微米橄欖石型LiFePO4的水熱合成優(yōu)化
孫孝飛 徐友龍,* 劉養(yǎng)浩 李 璐
(1西安交通大學(xué),電子陶瓷與器件教育部重點(diǎn)實(shí)驗(yàn)室,西安710049;2西安交通大學(xué),國(guó)際電介質(zhì)研究中心,西安710049; 3西安交通大學(xué)化學(xué)系,西安710061)
1 Introduction
Rechargeable lithium-ion batteries(LIBs)are now widely used in laptops,cell phones,cameras,and other portable electronic devices.At the same time,they are also considered to have broad applications in energy storage systems for high efficiency energy transmission and distribution as the fossil fuels have limited resources and severe environmental pollutions.1,2For instance,lithium-ion battery is the most hopeful energy storage technology for electric vehicles(EVs)including hybrid electric vehicles(HEVs)and plug-in hybrid electric vehicles (PHEVs).Since the successful commercialization of carbon as the anode by Sony Corporation in 1992,3world-wide research has been dedicated to develop high-performance cathode materials.
Olivine lithium iron phosphate(LiFePO4)is one of the most promising cathode materials for LIBs because of its intrinsic thermal stability,high theoretical capacity(170 mAh·g-1),environmental benignity,and cost effectiveness.1,4Since first studied by Padhi et al.5in 1997,a lot of effort has been put on this material to find new synthesis routes6,7and to understand the relationship between the physicochemical properties and the crystal structures,8-13so as to improve its electrochemical performance.14The main obstacle hindering the large-scale commercialization of LiFePO4in EVs/HEVs/PHEVs is the low conductivity that constrains its high-rate performance.From ab-initio calculation,Morgan et al.15proposed that Li+diffuses preferentially via a one-dimensional channel along[010]direction,which was further confirmed computationally by Islam et al.16and experimentally by Nishimura et al.17Therefore,particle size plays an important role and nanoparticles are no doubt beneficial for lithium migration.14It has been found that during electrochemical charge/discharge,the solid-solution phase increases with the reduction of particle size.18,19Recently,Malik?s computation revealed the particle size dependence of Li+diffusivity which can be easily blocked in large particle LiFePO4.20Therefore,nano-sized LiFePO4is intentionally made to shorten Li+diffusion path.21,22Besides,surface coating23and bulk doping24are employed to improve the electrical conductivity and ultimately the battery performance of LiFePO4.
However,the tap density of nano materials is lower comparing with that of micro-sized counterparts.Therefore,the volumetric capacity as well as the energy density and power density are decreased.25An ideal case would be having good performance while still keeping particle size in micro scale.Unfortunately,there is no such material reported yet due to the above assumptions.Furthermore,the pathological underneath mechanism that large particle LiFePO4has poorer electrochemical performance is still unclear.We think a reliable groundwork of preparing a representative LiFePO4with single olivine phase, appropriate particle size,uniform morphology,and homogeneous particle distribution is of great importance to start investigation on such fundamental mechanism.Hydrothermal synthesis has been successfully used to prepare olivine LiMPO4(M=Fe,Mn,Co,etc.)family compounds as an effective and low cost method.26,27Its specialty in easy control of particle size and morphology makes it facile in studying the structure and property of LiFePO4.28But a systematic investigation is still needed to fully understand the influence of synthesis condition on the structure and property of the final product.29
2 Experimental
2.1 Material preparation
A series of LiFePO4with different particle sizes and morphologies were prepared hydrothermally by adjusting the synthesis parameters.The starting materials of LiOH·H2O(>98%, Alfa Aesar),FeSO4·7H2O(>99%,Alfa Aesar),and H3PO4(>99%,Aldrich)were mixed together in de-ionized water by a molar ratio of 3:1:1,a little citric acid(>99.5%,Aldrich)was added to prevent oxidation of Fe2+.The pH value was controlled by LiOH or citric acid.The mixture was then transferred into a Teflon-lined stainless steel autoclave which was sealed into an oven.Different temperatures and heating times were implemented to modify the particle size and morphology of LiFePO4.After heat treatment,the products were washed and filtered for several times.The final samples were collected by drying at 80°C under vacuum for 12 h.
2.2 Characterization
Powder X-ray diffraction(XRD)was conducted to identify the phase structures on a Rigaku diffactometer with Cu Kαradiation at the 2θ range of 10°-80°.The particle morphology was observed by a JEOL 6320FV field emission scanning electron microscopy(FE-SEM)with an accelerating voltage of 5 kV.
2.3 Battery performance
Swagelok half cells using lithium metal as the anodes were assembled in an Argon filled glove box.The cathodes were consisted of LiFePO4,super P,and polytetrafluoroethylene(PTFE) in a mass ratio of 80:15:5.The electrolyte was 1 mol·L-1LiPF6in ethylene carbonate/dimethyl carbonate(EC/DMC,1:1)and the separator was Celgard 2500.The batteries were cycled with constant currents on anArbin BT2000 at room temperature.
3 Results and discussion
3.1 Hydrothermal synthesis
In this work,the influences of precursor concentration,pH value,hydrothermal temperature,and heating time on the particle size and morphology of LiFePO4were investigated.
3.1.1 Precursor concentration
The concentration of FeSO4·7H2O was altered to 0.16,0.32, 0.48,and 0.60 mol·L-1while the molar ratio of Li+:Fe2+:PO34-was kept stoichiometrically at[Li+]:[Fe2+]:[PO34-]=3:1:1.The pH value was not changed intentionally,and was 7.87,6.93, 6.85,and 6.89 respectively after mixing.The mixture was heated at 190°C for 60 h.No detective impurities can be found by X-ray diffraction in Fig.1 for all the 4 samples.The difference of peak intensities among these samples is attributed to different atom ordering and preferred orientation in the crystals as can be seen by SEM images in Fig.2.The particle grows bigger when the precursor concentration is increased.At low concentration of[Fe2+]=0.16 mol·L-1,the primary particles are about 200-300 nm and aggregate to flower-like secondary particles with a typical size of~0.7 μm.When the concentration is increased to[Fe2+]=0.32 mol·L-1,the particle size is~1.8 μm with platelet-like morphology.Larger particles with sizes of~2.6 and~3.0 μm are prepared respectively when the concentration is further increased to[Fe2+]=0.48 mol·L-1and[Fe2+]= 0.60 mol·L-1.Moreover,the platelets are grown thicker to form diamond shapes,and the particle distribution gets more homogeneous.According to first principle calculation,the growing particles exhibit(010),(100),and(101)faces.30As identified by Chen et al.,31the large diamond surface corresponds to ac plane that is perpendicular to(010).When the diamond-shaped platelets grow thicker and bigger at higher concentrations,such preferred orientation generates higher(020) intensity on XRD patterns.Since Li+diffuses in a one-dimension channel along(010)direction,the thick profiles along (010)will increase the Li+diffusion path,and eventually degrade the electrochemical performance of LiFePO4.In case of [Fe2+]=0.48 mol·L-1,it shows slightly different relative peak intensity in X-ray diffraction because of partial morphology distortion to elongated hexagonal-prisms as shown in Fig.2(c).30It is also interesting to see small pores on the particle surface of some samples.The detailed mechanism is still unclear and needs further investigation.

Fig.1 XRD patterns of LiFePO4synthesized at different concentrations
3.1.2 pH value
The starting materials were kept at[Fe2+]=0.60 mol·L-1with a molar ratio of[Li+]:[Fe2+]:[PO3-4]=3:1:1.LiOH and citric acid were used to control the pH values at 10.05,7.05,and 4.50,respectively.The mixtures were heated at 190°C for 60 h.The initial pH value was about 6.50 when the precursors were mixed together.The XRD patterns and SEM images are shown in Fig.1([Fe2+]=0.60 mol·L-1)and Fig.2(d),respectively.The particle size is~3.0 μm and the morphology looks like a thick diamond.In the basic solution(pH=10.05),impurities of Li3PO4and Fe3O4are observed by X-ray diffraction in Fig.3.In order to adjust the pH value,large amount of LiOH is added and there is much lithium excess in the precursors.It is then possible to produce Li3PO4during hydrothermal synthesis,and the residual iron can also be oxidized.The typical particle size is about 0.5 μm as shown in Fig.4(a).Yu et al.32also found Li3PO4as an impurity in hydrothermally synthesized LiFePO4with extra lithium in the precursors,which was removed by washing the sample in a neutral buffer solution.When the synthesis solution is adjusted to neutral at pH=7.05,it results in pure-phase LiFePO4with a diamond shape,and the particle size is about 2.3 μm.It is noteworthy that the particles grow dramatically larger to~16.5 μm without any detectable impurities when the pH value is decreased to 4.50.The morphology is not homogeneous either(Fig.4(c)).Some are large bulks while others are agglomerates of needle-like particles.Therefore,the particles are grown bigger at lower pH which is consistent with ab-initio calculation by Wang et al.33that LiFePO4particles can be modified to large plates at lower pH value during solvent synthesis.One should also note that the large content citric acid might play a certain role as well during the particle formation of Fig.4(c).
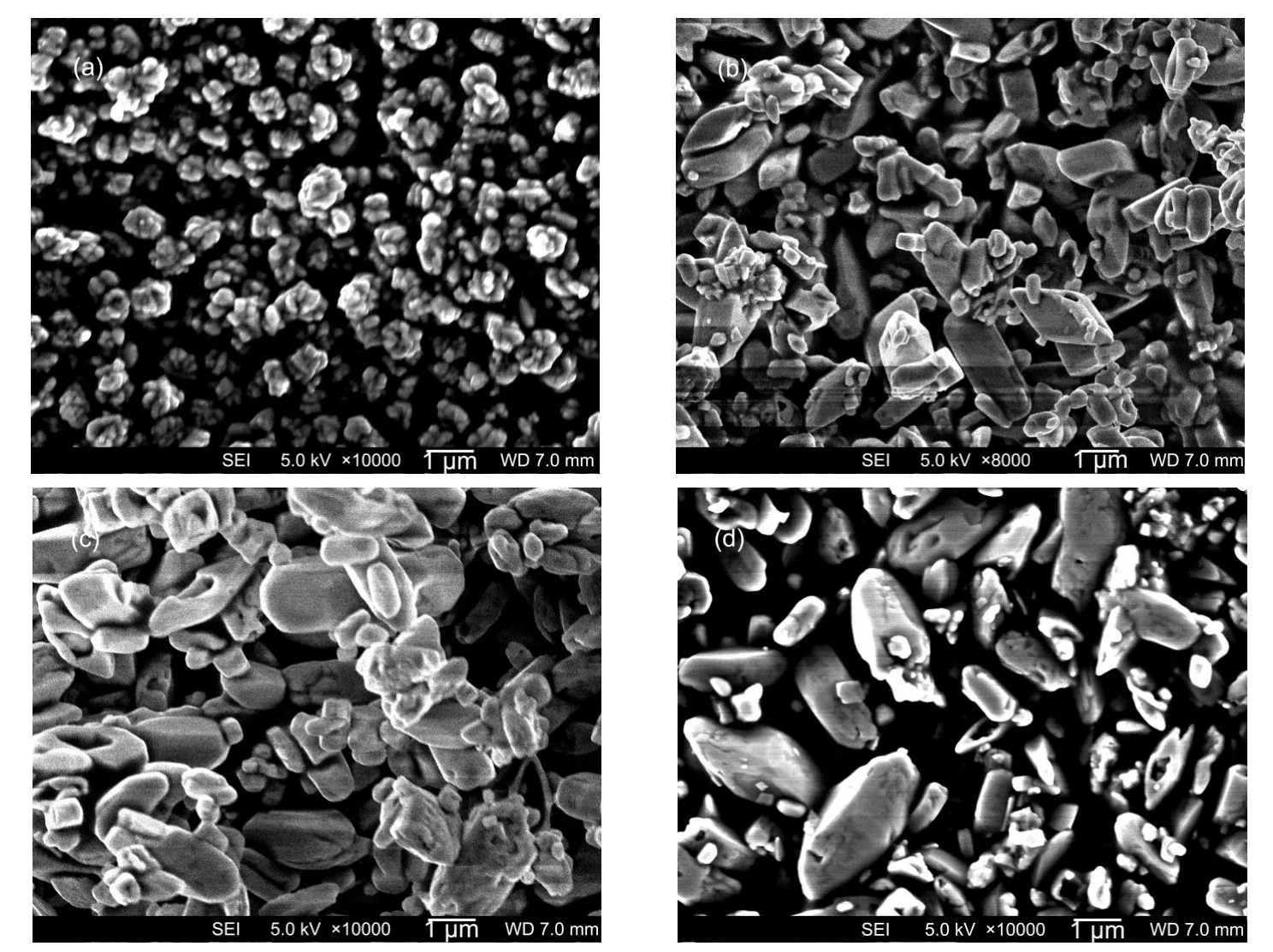
Fig.2 SEM images of LiFePO4synthesized at different concentrations[Fe2+]/(mol·L-1):(a)0.16,(b)0.32,(c)0.48,(d)0.60

Fig.3 XRD patterns of LiFePO4synthesized at different pH values (●)Li3PO4(JCPDS 74-0358),(?)Fe3O4(JCPDS 75-0033)

Fig.4 SEM images of LiFePO4synthesized at different pH values pH:(a)10.05,(b)7.05,(c)4.50
3.1.3 Hydrothermal temperature
In this group of experiments,the concentration was kept constant at[Fe2+]=0.60 mol·L-1with a molar ratio of[Li+]:[Fe2+]: []=3:1:1.The pH value was not changed when the precursors were mixed together,which was about 6.50.The mixture was heated at 170,190,and 220°C respectively for the same time of 60 h to find an optimum temperature of preparing homogeneous micro-sized LiFePO4.
As Dokko et al.34has pointed out,170°C is high enough to get phase-pure olivine LiFePO4(see Fig.5),but SEM picture in Fig.6(a)indicates that the particle size is only~0.9 μm.The particles are grown to~3.0 μm at 190°C as already discussed in Fig.2(d).However,further increasing the temperature to 220°C has little influence on modifying the particle size and morphology(Fig.6(b)).Ellis et al.35also used hydrothermal method to synthesize LiFePO4and found that the particles prepared at 190°C were bigger than that at 140°C,but they had no clue of further increasing the temperature.
3.1.4 Heating time
The heating time was set as 3,6,12,20,and 60 h respectively while the heat temperature was kept at 190°C.Other synthesis parameters were the same as in part 3.1.3.At 190°C, phase-pure LiFePO4could be obtained after 3 h(Fig.7),the longer time was then used to modify the particle size and morphology as shown in Fig.8.The typical particle size is only~0.6 μm when t=3 h.When it is prolonged to 6,12,and 20 h,the particle size increases to~1.5,~2.2,and~2.6 μm respectively and finally reaches~3.0 μm(t=60 h)in Fig.2(d).It seems that longer time is helpful for particle growth,but the electrochemical performance and the synthesis cost should also be considered in large-scale production.Therefore,a better way of synthesizing high performance large particle LiFePO4is combining all these factors together to get a group of optimized param-eters with a compromise as will be discussed later.
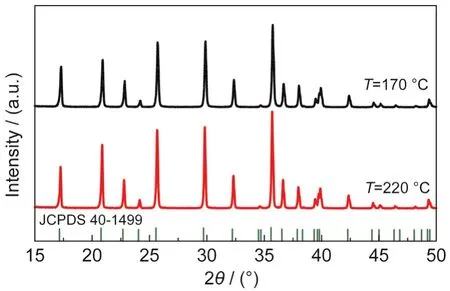
Fig.5 XRD patterns of LiFePO4synthesized at different temperatures
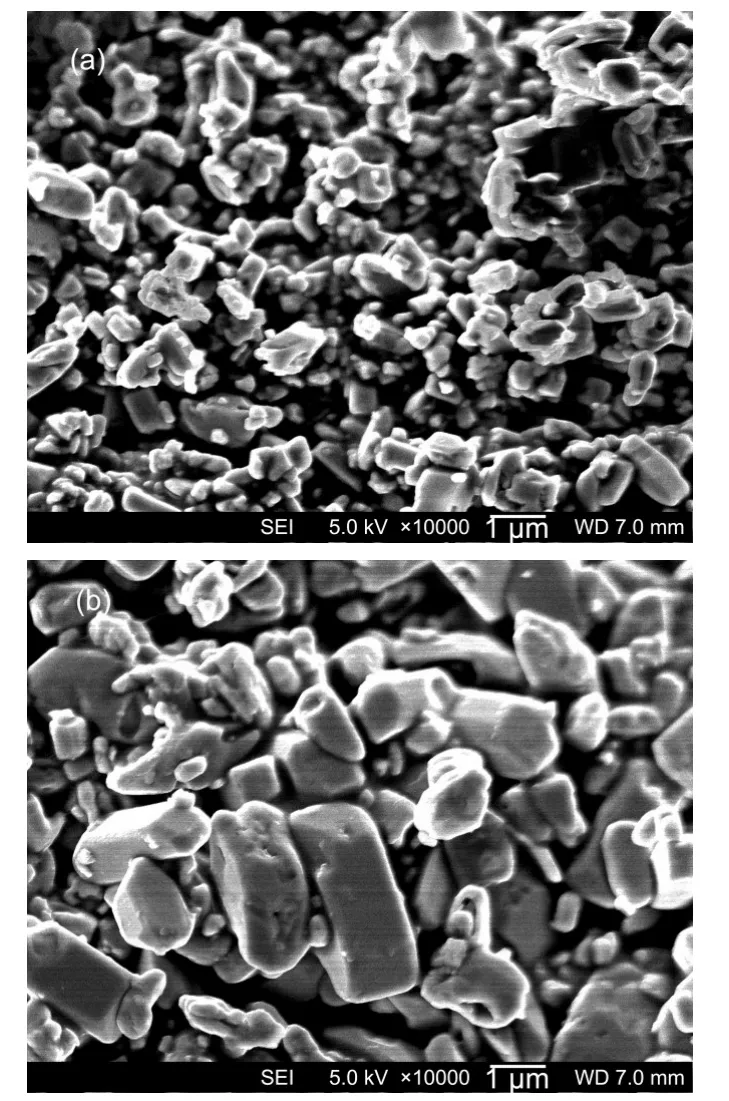
Fig.6 SEM images of LiFePO4synthesized at different temperatures T/°C:(a)170,(b)220
3.2 Electrochemical performance
Four representatives of the above LiFePO4with different sizes and morphologies were selected to study their electrochemical performance:PS0.7([Fe2+]=0.16 mol·L-1,Fig.2(a)),PS2.3 (pH=7.05,Fig.4(b)),PS3.0([Fe2+]=0.60 mol·L-1,Fig.2(d))and PS16.5(pH=4.50,Fig.4(c)),where PS stands for particle size and the number shows its value of~0.7,~2.3,~3.0,and~16.5 μm,respectively.

Fig.7 XRD patterns of LiFePO4synthesized for different heating times
Swagelok prototype batteries were assembled in an Ar-filled glove box and were cycled on an Arbin BT2000 between 2.0-4.3V(vs Li/Li+hereafter)at room temperature.The charge/ discharge curves are plotted in Fig.9(a)at a constant current density of 0.1C(1C=170 mA·g-1).All of them have the similar discharge plateau at 3.4 V that is characteristic of LiFePO4. The specific discharge capacities are 152,146,and 142 mAh· g-1when the particle sizes are 0.7,2.3,and 3.0 μm,respectively.It decreases dramatically to 80 mAh·g-1for PS16.5.One should also note the small discharge plateau at 2.5 V for PS0.7 which comes from reduction of Fe2O3according to Dokko et al.36Amorphous Fe2O3could be formed during hydrothermal synthesis in high temperature aqueous solutions.With only a little quantity,it cannot be detected by XRD.
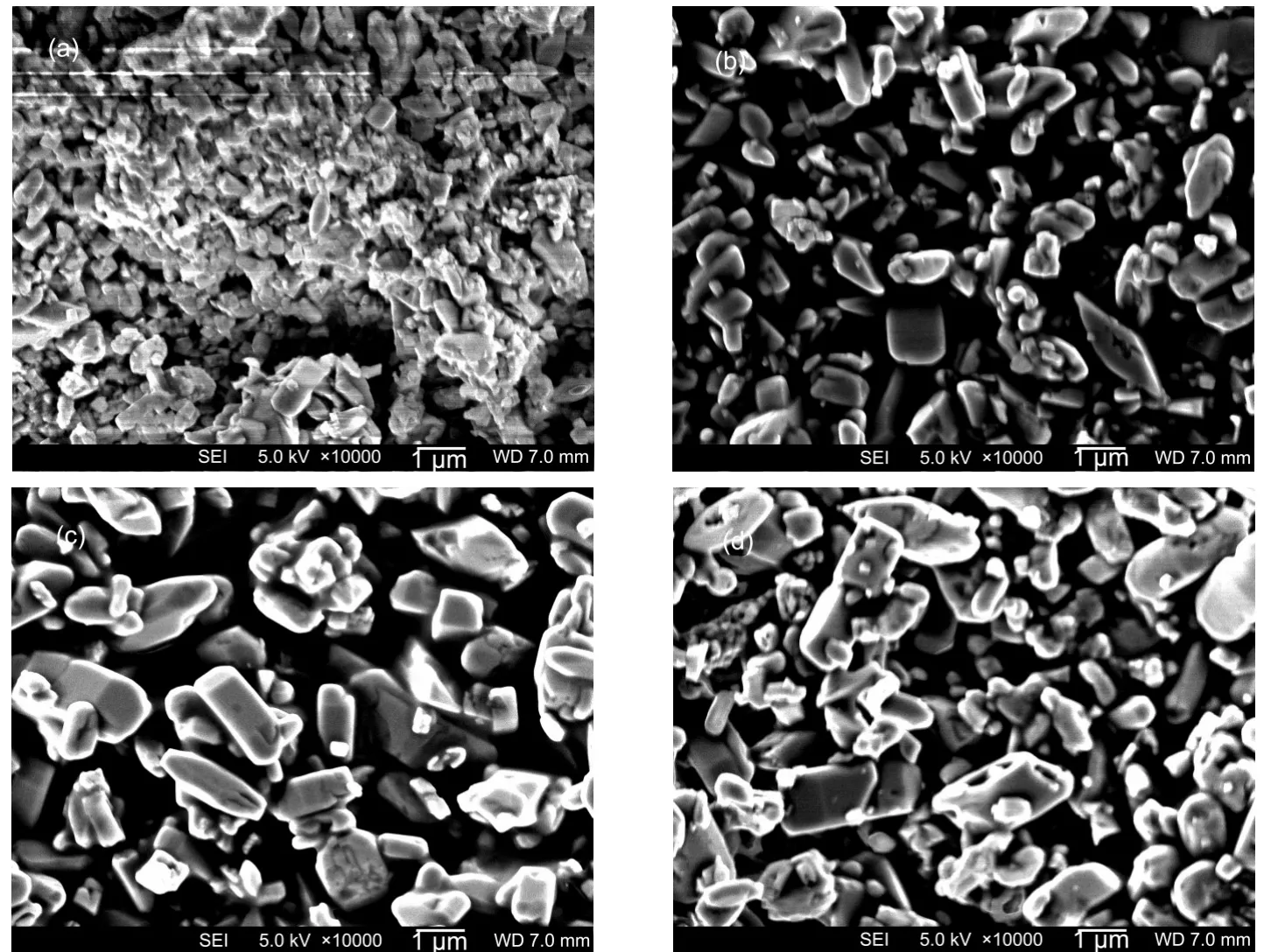
Fig.8 SEM images of LiFePO4synthesized for different heating times t/h:(a)3,(b)6,(c)12,(d)20

Fig.9 Charge/discharge curves at 0.1C rate(a)and constant current cyclings at 1C rate(b)of LiFePO4with different particle sizesContinuous lines and dotted lines in(a)correspond to the first and fifth cycles,respectively.The potential window in(b)is 2.0-4.3 V(vs Li/Li+).All tests are performed at room temperature and the cathodes are composed of 80%(w)active material,15%(w)conducting carbon(super P),and 5%(w)binder(PTFE).
The cells were then cycled at a constant current of 1C for 100 cycles between 2.0 and 4.3 V.No relaxation was allowed during cycling and the results are shown in Fig.9(b).LiFePO4with particle size of 0.7 μm has the highest initial capacity of 105 mAh·g-1and the highest capacity retention of 94.6%after 100 cycles,while PS16.5 has the lowest capacity of 40 mAh· g-1with the capacity retention of 82%.The performance difference between PS2.3 and PS3.0 is not significant at the beginning where they have a similar capacity of 92 mAh·g-1,but PS3.0 loses more capacity after 100 cycles with a capacity retention of only 77.2%(vs 83.77%for PS2.3).The results are in agreement with the charge/discharge curves.Remember that there is no modification such as surface coating or bulking doping for these pristine samples,both Fig.9(a)and Fig.9(b)demonstrate the intrinsic degradation of electrochemical performance in large particle LiFePO4that deserves further investigation.
3.3 Optimized micro LiFePO4
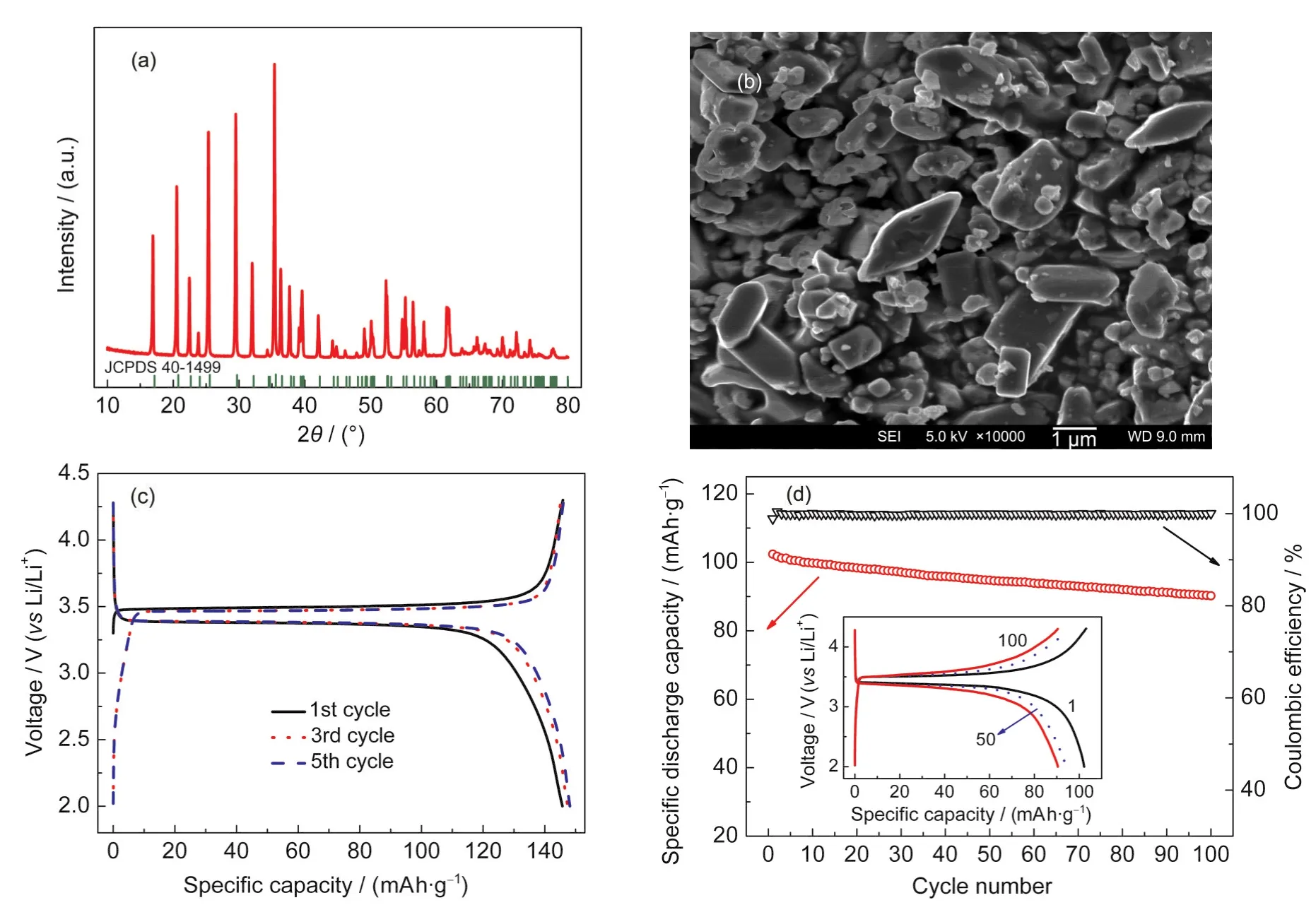
Fig.10 Optimized micro-sized LiFePO4as a cathode for LIBs(a)and(b)are the XRD pattern and SEM image of hydrothermally prepared LiFePO4respectively,(c)is the charge/discharge curve at 0.1C rate and(d)is the cycling performance at 1C rate between 2.0 and 4.3 V(vs Li/Li+).The inset in(d)shows the charge/discharge curves of the 1st,50th,and 100th cycles respectively along with cycling.All tests are performed at room temperature and the cathode composition is LiFePO4/super P/PTFE of 80/15/5(mass ratio).
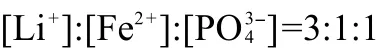
Based on the above results,an optimized synthesis condition for micro-sized LiFePO4was chosen as follows:[Fe2+]=0.4 mol· L-1with a stoichiometric ratio of ,the pH value was adjusted to 7.05 by a little LiOH,and the mixture was hydrothermally heated at 200°C for 24 h.Phase-pure olivine LiFePO4is prepared as indicated by XRD in Fig.10(a). The SEM image in Fig.10(b)shows that the particles are mainly~2 μm size with distorted thick diamond shapes that are typical for hydrothermally synthesized LiFePO4as discussed before.Moreover,the particle distribution in concern of size and morphology is more homogeneous than the aforementioned samples.The charge/discharge curves of this optimized LiFe-PO4at a constant current of 0.1C are shown in Fig.10(c).Its initial specific discharge capacity is 146 mAh·g-1and a very slight discharge plateau at 2.5 V of Fe2O3could still be found in the first cycle but seems disappeared along with cycling.37The specific discharge capacity of the fifth cycle is stabilized at 148 mAh·g-1with a mono plateau at 3.4 V.When the charge/ discharge rate is changed to a higher current of 1C(Fig.10(d)), the capacity decreases dramatically to 102 mAh·g-1and decays to 90 mAh·g-1after 100 cycles with a retention of 88.24%.It follows well with the above performance trend in micro-sized LiFePO4and lies between sub-micro particles and large micro particles.Without any modification(coating/doping),the capacity is reasonable and kind of acceptable but the rate and cycle performance still need to be enhanced.Moreover,the inset of Fig.10(d)indicates that the charge/discharge curves are slightly transformed to slope-like profiles with increased polarization along with cycling.Therefore,it is a good candidate for investigating the plausible mechanism of performance degradation in large particle LiFePO4.If the electrochemical property LiFePO4with such particle size could be improved,the practical performance especially the volumetric performance of LIBs as well as that of the end applications such as HEVs,PHEVs,and EVs could be much elevated.
4 Conclusions
The particle size of olivine LiFePO4increases with the increase of precursor concentration,heat temperature and heating time but with the decrease of pH value during hydrothermal synthesis.The specific capacity drops from 152 to 80 mAh·g-1at 0.1C rate and from 105 to 40 mAh·g-1at 1C rate when the particle size is increased from 0.7 to 16.5 μm demonstrating the intrinsic electrochemical performance degradation in large particle LiFePO4.An optimized 2 μm LiFePO4with relatively homogeneous size and morphology distribution shows reasonable capacity without any modification such as surface coating or bulk doping.Further work is undergoing to understand the underneath mechanism of performance loss in large particle LiFePO4and to improve its battery performance accordingly so as to get high energy/power density cathodes for lithium ion batteries in high efficiency energy storage applications.
(1)Whittingham,M.S.Chem.Rev.2004,104,4271.doi:10.1021/ cr020731c
(2) Yang,Z.;Liu,J.;Baskaran,S.;Imhoff,C.;Holladay,J.D. Journal of the Minerals,Metals and Materials Society 2010,62, 14.
(3) Ozawa,K.Solid State Ionics 1994,69,212.doi:10.1016/0167-2738(94)90411-1
(4) Ellis,B.L.;Lee,K.T.;Nazar,L.F.Chem.Mater.2010,22,691. doi:10.1021/cm902696j
(5) Padhi,A.K.;Nanjundaswamy,K.S.;Goodenough,J.B. J.Electrochem.Soc.1997,144,1188.doi:10.1149/1.1837571
(6) Jugovic,D.;Uskokovic,D.J.Power Sources 2009,190,538. doi:10.1016/j.jpowsour.2009.01.074
(7) Franger,S.;Le Cras,F.;Bourbon,C.;Rouault,H.J.Power Sources 2003,119-121,252.
(8) Cabana,J.;Shirakawa,J.;Chen,G.Y.;Richardson,T.J.;Grey, C.P.Chem.Mater.2010,22,1249.doi:10.1021/cm902714v
(9)Ong,S.P.;Wang,L.;Kang,B.;Ceder,G.Chem.Mater.2008, 20,1798.doi:10.1021/cm702327g
(10) Recham,N.;Casas-Cabanas,M.;Cabana,J.;Grey,C.P.;Jumas, J.C.;Dupont,L.;Armand,M.;Tarascon,J.M.Chem.Mater. 2008,20,6798.doi:10.1021/cm801817n
(11) Zhou,F.;Maxisch,T.;Ceder,G.Phys.Rev.Lett.2006,97,4.
(12)Yamada,A.;Koizumi,H.;Nishimura,S.I.;Sonoyama,N.; Kanno,R.;Yonemura,M.;Nakamura,T.;Kobayashi,Y.Nat. Mater.2006,5,357.doi:10.1038/nmat1634
(13) Delacourt,C.;Poizot,P.;Tarascon,J.M.;Masquelier,C.Nat. Mater.2005,4,254.doi:10.1038/nmat1335
(14) Kang,B.;Ceder,G.Nature 2009,458,190.doi:10.1038/ nature07853
(15) Morgan,D.;Van der Ven,A.;Ceder,G.Electrochem.Solid-State Lett.2004,7,A30.
(16) Islam,M.S.;Driscoll,D.J.;Fisher,C.A.J.;Slater,P.R.Chem. Mater.2005,17,5085.doi:10.1021/cm050999v
(17) Nishimura,S.I.;Kobayashi,G.;Ohoyama,K.;Kanno,R.; Yashima,M.;Yamada,A.Nat.Mater.2008,7,707.doi: 10.1038/nmat2251
(18) Kobayashi,G.;Nishimura,S.I.;Park,M.S.;Kanno,R.; Yashima,M.;Ida,T.;Yamada,A.Adv.Funct.Mater.2009,19, 395.doi:10.1002/adfm.v19:3
(19) Gibot,P.;Casas-Cabanas,M.;Laffont,L.;Levasseur,S.; Carlach,P.;Hamelet,S.;Tarascon,J.M.;Masquelier,C.Nat. Mater.2008,7,741.doi:10.1038/nmat2245
(20) Malik,R.;Burch,D.;Bazant,M.;Ceder,G.Nano Lett.2010, 10,4123.doi:10.1021/nl1023595
(21) Delacourt,C.;Poizot,P.;Levasseur,S.;Masquelier,C. Electrochem.Solid-State Lett.2006,9,A352.
(22) Herle,P.S.;Ellis,B.;Coombs,N.;Nazar,L.F.Nat.Mater. 2004,3,147.doi:10.1038/nmat1063
(23) Herstedt,M.;Stjerndahl,M.;Nytén,A.;Gustafsson,T.; Rensmo,H.;Siegbahn,H.;Ravet,N.;Armand,M.;Thomas,J. O.;Edstr?m,K.Electrochem.Solid-State Lett.2003,6,A202.
(24) Chung,S.Y.;Bloking,J.T.;Chiang,Y.M.Nat.Mater.2002,1, 123.doi:10.1038/nmat732
(25)Oh,S.W.;Bang,H.J.;Myung,S.T.;Bae,Y.C.;Lee,S.M.; Sun,Y.K.J.Electrochem.Soc.2008,155,A414.
(26) Chen,J.;Vacchio,M.J.;Wang,S.;Chernova,N.;Zavalij,P.Y.; Whittingham,M.S.Solid State Ionics 2008,178,1676.doi: 10.1016/j.ssi.2007.10.015
(27) Yang,S.;Zavalij,P.Y.;Whittingham,M.S.Electrochem. Commun.2001,3,505.doi:10.1016/S1388-2481(01)00200-4
(28) Chen,G.;Song,X.;Richardson,T.J.J.Electrochem.Soc.2007, 154,A627.
(29) Zhao,H.C.;Song,Y.;Guo,X.D.;Zhong,B.H.;Dong,J.;Liu, H.Acta Phys.-Chim.Sin.2011,27,2347.[趙浩川,宋 楊,郭孝東,鐘本和,董 靜,劉 恒.物理化學(xué)學(xué)報(bào),2011,27, 2347.]doi:10.3866/PKU.WHXB20110905
(30) Fisher,C.A.J.;Islam,M.S.J.Mater.Chem.2008,18,1209. doi:10.1039/b715935h
(31) Chen,G.;Song,X.;Richardson,T.J.Electrochem.Solid-State Lett.2006,9,A295.
(32) Yu,D.Y.W.;Donoue,K.;Kadohata,T.;Murata,T.;Matsuta,S.; Fujitani,S.J.Electrochem.Soc.2008,155,A526.
(33) Wang,L.;Zhou,F.;Meng,Y.S.;Ceder,G.Phys.Rev.B 2007, 76,165435.doi:10.1103/PhysRevB.76.165435
(34)Dokko,K.;Koizumi,S.;Kanamura,K.Chem.Lett.2006,35, 338.doi:10.1246/cl.2006.338
(35)Ellis,B.;Kan,W.H.;Makahnouk,W.R.M.;Nazar,L.F. J.Mater.Chem.2007,17,3248.doi:10.1039/b705443m
(36) Dokko,K.;Shiraishi,K.;Kanamura,K.J.Electrochem.Soc. 2005,152,A2199.
(37) Sun,X.;Xu,Y.Mater.Lett.2012,84,139.doi:10.1016/ j.matlet.2012.06.053

