Shuanghuanglian injection downregulates nuclear factor-kappa B expression in mice with viral encephalitis*★
Naibing Gu, Ye Tian, Zhengli Di, Caiping Han, Hui Lei, Gejuan Zhang
Department of Neurology, Xi’an Center Hospital, Xi’an 710003, Shaanxi Province, China
Shuanghuanglianinjection downregulates nuclear factor-kappa B expression in mice with viral encephalitis*★
Naibing Gu, Ye Tian, Zhengli Di, Caiping Han, Hui Lei, Gejuan Zhang
Department of Neurology, Xi’an Center Hospital, Xi’an 710003, Shaanxi Province, China
A mouse model of viral encephalitis was induced by intracranial injection of a Coxsackie virus B3 suspension. Quantitative real-time reverse transcription-PCR and western blot assay were applied to detect mRNA and protein expression of intelectin-2 and nuclear factor-kappa B in the viral encephalitis and control groups. Nuclear factor-kappa B and intelectin-2 mRNA and protein expression were significantly increased in mice with viral encephalitis. After intraperitoneal injection ofShuanghuanglianat a dose of 1.5 mg/kg for 5 successive days, intelectin-2 and nuclear factor-kappa B protein and mRNA expression were significantly decreased. To elucidate the relationship between intelectin-2 and nuclear factor-kappa B, mice with viral encephalitis were administered an intracerebral injection of 107pfu recombinant lentivirus expressing intelectin shRNA. Both protein and mRNA levels of intelectin and nuclear factor-kappa B in brain tissue of mice were significantly decreased. Experimental findings suggest thatShuanghuanglianinjection may downregulate nuclear factor-kappa B productionviasuppression of intelectin production, thus inhibiting inflammation associated with viral encephalitis.
intelectin; nuclear factor-kappa B; viral encephalitis; short hairpin RNA;Shuanghuanglianinjection; mice; lentivirus; nervous system disease; traditional Chinese medicine; neural regeneration
Research Highlights
(1) Expression of intelectin-2 and nuclear factor-kappa B mRNA and protein was significantly increased in brain tissue of mice with viral encephalitis.
(2)Shuanghuanglianinjection may downregulate intelectin-2 and nuclear factor-kappa B mRNA and protein expression in brains of viral encephalitis mice.
(3) Intelectin shRNA also decreased intelectin-2 and nuclear factor-kappa B mRNA and protein expression in brains of viral encephalitis mice.
(4) Intelectin is a potential target for the treatment of viral encephalitis.
(5)Shuanghuanglianadministration can downregulate nuclear factor-kappa B productionviasuppression of intelectin production, thus inhibiting inflammation associated with viral encephalitis.
Abbreviations
NF-κB, nuclear factor-kappa B; shRNA, short hairpin RNA
INTRODUCTlON
Encephalitis is of public health importance worldwide because of its high morbidity and mortality. However, many details regarding its epidemiology have yet to be elucidated[1]. We observed that nuclear factor-kappa B (NF-κB) expression was significantly increased in mice with viral encephalitis, andShuanghuanglianinjection significantly decreased NF-κB protein and mRNA levels[2]. The inhibitory effect was more significant with prolonged intervention duration and increased treatment dose[2]. These findings suggested thatShuanghuanglianinjection could be therapeutic for viral encephalitis by reducing the expression of NF-κB in a time- and dose-dependent manner[2]. However, the underlying mechanisms of the inhibitory effect ofShuanghuanglianinjection on NF-κB expression in mice with viral encephalitis remain unclear.
Previous studies showed that intelectin was upregulated in a mouse model of asthma and was required for the production of interleukin-13-induced monocyte chemotactic protein-1 and -3 in mouse lung epithelial cells and contributed to allergic airway inflammation[3]. This indicated that intelectin induced the upregulation of cytokines. Based on a preliminary study, we observed that intelectin and NF-κB expression were significantly increased in mice with viral encephalitis. We hypothesized that intelectin played a role in brain inflammation by regulating cytokine expression.
To test our hypothesis, we examined the kinetics of intelectin and NF-κB expression in a mouse model of viral encephalitis. We administered injection ofShuanghuanglianor recombinant lentivirus expressing intelectin short hairpin RNA (shRNA) to mice with viral encephalitis to determine whether intelectin is involved in the upregulation of NF-κB and brain inflammation.
RESULTS
Quantitative analysis of experimental animals
The study used 60 rats and consisted of three parts. First, 20 mice were equally divided into a viral encephalitis model (n=10) or control group (n=10). Second, 20 mice induced for viral encephalitis were equally divided into a model group andShuanghuangliantreatment group. Third, 20 mice with viral encephalitis were equally divided into a model group and shRNA group. Except for the control group, other groups of mice were administered an intracranial injection with Coxsackie virus B3 suspension to induce viral encephalitis. Mice in theShuanghuangliangroup and shRNA group were treated withShuanghuanglianinjection or lentivirus expressing intelectin shRNA, respectively. A total of 60 mice were involved in the final analysis.
lntelectin-2 and NF-κB were significantly upregulated in the brain of mice with viral encephalitis
We determined the level of intelectin-2 and NF-κB expression in mouse brain after induction of viral encephalitis with Coxsackie virus B3 encephalitis by quantitative real-time reverse transcription-PCR and western blot analysis. Intelectin-2 transcripts were significantly increased in viral encephalitis mice compared with controls (P<0.05; Figure 1). Western blot using an antibody that recognizes identical sequences in intelectin-1 and -2 showed that intelectin protein was significantly increased in mice with viral encephalitis (Figure 1). Similar to intelectin-2 RNA and protein expression, levels of NF-κB transcripts and protein were also increased (P<0.05; Figure 2). Our findings indicated that intelecin-2 and NF-κB mRNA and protein expression were upregulated in the brain tissue of mice with viral encephalitis.
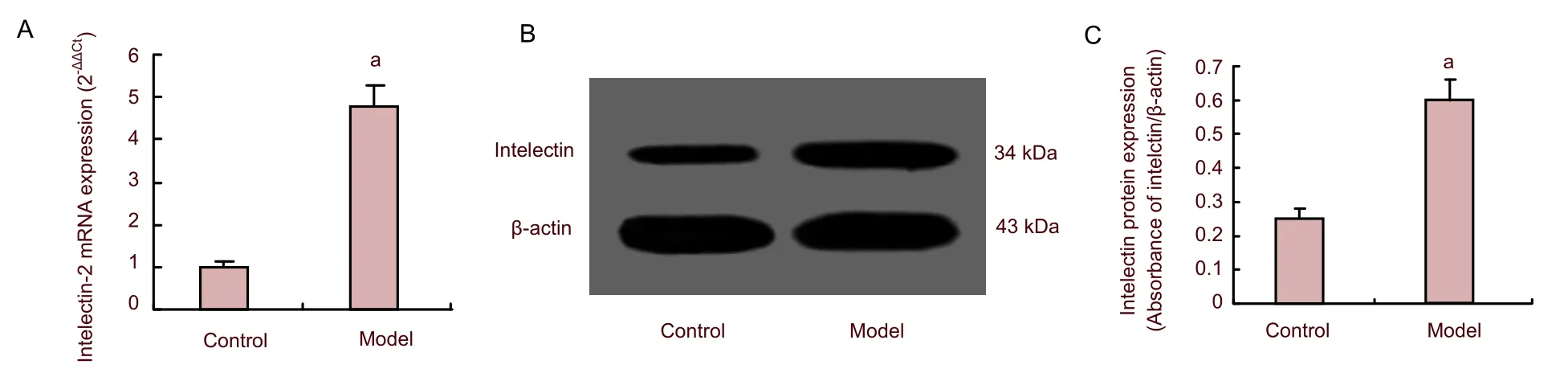
Figure 1 Expression of intelectin-2 mRNA (A: quantitative real-time reverse transcription-PCR) and protein (B, C: western blot assay) in the brain of viral encephalitis (model group) and normal (control group) mice.

Figure 2 Expression of nuclear factor-kappa B (NF-κB) mRNA (A: quantitative real-time reverse transcription-PCR) and protein (B, C: western blot assay) in the brain of viral encephalitis (model group) and normal (control group) mice.
Shuanghuanglian injection significantly downregulated intelectin-2 and NF-κB expression in the brain of mice with viral encephalitis
We next determined whether intelectin was decreased followingShuanghuanglianinjection. After induction of viral encephalitis, mice were administeredShuanghuanglianinjection intraperitoneally at a dose of 1.5 mg/kg, once daily, until sacrifice on day 5. We determined intelectin-2 and NF-κB expression by quantitative real-time reverse transcription-PCR and western blot analysis. Intelectin-2 mRNA and protein levels were markedly decreased in mice afterShuanghuanglianinjection compared with the model group (P<0.05; Figure 3). Simultaneously, protein and mRNA expression levels of NF-κB were significantly downregulated afterShuanghuanglianinjection (P<0.05; Figure 4). This suggested that intelectin-2 and NF-κB were downregulated in mice afterShuanghuangliantreatment.
lntelectin shRNA treatment significantly decreased intelectin-2 and NF-κB expression in the brain of mice with viral encephalitis
Based on the above results, we designed the third experimental study to verify the relationship between intelectin and NF-κB. To address this question, we administered intracerebral injection of 107pfu recombinant lentivirus expressing intelectin shRNA to mice 24 hours after infection with Coxsackie virus B3. Two days after lentivirus injection, mice were sacrificed to harvest brain tissues. Intelectin shRNA transfection inhibited the increase of intelectin-2 and NF-κB transcript and protein levels compared with the model group (P<0.05; Figures 5, 6). Histological analysis revealed that NF-κB positive cells were markedly decreased after intelectin shRNA transfection (P<0.05; Figure 7). This indicated that intelectin was required for the upregulation of NF-κB, and further verified that intelectin is involved in brain inflammation.
DISCUSSION
Intelectin was first reported to be expressed in small intestinal Paneth cells in mice[4]. There are two intelectin genes, intelectin-1 and -2, in mice and humans. These genes are highly homologous to a Xenopus oocyte granule lectin[5-6].
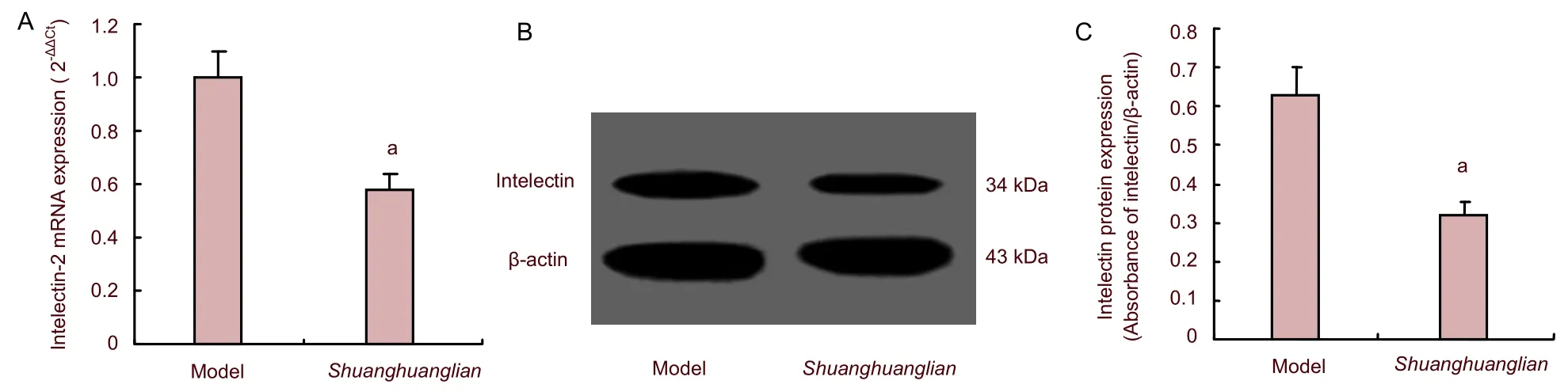
Figure 3 Expression of intelectin-2 mRNA (A: quantitative real-time reverse transcription-PCR) and protein (B, C: western blot assay) in mice with viral encephalitis (model group) and Shuanghuanglian group.
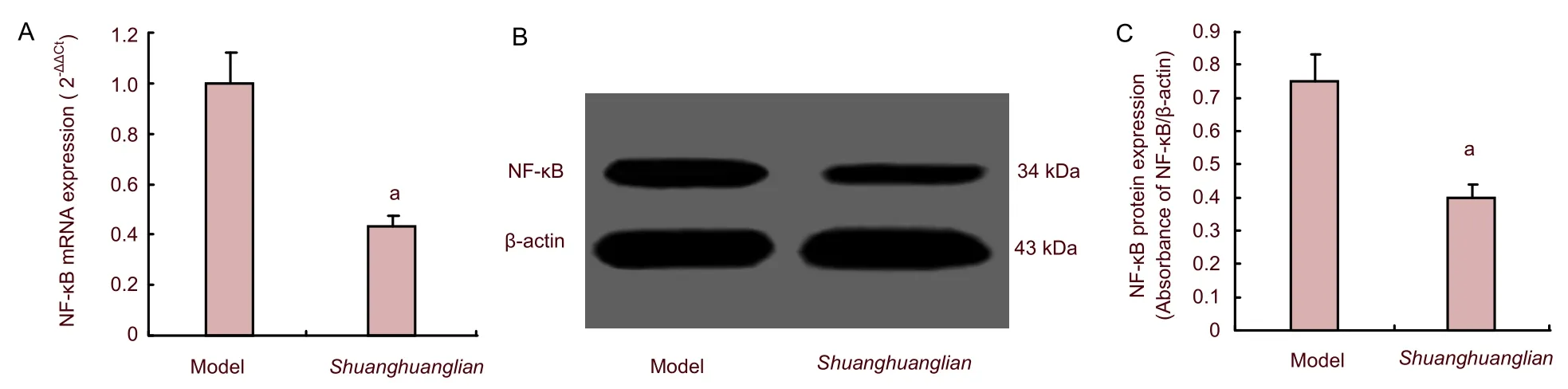
Figure 4 Expression of nuclear factor-kappa B (NF-κB) mRNA (A: quantitative real-time reverse transcription-PCR) and protein (B, C: western blot assay) in mice with viral encephalitis (model group) and Shuanghuanglian group.
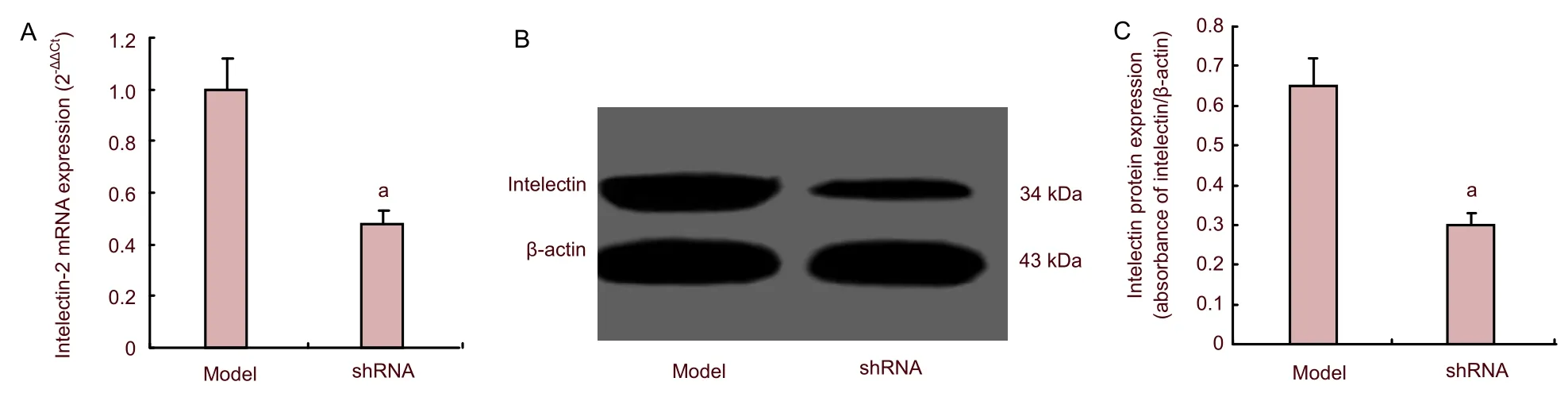
Figure 5 Expression of intelectin-2 mRNA (A: quantitative real-time reverse transcription-PCR) and protein (B, C: western blot assay) in mice with viral encephalitis (model group) and shRNA group.

Figure 6 Expression of nuclear factor-kappa B (NF-κB) mRNA (A: quantitative real-time reverse transcription-PCR) and protein (B, C: western blot assay) in mice with viral encephalitis (model group) and shRNA group.
The cDNA sequences for mouse intelectin-1 and -2 have 94% homology[6]. Human intelectin-1 is also known as omentin[7]and as intestinal lactoferrin receptor[8]. Since it is found in human omental adipose tissue and can bind to human lactoferrin, human intelectin-1 has been described as a soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell walls, indicating it may play a role in immune defense against bacteria[9].
NF-κB is a critical protein in the pathogenesis of inflammation, and NF-κB-targeted therapeutics may be effective in treating inflammatory diseases[10-11]. NF-κB is activated in mouse brain following reovirus infection, which is required for Bid cleavage and subsequent proapoptotic signaling[12-13]. Coxsackie virus B3 is a strong virulent pathogen that has been associated with serious diseases including myocarditis and encephalitis[14-15].
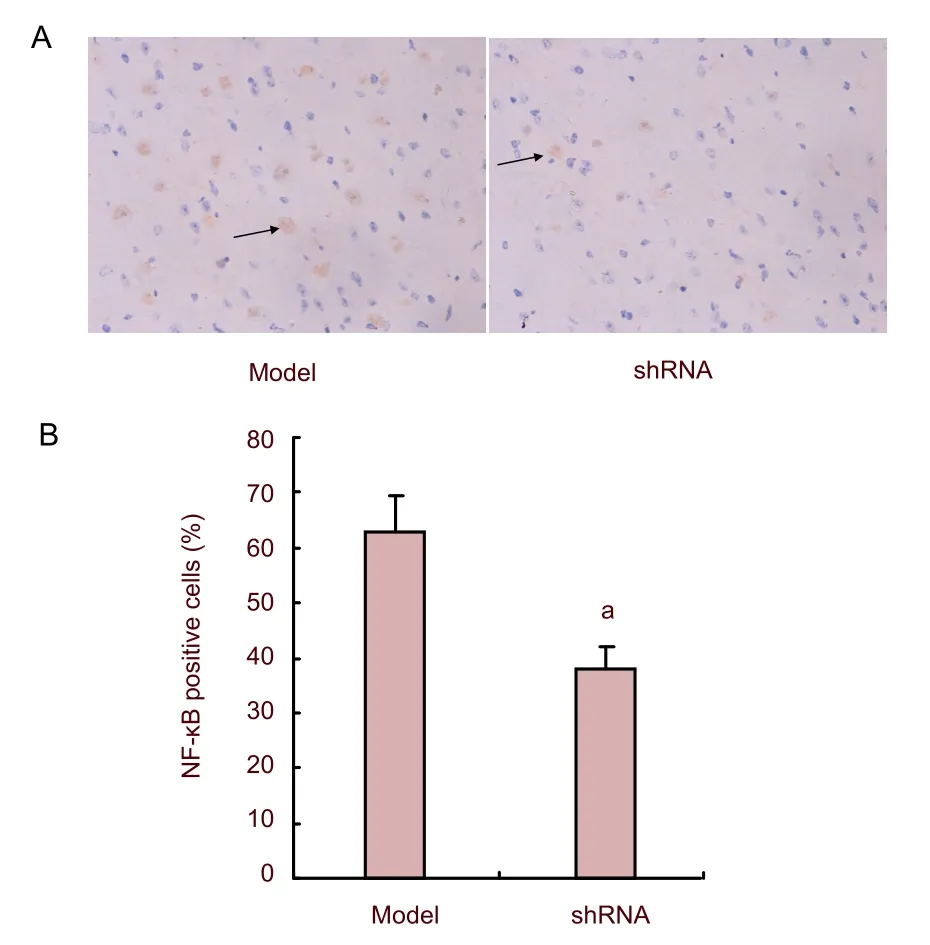
Figure 7 Effect of intelectin short hairpin RNA (shRNA) on nuclear factor-kappa B (NF-κB) positive cell expression in the brain of mice with viral encephalitis.
Previous studies indicated thatShuanghuanglianinjection has a vital antiviral role in inflammatory diseases[16-17]. Consistent with this, our previous results demonstrated thatShuanghuanglianinjection inibited NF-κB expression[2]; however, the related mechanism by whichShuanghuangliandownregulated NF-κB expression is unclear. To explore this mechanism, we designed the following experiments. First, we detected the level of intelectin-1 and -2 and NF-κB in the brains of mice with viral encephalitis and the control group.
Second, we assessed the inhibitory effect ofShuanghuanglianon the expression of intelectin-1 and -2 as well as NF-κB. Third, we observed expression of intelectin-1 and -2 as well as NF-κB in the brain of mice with viral encephalitis and treated with intelectin shRNA. We also analyzed the numbers of NF-κB-positive cells and levels of NF-κB proteins by western blot assays and quantitative real-time PCR.
We demonstrated that intelectin-2 and NF-κB were upregulated in the brain of mice with viral encephalitis compared with the control group. Protein and mRNA expression of NF-κB and intelectin-2 were simultaneously suppressed following treatment withShuanghuanglianinjection compared with the model group. These results suggestedShuanghuanglianinjection might decrease NF-κB expression levelviainhibition of intelectin-2 production. Recombinant lentivirus expressing intelectin shRNAviaintracerebral injection in mice with viral encephalitis downregulated protein and mRNA levels of intelectin and NF-κB. We also observed that NF-κB-positive cells were decreased in the shRNA group compared with the model group.
These results suggested thatShuanghuanglianinjection exerted a therapeutic effect against viral encephalitis by inhibiting the activation of NF-κB through modulating NF-κB gene expression. In addition,Shuanghuanglianinjection decreased the expression of NF-κB by lowering the expression of intelectin-2. The study also indicated that intelectin might be involved in the pathogenesis of viral encephalitis in mice. Consistent with this, we previously observed that intelectin was required for increased levels of inflammatory factors in the genesis and development of asthmatic airway inflammation[3].
In summary, this study indicated thatShuanghuanglianinjection exerted antiviral effects through modulating NF-κB gene expression. This also supports a role for intelectin in increased expression of NF-κB and brain inflammation in mice with viral encephalitis. The results indicate thatShuanghuanglianinjection may downregulate NF-κB expressionviasuppression of intelectin production. Thus, intelectin could be a candidate target for the treatment of viral encephalitis. Further investigations of the therapeutic utility of intelectin intervention in animal models of viral encephalitis are warranted.
MATERlALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The experiments were performed at the Central Laboratory of the Fourth Military Medical University of Chinese PLA between March 2009 and May 2011.
Materials
Sixty healthy, 4-week-old, BALB/c mice, male, weighing 20±2 g, were provided by the Laboratory Animal Center of the Fourth Military Medical University, China (license No. 08-33). The mice were housed at 22±2°C, with 50±5% humidity, and were allowed free access to water and chow. All experimental protocols were conducted in accordance with theGuidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[18].
Methods
Establishment of a mouse model of viral encephalitis
The modeling procedure was previously described[19-20]. In brief, following anesthesia with ether, the midpoint between the right eye and ear of mice was selected as the injection site. A sharp needle, 0.4 mm in diameter, was inserted perpendicularly into the brain at a depth of about 3 mm. Mice in the viral encephalitis group were intracranially injected with 20 μL of 4.5×108pfu of a Coxsackie virus B3 suspension (Wuhan Institute of Virology, Chinese Academy of Sciences, Hubei Province, China), which was propagated in HeLa cells (Tongji Medical College, Huazhong University of Science and Technology, China) and titered as previously described[21]. Mice from the control group were injected with 20 μL of saline. Clinical manifestations were monitored daily and scored as follows[22]: 0, no detectable sign of disease; 1, ruffled fur; 2, slightly hunched back and ruffled fur; 3, very hunched back and lethargy; and 4, death. Mice that were successfully infected and scored between 1–3 points were included in the study.
Shuanghuanglian treatment
After establishment of the viral encephalitis model, some mice were intraperitoneally injected withShuanghuanglianat dose of 1.5 mg/kg[2](a compound powder for injection; main components include Honeysuckle Flower, Radix Scutellariae and Fructus Forsythiae; National Medicine Permit No. Z23021513; Harbin Pharmaceutical Co., Ltd., Harbin, Heilongjiang Province, China), once daily, until sacrifice (a total of 5 days). Mice from the model group were injected with 20 μL of saline, once daily. The two groups were sacrificed on day 5.
Administration of lentivirus expressing intelectin shRNA
Mice in the shRNA group were administered an intracerebral injection of 107pfu recombinant lentivirus expressing intelectin shRNA (Tongji Medical College, Huazhong University of Science and Technology, China) 1 day after injection with Coxsackie virus B3 suspension. Intelectin shRNA significantly inhibited the basal level of both intelectin-1 and -2 transcripts[3]. The control model group was administered an empty plasmid. The two groups were sacrificed on day 3.
Tissue specimens
After abdominal anesthesia by chloral hydrate, mice brains were harvested. Brain tissues were incubated in 4% formalin for 24 hours for immunohistochemistry. Mice brains were isolated and stored at –80°C until used for western blot analysis.
Quantitative real-time reverse transcription-PCR for intelecin-2 and NF-κB mRNA expression
Total RNA from mouse brains was isolated using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA). First-strand cDNA synthesis was performed using PrimeScript RT reagent kit (Takara, Tokyo, Japan). SYBR Green real-time PCR was performed for mouse intelectin-2 and NF-κB using Perfect Real Time kit (Takara). Real-time PCR was performed using SYBR Premix Ex Taq polymerase (Takara) and Rotor-Gene 3000 (Corbett Research, Sydney, Australia). Two-step PCR amplification conditions are as follows: step 1: pre-denaturing, (95°C for 10 seconds, 20°C/s, 1 cycle); step 2: PCR reaction (95°C for 5 seconds, 20°C/s; 60°C for 20 seconds, 20°C/s; 40 cycles). The cycle threshold of each mouse gene transcript was normalized to the Ct of mouse β-actin. Fold differences were determined by the 2-ΔΔCtmethod[23]. The primer sequences are as follows:
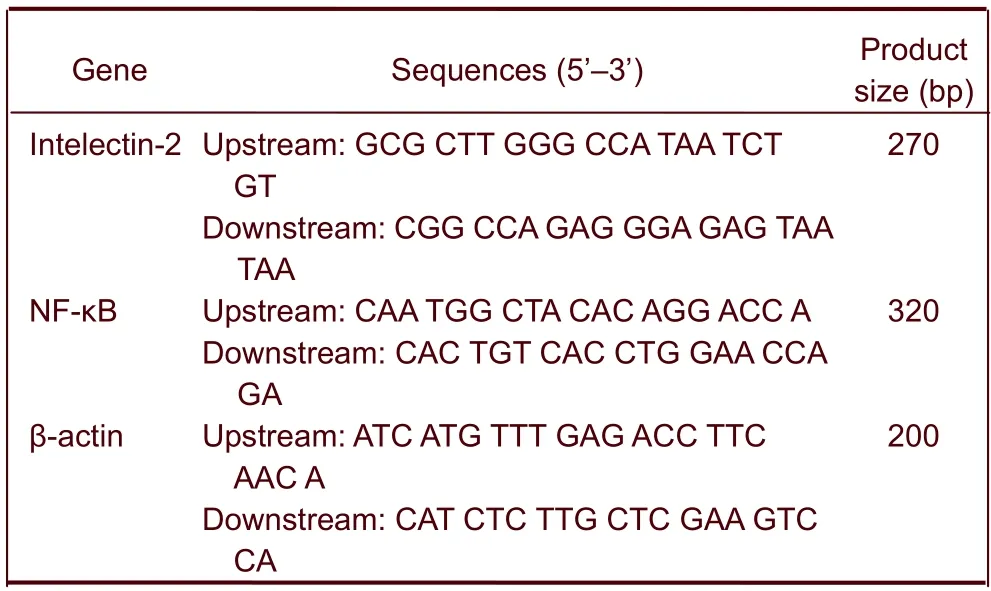
Gene Sequences (5’–3’) Product size (bp) Intelectin-2Upstream: GCG CTT GGG CCA TAA TCT GT Downstream: CGG CCA GAG GGA GAG TAA TAA 270 NF-κB Upstream: CAA TGG CTA CAC AGG ACC A Downstream: CAC TGT CAC CTG GAA CCA GA 320 β-actin Upstream: ATC ATG TTT GAG ACC TTC AAC A Downstream: CAT CTC TTG CTC GAA GTC CA 200
Western blot analysis for intelecin-2 and NF-κB protein expression
Mouse brain tissue was harvested at the indicated time points, washed twice with cold PBS, and lysed in Mammalian Protein Extraction Reagent with phenylmethylsulfonyl fluoride (Pierce Biotechnology Inc., Rockford, IL, USA). Protein concentrations were determined using the Bradford method[24]. After 15 minutes on ice, lysates were centrifuged at 15 000×gfor 15 minutes to remove insoluble material. 50 μg of protein samples were resolved by 10% SDS-PAGE, transferred and immuno-probed with chicken anti-mouse monoclonal antibody against intelectin (1:750), and incubated for 12 hours at 4.0°C. The intelectin antibody (Division of Veterinary Clinical Sciences, University of Edinburgh, Roslin, UK) was raised against a peptide containing a sequence that is completely conserved between mouse intelectin-1 and -2[25]. The antibody was detected using peroxidase-conjugated rabbit anti-chicken IgY (1:5 000; Sigma, St. Louis, MO, USA; incubating for 1 hour at room temperature) followed by ECL (Pierce Biotechnology). Blots were stripped and then reprobed for rabbit anti β-actin polyclonal antibody (1:1 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). For NF-κB western blot analysis, rabbit anti-mouse NF-κB monoclonal antibody (1:250; Serotec, Oxford, UK) was used. The antibody was detected with peroxidase-conjugated goat anti-rabbit IgG (1:1 000; Zhongshan Golden Bridge Biotechnology, Beijing, China). Densitometry was performed using Image J (National Institutes of Health, Bethesda, MD, USA) and the protein levels of intelectin and NF-κB were indexed to β-actin (data were shown as relative absorbance).
Immunohistochemistry for NF-κB-positive cells in brain tissues
Mouse brain tissue was fixed for 5 minutes by instillation of 4% paraformaldehyde-PBS (Sigma) overnight and cut to 2-mm-thick coronal slices. Slices including the injection site were embedded in paraffin and further sliced at a thickness of 5 μm. In accordance with the streptavidin-biotin-peroxidase complex method[26], 5-μm-thick sections were used for immunohistochemistry with rabbit anti-mouse monoclonal antibody against NF-κB (1:250; Serotec; incubating for 12 hours at 4.0°C). Peroxidase-conjugated goat anti-rabbit IgG (1:1 000; Zhongshan Golden Bridge Biotechnology) was used as the secondary antibody (incubating for 30 minutes at 37.0°C). Antibodies were detected using the DAB kit (Zhongshan Golden Bridge Biotechnology) as directed by the manufacturer. Finally, the distribution of NF-κB-positive cells was observed using a light microscope (BH-2; Olympus, Tokyo, Japan).
Five visual fields (× 400) from each of five sections from each mouse were randomly selected for quantification of NF-κB-positive cells and total cells. The mean values were also calculated. The ratio of NF-κB-positive cells was calculated as the mean number of NF-κB-positive cells/the total number of cells observed. As a negative control, PBS was used in place of the primary antibody, and identical procedures were performed on serial sections[27].
Statistical analysis
Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Experimental data are presented as mean±SEM. Differences between groups were assessed by two-samplet-test. APvalue <0.05 was considered statistically significant.
Funding:This study was funded by the Health Research Fund from the Health Department of Shaanxi Province, China, No. 04015.
Author contributions:This study was designed and performed by Naibing Gu, and completed with the technical support of Gejuan Zhang and Hui Lei. The data were analyzed by Ye Tian. The manuscript was written by Zhengli Di, and revised by Caiping Han.
Conflicts of interest:None declared.
Ethical approval:This study was permitted by the Animal Ethics Committee of Fourth Military Medical University of Chinese PLA.
Author statements:The manuscript is original, has not been submitted to or is not under consideration by another publication, has not been previously published in any language or any form, including electronic, and contains no disclosure of confidential information or authorship/patent application disputations.
[1] Granerod J, Crowcroft NS. The epidemiology of acute encephalitis. Neuropsychol Rehabil. 2007;17(4-5):406-428.
[2] Tian Y, Han CP, Gu NB, et al. Inhibitory effects of Shuanghuanglian injection on nuclear factor-kappa B expression in mice with viral encephalitis in a time- and dose-dependent manner. Neural Regen Res. 2011;6(24): 1865-1869.
[3] Gu N, Kang G, Jin C, et al. Intelectin is required for IL-13-induced monocyte chemotactic protein-1 and -3 expression in lung epithelial cells and promotes allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;298(3):L290-296.
[4] Komiya T, Tanigawa Y, Hirohashi S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem Biophys Res Commun. 1998;251 (3):759-762.
[5] Lee JK, Schnee J, Pang M, et al. Human homologs of the Xenopus oocyte cortical granule lectin XL35. Glycobiology. 2001;11(1):65-73.
[6] Pemberton AD, Knight PA, Gamble J, et al. Innate BALB/c enteric epithelial responses to Trichinella spiralis: inducible expression of a novel goblet cell lectin, intelectin-2, and its natural deletion in C57BL/10 mice. J Immunol. 2004;173(3):1894-1901.
[7] Sch?ffler A, Neumeier M, Herfarth H, et al. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim Biophys Acta. 2005;1732(1-3):96-102.
[8] Suzuki YA, Shin K, L?nnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40(51):15771-15779.
[9] Tsuji S, Uehori J, Matsumoto M, et al. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem. 2001;276(26):23456-23463.
[10] Sarkar FH, Li Y, Wang Z, et al. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol. 2008;27(5):293-319.
[11] Lin Y, Bai L, Chen W, et al. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010; 14(1):45-55.
[12] yler KL, Leser JS, Phang TL, et al. Gene expression in the brain during reovirus encephalitis. J Neurovirol. 2010;16 (1):56-71.
[13] Danthi P, Pruijssers AJ, Berger AK, et al. Bid regulates the pathogenesis of neurotropic reovirus. PLoS Pathog. 2010; 6:e1000980.
[14] Gao G, Zhang J, Si X, et al. Proteasome inhibition attenuates coxsackievirus-induced myocardial damage in mice. Am J Physiol Heart Circ Physiol. 2008;295(1): H401-408.
[15] Feuer R, Ruller CM, An N, et al. Viral persistence and chronic immunopathology in the adult central nervous system following Coxsackievirus infection during the neonatal period. J Virol. 2009;83(18):9356-9369.
[16] Kong XT, Fang HT, Jiang GQ, et al. Treatment of acute bronchiolitis with Chinese herbs. Arch Dis Child. 1993; 68(4):468-471.
[17] Chen X, Howard OM, Yang X, et al. Effects of Shuanghuanglian and Qingkailing, two multi-components of traditional Chinese medicinal preparations, on human leukocyte function. Life Sci. 2002;70(24):2897-2913.
[18] The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestion for the Care and Use of Laboratory Animals. 2006-09-30.
[19] Meyding-Lamadé U, Haas J, Lamadé W, et al. Herpes simplex virus encephalitis: long-term comparative study of viral load and the expression of immunologic nitric oxide synthase in mouse brain tissue. Neurosci Lett. 1998;244 (1):9-12.
[20] Schubert S, M?ller-Ehrlich K, Singethan K, et al. A mouse model of persistent brain infection with recombinant Measles virus. J Gen Virol. 2006;87(Pt 7):2011-2019.
[21] Feuer R, Mena I, Pagarigan RR, et al. Coxsackievirus B3 and the neonatal CNS: the roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination, and disease. Am J Pathol. 2003;163(4):1379-1393.
[22] Burrer R, Buchmeier MJ, Wolfe T, et al. Exacerbated pathology of viral encephalitis in mice with central nervous system-specific autoantibodies. Am J Pathol. 2007;170(2): 557-566.
[23] Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402-408. [24] St?hlberg A, Aman P, Ridell B, et al. Quantitative real-time PCR method for detection of B-lymphocyte monoclonality by comparison of kappa and lambda immunoglobulin light chain expression. Clin Chem. 2003;49(1):51-59.
[25] French AT, Bethune JA, Knight PA, et al. The expression of intelectin in sheep goblet cells and upregulation by interleukin-4. Vet Immunol Immunopathol. 2007;120(1-2): 41-46.
[26] Zheng LF, Wang R, Xu YZ, et al. Calcitonin gene-related peptide dynamics in rat dorsal root ganglia and spinal cord following different sciatic nerve injuries. Brain Res. 2008; 1187:20-32.
[27] Yi XN, Zheng LF, Zhang JW, et al. Dynamic changes in Robo2 and Slit1 expression in adult rat dorsal root ganglion and sciatic nerve after peripheral and central axonal injury. Neurosci Res. 2006;56(3):314-321.
10.3969/j.issn.1673-5374.2012.33.004 [http://www.crter.org/nrr-2012-qkquanwen.html]
Gu NB, Tian Y, Di ZL, Han CP, Lei H, Zhang GJ. Shuanghuanglian injection downregulates nuclear factor-kappa B expression in mice with viral encephalitis. Neural Regen Res. 2012;7(33):2592-2599.
Naibing Gu★, Master, Attending physician, Department of Neurology, Xi’an Center Hospital, Xi’an 710003, Shaanxi Province, China
Ye Tian, Ph.D., Chief physician, Department of Neurology, Xi’an Center Hospital, Xi’an 710003, Shaanxi Province, China chhty@sina.com
2012-08-01
2012-10-10 (N20120530001/WLM)
We are very grateful to Dr. Alan Pemberton (Division of Veterinary Clinical Sciences, University of Edinburgh, Roslin, UK) for kindly providing the intelectin antibody.
(Edited by Yang Y/Song LP)
- 中國神經(jīng)再生研究(英文版)的其它文章
- Electroacupuncture improves neuropathic pain Adenosine, adenosine 5’-triphosphate disodium and their receptors perhaps change simultaneously☆
- Underlying mechanism of protection from hypoxic injury seen with n-butanol extract of Potentilla anserine L. in hippocampal neurons***☆
- Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice**★
- Acupuncture inhibits cue-induced heroin craving and brain activation**★
- Puerarin prevents high glucose-induced apoptosis of Schwann cells by inhibiting oxidative stress*★
- Heat-sensitive moxibustion attenuates the inflammation after focal cerebral ischemia/ reperfusion injury*☆

