Underlying mechanism of protection from hypoxic injury seen with n-butanol extract of Potentilla anserine L. in hippocampal neurons***☆
Xiaojing Qin, Lingzhi Li, Qi Lv, Baoguo Yu, Shuwang Yang, Tao He, Yongliang Zhang,
1 Department of Pathology, Affiliated Hospital of Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
2 Department of Medicinal Chemistry, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
3 Tianjin Key Laboratory of Occupational and Environmental Hazard Biomarkers, Tianjin 300162, China
4 Department of Central Laboratory, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
5 Department of Rescue Medicine, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
6 Department of Postgraduate, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
7 Ministry of Scientific Research, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
Underlying mechanism of protection from hypoxic injury seen with n-butanol extract ofPotentilla anserineL. in hippocampal neurons***☆
Xiaojing Qin1, Lingzhi Li2,3, Qi Lv4, Baoguo Yu5, Shuwang Yang6, Tao He1, Yongliang Zhang3,7
1Department of Pathology, Affiliated Hospital of Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
2Department of Medicinal Chemistry, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
3Tianjin Key Laboratory of Occupational and Environmental Hazard Biomarkers, Tianjin 300162, China
4Department of Central Laboratory, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
5Department of Rescue Medicine, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
6Department of Postgraduate, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
7Ministry of Scientific Research, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
The alcohol and n-butanol extract ofPotentilla anserineL. significantly protects myocardium from acute ischemic injury. However, its effects on rat hippocampal neurons and the mechanism of protection remain unclear. In this study, primary cultured hippocampal neurons from neonatal rats were incubated in 95% N2and 5% CO2for 4 hours. Results indicated that hypoxic injury decreased the viability of neurons, increased the expression levels of caspase-9 and caspase-3 mRNA, as well as cytochrome c, Caspase-9, and Caspase-3 protein. Pretreatment with 0.25, 0.062 5, 0.015 6 mg/mL n-butanol extract ofPotentilla anserineL. led to a significant increase in cell viability. Expression levels of caspase-9 and caspase-3 mRNA, as well as cytochrome c, Caspase-9, and Caspase-3 protein, were attenuated. The neuroprotective effect of n-butanol extract ofPotentilla anserineL. was equivalent to tanshinone IIA. Our data suggest that the n-butanol extract ofPotentilla anserineL.could protect primary hippocampal neurons from hypoxic injury by deactivating mitochondrial cell death.
n-butanol extract ofPotentilla anserineL.; neuron; hypoxia; mitochondria injury; cytochrome c; caspase; neural regeneration
Research Highlights
(1) 0.25, 0.062 5, and 0.015 6 mg/mL n-butanol extract ofPotentilla anserineL. increased the viability ofin vitrohypoxic hippocampal neurons from neonatal rats.
(2) 0.25, 0.062 5, and 0.015 6 mg/mL n-butanol extract ofPotentilla anserineL. attenuated the expression of caspase-9 and caspase-3 in hypoxic hippocampal neurons.
(3) 0.25, 0.062 5, and 0.015 6 mg/mL n-butanol extract ofPotentilla anserineL. decreased the release of cytochrome c in hypoxic hippocampal neurons.
Abbreviations
MAP2, microtubule-associated protein 2; MPTP, mitochondrial permeability transition pore
lNTRODUCTlON
Neurons die from hypoxia or hypoxia-ischemia much faster than other cell types[1]. Extensive studies have indicated that mitochondrial injury is the central cause of hypoxic brain injury[2-4]. After hypoxia, cytochrome c in the mitochondria is released, and results in the opening of the mitochondrial permeability transition pore[5-6], thus triggering the caspase cascade. Caspase-9 is the major initiator caspase of the intrinsic mitochondrial apoptotic pathway[7-8]. Caspase-3 acts as the final executor of cell death and is also activated in hypoxic neurons[9-10]. Caspase inhibitors can reduce hypoxia or hypoxia-ischemia induced neuronal death[11-13].
Potentilla anserinaL., commonly called the monorchid herminium herb, belongs to the Rosaceae family and contains polysaccharides, amylum, fatty acids, essential amino acids, and vitamins.Potentilla anserinaL. possesses a high medical and nutritional value, and has been used as a crude drug and a Chinese herbal medicine in Tibet, China. Recent studies have shown thatPotentilla anserinaL. strengthens immunity, exhibits anti-oxidative activity, and anti-hypoxic properties[14-16]. A previous study showed that the alcohol extract ofPotentilla anserinaL. could protect myocardium cells from ischemic or ischemic/reperfusion injuryin vitroandin vivo[17-19]. In particular its n-butanol extract, an effective part of the alcohol extract, could remarkably protect the myocardium from acute ischemic injury[20-21].
However, its effects on rat hippocampal neurons and the mechanism of this protection are not yet well understood. In the present study, we investigated the effects of the n-butanol extract ofPotentilla anserinaL. on hypoxic injury induced by low oxygen density in primary hippocampal neurons. The effects ofPotentilla anserinaL. were then compared with tanshinone IIA, which has been shown to be neuroprotective[22-27].
RESULTS
Morphology of primary cultured hippocampal neurons
After 7 days in culture, neurons were plump, strongly refractory, displayed central cell nuclei and nucleoli were clearly visible. Neuronal processes were interwoven into a thick network (Figure 1A).
Microtubule-associated protein 2 (MAP2) is an abundant neuronal cytoskeletal protein that binds to tubulin and stabilizes microtubules[28]. MAP2 is essential for the development and maintenance of neuronal morphology[29]. MAP2 was abundantly expressed in hippocampal neurons, and seldom expressed in gliocytes. The purity of primary cultured hippocampal neurons was identified by immunocytochemistry using MAP2. Results showed that the proportion of positively stained cells reached 75.2 ± 8.1% (Figure 1B). These cells were then used for subsequent research.
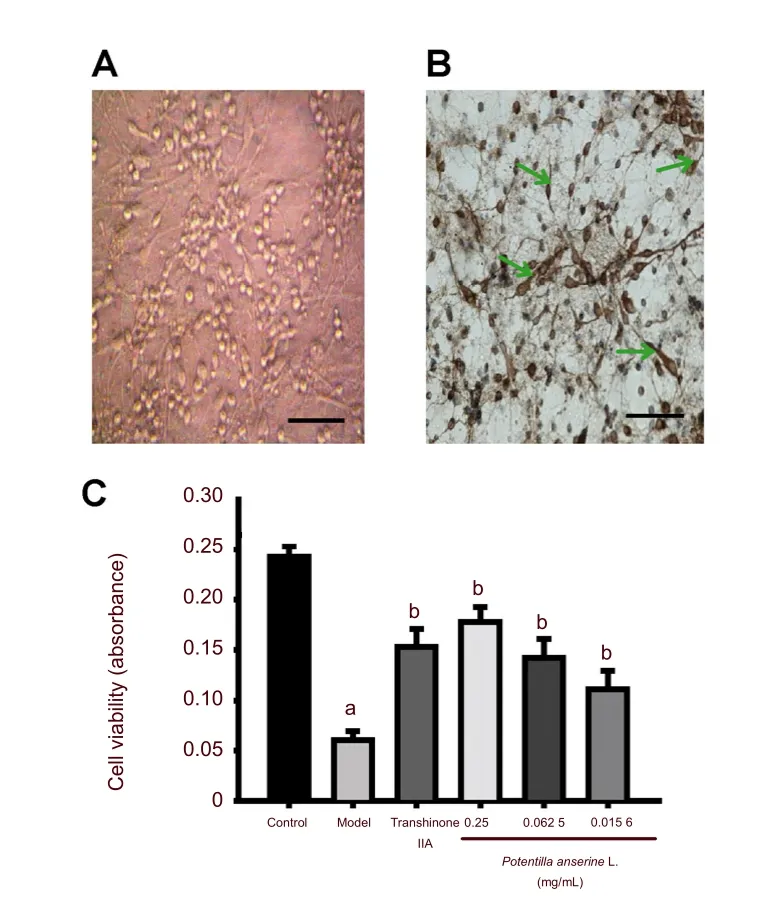
Figure 1 Effects of the n-butanol fraction of Potentilla anserine L. on the viability of hypoxic hippocampal neurons. Primary cultured hippocampal neurons were pre-incubated with different concentrations of Potentilla anserine L. (0, 0.25, 0.062 5, 0.015 6 mg/mL) for 24 hours and then exposed to 0.1% desired oxygen concentration for 4 hours. The neurons in the control group were not treated with Potentilla anserine L. and hypoxia.
Pretreatment with n-butanol extract of Potentilla anserine L. significantly increased cell viability in hypoxic hippocampal neurons
Cell viability was verified by MTT assay. Hypoxia led to a decrease in neuron cell viability (P< 0.01,versusthe control group). Decreased neuronal viability was suppressed by pretreatment with the n-butanol extract ofPotentilla anserineL. (P< 0.01,versusthe model group). The 0.25 mg/mL dosage group showed increasing viability compared with 0.062 5 mg/mL dosage groups (P< 0.05). Moreover, pretreatment with tanshinone IIA could also increase the viability of hippocampal neurons under hypoxia (P< 0.01,versusthe model group; Figure 1C).
n-butanol extract of Potentilla anserine L. significantly decreased the release of cytochrome c and attenuated the expression of caspase-9 and caspase-3 in hypoxic hippocampal neurons
Reverse transcription-PCR results showed that the expression levels of caspase-9 and caspase-3 mRNA were very low in the control group. Hypoxia strongly induced the activation of caspase-9 and caspase-3 mRNA in hippocampal neurons (P< 0.01,versusthe control group). Each dosage ofPotentilla anserineL. extract could significantly reduce the expression of caspase-9 and caspase-3 mRNA in hypoxic-neurons (P< 0.01,versusthe model group; Figure 2). pretreatment with tanshinone did not decrease the expression of cytochrome c (P< 0.01; Figure 3).
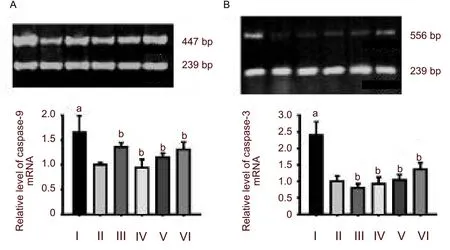
Figure 2 Change in caspase-9 (A) and caspase-3 (B) mRNA levels in hippocampal neurons.
Western blot revealed that protein expression levels of cytochrome c, Caspase-9 and Caspase-3 were very low in the control group. Hypoxia strongly induced the expressions of cytochrome c, Caspase-9 and Caspase-3 in hippocampal neurons (P< 0.01,versusthe control group). Pretreatment withPotentilla anserineL. extract or tanshinone IIA could significantly decrease the expression of Caspase-9 and Caspase-3 (P< 0.01 or 0.05,versusthe model group; Figure 3). However,
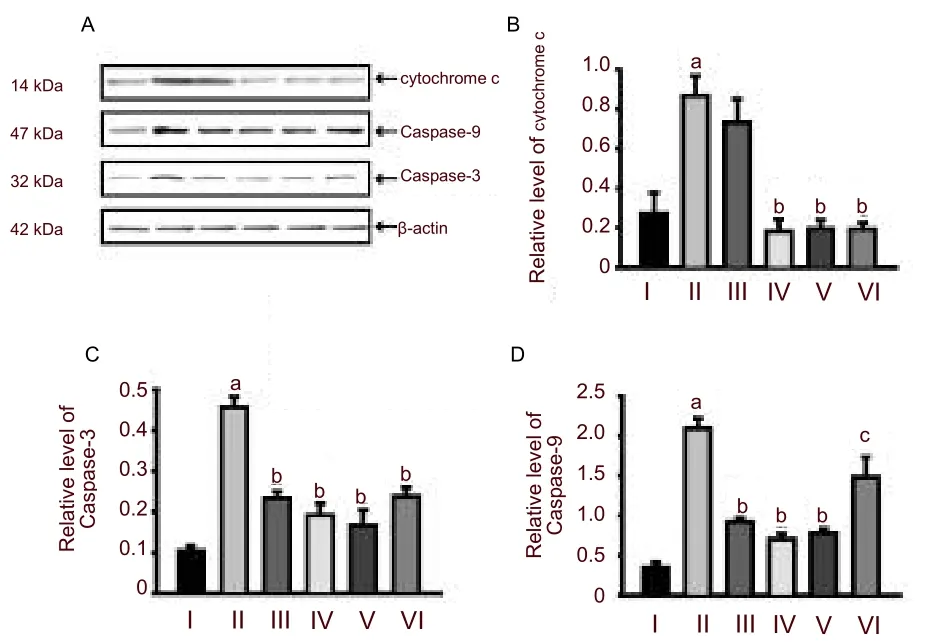
Figure 3 Change in cytochrome c, Caspase-9 and Caspase-3 expression in hippocampal neurons analyzed using western blot assay.
DlSCUSSlON
Mitochondrial injury is a major contributor to hypoxic brain injury as it connects upstream and downstream signal transmission. After hypoxia, electron transfer in the mitochondrial respiratory chain is hindered and energy metabolism is obstructed. Cytochrome c in the mitochondria is released and reactive oxygen species are generated, resulting in the opening of the mitochondrial permeability transition pore. The released cytochrome c from the mitochondria induces cell death in two ways. First, it triggers the caspase cascade.
Caspases, cysteine-dependent protein kinases, are the most important enzymes in cell death. Cytochrome c can associate with apoptosis protease activating factor and pro-caspase 9, and triggers the activation of caspase-3 and apoptosis[30-31]. Caspase-3 can specifically cleave Bcl-2, resulting in the loss of inhibitory effect on mitochondrial permeability transition pore, thus releasing more cytochrome c[32-34]. Second, it interrupts the electron transport chain, and thereby inhibits oxidative phosphorylation; this generates oxygen free radicals and results in a lack of ATP and eventually cell death. The caspase-dependent pathway is the faster process leading to cell death. In addition, hypoxic stress also leads to activation of caspase-8, which elicits the release of cytochrome c into the cytosol and activates the release of other caspases. This process initiates internucleosomal DNA fragmentation and results in apoptosis[35-36].
Mitochondrial cell death can occur due to apoptosis and necrosis. Hypoxic/ischemic brain injury induces cell death in neurons by apoptotic and necrotic mechanisms[37].
Apoptosis and necrosis are initially identified by two different modes, based on the morphological criteria. Caspases are essential for the execution steps in apoptosis[38]. Caspase-9 is the major initiator caspase of the intrinsic mitochondrial apoptotic pathway, and its inhibition in the brain by LEHD-CHO (Caspase-9 inhibitor) has been demonstrated to have a neuroprotective effect. Caspase-9 is important in the pathophysiology of hypoxic/ischemic neuronal destruction in newborn rats[39].
Caspase-3 acts as the final executor of cell death and is also activated in hypoxic neurons. Caspases may also be expressed in the context of necrotic cell death[40]. Necrosis is generally considered to occur because of an external stimulus (changes in ion flux), but recent studies have shown that neuronal death after oxygen ion flux is far from passive cell swelling and dissolution, but requires an orderly activated cell death program. Necrosis may be triggered by mitochondrial dysfunction, subsequently leading to the release of cytochrome c and the activation of the caspase system. This means that apoptosis and necrosis have the same final pathway. This involves mitochondrial dysfunction caused by cytochrome c release followed by the activation of Caspase-9 and Caspase-3 by cytochrome c, along with apoptosis protease activating factor-1. This activation is followed by hydrolysis, and finally the activation of the caspase kinase system occurs. However, in the process of apoptosis, gene expression and protein synthesis requires a large of amount energy. During hypoxia, ischemia, or any other low-energy state, the huge amount of energy required for protein synthesis cannot be met. By contrast, caspases exist in normal cells, and activation requires only a small amount of energy; therefore, in hypoxia, necrosis may be the main cause of cell death[41].
In this study, expression levels of caspase-9 mRNA and caspase-3 mRNA were very low in the control group. Hypoxia strongly induced the activation of caspase-9 and caspase-3 in neurons. However, pretreatment withPotentilla anserineL. extract significantly reduced the expression of caspase-9 mRNA and caspase-3 mRNA in hypoxic-neurons. Likewise, similar results were observed for the expression of Caspase-9 and Caspase-3 protein. Pretreatment withPotentilla anserineL. extract also significantly decreased the release of cytochrome c into the cytosol. These findings suggested that the n-butanol extract ofPotentilla anserineL.could protect primary hippocampal neurons from hypoxic injury by attenuating mitochondrial cell death. Oxygen free radicals are a main cause of mitochondrial injury. Our previous studies demonstrate thatPotentilla anserinaL. exhibits anti-oxidative activity[16,20]. This activity may contribute to the mitochondrial protective effect ofPotentilla anserinaL.
In summary, our findings demonstrate that the n-butanol extract ofPotentilla anserineL. has a neuroprotective effect on hypoxic injury in primary hippocampal neurons. The possible mechanism is as follows: the n-butanol extract ofPotentilla anserineL. protects mitochondrial function by attenuating the release of cytochrome c into the cytosol, and thereby inhibits the caspase cascade pathway. This prevents cell death. These findings provide a theoretical basis for developing the n-butanol extract ofPotentilla anserineL. as a neuroprotective agent.
MATERlALS AND METHODS
Design
A cytological comparison study.
Time and setting
Experiments were performed at the Department of Medicinal Chemistry, Medical College of Chinese People’s Armed Police Forces, Tianjin, China from May 2008 to January 2011.
Materials
Animals
Fifty pregnant Sprague-Dawley clean grade rats were purchased from the Laboratory Animal Center of Academy of Military Medical Sciences, China, permission No. SCXK (Army) 2002-001. Neonatal mice (within 24 hours of birth) were used for this study. All experiments were performed in accordance with theGuidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[42].
Drugs
Potentilla anserine L.was purchased from Qinghai Institute of Chinese Medicine, China. The n-butanol extract ofPotentilla anserineL.was extracted as previously described[20].Five compounds have been isolated from the extract, which were considered as contributors to the protective function of anti-hypoxia in neurons. They are adenosine, daidzin, puerarin, 3’-methoxypuerarin and daidzein 8-C-apiosyl glucoside.
Tanshinone IIA (Huike Botanical Development Co., Shaanxi, China) with a purity of more than 98%, was dissolved in 0.1% dimethyl sulfoxide and made up to a concentration of 20 mg/mL in D-Hank’s medium (Gibco, Grand Island, NY, USA).
Methods
Primary hippocampal neuron cultures
Sprague-Dawley neonatal rats were anesthetized with diethyl ether and disinfected with 75% alcohol. Primary hippocampal neurons were prepared from the hippocampus under sterile conditions[43]. Neurons were suspended in a culture medium that contained DMEM-F12 (Gibco), fetal bovine serum, mycillin, and glucose (4 × 105cell/mL), and then plated onto poly-D-lysine-coated 60 mm dishes. The medium was changed after 48 hours by replacing the fetal bovine serum with N2 (Gibco), and half of the medium was replaced every 3 days. The cells were cultured in a CO2incubator at 37°C and 5% CO2. After 7 days in culture, observation under a phase-contrast microscope (Olympus, Tokyo, Japan) demonstrated that cells were predominantly neurons (> 96%). All experiments were performed after cells were cultured for 7 days.
The purity of primary cultured hippocampal neurons identified by immunocytochemical analysis
Cultured cells were fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100, and blocked in 5% bovine serum albumin. MAP2 was detected with rabbit anti-MAP2 polyclonal antibody (1:100; Cell Signaling Technology, Beverly, MA, USA), and primary antibodies were incubated overnight at 4°C, followed by goat anti-rabbit secondary antibodies (Invitrogen, Grand Island, NY, USA). 3,3-diaminobenzidine was then used to visualize immunohistochemical staining. Cell nuclei were then counterstained with hematoxylin. Images were obtained with an Olympus BX51 microscope (Olympus) and the proportion of positive staining cells was analyzed with Image-Pro plus 5.1 software (Bethesda, MD, USA)[28-29].
Grouping and intervention
Culture dishes were randomly divided into the control, hypoxic injury model, and the 0.25, 0.062 5, and 0.015 6 mg/mL of n-butanol extract ofPotentilla anserineL. groups. These concentrations were chosen based on previous studies[20]. Tanshinone IIA, a positive control, was preincubated before hypoxia at a working concentration of 0.2 mg/mL. After 7 days of culture, control dishes were kept in normoxic conditions.
D-Hank’s medium with different concentrations of the extract was used for the hypoxic injury model group and the intervention group. D-Hank’s medium with extract was initially placed in a hypoxic environment (95% N2, 5% CO2) for 30 minutes and then replaced with normal medium. The model and intervention groups were then exposed to a 95% N2, 5% CO2air mixture for 4 hours.
Viability of hippocampal neurons determined using MTT assay
Neuronal viability was assessed using MTT assay as previously described[44], with some modifications. The yellow MTT was reduced to a purple formazan by mitochondrial dehydrogenase in live cells. Briefly, a total of 5 mg/mL MTT was added to each well (final concentration was 1 mg/mL) and another 4 hours of incubation at 37°C was conducted. The assay was stopped by the addition of a 100 μL lysine buffer (20% SDS in 50% N, N-dimethylformamide, pH 4.7). Absorbance value was measured at 570 nm with ELX-800 microplate assay reader (BioTek, Winooski, VT, USA).
Determination of caspase-9, caspase-3 mRNA inhippocampal neurons by reverse transcription-PCR
The total RNA were extracted from neurons using TRIzol reagent (Gibco), and its purity and integrity were measured. The primers (Invitrogen) were as follows:caspase-9, F: 5’-CCC GTG AAG CAA GGA TTT-3’, R:5’-TCT GTG GGT CTG GGA AGC-3’; caspase-3, F:5’-GCA CTG GAA TGT CAG CTC GCA A-3’, R:5’-GCC ACC TTC CGG TTA ACA CGA C-3’; β-actin, F: 5’-TGA TGA CAT CAA GAA GGT GGT GAA G-3’, R: 5’-TCC TTG GAG GCC ATG TAG GCC AT-3.’
Reverse transcription-PCR was performed using a TaKaRa RNA PCR Kit (AMV) Version 3.0 (TaKaRa, Otsu, Japan). Products were visualized with ethidium bromide staining. The relative expression of caspase-9, and caspase-3 mRNA was given as the ratio of the target mRNA absorbance value to the β-actin absorbance value. The absorbance of each band was analyzed with Gel-pro 3.0 (Bethesda).
Western blot analysis of cytochrome c, Caspase-9, and Caspase-3 protein expression in hippocampal neurons
Cells were lysed in ice-cold lysis buffer. After centrifugation, the supernatants were collected. Protein samples were then run on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride container. Membranes were blocked with 5% fat-free milk powder in Tris-buffered saline, incubated at 4°C overnight with mouse anti-rat cytochrome c (1:1 000), Caspase-3 (1:1 000), Caspase-9 (1:1 000), and β-actin (1:1 000) monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). This incubation was followed by probing with goat anti-mouse secondary antibody (1:1 000, Santa Cruz) at 37°C for 1 hour. The protein was visualized with enhanced chemiluminescence solution, scanned, photographed, and the target protein absorbance value to the β-actin absorbance value was analyzed with a gel-image analytical system, Gel-pro 3.0 (Bethesda).
Statistical analysis
Statistical analyses were performed using SPSS 10.0 (SPSS, Chicago, IL, USA) and the results were expressed as mean ± SEM. Differences between the means were determined by one-way analysis of variance followed by a Student-Newman-Keuls test for multiple comparisons. A value ofP <0.05 was considered significant.
Funding: This research was supported by the National Natural Science Foundation of China, No. 30672774 and No. 81073152, and the Great Program of Science Foundation of Tianjin, No. 10JCZDJC21100.
Author contributions: Lingzhi Li and Yongliang Zhang were in charge of funding and authorized this study. Xiaojing Qin conducted experiments, conceived and designed the study, and wrote the manuscript draft. Qi Lv contributed to the data analysis and revised the manuscript. Baoguo Yu, Shuwang Yang and Tao He assisted with the experiments.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Care and Research Committee of Tianjin, China.
Author statements: The manuscript is original, has not been submitted to or is not under consideration by another publication, has not been previously published in any language or any form, including electronic, and contains no disclosure of confidential information or authorship/patent application disputations.
[1] Yakovlev AG, Faden AI. Mechanisms of neural cell death:implications for development of neuroprotective treatment strategies. NeuroRx. 2004;1(1):5-16.
[2] Tamm C, Sabri F, Ceccatelli S. Mitochondrial-mediated apoptosis in neural stem cells exposed to manganese. Toxicol Sci. 2008;101(2):310-320.
[3] Soustiel JF, Larisch S. Mitochondrial damage: a target for new therapeutic horizons. Neurotherapeutics. 2010;7(1):13-21.
[4] Zhu M, Li MW, Tian XS, et al. Neuroprotective role of delta-opioid receptors against mitochondrial respiratory chain injury. Brain Res. 2009;1252:183-191.
[5] Lim ML, Lum MG, Hansen TM, et al. On the release of cytochrome c from mitochondria during cell death signaling. J Biomed Sci. 2002;9(6 Pt 1):488-506.
[6] Li S, Dong P, Wang J, et al. Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Lett. 2010;298(2):222-230.
[7] Kuida K. Caspase-9. Int J Biochem Cell Biol. 2000;32(2):121-124.
[8] Delivoria-Papadopoulos M, Ashraf QM, Mishra OP. Effect of hypoxia on the expression of procaspase-9 and procaspase-3 in neuronal nuclear, mitochondrial and cytosolic fractions of the cerebral cortex of newborn piglets. Neurosci Lett. 2008;438(1):38-41.
[9] Parikh NA, Katsetos CD, Ashraf QM, et al. Hypoxia-induced caspase-3 activation and DNA fragmentation in cortical neurons of newborn piglets: role of nitric oxide. Neurochem Res. 2003;28(9):1351-1357.
[10] Springer JE, Nottingham SA, McEwen ML, et al. Caspase-3 apoptotic signaling following injury to the central nervous system. Clin Chem Lab Med. 2001;39(4):299-307.
[11] Peng H, Sola A, Moore J, et al. Caspase inhibition by cardiotrophin-1 prevents neuronal death in vivo and in vitro. J Neurosci Res. 2010;88(5):1041-1051.
[12] Chiang MC, Ashraf QM, Mishra OP, et al. Mechanism of DNA fragmentation during hypoxia in the cerebral cortex of newborn piglets. Neurochem Res. 2008;33(7):1232-1237.
[13] Lang-Rollin IC, Rideout HJ, Noticewala M, et al. Mechanisms of caspase-independent neuronal death:energy depletion and free radical generation. J Neurosci. 2003;23(35):11015-11025.
[14] Wei W, Li GC, Gong HY, et al. The anti-hypoxia effects ofpotentilla anserineL. polysaccharide. Wujing Yixueyuan Xuebao. 2010;19(5):345-347.
[15] Hui J, Shang DJ, Li QW. Effect of Potentilla anserina L.in xizang on anti-fatigue and hypoxia tolerance in mice. Yingyang Xuebao. 2003;25(2):218-219.
[16] Li LZ, Zhang L, Gong HY, et al. Study on anti-hypoxia and anti-oxidation effects ofPoteutilla anserinaL. alcohol extract. Zhongguo Shipin Weisheng Zazhi. 2005;17(4):306-309.
[17] Li JY, Li LZ, Zhang YL, et al. Protective effect of Poteutilla anserina . on hypoxia injury in neonatal rats cardiomyocytes. Zhongguo Xinyao Zazhi. 2007;16(12):944-946.
[18] Ye L, Chen Y, Li LZ, et al. Protective effect of alcohol extract of Poteutilla anserina L. on acute myocardial ischemin/reperfusion injury in mice. Zhongcaoyao. 2009; 40(5):774-777.
[19] Lv Q, Qin XJ, Zhang XN, et al. Effects of Poteutilla anserina L. on acute ischemic myocardiac muscle and the differerntially expressed proteins in serum in rats. Wujing Yixueyuan Xuebao. 2011;20(6):429-433,444.
[20] Li JY, Li Y, Gong HY, et al. Protective effects of n-butanol extract of Potentilla anserina on acute myocardial ischemic injury in mice. Zhong Xi Yi Jie He Xue Bao. 2009; 7(1):48-52.
[21] Li JY, Li Y, Gong HY, et al. Effects of n-butanol extract of Potentilla anserina L. on expressions of caspase-9/3 mRNA in mice injured by acute myocardial ischemi. Zhongguo Zhongxiyi Jiehe Jijiu Zazhi. 2009;16(1):18-21.
[22] Han JY, Fan JY, Horie Y, et al. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008;117(2):280-295.
[23] Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27(1):133-148.
[24] Takahashi K, Ouyang X, Komatsu K, et al. Sodium tanshinone IIA sulfonate derived from Danshen (Salvia miltiorrhiza) attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac cells. Biochem Pharmacol. 2002;64(4):745-749.
[25] Wu TW, Zeng LH, Fung KP, et al. Effect of sodium tanshinone IIA sulfonate in the rabbit myocardium and on human cardiomyocytes and vascular endothelial cells. Biochem Pharmacol. 1993;46(12):2327-2332.
[26] Lam BY, Lo AC, Sun X, et al. Neuroprotective effects of tanshinones in transient focal cerebral ischemia in mice. Phytomedicine. 2003;10(4):286-291.
[27] Dong K, Xu W, Yang J, et al. Neuroprotective effects of Tanshinone IIA on permanent focal cerebral ischemia in mice. Phytother Res. 2009;23(5):608-613.
[28] Burtelow MA, Longacre TA. Utility of microtubule associated protein-2 (MAP-2) immunohistochemistry for identification of ganglion cells in paraffin-embedded rectal suction biopsies. Am J Surg Pathol. 2009;33(7):1025-1030.
[29] Kühn J, Meissner C, Oehmichen M. Microtubuleassociated protein 2 (MAP2)--a promising approach to diagnosis of forensic types of hypoxia-ischemia. Acta Neuropathol. 2005;110(6):579-586.
[30] Zou H, Henzel WJ, Liu X, et al. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90(3):405-413.
[31] Zou H, Li Y, Liu X, et al. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274(17):11549-11556.
[32] Chen Q, Gong B, Almasan A. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000;7(2):227-233.
[33] Liu WB, Zhou J, Qu Y, et al. Neuroprotective effect of osthole on MPP+-induced cytotoxicity in PC12 cells via inhibition of mitochondrial dysfunction and ROS production. Neurochem Int. 2010;57(3):206-215.
[34] de Moissac D, Gurevich RM, Zheng H, et al. Caspase activation and mitochondrial cytochrome C release during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol. 2000;32(1):53-63.
[35] Nagarajah NS, Vigneswaran N, Zacharias W. Hypoxiamediated apoptosis in oral carcinoma cells occurs via two independent pathways. Mol Cancer. 2004;3(1):38.
[36] Inaba S, Eguchi T, Motegi A, et al. The cytotoxic macrolide FD-891 induces caspase-8-dependent mitochondrial release of cytochrome c and subsequent apoptosis in human leukemia Jurkat cells. J Antibiot (Tokyo). 2009; 62(9):507-512.
[37] Northington FJ, Ferriero DM, Martin LJ. Neurodegeneration in the thalamus following neonatal hypoxia-ischemia is programmed cell death. Dev Neurosci. 2001;23(3):186-191.
[38] Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1-16.
[39] Feng Y, Fratkin JD, LeBlanc MH. Inhibiting caspase-9 after injury reduces hypoxic ischemic neuronal injury in the cortex in the newborn rat. Neurosci Lett. 2003;344(3): 201-204.
[40] Graeber MB, Moran LB. Mechanisms of cell death in neurodegenerative diseases: fashion, fiction, and facts. Brain Pathol. 2002;12(3):385-390.
[41] Niquet J, Baldwin RA, Allen SG, et al. Hypoxic neuronal necrosis: protein synthesis-independent activation of a cell death program. Proc Natl Acad Sci U S A. 2003; 100(5):2825-2830.
[42] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[43] May PC, Robison PM. GYKI 52466 protects against non-NMDA receptor-mediated excitotoxicity in primary rat hippocampal cultures. Neurosci Lett. 1993;152(1-2):169-172.
[44] Xu Y, Zhang Q, Yu S, et al. The protective effects of chitooligosaccharides against glucose deprivationinduced cell apoptosis in cultured cortical neurons through activation of PI3K/Akt and MEK/ERK1/2 pathways. Brain Res. 2011;1375:49-58.
10.3969/j.issn.1673-5374.2012.33.002 [http://www.crter.org/nrr-2012-qkquanwen.html]
Qin XJ, Li LZ, Lv Q, Yu BG, Yang SW, He T, Zhang YL. Underlying mechanism of protection from hypoxic injury seen with
n-butanol extract of Potentilla anserine L. in hippocampal neurons. Neural Regen Res. 2012;7(33):2576-2582.
Xiaojing Qin☆, M.D., Department of Pathology, Affiliated Hospital of Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China
Yongliang Zhang, M.D., Professor, Tianjin Key Laboratory of Occupational and Environmental Hazard Biomar kers, Tianjin 300162, China;Ministry of Scientific Research, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China ; Lingzhi Li, M.D., Professor, Department of Medicinal Chemistry, Logistics University of Chinese People’s Armed Police Forces, Tianjin 300162, China; Tianjin Key Laboratory of Occupational and Environmental Hazard Biomarkers, Tianjin 300162, China
zhang78127@tom.com;
llzhx@yahoo.cn
2012-03-31
2012-07-26
(N20111013001/YJ)
(Edited by Liu ZX, Yang WM/Yang Y/Wang L)
- 中國神經(jīng)再生研究(英文版)的其它文章
- Electroacupuncture improves neuropathic pain Adenosine, adenosine 5’-triphosphate disodium and their receptors perhaps change simultaneously☆
- Shuanghuanglian injection downregulates nuclear factor-kappa B expression in mice with viral encephalitis*★
- Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice**★
- Acupuncture inhibits cue-induced heroin craving and brain activation**★
- Puerarin prevents high glucose-induced apoptosis of Schwann cells by inhibiting oxidative stress*★
- Heat-sensitive moxibustion attenuates the inflammation after focal cerebral ischemia/ reperfusion injury*☆

