Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
XU Qinqin (許勤勤), LIU Zhenhua (劉振華), SUN Yisheng (孫一晟), DING Zhongjie (丁忠杰),Lü Longxian (呂龍賢) and LI Yongquan (李永泉)**
College of Life Sciences, Zhejiang University, Hangzhou 310058, China
Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
XU Qinqin (許勤勤), LIU Zhenhua (劉振華), SUN Yisheng (孫一晟), DING Zhongjie (丁忠杰),Lü Longxian (呂龍賢) and LI Yongquan (李永泉)**
College of Life Sciences, Zhejiang University, Hangzhou 310058, China
Cordyceps ophioglossoides is a valuable traditional medicinal material. We have found that intracellular polysaccharide (IPS) is the major biologically active ingredient in Cordyceps ophioglossoides. This study is the first time to optimize the yield of IPS from Cordyceps ophioglossoides. The optimal medium for IPS production consists of glucose 54.50 g·L-1, yeast powder 25.50 g·L-1, NaH2PO40.4 g·L-1and K2HPO40.4 g·L-1. The suggested culture conditions are 24 °C, initial pH 4.5 with a rotary speed of 120 r·min-1for 168 h. The yield of IPS is 737.93 mg·L-1,which is 50% higher than the yield under the conditions prior to optimization. The anti-oxidative activities of IPS in Cordyceps ophioglossoides L2 are also characterized using various in vitro assay. The anti-oxidative activity may explain the reason why IPS from Cordyceps ophioglossoides can be used to fight against neurodegenerative diseases and menopausal symptoms.
antioxidant activity, Cordyceps ophioglossoides L2, intracellular polysaccharide, submerged culture,optimization
1 INTRODUCTION
Cordyceps ophioglossoides is a well-known fungus of the genus Cordyceps in the family of Clavicipitaceae.Some species of Cordyceps have been used as traditional medicine in China, Japan and Korea for hundreds of years. For instance, the aqueous extract of Cordyceps ophioglossoides has been used for women suffering massive postpartum vaginal bleeding in China[1, 2]. Some pharmacological effects of the cultured mycelia, including antitumor activity, estrogenic activity and anti-ageing activity, have been discovered in the past few decades [3-5]. Moreover, it has been reported that a protein-bound polysaccharide from Cordyceps ophioglossoides has antitumoral activity [6-8].
Polysaccharide is an important active component in Cordyceps which has multiple pharmacological activities including antitumor activity [9], antiinflammatory activity [10], immunopotentiation [11, 12],hypoglycemic activity [11] and hypocholesterolemic effect [13]. Polysaccharide protects neuronal cells against the free radical-induced cellular toxicity [14]and stimulates steroidogenesis [15]. It has also been demonstrated that the polysaccharides isolated from the mycelia of fungi have potential antioxidant properties [16]. Research on anti-oxidative activities from herbs has attracted much interest due to their high capacity in scavenging free radicals [17, 18]. These natural antioxidants not only protect food lipids from oxidation, but may also provide health benefits in preventing damages due to free radicals produced by biological degeneration [19, 20].
Cordyceps ophioglossoides is difficult to obtain and the collection might destroy the balance of the natural microenvironments. Cordyceps ophioglossoides L2, a strain of Cordyceps ophioglossoides, has been isolated from the fruiting body of Cordyceps ophioglossoides in our lab [21]. The culture conditions of many fungi have been optimized in order to obtain higher polysaccharide production in submerged culture[22-24], but the optimization of polysaccharide production from submerged culture of Cordyceps ophioglossoides has not been demonstrated. The determination of the antioxidant activity of polysaccharide from Cordyceps ophioglossoides has never been reported.
Here, we report the optimization of medium composition and cultivation conditions for intracellular polysaccharides (IPS) production from Cordyceps ophioglossoides L2 in submerged culture on the basis of the one-factor-at-a-time method and orthogonal matrix method. Furthermore, we evaluate the antioxidant activities of the polysaccharides in vitro by using 1, 1-diphenyl-2-picrylhydracyl (DPPH) radical scavenging assay, superoxide radical scavenging assay,and hydroxyl radical scavenging assay.
2 MATERIALS AND METHODS 2.1 Chemicals
Butylated hydroxytoluene (BHT) and tertbutylhydroquinone (TBHQ) were purchased fromBeijing Superior Chemical and Instrument Co., Ltd.(Beijing, China). 1, 1-Diphenyl-2-picrylhydrazyl (DPPH)was purchased from Sigma-Aldrich (St. Louis, MO,USA). L-ascorbic acid was purchased from Guangdong Guanghua Chemical Factory Co., Ltd. (Shantou,Guangdong, China). Gallic acid monohydrate was obtained from Sinopharm Chemical Reagent Co., Ltd.(Shanghai, China). All the other chemicals were analytical reagent grade and purchased from local chemical suppliers in China.
2.2 Microorganism and liquid culture
Cordyceps ophioglossoides L2 was maintained on potato sucrose agar (PSA) slant. The slant was incubated at 25 °C for 10 days before being stored at 4°C. Sub-culture was required every two months. The liquid medium for seed growth contained saccharine 30.0 g·L-1, yeast powder 5.0 g·L-1, peptone 5.0 g·L-1,MgSO4·7H2O 1.0 g·L-1and KH2PO40.5 g·L-1. After cultivation for 6 days at 24 °C on a rotary shaker (170 r·min-1), the seed was inoculated into the 200 ml of submerged medium (sucrose 66.0 g·L-1, yeast powder 10.0 g·L-1, silkworm chrysalises digest 30.0 g·L-1,MgSO4·7H2O 0.4 g·L-1, and KH2PO40.4 g·L-1) and incubated in 1 L Erlenmeyer flasks at 23-25 °C for 90 h as described by Xu et al [21].
2.3 Preparation of intracellular polysaccharide
The intracellular polysaccharide (IPS) used for anti-oxidative activity study was prepared as described by Xiao et al. [24] with some modifications: dry powder of mycelia was resuspended in 10 volumes of distilled water and sonicated at room temperature and actual sonic power of 300 W for 15 min. Then the extraction was processed at 95 °C for 1 h. The supernatant was collected by centrifugation (6000 r·min-1, 10 min),and the extraction was repeated twice. The supernatants were combined and concentrated in a rotary evaporator under reduced pressure, and then 95% ethanol was added to the final concentration of 70% (by volume). The mixture was stored at 4 °C for 24 h. The precipitate was centrifuged at 10000 r·min-1for 10 min and lyophilized. The crude IPS powder was then used for the determination of anti-oxidative activities in vitro. IPS1 was prepared from the mycelia cultured under the conditions reported by Xu et al. [21] and ISP2 was extracted from the mycelia after optimized in this study.
2.4 Measurement of mycelia biomass and IPS content
The mycelia cultured in various culture conditions were harvested by centrifugation at 6000 r·min-1for 10 min, and lyophilized in a vacuum freeze-drying machine to a constant mass. The contents of IPS were determined by the phenol-sulfuric acid method [25].
2.5 Experimental design for optimization of IPS production
Using the one-factor-at-a-time method, nine factors considered for the design were carbon resource,nitrogen resource, and ratio of carbon to nitrogen resource, mineral ions, temperature, initial pH value,medium capacities, rotary speed and culture time.According to the results of the single factor experiment, the orthogonal L9(34) was used to obtain the optimal culture conditions in submerged cultures. The levels of factors are listed in Table 1.
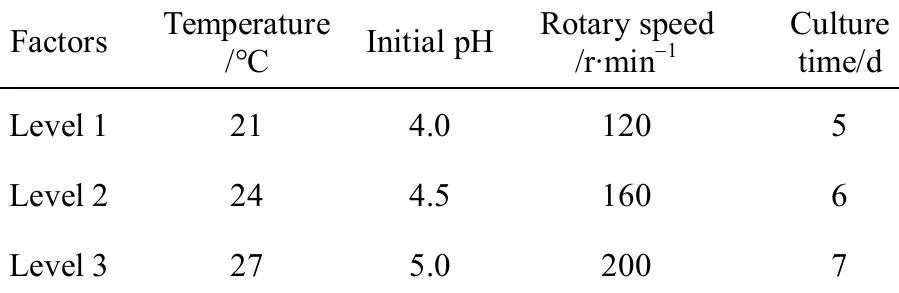
Table 1 Orthogonal design for optimization of production of IPS in shake flask culture
2.6 Anti-oxidative activity measurement
2.6.1 1,1-Diphenyl-2-picrylhydracyl radical scavenging assay
The free radical scavenging activity of IPS toward 1, 1-diphenyl-2-picryl-hydrazil (DPPH) was examined according to the method reported by Shimada et al [26]. 2 ml of 0.2 mmol·L-1DPPH ethanol solution was mixed with 2 ml of various concentrations of IPS solution at room temperature. After 30 min, the optical absorbance was measured at wavelength 517 nm. Low absorbance of the reaction mixture indicates higher free radical scavenging activity.The scavenging of DPPH radical in percentage was calculated by the following equation: scavenging activity (1 A1/A0)×100%, where A0is the absorbance of the blank reaction and A1is the absorbance in the presence of IPS. BHT and TBHQ were used as positive controls.
2.6.2 Superoxide radical scavenging assay
The scavenging of superoxide by antioxidants was estimated by the inhibition of pyrogallol autoxidation as described previously [27] with some modifications. In brief, 5 ml of 50 mmol·L-1Tris-HCl buffer(pH 8.2) and 3.8 ml of various concentrations of IPS solution were incubated at 25 °C. After 10 min 200 μl of pyrogallol was added. After 5 min, the optical absorbance at wavelength 322 nm was monitored for 4 min. The autoxidation speed was expressed as ?A per minute. The percentage inhibition of superoxide anion radical scavenging was calculated using the following formula: inhibition pyrogallol autoxidation= (1 A1/A0)×100%, where A0is the autoxidation speed of blank control without the tested samples and A1is the autoxidation speed in the presence of the tested samples. Ascorbic acid was used as a positive control.
2.6.3 Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity was measured according to the method described by Smirnoff and Cumbes [28] with minor modifications. The reaction mixture (3 ml) containing 0.2 mmol·L-1EDTA-Fe(3.0 ml), 3.0 mmol·L-1H2O2and 2.0 mmol·L-1salicylic acid was incubated in a 37 °C water bath for 15 min and was then added to 0.8 ml sample at different concentrations for 60 min at 37 °C. Hydroxyl radicals were detected by monitoring optical absorbance at wavelength 510 nm. Results were calculated with the following equation: scavenging effect(1- A1/A0)×100%, where A0is the absorbance of the control reaction and A1is the absorbance in the presence of IPS. Ascorbic acid was used as a positive control.
2.7 Statistical analysis
All data in this research are presented as mean ±standard deviation.
3 RESULTS AND DISCUSSION
3.1 Effects of medium compositions on IPS production in Cordyceps ophioglossoides L2
Four different carbon sources were selected to find the suitable medium source for the production of IPS in Cordyceps ophioglossoides L2. As showed in Table 2, Cordyceps ophioglossoides L2 grew normally in the medium with glucose, galactose or sucrose, but not in the one with starch, indicating that the fungus poorly utilized complicated carbon source. The yield of mycelia biomass was nearly the same in the medium with glucose or sucrose, while glucose was more favorable for the polysaccharide production. This is consistent with the studies that most of the polysaccharides from Cordyceps were mainly composed of glucose [29, 30].It is reported that Cordyceps ophioglossoides can produce extracellular β-glucans, which is synthesized from glucose [3, 6]. This result suggests that monosaccharide is the best carbon source for Cordyceps ophioglossoides L2 to produce IPS.
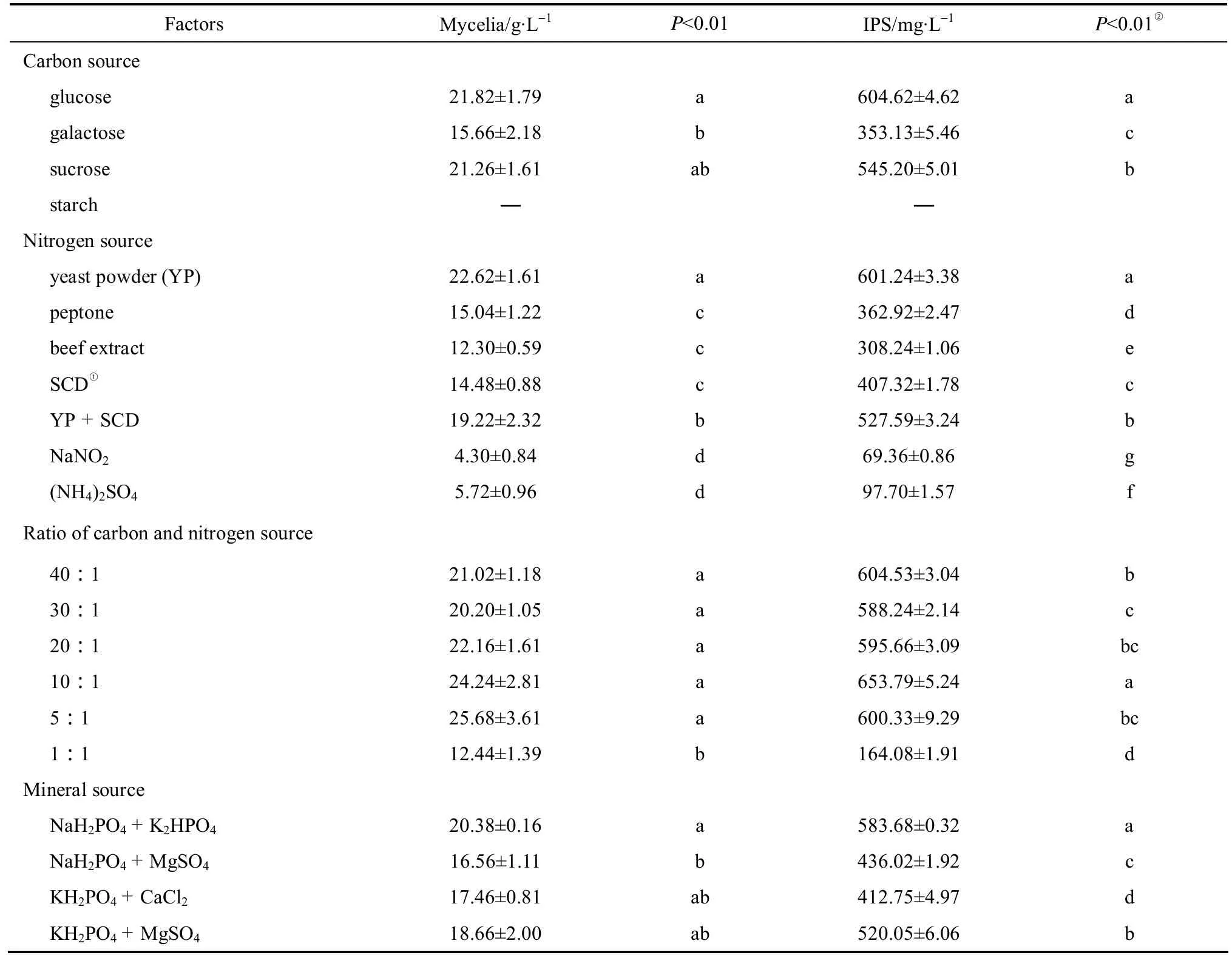
Table 2 Effects of culture medium on mycelia biomass and IPS production by Cordyceps ophioglossoides L2 in submerged culture
Organic nitrogen sources were more favorable for production of both mycelia biomass and IPS than inorganic ones. It is consistent with previous studies on Cordyceps as organic nitrogen sources contain not only proteins and amino acids, but also growth factors as well as many other ingredients that are necessary for growth [31]. Among the seven nitrogen sources used, yeast powder was the best (Table 2). Both mycelia biomass and polysaccharide produced by Cordyceps ophioglossoides L2 using yeast powder were significantly higher than those using other nitrogen sources.
The study of C/N ratio based on carbon and nitrogen elements was performed as previously described[31]. Generally, the demand of carbon sources was higher than that of nitrogen sources, since the former could act as the sources of both energy and cytoskeleton. As shown in Table 2, the highest yield of IPS appeared in the culture using C/N ratio at 10︰1 while the highest biomass was at 5︰1. This may be because the higher ratio of nitrogen resources was favorable for cell growth but unfavorable for accumulation of metabolites. Therefore, the optimal C/N ratio was fixed at 10︰1 to produce IPS in Cordyceps ophioglossoides L2.
Four groups of mineral ion combinations were selected to study the effect of various mineral sources on IPS production. The optimal combination was NaH2PO4and K2HPO4(Table 2). This result was consistent with the theory that Ca2+accumulation could inhibit the synthesis of fungal biopolymers through an effect on enzymes [32]. The high consistency between the yields of mycelia and polysaccharide was also observed in this one-factor-at-a-time experiment. Based on the above results, we chose the medium composition similar to that optimized for mycelia with a slight modification: glucose 54.50 g·L-1, yeast powder 25.50 g·L-1, NaH2PO40.4 g·L-1and K2HPO40.4 g·L-1.
3.2 Effect of culture conditions on IPS production in Cordyceps ophioglossoides L2
In order to investigate the effect of initial pH on IPS production, Cordyceps ophioglossoides L2 was cultivated with initial pH from 3.0 to 9.0. The maximum IPS production was observed at pH 4.0 (Table 3)while the optimal pH value for growth was 5.0. The pH value of the media was increased to around 8 at the end of the fermentation, which implies that Cordyceps ophioglossoides can grow on media with different pH levels and that a low initial pH is better for the production of IPS. It is consistent with the result reported by Hsieh et al. that acidic pH was more suitable for mycelia and polysaccharide production of Cordyceps sinensis [33]. Cultivation with a pH of 9.0 yielded almost no growth (data not shown).
To determine the optimal temperature for IPS production, this organism was cultivated at various temperatures. The optimum temperature was found to be at 25 °C (Table 3). This result was consistent with the optimum temperature for mycelia production.Several volumes (60 to 250 ml medium in 500 ml container) were studied to find the optimal media capacity (data not shown). IPS production under different (125 to 175 ml medium in 500 ml container) media capacities was similar at all levels, which is contrast to the study by Meng et al [16]. Therefore, we chose 125 ml medium in 500 ml container for the next step of orthogonal array designs. The maximum mycelia and IPS production was obtained in rotary speed of 120 r·min-1. Because media capacity and rotary speed are closely related to oxygen supply, these results suggested that mycelia growth and IPS production by Cordyceps ophioglossoides L2 required low concentrations of oxygen, which was likely determined by the natural environment of this fungus. The optimal culture time was 168 h, which was longer than that required for the production of mycelia. This indicates that the IPS of Cordyceps ophioglossoides L2 may accumulate in the later growth phases and that longer culture time may increase IPS production.
3.3 Cultivation condition optimization for IPS production using orthogonal array design
Based on the above results, a four-factor-threelevel orthogonal test was designed as showed in Table 3 to optimize the cultivation conditions for IPS production. Orthogonal array design was used to narrow the optimal range of cultivation conditions and to find correlation between different culture conditions. The orthogonal results shown in Table 4 reveal that the yield of IPS most significantly depends on temperature. High consistency is found in the yield between the mycelia and IPS under each culture condition except culture time. The effect of the culture conditions on IPS production is ordered as follows: temperature >culture time > initial pH > rotary speed. For mycelia,it is temperature > initial pH > culture time > rotary speed. The optimal culture condition for IPS production is as follows: temperature 24 °C, initial pH 4.5, rotary speed 120 r·min-1and culture time 168 h. Under the optimal conditions, IPS content is 737.93 mg·L-1,which is 50% higher than that under the non-optimized conditions.
3.4 Antioxidant activities of IPS from Cordyceps ophioglossoides L2
The capacity of polysaccharide from mushrooms to scavenging free radicals related to various diseases has been the topic of several studies [34-37]. Antioxidants are well known for playing important roles in the human metabolic system and for protecting against cardiovascular and neurodegenerative disease [38-40].In this study, the antioxidant activities of the IPS from Cordyceps ophioglossoides L2 were evaluated in vitro by using 1,1-diphenyl-2-picrylhydracyl (DPPH) radical scavenging assay, superoxide radical scavenging assay and hydroxyl radical scavenging assay. Compared with other methods, the model of scavenging the stable DPPH radical is a widely used method to evaluate natural antioxidant activities in a relatively short time.The DPPH radical scavenging activity of the IPS is evident in all of the tested concentrations, although it is lower than that of TBHQ and BHT (Fig. 1). The scavenging ability of IPS from Cordyceps ophioglossoides L2 is concentration dependent. The percentage of DPPH radical scavenging activity at 200 μl volume of IPS is 80%. EC50 value of 543.25 mg·L-1is lower than the value from mycelia of Ganoderma tsugae Murrill reported by Mau et al [41].

Table 3 Effects of cultivation conditions on mycelia biomass and IPS production by Cordyceps ophioglossoides L2 in submerged culture
Among different reactive oxygen species (ROS),superoxide is a relatively weak oxidant. However, it may decompose to form stronger ROS, such as hydroxyl radical, which may participate in lipid peroxidation and thereafter damage cellular membranes. To quantify the extent of superoxide scavenging activity, we determined the inhibition of pyrogallol autoxidation.The changes in color represent the content of superoxide radicals and indicate the antioxidant activity of the sample [42]. A 20% to 80% inhibition of pyrogallol autoxidation was detected at the various concentrations IPS added and had EC50 value of 270.04 mg·L-1(Fig. 2). There is a semi-linear relationship betweenthe concentration and activity.
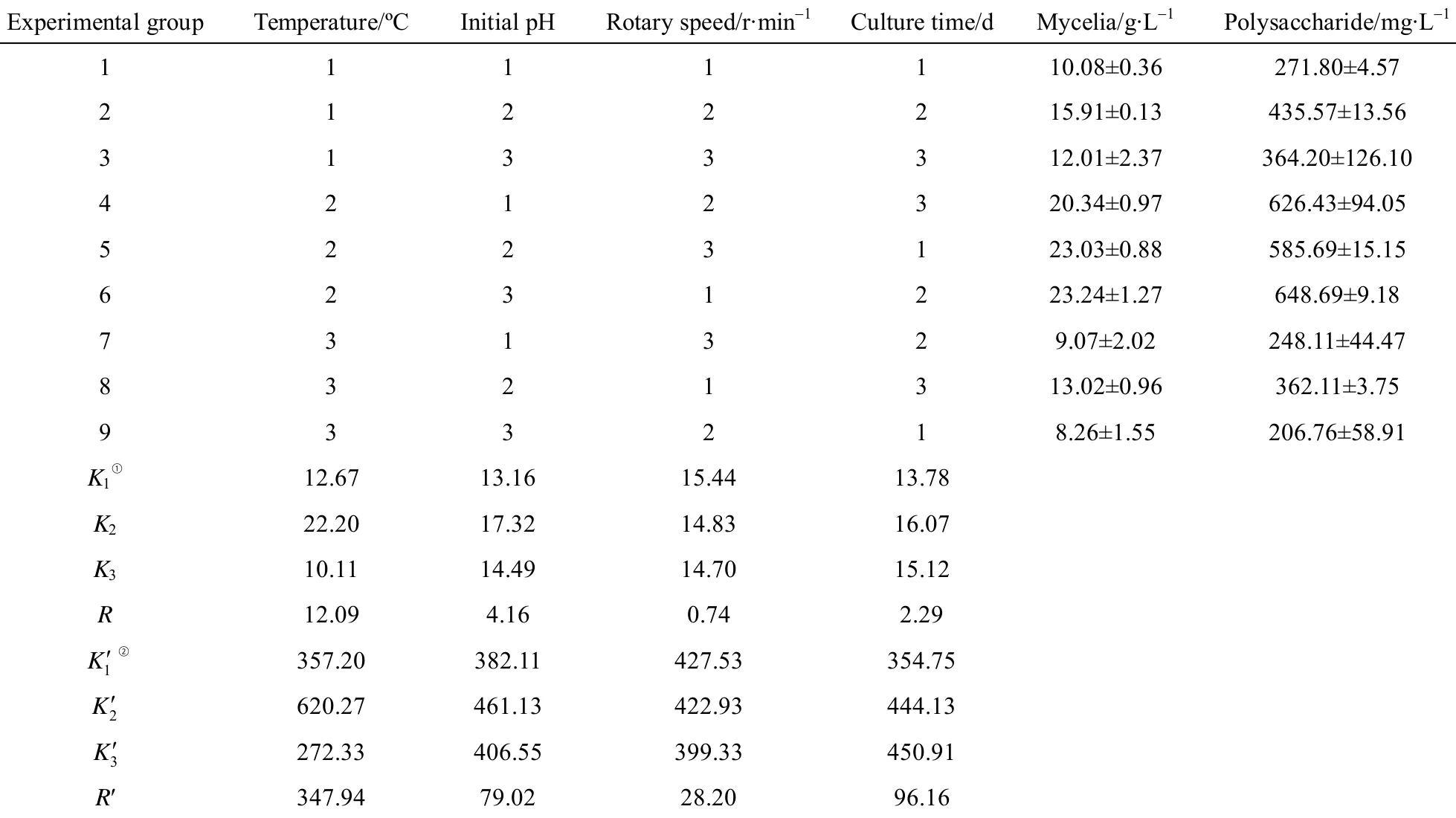
Table 4 Results of orthogonal experiments for culture conditions optimization of production of IPS
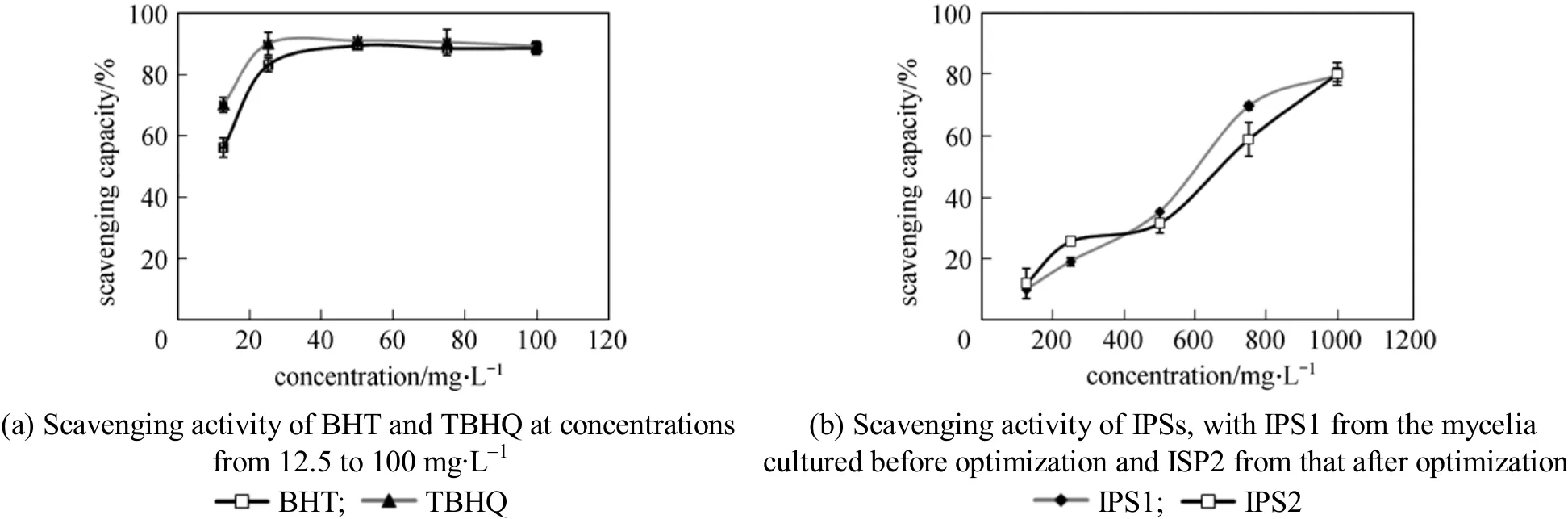
Figure 1 Scavenging effect on DPPH of intracellular polysaccharide (IPS) from mycelia of Cordyceps ophioglossoides L2
IPS exhibited scavenging activity on hydroxyl radical in a concentration dependent manner (Fig. 3).The scavenging ratios were improved with the increase of extracts concentration. The highest value(49.3%) was lower than ascorbic acid in two other systems. EC50value in scavenging abilities on hydroxyl radical was greater than 2883.94 mg·L-1. EC50values obtained from these assay implied that IPS from Cordyceps ophioglossoides L2 has good antioxidant properties although weak on hydroxyl radical.This suggests that the IPS in various solvents shows significant differences in scavenging activity. Although it is lower than positive control, it is more effective as a crude IPS. It is much higher than the antioxidant activities of hot water extracts from Ling chih and Agrocybe cylindracea (32.3% and 44.3% at 5 mg·ml-1)described by Mau and Tsai [41, 43]. It can be found that the anti-oxidative activity has no difference between the IPS produced under the optimized culture conditions or under non-optimized conditions in all three anti-oxidative activity screening systems. Therefore the optimization of culture conditions increases the content of IPS without affecting its anti-oxidative activity. The IPS from cultured mycelia has direct and potential anti-oxidative activities and optimization is meaningful. There may be some interactions and synergistic effects between different types of polysaccharide.The total anti-oxidative activity is often more meaningful to evaluate health benefits because of the cooperative action of antioxidants as reported by Celik et al[44]. Furthermore, the use of total IPS is more economical than the use of single compounds or synthetic chemistry.
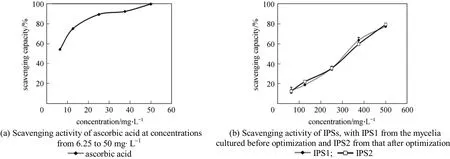
Figure 2 Scavenging effect on superoxide radical of intracellular polysaccharide (IPS) from mycelia of Cordyceps ophioglossoides L2

Figure 3 Scavenging effect on hydroxyl radical of intracellular polysaccharide (IPS) from mycelia of Cordyceps ophioglossoides L2
4 CONCLUSIONS
This research provides the optimization of submerged culture to produce IPS from Cordyceps ophioglossoides L2 and the method may be used in large scale extraction or used as an example for culturing other species of Cordyceps to produce IPS.Moreover, for the first time, we have demonstrated that the IPS of cultured Cordyceps ophioglossoides has anti-oxidative activity. These results may contribute to impart the use of IPS of cultured from Cordyceps ophioglossoides within the human metabolic system to fight against neurodegenerative diseases and menopausal symptoms.
ACKNOWLEDGEMENTS
We are thankful to China Scholarship Council for financial support of this study and we would like to thank our department external guide Dr. Chris Wood for his editing of this manuscript.
1 Zang, M., Xu, X.R., Ji, D.Q., “Yunnan economic fungus data”, Acta Botanica Yunnanica, 1, 28-34 (1975). (in Chinese)
2 Liu, B., Medical Fungi of China, 3rd ed., People’s Publisher of Shanxi, China (1984). (in Chinese)
3 Yamada, H., Kawaguchi, N., Ohmori, T., Takeshita, Y., Taneya, S.,Miyazaki, T., “Structure and antitumor activity of an alkali-soluble polysaccharide from Cordyceps ophioglossoides”, Carbohydr. Res.,125 (1), 107-115 (1984).
4 Kawagishi, H., Okamura, K., Kobayashi, F., Kinjo, N., “Estrogenic sbustances from the mycelia of medicinal fungus Cordyceps ophioglossoides (Ehrh.) Fr. (ascomycetes)”, Int. J. Med. Mushrooms,6, 249-251 (2004).
5 Jin, D.Q., Park, B.C., Lee, J.S., Choi, H.D., Lee, Y.S., Yang, J.H.,Kim, J.A., “Mycelial extract of Cordyceps ophioglossoides prevents neuronal cell death and ameliorates beta-amyloid peptide-induced memory deficits in rats”, Biol. Pharm. Bull., 27 (7), 1126-1129 (2004).
6 Ohmori, T., Tamura, K., Tsuru, S., Nomoto, K., “Antitumor activity of protein-bound polysaccharide from Cordyceps ophioglossoides in mice”, Jpn. J. Cancer Res., 77 (12), 1256-1263 (1986).
7 Ohmori, T., Tamura, K., Fukui, K., Kawanishi, G., Mitsuyama, M.,Nomoto, K., Miyazaki, T., “Isolation of galactosaminoglycan moiety(CO N) from protein-bound polysaccharides of Cordyceps ophioglossoides and its effects against murine tumors”, Chem.Pharm. Bull., 37, 1019-1022 (1989).
8 Ohmori, T., Tamura, K., Wakaiki, A., Kawanishi, G., Tsuru, S., Yadomae,T., Nomoto, K., “Dissociation of a glucan faction (CO-1) from proteinbound polysaccharide of Cordyceps ophioglossoides and analysis of its antitumor effect”, Chem. Pharm. Bull., 36, 4512-4518 (1988).
9 Chen, Y.J., Shiao, M.S., Lee, S.S., Wang, S.Y., “Effects of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells”, Life. Sci., 60, 2349-2359 (1997).
10 Yu, R., Song, L., Zhao, Y., Bin, W., Wang, L., Zhang, H., Wu, Y., Ye,W., Yao, X., “Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris”, Fitoterapia, 75, 465-472(2004).
11 Hsu, T.H., Shiao, L.H., Hsieh, C., Chang, D.M., “A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom Dong Chong Xia Cao, its counterfeit and mimic, and fermented mycelium of Cordyceps sinensis”, Food Chem.,78, 463-469 (2002).
12 Moradali, M.F., Mostafavi, H., Ghods, S., Hedjaroude, G.A., “Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi)”, Int. Immunopharmacol., 7, 701-724 (2007).
13 Koh, J.H., Kim, J.M., Chang, U.J., Suh, H.J., “Hypocholesterolemic effect of hot-water extract from mycelia of Cordyceps sinensis”, Biol.Pharm. Bull., 26, 84-87 (2003).
14 Li, S.P., Zhao, K.J., Ji, Z.N., Song, Z.H., Dong, T.T.X., Lo, C.K., Cheung,J.K.H., Tsim, K.W.K., “A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury”, Life Sci., 73, 2503-2513 (2003).
15 Huang, B.M., Ju, S.Y., Wu, C.S., Chuang, W.J., Sheu, C.C., Leu, S.F.,“Cordyceps sinensis and its fractions stimulate MA-10 mouse Leydig tumor cell steroidogenesis”, J. Androl., 22, 831-837 (2001).
16 Meng, F., Liu, X., Jia, L., Song, Z., Deng, P., Fan, K., “Optimization for the production of exopolysaccharides from Morchella esculenta SO-02 in submerged culture and its antioxidant activities in vitro”,Carbohydr. Polym., 79, 700-704 (2010).
17 Stanisavljevi?, I., Stoji?evi?, S., Veli?kovi?, D., Veljkovi?, V., Lazi?,M., “Antioxidant and antimicrobial activities of Echinacea (Echinacea purpurea L.) extracts obtained by classical and ultrasound extraction”, Chin. J. Chem. Eng., 17 (3), 478-484 (2009).
18 Karabegovi?, I., Nikolova, M., Veli?kovi?, D., Stoji?evi?, S.,Veljkovi?, V., Lazi?, M., “Comparison of antioxidant and antimicrobial activities of methanolic extracts of the Artemisia sp. recovered by different extraction techniques”, Chin. J. Chem. Eng., 19 (9),504-511 (2011).
19 Shahidi, F., Wanasundara, P.K.J.P.D., “Phenolic antioxidants”, Crit.Rev. Food Sci., 32, 67-103 (1992).
20 Hu, C., Kitts, D.D., “Dendelion (Taraxacum officinale) flower extract suppresses both reactive oxygen species and nitric oxide and prevents lipid oxidation in vitro”, Phytomedicine, 12 (8), 588-597 (2005).
21 Xu, Q., Lü, L., Chen, S., Zheng, G., Zheng, J., Li, Y., “Isolation of Cordyceps ophioglossoides L2 from fruit body and optimization of fermentation conditions for its mycelial growth”, Chin. J. Chem.Eng., 17 (2), 278-285 (2009).
22 Xiao, J.H., Chen, D.X., Wan, W.H., Hu, X.J., Qi, Y., Liang, Z.Q.,“Enhanced simultaneous production of mycelia and intracellular polysaccharide in submerged cultivation of Cordyceps jiangxiensis using desirability functions”, Process Biochem., 41, 1887-1893 (2006).
23 Luo, J.G., Liu, J., Ke, C.L, Qiao, D.L., Ye, H., Sun, Y., Zeng, X.X.,“Optimization of medium composition for the production of exopolysaccharides from Phellinus baumii Pilát in submerged culture and the immuno-stimulating activity of exopolysaccharides”,Carbohydr. Polym., 78, 409-415 (2009).
24 Xiao, J.H., Chen, D.X., Xiao, Y., Liu, J.W., Liu, Z.L.,Wan,W.H.,Fang, N., Tan, B.B., Liang, Z.Q., Liu A.Y., “Optimization of submerged culture conditions for mycelial polysaccharides production in Cordyceps pruinosa”, Process Biochem., 9, 2241-2247 (2004).
25 Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F.,“Colorimetric method for determination of sugars and related substances”, Anal. Chem., 28, 350-356 (1956).
26 Shimada, K., Fujikawa, K., Yahara, K., Nakamura, T., “Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion”, J. Agric. Food Chem., 40 (6), 945-948 (1992).
27 Marklund, S., Marklund, G., “Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase”, Eur. J. Biochem., 47, 469-474 (1974).
28 Smirnoff, N., Cumbes, Q.J., “Hydroxyl radical scavenging activity of compatible solutes”, Phytochemistry, 28, 1057-1060 (1989).
29 Kim, S.Y., Park, S.K., Park, H.K., Kim, S.W., “Compositional sugar analysis of antitumor polysaccharides by high performance liquid chromatography and gas chromatography”, Arch. Pharm. Res., 17,337-342 (1994).
30 Yu, K.W., Suh, H.J., Bae , S.H., Lee, C.S., Kim, S.H., Yoon, C.S.,“Chemical properties and physiological activities of stromata of Cordyceps militaris”, J. Microbiol. Biotechnol., 11, 266-274 (2001).
31 Dong, C.H., Yao, Y.J., “Nutritional requirements of mycelial growth of Cordyceps sinensis in submerged culture”, J. Appl. Microbiol., 99(3), 483-492 (2005).
32 Papagianni, M., “Fungal morphology and metabolite production in submerged mycelial processes”, Biotechnol. Adv., 22, 189-259 (2004).
33 Hsieh, C., Tsai, M.J., Hsu, T.H., Chang, D.M., Lo, C.T., “Medium optimization for polysaccharide production of Cordyceps sinensis”,Appl. Biochem. Biotechnol., 120, 145-157 (2005).
34 Deng, P., Zhang, G., Zhou, B., Lin, R., Le, J., Fan, K., Liu, X., Wang,G., Wang, L., Zhang, J., “Extraction and in vitro antioxidant activity of intracellular polysaccharide by Pholiota adiposa SX-02”, J. Biosci Bioeng., 111, 50-54 (2011).
35 Xiao, J.H., Chen, D.X., Liu, J.W., Liu, Z.L., Wan, W.H., Fang, N.,Xiao, Y., Qi, Y., Liang, Z.Q., “Optimization of submerged culture requirements for the production of mycelial growth and exopolysaccharide by Cordyceps jiangxiensis JXPJ 0109”, J. Appl. Microbiol., 96, 1105-1116 (2004).
36 Kim, S.W., Xu, C.P., Hwang, H.J., Choi, J.W., Kim,C.W., Yun, J.W.,“Production and characterization of exopolysaccharides from an enthomopathogenic fungus Cordyceps militaris NG 3”, Biotechnol.Progr., 2, 428-435 (2003).
37 Wang, X., Liang, Q.M., Li, T.T., Zhi, R., Zhang, N., Lu, J.H., “Study on extraction and antioxidation of Marasmius androsaceus mycelium polysaccharides”, Food. Sci. Technol., 12, 80-83 (2006).
38 Vinson, J.A., Hao, Y., Su, X., Zubik, L., “Phenol antioxidant quantity and quality in foods: vegetables”, J. Agric. Food Chem., 46, 3630-3634(1998).
39 Enayat, S., Banerjee, S., “Comparative antioxidant activity of extracts from leaves, bark and catkins of Salix aegyptiaca sp”, Food Chem., 116, 23-28 (2009).
40 Juan, M.Y., Chou, C.C., “Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715”, Food Microbiol., 27,586-591 (2010).
41 Mau, J.L., Tsai, S.Y., Tseng, Y.H., Huang, S.J., “Antioxidant properties of hot-water extracts from Ganoderma tsugae Murrill”,LWT-Food Sci. Technol., 38, 589-597 (2005).
42 Chen, Y., Xie, M.Y., Nie, S.P., Li, C., Wang, Y.X., “Purification,composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum”, Food Chem., 107,231-241 (2008).
43 Tsai, S.Y., Huang, S.J., Mau, J.L., “Antioxidant properties of hot-water extracts from Agrocybe cylindracea”, Food Chem., 98, 670-677 (2006).
44 Celik, S.E., Ozyurek, M., Guclu, K., Apak, R., “Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods”,Talanta, 81, 1300-1309 (2010).
2011-05-11, accepted 2011-08-26.
* Supported by the National High Technology Research and Development Program of China (2007AA021506), the Natural Science Foundation of Zhejiang Province (R207609), and the Research Project of Science and Technology of Zhejiang Province, China (2005C23027).
** To whom correspondence should be addressed. E-mail: lyq@zju.edu.cn
 Chinese Journal of Chemical Engineering2012年2期
Chinese Journal of Chemical Engineering2012年2期
- Chinese Journal of Chemical Engineering的其它文章
- A Pilot-scale Demonstration of Reverse Osmosis Unit for Treatment of Coal-bed Methane Co-produced Water and Its Modeling*
- ECT Image Analysis Methods for Shear Zone Measurements during Silo Discharging Process*
- Temperature-triggered Protein Adsorption and Desorption on Temperature-responsive PNIPAAm-grafted-silica: Molecular Dynamics Simulation and Experimental Validation*
- Adsorptive Thermodynamic Properties and Kinetics of trans-1,2-Cyclohexandiol onto AB-8 Resin
- Tracking Submicron Particles in Microchannel Flow by Microscopic Holography*
- Oxidation of p/o-Cresols to p/o-Hydroxybenzaldehydes Catalyzed by Metalloporphyrins with Molecular Oxygen*
