Submicron γ-LiAlO2 Powder Synthesized from Boehmite*
CHENG Jian (程健)**, GUO Liejin (郭烈錦), XU Shisen (許世森), ZHANG Ruiyun (張瑞云) and LI Chen (李晨)
1 School of Energy and Power Engineering, Xi’an Jiaotong University, Xi’an 710049, China
2 Huaneng Clean Energy Technology Institute, Beijing 100086, China
1 INTRODUCTION
Molten carbonate fuel cell (MCFC), which is a type of high temperature fuel cell, has attained prominence in recent years because of its high efficiency,high tolerance to impurities, and flexibility in selection of fuels [1, 2]. The MCFC shows a great potential as an alternative for conventional thermal power plants [3].
The matrix, one of the components of MCFC,plays an important role in supporting the electrolyte in its pores to form a paste-like structure [4] for ionic transport and reactant gas separation. High total porosity, narrow pore size distribution, stable electrolyte retention, and mechanical strength are required with matrix for MCFC application. The compound γ-LiAlO2has good thermophysical, chemical, and mechanical stability properties at high temperature [5]. In fact,γ-LiAlO2remains stable [6] in molten alkali carbonate melts at the typical MCFC operating temperature (650 °C)[7], and is a widely used matrix material in MCFCs.
The electrolyte matrix of MCFC is required to have high porosity (50%-70%) and a sharp pore-size distribution (0.1-0.3 μm) for stable electrolyte retention by capillary action [8]. As the raw material for preparing the electrolyte matrix, γ-LiAlO2is required to have a specific area of over 20 m2·g-1and an average particle diameter of less than 0.2 μm.
Conventionally, γ-LiAlO2is prepared by solid state method, molten salt hydrolysis and sol-gel method [9-11]. Additional new routes such as combustion synthesis method are attributed to solid state method. The solid-state method is the simplest one,but the particles recombine easily and the particle size of the product powder is affected by the choice of reactant materials. The molten salt hydrolysis method produces γ-LiAlO2with low purity, and the sol-gel method encounters difficulty in controlling the morphology of the particles. All of these methods need a reagent to disperse the reactants. Chen et al. [12] used ethanol to disperse pure γ-Al2O3and Li2CO3, and then ball-milled, dried and calcined the resulting mixture to obtain γ-LiAlO2powder with an average diameter of 0.3 μm. Wang and Zhao [13] used citric acid to disperse LiNO3and Al(NO3)3·9H2O, but the nitrate radical introduced in this method may cause erosion and NO2emission. However, all of the above mentioned syntheses employ aluminum nitrate or high-purity γ-Al2O3(relatively expensive) and dispersed by alcoholic solvents. In these regards, boehmite (AlOOH·nH2O)seemed to be more practical because of being relatively cheap and its high dispersibility in water.
In this work, we used water as the dispersant, and then put LiOH and a highly water-dispensable material, boehmite, into the dispersant to form a mixture which is dried and calcined subsequently to synthesize submicron γ-LiAlO2powder. Analytical techniques,such as thermogravimetric analysis (TGA), X-ray diffraction (XRD) and scanning electron microscopy(SEM), were employed to study the composition and morphology of the synthesized powder.
2 EXPERIMENTAL
2.1 Preparation of lithium aluminate
In order to find a simple and environmentally friendly way to synthesize γ-LiAlO2powders with fine crystal structure and high purity, four samples of γ-LiAlO2powder were prepared using different materials and four methods mentioned above.
2.1.1 Sample 1
Sample 1 was prepared by the sol-gel method. In this procedure, 0.1 mol lithium nitrate and 0.1 mol aluminum nitrate were dissolved in 200 ml distilled water [12], and 20.14 g citric acid was added to the solution. Ammonium hydroxide was slowly added to adjust the pH of the solution into the range of 8-9 and to stabilize the nitrate-citrate solution. During the procedure, the solution was kept at room temperature and continuously stirred, and a transparent and homogeneous sol was obtained. The sol was kept at 80 °C to produce a gel, which was subsequently distilled at 100 °C for 12 h and evaporated to condense at 70 °C to yield a yellow dope liquid with strong acidity that would erode a stainless steel spoon. Some brown and noxious gas was released during the course of evaporation. The yellow liquid was dried in an oven at 110 °C for 24 h, and a light green powder was obtained. The light green powder was calcined at 900 °C for 6 h to yield a white powder.
2.1.2Sample 2
Sample 2 was also prepared by the sol-gel method. However, AlCl3and Li2CO3were used as Al and Li sources instead to reduce the acidity of the liquid. In this procedure, 13.3 g AlCl3and 3.7 g Li2CO3were dissolved in 200 ml distilled water, and 20.14 g citric acid was added to the solution. Ammonium hydroxide was slowly added to adjust pH of the solution into the range of 8-9 and to stabilize the nitrate-citrate solution. Then the solution experienced the same process as Sample 1 to get a white powder.
2.1.3Sample 3
Solid-state method was employed. In this procedure, 7.4 g Li2CO3(A.R. grade) and 10.2 g γ-Al2O3were dispersed in 70.4 g ethanol [13]. The mixture was ball-ground for 24 h, dried at 80 °C for 24 h and calcined at 900 °C for 5 h to produce the γ-LiAlO2powder. The γ-Al2O3was of analytical grade with average particle size of 20-30 nm, specific area over 100 m2·g-1,pore volume of 0.4 cm3·g-1. The γ-Al2O3reactant was product from Shanghai Reagent Factory of China Pharmaceutical Group.
2.1.4Sample 4
Sample 4 was also prepared by the solid-state method. In this procedure, 12 g LiOH was dissolved in 100 ml distilled water, and then 30 g boehmite(AlOOH·nH2O) was added to the LiOH solution to obtain a mixture. The mixture was stirred at 80 °C to get a well-dispersed sol. The water in the sol was evaporated at 80 °C to yield a primary product that was subsequently calcined at 900 °C for 2 h to yield the γ-LiAlO2powder. The boehmite has average particle size of 20-30 nm, specific area over 100 m2·g-1, pore volume of 0.4 cm3·g-1, and over 95% dispersible in the water.
All of the chemicals except boehmite were of analytical grade and were used without further purification. The boehmite powder was purchased from Tianjin Chemist Scientific Co., China.
2.2 Characterization of lithium aluminate powders
Phase purity of the powders obtained is an important criterion [14] for the selection of the γ-LiAlO2powder synthetic production process. The phase structures of the four samples were characterized by an X-ray diffractometer (D/max-3A, Rigaku, Japan)with Cu Kαradiation. Fig. 1 and Table 1 present the XRD analysis results. The BET surface area analyzer(Physisorption analyzer, ASAP-2020, Micromeritics,USA) was employed to measure the specific surface area of the prepared lithium aluminate powders. The comparison of various synthesis routes and their effects on BET surface area is shown in Table 2.

Table 1 γ-LiAlO2 compounds in the samples obtained by different methods
For the XRD patterns of Sample 1 and Sample 2,a large amount of γ-LiAlO2phase, as well as some of LiAl5O8phase, is observed. The result is similar to the results reported by Khomaneet al[15]. The XRD patterns of Sample 3 and Sample 4 show that the main component is γ-LiAlO2together with Li2CO3and LiAl5O8[16]. In the XRD pattern of Sample 4, the X-ray diffraction peaks of γ-LiAlO2phase are more intense and narrower than those of other samples.
Table 1 shows that the powder in Sample 4 possesses a higher purity of γ-LiAlO2phase than that of other samples. The comparison of four sample powders shows that the crystals of Sample 4 prepared from boehmite and LiOH are small and well defined.
A high specific surface area denotes the increased chemical reactivity for oxidation, reduction, catalysis,tendency for compound formationetc. Considering that the synthesis routes of the γ-LiAlO2powders were only chemical methods, the crystalline type of the four samples is as unique as those obtained from X-ray diffraction analysis. Therefore, as for the same type of material, the greater the surface area is, the smaller the particle size will be.
The nitrogen adsorption (Physisorption analyzer,ASAP-2020) BET surface area of the lithium aluminate sample prepared using boehmite and LiOH was 22 m2·g-1, which was higher than that of Samples 1 and 2 prepared by the sol-gel method and Sample 3 prepared by the solid-state method, due to smaller crystalline sizes (Table 2). Thus γ-LiAlO2materials prepared by boehmite are expected to have better chemical reactivity than that prepared by other methods.
The method to prepare γ-LiAlO2using boehmite is simple, low-cost and environmentally friendly. In addition, sample synthesized by this method provides the best product with the maximum content of γ-LiAlO2, as observed from Table 2. So this method seems to be the best technique to prepare γ-LiAlO2.
Other several analysis techniques were adopted to characterize the structural and morphological properties of the γ-LiAlO2powders prepared at different temperature. The crystalline phases of the powders prepared at different temperatures were determined by XRD (D/max-3A) with radiation of Cu Kαat the scanning speed of 2°·min-1. The mass and heat rate change of the primary product were analyzed by a TGA/DSC

Table 2 Comparative results of LiAlO2 products obtained through various methods

Figure 2 XRD patterns for the primary product obtained from boehmite and LiOH at 80 °C1—Li2Al4 (CO3)(OH)12 (JCPDS 37-0728); 2—Li2CO3 (JCPDS 22-1141); 3—LiOH·H2O (JCPDS 24-0619)
analyzer (SDT-Q600, TA, USA) with a heating rate of 5 °C·min-1and a protective nitrogen atmosphere. SEM(Philips 515, Eindhoven, Netherlands) was used to study the morphology of the sintered powders. A laser particle-size analyzer (S3500, Microtrac, USA) was used to measure the particle size distribution of the lithium aluminate powders.
3 RESULTS AND DISCUSSION
3.1 Phase and particle morphology
Figure 2 shows the XRD patterns of the primary product obtained from the reactant mixtures evaporated and dried at 80 °C. Three different phases were found in the XRD pattern of the product, with Li2Al4(CO3)(OH)12·3H2O (lithium aluminate carbonate hydroxide hydrate) being the major phase and Li2CO3and LiOH·H2O being the minor ones.
Tallant and Eatough [17] found that Li2Al4(CO3)(OH)12·3H2O was stable below 175 °C and lost water and carbon dioxide to transform to Al2O3and Li2Al4O7(CO2)0.1·10.5H2O at 275 °C. In order to study the γ-LiAlO2powders transformed from Li2Al4(CO3)(OH)12·3H2O, the primary product powders synthesized from AlOOH and LiOH were heated at 130, 180, 300, 600, 800, 900 or 950 °C, each for 3 h.Fig. 3 presents the comparison of the X-ray diffractograms of the primary products heated at 130 °C and 180 °C for 3 h, and Fig. 4 gives the diffractograms of the primary products heated at different temperatures from 300 °C to 950 °C respectively for 3 h.

Figure 3 XRD patterns of powders obtained from the primary products heated at 130 °C and 180 °C1—β-LiAlO2; 2—Li2Al4O7 (CO2)0.1·10.5H2O; 3—Li2CO3

Figure 4 XRD patterns of powders obtained from the primary products heated at different temperature1—γ-LiAlO2 (JCPDS 38-1461); 2—Li2CO3 (JCPDS 22-1141); 3—LiAl5O8 (JCPDS 38-1425); 4—β-LiAlO2 (JCPDS 33-0785)
The XRD patterns in Fig. 3 show that the main components are Li2Al4O7(CO2)0.1·10.5H2O and Li2CO3at 130 °C, while at 180 °C, the main components are β-LiAlO2and Li2Al4O7(CO2)0.1·10.5H2O. The low intensity and broad diffraction peaks of the XRD patterns indicate that the powders must consist of abundant non-crystalloid phase [18]. From the crystal phase change in Figs. 2 and 3, we could conclude that when heated Li2Al4(CO3)(OH)12would lose water and CO2and transform into Li2Al4O7(CO2)0.1·10.5H2O and β-LiAlO2phases, and CO2was adsorbed on the surface of LiOH particles to form Li2CO3.
Figure 4 shows the XRD patterns for the primary product powders heated at different temperatures. The diffraction peaks gradually became sharper and narrower with increasing temperature, as more quantities of crystals were generated above 300 °C and the content of γ-LiAlO2reached a highest value at 950 °C.The trend of the composition change for the calcined primary product is shown in Fig. 4. In the course of heating, Li2Al4O7(CO2)0.1·10.5H2O lost water and CO2to generate β-LiAlO2[19], which further transformed into γ-LiAlO2. A small amount of LiAl5O8was observed when the baking temperature was above 900 °C. Crystals of aluminum compounds other than LiAlO2were not found in the XRD patterns during the primary product calcining. The absence of XRD signals from other crystalline compounds of aluminum might be due to the other aluminum compounds being non-crystalline,so that the diffraction peaks were not distinct.
Using the integrated intensity of content phase diffraction peaks of products at different sintering temperature in Fig. 4, the relative phase content is calculated as showed in Table 3.

Table 3 The relative content of products at different sintering temperature
Clearly seen from Table 3, most β-LiAlO2was transformed into γ-LiAlO2starting from the temperature of 800 °C, and the relative content of γ-LiAlO2in sintering products reached 98.27% at 950 °C. The relative content of γ-LiAlO2and a small amount of Li2CO3in the sintering products at 900 °C and 950 °C had little difference. Since molten Li2CO3can be used as electrolyte and kept in the matrix pores of MCFC,small amount of Li2CO3in γ-LiAlO2has little effect on the performance of the matrix. Therefore, the optimal sintering temperature turned out to be 900 °C.
Figure 5 shows the SEM images of the primary products calcined at 800 °C and 900 °C for 3 h at each temperature. The calcined product at 800 °C appears to be irregular and heterogeneous, with multiple particles coalesced to form a mass. The particles became well-distributed and loose at 900 °C with an average diameter of 0.2 μm, which is smaller than that recorded in other papers [10-13].
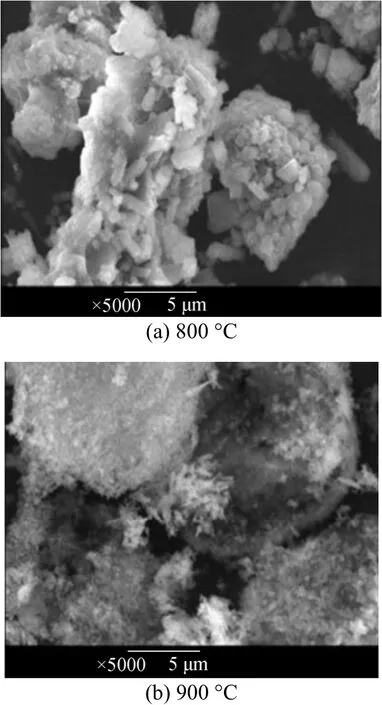
Figure 5 Patterns of the powders sintered from boehmite and LiOH at (a) 800 °C and (b) 900 °C
The electron micrographs of powders at different temperature in Fig. 5 shows that as the temperature increases, the agglomeration gradually disappears, and the γ-LiAlO2particle crystal becomes more stable and smaller in size. This is the same with the trends that the diffraction peak of γ-LiAlO2becomes sharper and narrower as the temperatures increases. The comparison result indicates that the property of γ-LiAlO2powder calcined at 900 °C is better than that at 800 °C.
3.2 γ-LiAlO2 formation mechanism
3.2.1Theoretical basis for the γ-LiAlO2 production process
The method of preparing γ-LiAlO2powder with boehmite and LiOH is considered to be a solid-state method. According to the theory of solid-state reactions, the activity of the aluminum compound is lower than that of the lithium compound, so that the reactions occur on the crystal surface and in the crystal lattice of the aluminum compound. Boehmite is a precursor to the production of the nanometer material Al2O3. With good reactivity, high specific area and smaller nanometer particle size, boehmite could disperse well in the water and react rapidly with Li+on the surface of AlOOH to generate Li2Al4(CO3)(OH)12. The mechanism of formation of the phase Li2Al4(CO3)(OH)12·3H2O may be explained by the fact that CO2in the air was adsorbed on the surface of AlOOH and reacted with LiOH in water during stirring to form Li2Al4(CO3)(OH)12·3H2O.The process can be expressed as:
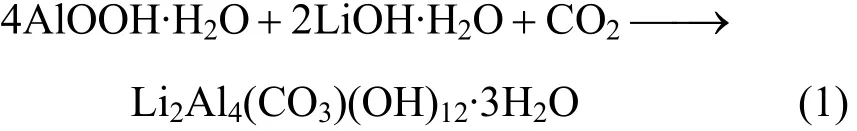
Li2Al4(CO3)(OH)12is unstable so that it loses water and CO2and transforms to β-LiAlO2and Li2CO3at the low temperature of 180 °C. As the temperature increasing and along with the lattice surface and internal state changing, the reactivity of Li2CO3increases continuously. When the reaction temperature reaches the melting point of Li2CO3(720 °C) , Li2CO3changes into a molten state, and its viscosity decreases, and then Li+is sucked into the gap of the Al compound crystal layer and reacts with Al3+to produce β-LiAlO2or γ-LiAlO2. Due to the fact that β-LiAlO2is a sub-stable phase and the reaction happens in a very high temperature, thus the reaction rate is relatively faster, producing directly the products γ-LiAlO2instead of the sub-stable γ-LiAlO2. A13+and Li+can exchange with each other to generate Li2Al4(CO3)(OH)12in the layer of crystal Li2Al4(CO3)(OH)12. The reaction occurs inside the crystal. Li2CO3reacts with aluminum compounds to generate γ-LiAlO2on the surface of the aluminum compounds. The transformation of LiOH to Li2CO3occurs from the surface to the interior of crystal.
3.2.2Experimental verification of the γ-LiAlO2 production process
The transformation process of γ-LiAlO2from the primary product can be verified by the TG/DSC curves (Fig. 6). Several reactions occurred when the primary product was heated.
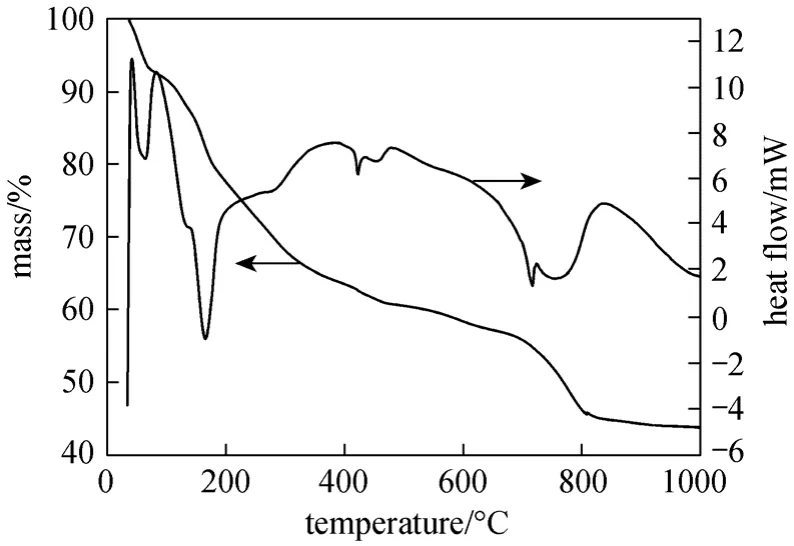
Figure 6 TG/DSC analysis curves of the primary product obtained from boehmite and LiOH
The TGA curve of the primary product shows four distinct steps. In the first step, mass loss occurred over a temperature range from room temperature to approximately 130 °C (11%), due to desorption of adsorbed water molecules, and LiOH·H2O and Li2Al4(CO3)(OH)12decomposed to emit water and CO2to generate Li2CO3and Li2Al4O7(CO2)0.1·10.5H2O. An endothermic event for mass loss and an exothermic event for crystallization transition are indicated by the DSC curve. In the second step, a sharp mass loss occurred around 180 °C and continued over the temperature range from 180 °C to 700 °C (33%).Li2Al4O7(CO2)0.1·10.5H2O decomposed completely to generate β-LiAlO2; endothermic and exothermic phenomena were also observed. In the third step, the mass loss was much less (about 11%) in the temperature range between 700 °C and 900 °C. An exothermic event started at 760 °C with a maximum at 850 °C,corresponding to the formation of γ-LiAlO2from β-LiAlO2, as confirmed by the XRD pattern in Fig. 4.Li2CO3could decompose at 730 °C, as shown by an endothermic peak in the DSC curve. Li2CO3could react with some Al compounds to generate γ-LiAlO2,leading to a sharp decrease in the content of Li2CO3.No significant mass loss was observed above 900 °C,suggesting a high thermal stability for the calcined product.
The particle size of the LiAlO2product is determined by the particle size of the aluminum compound.Boehmite is considered to be a suitable material for producing well-distributed submicron γ-LiAlO2. A small amount of Li2CO3and a small amount of LiAl5O8have no impact on the performance of γ-LiAlO2, which indicates that the optimal temperature for preparing γ-LiAlO2is 900 °C.
3.3 Test of γ-LiAlO2 performance in MCFC
The matrix of the MCFC was prepared with the γ-LiAlO2powder [20] and other materials such as polyvinyl butyral, butanone, absolute ethanol and cyclohexanone [21]. The composition of the tape-casting slurry of the γ-LiAlO2matrix was shown in Table 4.All materials were mixed and ground in a ball mill for30 to 40 h to obtain well-proportioned slurry, which was tape cast to produce a matrix film of 0.1 mm thickness. Two pieces of the matrices were heated at 80 °C and pressed at 3 MPa to get one dense matrix for the thickness of 0.5 mm.
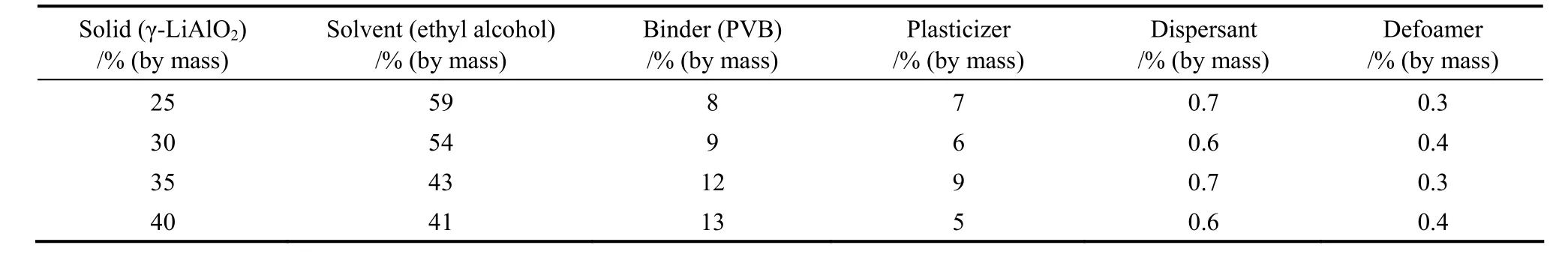
Table 4 Tape-casting slurry composition of the γ-LiAlO2 matrix
Figure 7 shows the SEM photographs of the surface of the matrix sintered at 700 °C for 3 h without impregnating the matrix with electrolytes under ambient pressure. The aperture of a single matrix is less than 5 μm when the organic material has been removed by oxidative combustion. In order to decrease the size of the aperture to yield a denser matrix, two pieces of matrix are compressed to a dense single piece at 120 °C under 2 MPa for 3 min.

Figure 7 The SEM photographs of the surfaces of the matrix sintered without impregnating with electrolyte
A single MCFC was assembled to test the performance of the matrix in preventing gas leakage. The MCFC consisted of sintered nickel electrodes, matrices, carbonate (Li2CO3∶K2CO3, 62%∶38%) as electrolyte films and stainless steel bipolar compounds.The available area of the MCFC is 100 cm2. The anode had a porosity of 60% (by volume) and a pore size in the range 3-6 μm. The cathode had a porosity of 65%(by volume) and a pore size in the range 6-12 μm.The bipolar plates performed as the current conductor and provided conduits for reactant gases flow. The bipolar plates were commonly made of stainless steel for high corrosion resistance and good surface contact resistance. Testing was conducted in a 12 cm×12 cm cell which had an electrochemical active area of 10 cm×10 cm. Two matrices and three carbonate sheets were stacked like a sandwich, which contacted with electrodes and stainless steel bipolar plates to assemble into a single MCFC as shown in Fig. 8.
The single MCFC was heated from room temperature to 650 °C under a specific heating program. The single MCFC sintering program was show in Table 5.
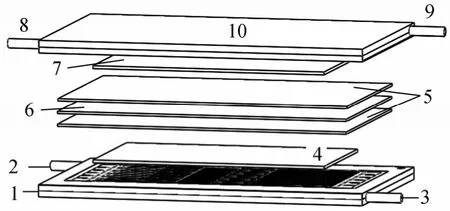
Figure 8 Schematic of a single MCFC1—cathode bipolar; 2—gas inlet; 3—gas outlet; 4—cathode electrode; 5—electrolyte; 6—matrix; 7—anode electrode; 8—gas outlet; 9—gas inlet; 10—anode bipolar
The organic materials were removed from the MCFC at 450 °C through vaporization and combustion. The carbonates were molten from 480 °C and were immersed in the micro-holes of the matrix with capillary force. A gas crossover measure [22] was carried out when the temperature of the single MCFC rose to 540 °C. The cathode entrance and the anode outlet were shut, and the nitrogen pressure of the anode entrance was gradually increased to test the gas crossover performance of the matrix. The gas crossover of the matrix occurs when gas is released from the cathode outlet. The pressure under which gas crossover occurs is the pressure required for gas leakage prevention. The test result showed that the gas crossover occurred when the nitrogen pressure reached 0.8 MPa,indicating that the matrix prepared from γ-LiAlO2powder could prevent gas leakage until 0.8 MPa.
Open circuit voltage appeared when H2was put into the anode, and air and CO2were put into the cathode. The performance of the MCFC was tested with an electric load above 650 °C when the reactant gas differential pressure was 0.2 MPa between the anode and the cathode.
A fuel cell testing device (FCTS, Arbin, USA)was used to measure the current and voltage of the single MCFC. The current and voltage of the electric load were 60 A and 20 V. The current and voltage of the single MCFC are shown in Fig. 9. The red and blue curves stood for the discharge voltage and current respectively. The discharge testing was executed in the program of constant current discharge from 1A to 9A.The voltage changed according to the discharge current. The discharge testing lasted for 150 min, and the results showed a maximum discharge current of 9A and a maximum open-circuit voltage above 1 V.During the course of the testing, the voltage of theMCFC remained stable when the MCFC discharged with different currents. The testing results demonstrated good performance of the matrix prepared from boehmite and γ-LiAlO2powder in preventing gas leakage,meeting the requirements of the MCFC.

Table 5 Sintering program of MCFC

Figure 9 Performance of a single MCFC with a matrix made of γ-LiAlO2 from boehmite
4 CONCLUSIONS
The present study suggests that γ-LiAlO2synthesized from boehmite and LiOH·H2O has excellent performance characteristics due to the high level of dispersion and reactivity of boehmite. Li2Al4(CO3)(OH)12is a component of the primary product derived from the sol of AlOOH and LiOH, which decomposes to produce Li2Al4O7(CO2)0.1·10.5H2O at 130 °C, and Li2Al4(CO3)(OH)12, which lose water and CO2to transform gradually into γ-LiAlO2within the temperature range of 130 °C to 900 °C. Phase and particle morphology analysis showed that the γ-LiAlO2prepared with boehmite has elevated purity and produced well-distributed particles. Compared with other methods for γ-LiAlO2powder synthesis, the approach to preparing γ-LiAlO2from boehmite and LiOH is the most promising because it is a simple, energy-saving and pollution-free method.
The matrix made of γ-LiAlO2powder using Boehmite show good performance in preventing gas leakage, and the γ-LiAlO2powder made from Boehmite is an appropriate material in the field of MCFCs.Preparing γ-LiAlO2with Boehmite is found to be an effective method.
It is believed that the gas barrier performance of electrolyte supporting plate and the electrochemical performance of the molten carbonate fuel cell could be largely improved through further optimizing the porosity of the electrolyte supporting plate.
1 Li, Y., Cao, G.Y., Yu, Q.C., “Daily operation optimization of a residential molten carbonate fuel cell power system using genetic algorithm”,J.Chem.Eng., 14 (3), 349-356 (2006). (in Chinese)
2 Yu, L.J., Yuan, J.Q., Cao, G.Y., Jiang X.M., “Numerical simulation of dynamic performance of the molten carbonate fuel cell”,J.Chem.Eng., 12 (2), 272-276 (2004). (in Chinese)
3 Xia, C., Li, Y., Tian, Y., Liu, Q., Zhao, Y., Jia, L., Li, Y., “A high performance composite ionic conducting electrolyte for intermediate temperature fuel cell and evidence for ternary ionic conduction”,J.Power Sources, 188 (1), 156-162 (2009).
4 Yuh, C., Johnsen, R., Farooque, M., Maru, H., “Status of carbonate fuel cell materials”,J.Power Sources, 56 (1), 1-10 (1995).
5 Lee, J.J., Choi, H.J., Hyun, S.H., Im, H.C., “Characteristics of aluminum reinforced LiAlO2matrices for molten carbonate fuel cells”,J.Power Sources, 179 (2), 504-510 (2008).
6 Terada, S., Nagashima, I., Higaki, K., Ito, K., “Stability of LiAlO2as electrolyte matrix for molten carbonate fuel cells”,J.Power Sources,75 (2), 223-229 (1998).
7 Suski, L., Tarniowy, M., “The phase stability of solid LiAlO2used for the electrolyte matrix of molten carbonate fuel cells”,J.Mater.Sci., 36 (21), 5119-5124 (2001).
8 Kim, S.D., Hyun, S.H., Shin, M.Y., Lim, T.H., Hong, S.A., Lim,H.C., “Phase and microstructure stabilities of LiAlO2in molten Li/Na carbonate for molten carbonate fuel cells”,J.Power Sources,143 (2), 24-29 (2005).
9 Sokolov, S., Stein, A., “Preparation and characterization of macroporous γ-LiAlO2”,J.Mater.Lett., 57 (22-23), 3593-3597 (2003).
10 Lin, H.X., Yi, B.L., Li, N.C., Kong, L.Y., Zhang, E.J., Qu, T.X.,Cheng, Y.C., Wang, F.X., “Investigation on fine α-LiAlO2powder for matrix material in MCFC by means of chloride synthesis”,J.Chin.Ceram.Soc., 27 (4), 452-460 (1999). (in Chinese).
11 Gao, Z.Q., Shen, X.D., Cui, S., Xiao, S., “Research on preparations of nano-LiAlO2micromerity”,J.Chin.Mater.Rev., 20 (11), 145-148(2006).
12 Chen, G., Fu, Y., Wang, H., Hu, K., “Research on the synthesis and reaction mechanism of sub-micron LiAIO2powders”,J.Chin.Inorg.Chem., 18 (2), 219 (2002). (in Chinese)
13 Wang, L., Zhao, M., “Synthesis of γ-LiAlO2ultra-fine powder by citrate method”,J.Chin.Ceram.Soc., 25 (5), 618-620 (1997). (in Chinese)
14 Kwon, S.W., Park, S.B., Seo, G., Hwang, S.T., “Preparation of lithium aluminateviapolymeric precursor routes”,J.Nucl.Mater., 257(2), 172-179 (1998).
15 Khomane, R.B., Agrawal, A., Kulkarni, B.D., “Synthesis and characterization of lithium aluminate nanoparticles”,Mater.Lett., 61(23-24), 4540-4544 (2007).
16 Kang, Y.C., Park, S.B., Kwon, S.W., “Preparation of submicron size gamma lithium aluminate particles from the mixture of alumina sol and lithium salt by ultrasonic spray pyrolysis”,J.Colloid.Interf.Sci.,182, 59-62 (1996).
17 Tallant, D.R., Eatough, M.O., “Thermal stability and decomposition kinetics of Li2Al4CO3(OH)12?3H2O”,J.Mater.Sci., 31 (16), 4321-4325(1996).
18 Chen, W., Yin, Z.L., Ma, Y.H., Wang, J., Liu, X.H., “Influence of sintering temperature to performance of pseudoboehmite gel particle”,J.Chin.Proc.Eng., 4 (8), 301-304 (2004). (in Chinese).
19 Kwon, S.W., Park, S.B., “Effect of precursors on the morphology of lithium aluminate prepared by hydrothermal treatment”,J.Mater.Sci., 35 (8), 1973-1978 (2000).
20 Kim, J.E., Patil, K.Y., Han, J., Yoon, S.P., Nam, S.W., Lim, T.H.,Hong, S.A., Kim, H., Lim, H.C., “Using aluminum and Li2CO3particles to reinforce theα-LiAlO2matrix for molten carbonate fuel cells”,Int.J.Hydrogen Energy, 34 (22), 9227-9232 (2009).
21 Seo, J.J., Kuk, S.T., Kim, K., “Thermal decomposition of PVB(polyvinyl butyral) binder in the matrix and electrolyte of molten carbonate fuel cells”,J.Power Sources, 69 (1-2), 61-68 (1997).
22 Li, Z., Lin, H.X., Yi, B.L., “Sintering behavior of porous α-lithium aluminate matrices in molten carbonate fuel cells at high temperature”,J.PowerSources, 164 (1), 24-32 (2007).
 Chinese Journal of Chemical Engineering2012年4期
Chinese Journal of Chemical Engineering2012年4期
- Chinese Journal of Chemical Engineering的其它文章
- HPLC Analysis of Egg Yolk Phosphatidylcholine by Evaporative Light Scattering Detector
- Removal of Organic Matter and Ammonia Nitrogen inAzodicarbonamide Wastewater by a Combination of Power Ultrasound Radiation and Hydrogen Peroxide*
- A Geometric Approach to Support Vector Regression and Its Application to Fermentation Process Fast Modeling*
- Three Dimensional Numerical Simulation of Convection-Condensation of Vapor with High Concentration Air in Tube with Inserts*
- Nanoparticle Migration in a Fully Developed Turbulent Pipe Flow Considering the Particle Coagulation*
- Regeneration of Spent Activated Carbon by Yeast and Chemical Method*
