fuel has a significant impact on structure,morphology,and performance of the synthesized materials.In this study,Li1.07Mn1.93O4(nLi/nMn=0.55,molar ratio)nanoflakes were synthesized by a gel-combustion method using polyvinylpyrrolidone(PVP)as the polymer chelating agent and fuel.PVP was employed due to its low toxicity and high aqueous solubility.It has been extensively used as a stabilizer and a structure-directing agent in nanotechnology because of its excellent adsorption ability.43,44Fu et al.44prepared PVP/LiCoO2nanofibers using an electrospinning route.Kanamura et al.45-48prepared Li4Ti5O12,LiCoO2,and LiMn2O4thin films by introducing PVP to a sol. The authors have synthesized sub-micron LiNi0.5Mn1.5O4with excellent high rate performance by the PVP-assisted gel-combustion method.49It is naturally guessed that PVP also can be used to prepare lithium manganese oxide nano-powders with high electrochemical performance.The molar ratio of Li/Mn(0.55)was chosen because Li doping has been proved to be a simplest but effective strategy to enhance the intrinsic structure stability during Li-ion insertion and extraction from the spinel framework.50,51
2 Experimental
Synthesis of Li1.07Mn1.93O4nanoflakes was carried out by dissolving PVP(AR),LiCH3COO·2H2O(AR),and Mn(CH3COO)2· 4H2O(AR)in deionized water with a Li/Mn molar ratio of 0.55.The molar ratio of PVP to total metal ions was fixed at 2.0.HNO3(AR)was added to the solution until the pH value of 3 was achieved.The mixture was stirred and heated at 90°C until viscous,and then the gel was dried at 110°C for 2 h.The resulting dried gel was heated in air on an electric hot plate to ignite a combustion reaction in several minutes.The obtained black powders were heated at 400°C for 3 h then calcinated at 700°C for 6 h to obtain well crystallized Li1.07Mn1.93O4.Finally, Li1.07Mn1.93O4was ground and passed through 300-mesh sieve.
Simultaneous thermogravimetric and differential thermal analyses(TG/DTA)measurements of the dried gel were carried out in America TA Instrument SDT 2960 Simultaneous DTA-TGA.Crucibles of alumina were used both for the specimen and for the reference material.The samples(about 10 mg) were heated from room temperature to 700°C in flowing air, with a heating rate of 10°C·min-1.
Powder X-ray diffraction measurement was performed on a Japan Rigaku D/Max-2500PC X-ray diffractometer using Cu Kαradiation.Morphological study was conducted using a FEI Nova NanoSEM 430 scanning electron microscope(SEM,Europe).To determine the chemical composition,Li and Mn concentrations in the lithium manganese oxide were measured by inductively coupled plasma(ICP,Optima 4300DV,PE Ltd.).
The electrochemical performance of the Li1.07Mn1.93O4nanoflakes as cathode was evaluated using a CR2025 coin cell with a lithium metal anode.The cathode was a mixture of active material/acetylene black/polyvinylidene fluoride(PVDF)with mass ratio of 80:10:10.The average loading density of active material was about 5 mg·cm-2.Celgard 2400 was used as separator and the electrolyte was 1 mol·dm-3LiPF6in a 1:1:1(volume ratio)mixture of ethylene carbonate(EC),dimethyl carbonate(DMC),and ethyl methyl carbonate(EMC).The cells were assembled in an argon filled glovebox.For comparison,a commercial material(produced by CITIC GUOAN Mengguli) with the same chemical composition was chosen and assembled similarly.Charge and discharge tests were performed at various current densities between 3.3 and 4.3 V by a LAND CT2001A battery test system at ambient temperature.Electrochemical impedance spectroscopy(EIS)studies were performed on the coin cell by a Princeton Applied Research PARSTAT2273 electrochemical measurement system.The cells were charged to 3.9 V and balanced for 12 h before EIS tests. The frequency ranged from 100 kHz to 0.1 Hz and the acoscillation amplitude was 10 mV.
3 Results and discussion
The pyrolysis process of the dried gel precursor was investigated by TG and DTA(Fig.1).The mass loss below 160°C can be ascribed to the evaporation of residual water.The small exothermic peak between 200 and 250°C can probably be explained by pyrolysis of acetates,and the broad exothermic peak between 250 and 350°C can probably be associated with pyrolysis of PVP.The precursor bloated and charred with giving out brown smoke along with the pyrolysis of PVP and then the polymer precursor automatically ignited and burned violently.This combustion reaction corresponds to the strong exothermic peak at about 400°C in the DTA curve and distinct mass loss in the TG curve.Because the metal ions had been mixed evenly at atomic level by chelating of PVP,the metal precursor formed basic spinel phase in situ during the combustion reaction.The combustion reaction was accompanied by violent gas evolution and the volume of the mixture bloomed up quickly.It means that the newly formed particles are compacted loosely which can prevent the particle growth in the subsequent heat treatment.No obvious mass loss is found after the combustion peak.However,for more gel precursor the remains of the combustion still needed to be calcinated at 400°C for 3 h for complete removal of the organic residue.Heat treating at 700°C is needed to improve the crystallinity and the electrochemical property of the as-prepared nanoparticles.
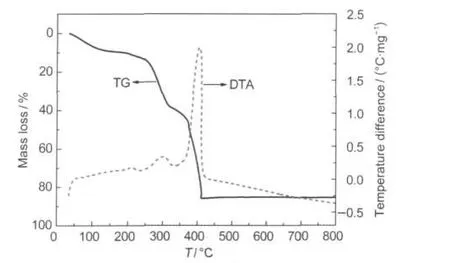
Fig.1 TG and DTAcurves of the dried gel precursors
The prepared and commercial lithium manganese oxides were analyzed by ICP and both identified as Li1.07Mn1.93O4(nLi/ nMn=0.55).
Fig.2 shows XRD patterns of the samples at different stages. It can be seen from Fig.2a that a spinel phase is formed by the combustion reaction of the gel precursor even in a very short period of time,though some impurities such as Mn3O4phase also exist.Fig.2b shows that very little impurities exist after calcination at 400°C for 3 h but the diffraction lines are still broad indicating a low crystallinity.After heat treatment at 700°C,the impurities completely disappear and the peaks become sharp indicating a higher crystallinity(Fig.2c).XRD analysis of the sample definitely indicates its spinel structure and the crystal structure is indexed to a cubic system with a lattice parameter a of 0.8227(2)nm,and then is defined to the space group Fd3m.
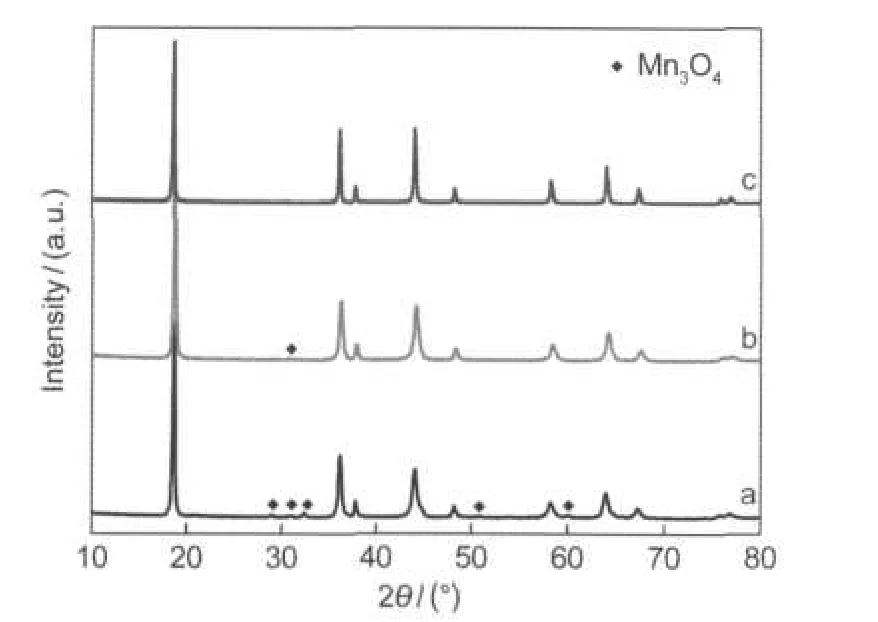
Fig.2 XRD patterns of the precursor and samples(a)burned precursor;(b)sample after calcination at 400°C; (c)sample after calcination at 700°C
Figs.3a and 3b show the SEM images of the as-prepared Li1.07Mn1.93O4with different magnifications.Under lower magnification,Fig.3a shows that most of the secondary particles are nanoflakes with several micrometers in size,about 100 nm in thickness and piled up loosely.Under higher magnification (Fig.3b),it can be seen that the nanoflakes consist of uniform nanocrystallites with a size of 100 nm or so.The unique thin flake-like morphology is different from those obtained via other methods.This may be related to good film-forming property of PVP.It may favor electrolyte penetration,thereby enabling better wetting of spinel cathode and faster Li-ion transfer at the interface.At the same time the nanosize of the primary crystallite makes the Li-ion and electron dissociating pathway inside the crystal shorter and then the cathode can behave better under high current densities.While the SEM images of the commercial Li1.07Mn1.93O4(Figs.3c&3d)demonstrate its secondary particle size of about 10 μm,and its primary particle size is about 300-500 nm,much bigger than that of the as prepared Li1.07Mn1.93O4.The subsequent electrochemical tests show that the Li1.07Mn1.93O4nanoflakes have excellent rate capability, which is much better than that of the commercial one.

Fig.3 SEM images of Li1.07Mn1.93O4(a,b)the nano-Li1.07Mn1.93O4;(c,d)the commercial Li1.07Mn1.93O4
The high rate capability of the Li1.07Mn1.93O4nanoflakes is presented in Fig.4 and Fig.5.Fig.4 shows the discharge curves of the Li1.07Mn1.93O4nanoflakes recorded at different discharge rates.The rate capability measurements were investigated by discharging from 0.5C to 40C.Charging was done at 1C for all the discharge rates except when discharging was done at 0.5C. In this case,charging was conducted at 0.5C.At 0.5C,4C, 10C,20C,and 40C,the discharge capacity was 115.4,115.3, 114.4,111.9,and 105.3 mAh·g?1,respectively.Moreover,the discharge profiles at high rates of 10C,20C,and 40C still have a relative flat discharge plateau.The maintained relative flat plateau in the high rate of 40C can sustain a constant output voltage.3
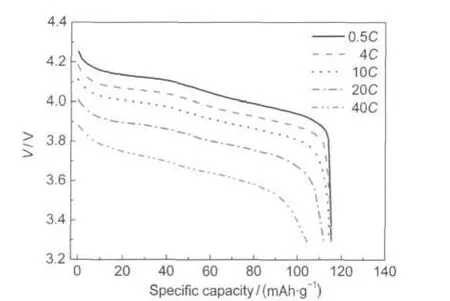
Fig.4 Discharge profiles of the nano-Li1.07Mn1.93O4at different rates between 3.3 and 4.3 V1C=120 mA·g-1
Fig.5 compares the rate capability of the Li1.07Mn1.93O4nanoflakes and the commercial Li1.07Mn1.93O4.The capacities of the Li1.07Mn1.93O4nanoflakes remain 99.9%at a discharge rate of 4C,99.1%at 10C,97.0%at 20C,and 91.2%at 40C,and those of the commercial Li1.07Mn1.93O4only remain 96.4%at discharge rate of 4C,92.0%at 10C,86.6%at 20C,and 74.9%at 40C,although the electrodes have approximate mass.The results indicate that the excellent rate capability of the as-prepared Li1.07Mn1.93O4nanoflakes is caused by the very small particle size but not by the thin electrode.
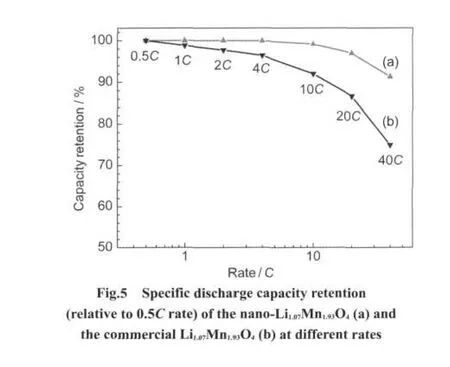
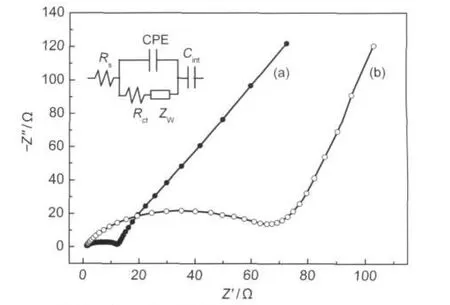
Fig.6 Electrochemical impedance spectra of the nano-Li1.07Mn1.93O4(a)and the commercial Li1.07Mn1.93O4(b) Rs:ohmic resistance;Rct:charge-transfer resistance;CPE:constant phase-angleelement;ZW:Warburg impedance;Cint:insertion capacitance
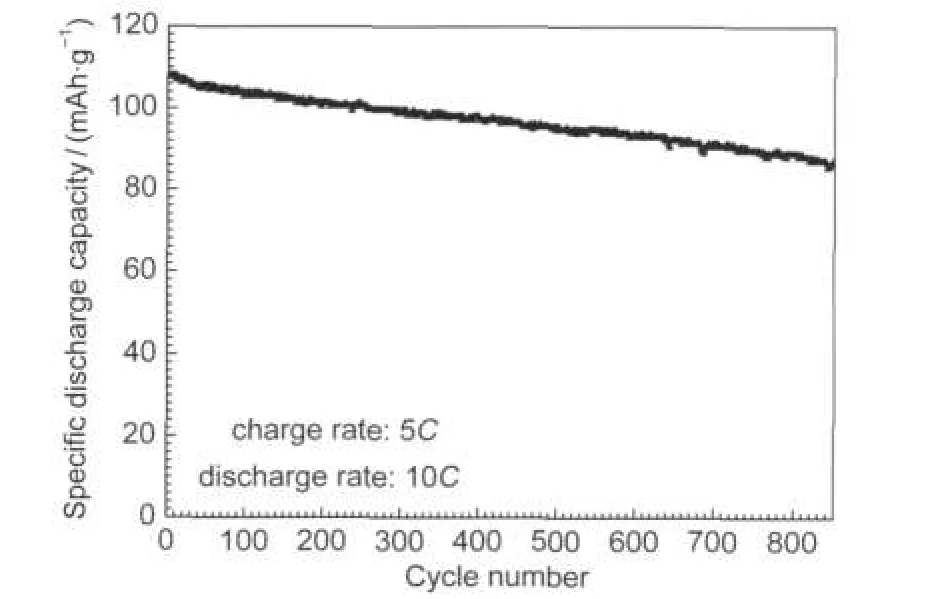
Fig.7 Cyclic performance of the nano-Li1.07Mn1.93O4at a high discharge rate of 10C
The electrochemical impedance spectra(EIS)of the nano-Li1.07Mn1.93O4/Li cell and commercial Li1.07Mn1.93O4/Li cell after 3 cycles at 3.9 V are shown in Fig.6.The Nyquist plots of two samples in Fig.6 show a semicircle followed by a sloping line at low frequencies and they were fitted with the equivalent circuit depicted in the inset of Fig.6.The result of fitting indicates that the Rctof Li1.07Mn1.93O4nanoflakes is 9.7 Ω,which is much less than that of commercial Li1.07Mn1.93O4(60.5 Ω).This means that the lithium insertion/extraction could be easily conducted in the nanoparticles.This result agrees well with the good rate capability of the Li1.07Mn1.93O4nanoflakes as demonstrated above.
The Li1.07Mn1.93O4nanoflakes has been cycled at a high discharge rate of 10C(charged at 5C rate)for 850 cycles to investigate whether the good rate capability can be retained on prolonged cycling.The result in Fig.7 shows an initial capacity of 107.9 mAh·g-1and the capacity retention is 81%after 850 cycles.It is speculated that the good cycling performance may be originated from the well-crystallized nanoscale cathode active particles which minimize structural distortion at the surface of the cathode.
4 Conclusions
Li1.07Mn1.93O4nanoflakes were synthesized by the novel gelcombustion method using PVP as the polymer chelating agent and fuel.TG and DTA results clarified the quick combustion process of the gel.XRD analysis indicated the as-prepared Li1.07Mn1.93O4was pure phase and highly crystallized.SEM images displayed that most of the secondary particles were nanoflakes with thickness of about 100 nm and the primary particle size was about 100 nm.Electrochemical tests showed that the Li1.07Mn1.93O4nanoflakes behaved an excellent rate capability and cycling performance as a cathode material for lithium ion batteries.The discharge capacities at 0.5C and 40C were 115.4 and 105.3 mAh·g-1,respectively.It maintained 81%of its initial capacity after 850 cycles when cycling at 10C rate.EIS tests showed that the charge transfer resistance of the Li1.07Mn1.93O4nanoflakes was smaller than that of the commercial Li1.07Mn1.93O4. This excellent performance of the Li1.07Mn1.93O4nanoflake in this work can be attributed to the small particle size and high crystallinity prepared by the PVP-assisted combustion method.
(1)Tarascon,J.M.;Armand,M.Nature 2001,414,359.
(2) Du Pasquier,A.;Huang,C.C.;Spitler,T.Journal of Power Sources 2009,186,508.
(3)Kudo,T.;Honma,I.;Matsuda,H.;Zhou,H.S.Nano Letters 2009,9,1045.
(4) Lanz,M.;Kormann,C.;Steininger,H.;Heil,G.;Haas,O.; Novak,P.Journal of the Electrochemical Society 2000,147, 3997.
(5) Lee,J.W.;Park,S.M.;Kim,H.J.Electrochemistry Communications 2009,11,1101.
(6)Lee,K.S.;Myung,S.T.;Bang,H.;Amine,K.;Kim,D.W.; Sun,Y.K.Journal of Power Sources 2009,189,494.
(7) Lim,S.;Cho,J.Electrochemistry Communications 2008,10, 1478.
(8)Ma,S.B.;Nam,K.W.;Yoon,W.S.;Bak,S.M.;Yang,X.Q.; Cho,B.W.;Kim,K.B.Electrochemistry Communications 2009,11,1575.
(9) Park,S.C.;Han,Y.S.;Kang,Y.S.;Lee,P.S.;Ahn,S.;Lee,H. M.;Lee,J.Y.Journal of the Electrochemical Society 2001,148, A680.
(10)Park,S.C.;Kim,Y.M.;Kang,Y.M.;Kim,K.T.;Lee,P.S.; Lee,J.Y.Journal of Power Sources 2001,103,86.
(11) Wang,X.Q.;Tanaike,O.;Kodama,M.;Hatori,H.Journal of Power Sources 2007,168,282.
(12)Yue,H.;Huang,X.;Lv,D.;Yang,Y.Electrochimica Acta 2009, 54,5363.
(13)Arico,A.S.;Bruce,P.;Scrosati,B.;Tarascon,J.M.;Van Schalkwijk,W.Nature Materials 2005,4,366.
(14) Bruce,P.G.;Scrosati,B.;Tarascon,J.M.Angewandte Chemie-International Edition 2008,47,2930.
(15) Chen,Z.Y.;Zhu,H.L.;Ji,S.;Linkov,V.;Zhang,J.L.;Zhu,W. Journal of Power Sources 2009,189,507.
(16)Kamarulzaman,N.;Yusoff,R.;Kamarudin,N.;Shaari,N.H.; Aziz,N.A.A.;Bustam,M.A.;Blagojevic,N.;Elcombe,M.; Blackford,M.;Avdeev,M.;Arof,A.K.Journal of Power Sources 2009,188,274.
(17)Ye,S.H.;Lv,J.Y.;Gao,X.P.;Wu,F.;Song,D.Y. Electrochimica Acta 2004,49,1623.
(18) Caballero,A.;Cruz,M.;Hernán,L.;Melero,M.;Morales,J.; Castellón,E.R.Journal of Power Sources 2005,150,192.
(19) Huang,Y.D.;Jiang,R.R.;Bao,S.J.;Dong,Z.F.;Cao,Y.L.; Jia,D.Z.;Guo,Z.P.Journal of Solid State Electrochemistry 2009,13,799.
(20) Shaju,K.M.;Bruce,P.G.Chemistry of Materials 2008,20, 5557.
(21) Vivekanandhan,S.;Venkateswarlu,M.;Satyanarayana,N. Journal of Alloys and Compounds 2007,441,284.
(22) Patey,T.J.;Buchel,R.;Nakayama,M.;Novak,P.Physical Chemistry Chemical Physics 2009,11,3756.
(23) Patey,T.J.;Buchel,R.;Ng,S.H.;Krumeich,F.;Pratsinis,S.E.; Novak,P.Journal of Power Sources 2009,189,149.
(24) Cabana,J.;Valdes-Solis,T.;Palacin,M.R.;Oro-Sole,J.; Fuertes,A.;Marban,G.;Fuertes,A.B.Journal of Power Sources 2007,166,492.
(25) Jiao,F.;Bao,J.L.;Hill,A.H.;Bruce,P.G.Angewandte Chemie-International Edition 2008,47,9711.
(26)Luo,J.Y.;Wang,Y.G.;Xiong,H.M.;Xia,Y.Y.Chemistry of Materials 2007,19,4791.
(27)Katakura,K.;Wada,K.;Kajiki,Y.;Yamamoto,A.;Ogumi,Z. Journal of Power Sources 2009,189,240.
(28) Luo,J.Y.;Cheng,L.;Xia,Y.Y.Electrochemistry Communications 2007,9,1404.
(29) Uchiyama,H.;Hosono,E.;Zhou,H.S.;Imai,H.Journal of Materials Chemistry 2009,19,4012.
(30) Fang,H.S.;Li,L.P.;Yang,Y.;Yan,G.F.;Li,G.S.Journal of Power Sources 2008,184,494.
(31) Jiang,C.H.;Dou,S.X.;Liu,H.K.;Ichihara,M.;Zhou,H.S. Journal of Power Sources 2007,172,410.
(32) Kim,D.K.;Muralidharan,P.;Lee,H.W.;Ruffo,R.;Yang,Y.; Chan,C.K.;Peng,H.;Huggins,R.A.;Cui,Y.Nano Letters 2008,8,3948.
(33) Fey,G.;Cho,Y.;Kumar,T.Materials Chemistry and Physics 2006,99,451.
(34)Liu,Q.G.;Yang,W.S.;Zhang,G.;Xie,J.Y.;Yang,L.L. Journal of Power Sources 1999,81,412.
(35)Fey,G.T.K.;Cho,Y.D.;Kumar,T.P.Materials Chemistry and Physics 2004,87,275.
(36) Kalyani,P.;Kalaiselvi,N.;Muniyandi,N.Journal of Power Sources 2002,111,232.
(37) Park,H.B.;Kim,J.;Lee,C.W.Journal of Power Sources 2001, 92,124.
(38) Subramania,A.;Angayarkanni,N.;Vasudevan,T.Materials Chemistry and Physics 2007,102,19.
(39)Wu,X.M.;Li,X.H.;Xiao,Z.B.;Liu,J.;Yan,W.B.;Ma,M.Y. Materials Chemistry and Physics 2004,84,182.
(40) Zhang,Y.;Shin,H.C.;Dong,J.;Liu,M.Solid State Ionics 2004,171,25.
(41)Amarilla,J.M.;Petrov,K.;Pico,F.;Avdeev,G.;Rojo,J.M.; Rojas,R.M.Journal of Power Sources 2009,191,591.
(42) Kovacheva,D.;Gadjov,H.;Petrov,K.;Mandal,S.;Lazarraga, M.G.;Pascual,L.;Amarilla,J.M.;Rojas,R.M.;Herrero,P.; Rojo,J.M.Journal of Materials Chemistry 2002,12,1184.
(43) Zhang,J.H.;Liu,J.B.;Wang,S.Z.;Zhan,P.;Wang,Z.L.; Ming,N.B.Adv.Funct.Mater.2004,14,1089.
(44) Fu,Y.S.;Chen,L.J.;Liao,J.D.;Chuang,Y.J.;Hsu,K.C.; Chiang,Y.F.J.Appl.Polym.Sci.2011,121,154.
(45) Kanamura,K.;Rho,Y.H.J.Electroanal.Chem.2003,559,69.
(46) Kanamura,K.;Rho,Y.H.J.Solid State Chem.2004,177,2094.
(47) Kanamura,K.;Rho,Y.H.Journal of Power Sources 2006,158, 1436.
(48)Kanamura,K.;Rho,Y.H.;Umegaki,T.Chem.Lett.2001,1322.
(49) Dai,K.H.;Mao,J.;Zhai,Y.C.Acta Phys.-Chim.Sin.2010,26, 2130.[代克化,毛 景,翟玉春.物理化學(xué)學(xué)報(bào),2010,26, 2130.]
(50) Hirose,S.;Kodera,T.;Ogihara,T.Journal of Alloys and Compounds 2010,506,883.
(51)Peng,Z.D.;Jiang,Q.L.;Du,K.;Wang,W.G.;Hu,G.R.;Liu, Y.X.Journal of Alloys and Compounds 2010,493,640.
July 18,2011;Revised:November 24,2011;Published on Web:December 5,2011.
High Rate Capability and Cycling Stability of Li1.07Mn1.93O4Nanoflakes Synthesized via Gel-Combustion Method
MAO Jing DAI Ke-Hua*ZHAI Yu-Chun*
(School of Materials and Metallurgy,Northeastern University,Shenyang 110004,P.R.China)
Li1.07Mn1.93O4nanoflakes were synthesized by a gel-combustion method using polyvinylpyrrolidone(PVP)as the polymer chelating agent and fuel.Thermogravimetric and differential thermal analyses(TG/DTA)were used to investigate the combustion process of the gel precursor.X-ray diffraction(XRD)analysis indicated that the as-prepared Li1.07Mn1.93O4was a pure,highly crystalline phase. Scanning electron microscopy(SEM)results showed that most of the secondary particles were nanoflakes, about 100 nm in thickness,and the primary particle of the nanoflakes was about 100 nm in size.Charge and discharge tests suggested that the Li1.07Mn1.93O4nanoflakes had excellent rate capability and good cycling stability.The initial discharge capacity was 115.4 mAh·g-1at a rate of 0.5C(1C=120 mAh·g-1)and the capacity was maintained at 105.3 mAh·g-1at the high discharge rate of 40C.When cycling at 10C,the material retained 81%of its initial capacity after 850 cycles.Electrochemical impedance spectroscopy (EIS)tests indicated that the charge-transfer resistance(Rct)of the Li1.07Mn1.93O4nanoflakes was much less than that of commercial Li1.07Mn1.93O4.
Lithium ion battery;Lithium manganese oxide;Combustion synthesis;Rate capability; Cycling stability
10.3866/PKU.WHXB201112052
*Corresponding authors.DAI Ke-Hua,Email:daikh@smm.neu.edu.cn;Tel/Fax:+86-24-83684943.ZHAI Yu-Chun,Email:zhaiyc@smm.neu.edu.cn
O646

