Adsorbents for Expanded Bed Adsorption: Preparation and Functionalization*
ZHAO Jun (趙珺), YAO Shanjing (姚善涇) and LIN Dongqiang (林東強(qiáng))
?
Adsorbents for Expanded Bed Adsorption: Preparation and Functionalization*
ZHAO Jun (趙珺), YAO Shanjing (姚善涇)**and LIN Dongqiang (林東強(qiáng))
Department of Chemical and Biochemical Engineering, Zhejiang University, Hangzhou 310027, China
Expanded bed adsorption (EBA), a promising and practical separation technique, has been widely studied in the past two decades. The development of adsorbents for EBA process is a challenging course, with the special design and preparation according to the target molecules and specific expanded bed systems. Many types of supporting matrices for expanded bed adsorbents have been developed, and their preparation methods are being consummated gradually. These matrices are activated and then coupled with ligands to form functionalized adsorbents, including ion-exchange adsorbents, affinity adsorbents, mixed mode adsorbents, hydrophobic charge induction chromatography adsorbents and others. In this review, the preparation of the matrices for EBA process is summarized, and the coupling of ligands to the matrices to prepare functionalized adsorbents is discussed as well.
expanded bed adsorption, matrix, preparation, functionalized adsorbent, chromatography, separation
1 INTRODUCTION
Chromatography technology, with high performance and efficiency, is one of the most powerful separation methods for the purification of bio-molecules in the biotechnological industry in the past few decades. Expanded bed adsorption (EBA) is a novel chromatographic technique integrating clarification, concentration, and initial purification into a single step [1]. It allows the capture of bio-molecules from particle- containing feedstock without prior removal of particulates, thus enabling clarification of a cell suspension or cell homogenate and the concentration of the desired product in one operation [1-3]. EBA processes use the specially designed adsorbents expanded in the column with an upward liquid phase, exhibiting as a perfectly classified fluidized bed, with a plug-flow profile and low back-mixing through the column [3, 4]. Compared with a packed-bed operation, the expanded bed avoids the shortcomings of small voidage of the bed, suitable for operation at high flow rate with relatively low pressure drop. Moreover, it allows cells and cell debris to pass through without blocking the bed, avoiding the contamination by these particulates [5]. EBA process, as a result, meets the high-throughput requirement of production, and is regarded as a promising and practical technique for industrial applications.
EBA systems require new types of adsorbent structures. Some parameters should be considered, such as size, density, ligand and adsorption capacity [6]. The EBA adsorbent matrices usually have regular spherical shape, relatively high density, proper size and size distribution, which would be suitable for high operation velocity to shorten the processing time [6-9]. The matrix beads with an appropriate distribution of size and/or density, grades in the expanded bed, with the larger/denser particles being near the bottom of the bed, and the smaller/lighter particles nearer the top [2] (Fig. 1). When the ratio of the maximum particle diameter to the minimum diameter is greater than 2.2, the effects of classification dominate over the tendency to mix, and thus a stable graded bed will be formed [10]. Furthermore, other parameters are required for EBA matrices, including high specific surface area, good mechanical strength, and chemical stability,.
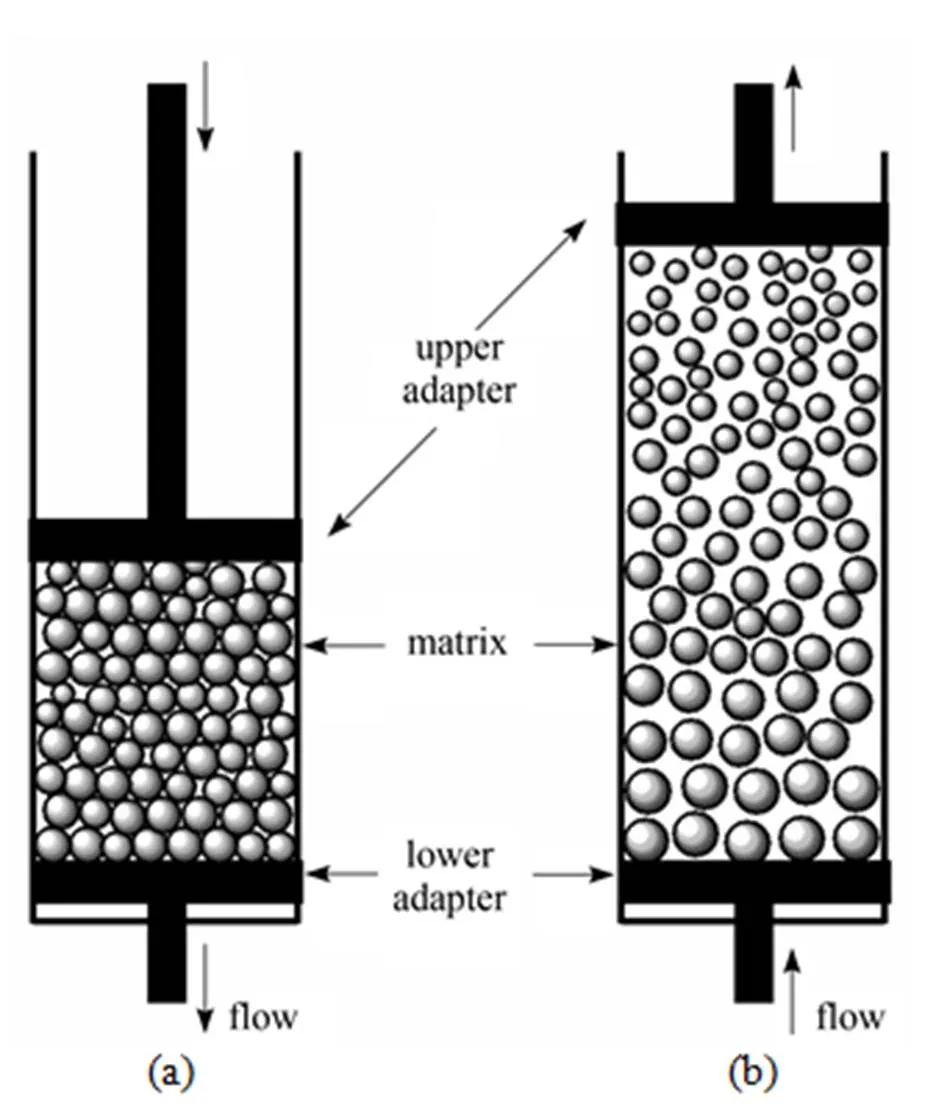
Figure 1 Comparison of a fixed bed (a) and a stable expanded bed (b)
The development of adsorbent for EBA process is a challenging course, with the special design for both matrix and ligand according to the target molecules and the specific expanded bed systems. Until now, various methods have been employed to produce the supporting matrix with excellent properties, in which the matrix is modified and then coupled with a great many ligands to form different functionalized adsorbents.
In the present work, the preparation of the matrices for EBA process will be summarized, and the coupling of ligands to the matrices to prepare functionalized adsorbents will be discussed as well.
2 MATRICES FOR EXPANDED BED
During the past decades, three types of matrices have been developed for EBA applications [11, 12]. The first is core type, with high-density particles as the cores covered with the gel layer. It can be divided into single-core type, with one particle core in the bead [shown in Fig. 2 (a)], and multi-core type, with agglomerate particulates in the bead [shown in Fig. 2 (b)]. The second is dispersing type, with densified- powder uniformly dispersed in the polymers or gels [shown in Fig. 2 (c)]. The third is the rigid porous beads without the polymer-coated layer, called integrity type matrices [shown in Fig. 2 (d)].
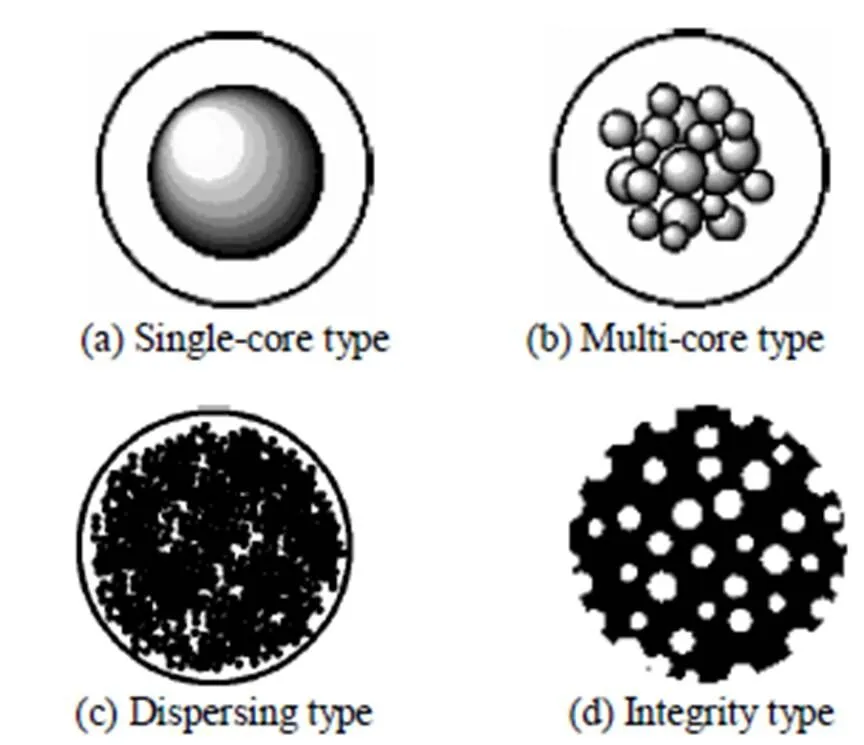
Figure 2 The types of matrices for EBA: single-core type, multi-core type, dispersing type, and integrity type
2.1 Matrices of core type
The inner cores of these matrices are high-density nonporous granules, usually with regular shape and good chemical stability. The rigid nonporous inorganic materials like stainless steel and glass bead are commonly used as the densified cores. The outer layers of the matrices are highly hydrophilic polymeric materials with porous or nonporous structure, such as the polysaccharides. The remarkable advantage of this matrix is that the mass transfer path is shortened owing to the addition of the large cores, and therefore, transport just occurs within the outer layers. Nowadays, most studies have been focused on developing matrices with high density and pellicular outer layer. The pellicular layer is favorable for the mass transfer, especially in the dynamic adsorption, but it may decrease the adsorption capacity.
A high-density matrix was exploited by P?lsson. [9], with a heavy core of stainless steel covered with a pellicular agarose layer, and two bead size fractions, the smaller one (32-75mm) and the larger (75-180mm) were sieved, with the density of 3.3 and 4.4 g·cm-3, respectively. The matrix gave stable expanded beds with good mass transfer behavior at the flow rate as high as 3000 cm·h-1. Miao. [13] prepared cellulose/stainless steel composite matrix with the mean particle size of 100 and 170mm, and the density of 1.2-1.8 g·cm-3, the largest of which was suitable for the flow rate of 1000 cm·h-1. Jahanshahi. [14] developed a polymer-coated adsorbent, with silica-zirconia particles coated by an agarose gel. This new exterior coating acted as a sieve reducing the non-specific binding of cell and cell debris without diminution of selective capture of target protein from complex feedstock.
The organic fluorides were developed as the core material for the matrix as well. McCreath. [15] prepared a heavy matrix of perfluoropolymer covered with polyvinyl alcohol (PVA). The matrix had a size range of 50-80mm and a density of 2.2 g·cm-3, showing fast adsorbing rate and convenient eluting and cleaning process.
2.2 Matrices of dispersing type
Compared with the core type matrix, the matrix of dispersing type has no obvious inner cores. The densified-powder is homogeneously dispersed in the polymer skeletons to form the organic/inorganic composite structure with non-covalent interactions. These densifiers, generally in several microns or smaller, do not aggregate to form obvious cores. The mass transfer occurs in the inner pores or channels throughout the whole bead, and the transfer path is hardly shortened, so that the mass transfer within the dispersing type matrix is slow. For this matrix, the micro-structure of the inner pores would show a significant effect on transport and adsorption. The general pore size is not very large, for example, the matrix prepared with regenerated cellulose has the mean pore diameter of 60-100 nm and the bovine serum albumin (BSA) adsorption capacity of 50-60 mg·ml-1, so that the dynamic adsorption capacity is much lower [13, 16-18]. The small pores are not conducive to the dynamic adsorption processes andcleaning. In order to increase the adsorbing rate and reduce the contamination by debris, porogenic agents are frequently used in the preparation process to enlarge the pore size and to improve the pore distribution [19]. Xia. [20] used gelatinized cassava starch as a porogenic agent to prepare a series of macroporous cellulose/ tungsten carbide composite matrices. When treated with boiling water and amylase, certain amount of large pores was formed inside the beads, with the diameter of about 1-3mm. The saturated adsorption capacity of BSA reached 97.1 mg·ml-1according to the adsorption equilibrium experiments [21]. Calcium carbonate was another significant porogenic agent commonly applied in the preparation of separation matrices [22-24]. These macroporous matrices allowed higher flow rate, and reduced diffusion limitations due to reduced transport length in the adsorbing layer [6].
2.3 Integrity type matrices
The integrity type matrices are the high-density rigid porous microspheres with high mechanical strength and good chemical stability, but without the polymer-coated layer. Porous glass beads were early applied to purify the monoclonal antibodies by Th?mmes[25]. This matrix had a uniform density and a size deviated from the Gaussian distribution, resulting in serious back mixing in the bed as the flow rate increased. Afterwards, the inert homogeneous inorganic oxides including zirconia, titania and hafnia are widely used instead. Porous zirconia is an attractive option for the production of dense adsorbent for EBA. It shows less Lewis acid-base interaction compared with titania, and is more stable in alkaline condition than silica [26]. However, due to the complex surface chemistry and the surface Lewis acid sites of the inorganic oxides, the matrix is short of specific adsorption sites, and thus, chemical modification is usually needed to improve the surface properties. Fluoride is mostly used as a hard Lewis base attenuating the Lewis acid-base interactions between the zirconia surface and carboxyl groups on the target protein surface. Griffith. [27, 28] developed the “fluoride-modified zirconia” (FmZr) matrix, which functioned as a mixed mode support and could retain proteins by cation-, anion-, and ligand-exchange mechanisms and did not suffer from ‘‘selection monotony’’ of ion-exchange phases.
Table 1 lists some matrices for EBA processes, several of which have been commercialized.
3 PREPARATION METHODS OF MATRIX BEADS
Preparation of matrix for EBA has been widely concerned, and various techniques have been applied. The process parameters are studied and accurately controlled to produce matrices with proper density and size distribution, which provide a good technical support for EBA matrices with excellent performance.
3.1 Reversed-phase suspension method
In reversed-phase suspension method, the densified- particles are uniformly mixed with the hydrophilic polymers (dispersed phase), and dispersed by comminution into the hydrophobic solvents (continuous phase) to form the embedded droplets. Solidification occurs to the droplets afterward by changing the temperature, polymerizing or crosslinking. The oils with high viscosity are preferred to use as the continuous phase since they increase the droplet stability by decreasing the droplet collision frequency [35]. A small amount of stabilizer is added, which forms an interfacial layer around the droplets, reducing the interfacial tension and the rate of coalescence and break-up [36]. Agitation is normally continued throughout the process to prevent settling or creaming. The size distribution of the final beads is dependent upon the balance between droplet break-up and coalescence, which can be controlled by the characteristics of continuous phase and dispersed phase, additionally the speed of agitator and the concentration of stabilizer used [35, 37].
Densifier is indispensable in the synthesis of matrix for EBA, and it is a distinguishing feature compared with the matrix for fixed bed. In order to increase the matrix density, various heavy micro-granulesare often selected as the densifier. These micro-granules are usually amorphous and suitable for production of the dispersing type matrix. However, for the core type matrix, larger spherical shape is preferred as the inner cores. Some metal or alloy powder is prepared by ball milling method, but the sphericity is not very good [38]. The oxides or ceramic granules, such as spherical zirconia powder [39-41], can be synthesized by emulsion synthesis method, and then, sintered to form the larger spheres. High temperature is frequently introduced in the sintering or fusing technology. For example, the spherical titanium dioxide powder is prepared by fusion of TiO2powders in the O2/H2flame [42], or by in-flight oxidation of titanium carbide powder in Ar/O2thermal plasma [43]. Many kinds of the densifier have been commercialized, which is beneficial to the preparation of EBA matrices.
As mentioned above, porogenic agents are also added to obtain porous beads, aiming to improve the mass-transfer characteristics. The general method is that the porogenic agent is mixed with the dispersed phase and densifier, suspended in the oil phase, and then solidified to form the composite beads. After dissolving or degrading the porogenic agents, the porous matrix is obtained. However, the enlargement of pores may decrease the mechanical strength, as a result, the matrix skeleton should have enough rigidity to prevent pore collapse at high flow rate. One convenient solution is crosslinking. The crosslinking procedure is usually carried out at the same time with the reversed-phase suspension process, called reversed- phase suspension crosslinking method. The commercialized StreamlineTMDEAE adsorbent, a composite crosslinked agarose gel based anion exchanger adsorbent, was prepared by this method [33]. The crosslinked matrix may have favorable mechanical strength and chemical stability for repeating use.
Reversed-phase suspension method is by far the most commonly used technique for the production of matrices for EBA, especially for the polysaccharide- skeleton based matrices. P?lsson. [9] reported the preparation process of agarose/stainless steel matrix. The stainless steel beads were thoroughly mixed with a heat agarose solution (4%, by mass), and then poured into paraffin oil with a small amount of Span 85. After 30 s the mixture was cooled to below 15°C and stirred for a further 2 min, forming a pellicular matrix with high density. A novel agarose/Nd-Fe-B alloy composite matrix was prepared by Tong and Sun [31]. They used soybean oil as the continuous phase containing 10 g·L-1Span 80, slowly added the mixture of agarose solution (4%, by mass) with the Nd-Fe-B alloy, and agitated at 1400 r·min-1for 30 min at 90°C. After cooling to 15°C and a further agitation for 30 min, the densified beads were recovered by the permanent magnet from the oil phase. The CELBEADS matrix reported by Pai. [44] was cellulose-based beads with a procedure involved cross-linking of cellulose in a porogen solvent and hardening of the dispersed beads in aqueous phase. Other agarose-based matrices for EBA were prepared in the similar process with a slight variation of process parameters [32, 45-47].
The cellulose-based matrices are produced by thermal regeneration method, including the procedures of alkalization, xanthation, acidification and desulfurization [18, 48]. Cellulose is alkalized in concentrated sodium hydroxide solution, and then squeezed, oxidized, and further reacted with carbon disulfide to form cellulose xanthate viscose [49]. This viscose is mixed thoroughly with densifier, dispersed in the oil phase, then treated with acid and heating, the cellulose- based composite microspheres are regenerated. Other solvents can also be used to prepare the regenerated cellulose, such as calcium thiocyanate [50] and-methylmorpholine--oxide (NMMO) [51, 52]. Various cellulose-based matrices for EBA have been produced now, including cellulose/titanium dioxide [16], cellulose/stainless steel [13], cellulose/nickel powder [17], cellulose/tungsten carbide [20, 21] and so on. These matrices present high porosity, proper density and appropriate size distribution, which show promising practicality in EBA processes.

Table 1 Some matrices for EBA
①Superficial velocity of irrigating buffers for a two-fold expansion.
②The large fraction of these matrices.
3.2 Emulsion synthesis method
4 FUNCTIONALIZED ADSORBENTS: MODIFICATION WITH LIGANDS
Various matrices have been produced nowadays and, the physical properties and the expansion characteristics are measured as well. These matrices, however, can not be directly applied in the expanded bed separation of bio-molecules, since they are usually poor in adsorption capacity, especially the specific adsorption of bio-molecules. Ligands should be coupled to the supporting matrices to prepare the functionalized adsorbents. The preparation processes generally include the steps of surface activation and ligand coupling, and sometimes the ligands are bonded through connecting-arms.
4.1 Matrix activation
Different from the silicon-based or man-made polymer matrices for fixed bed, the skeleton of the matrices for EBA are mostly polysaccharides, which are rich in hydroxyl groups. Therefore, nearly all the modifications are based on the hydroxyl groups on the polysaccharide skeleton of the matrices. Some reactive intermediates are prepared with bifunctional reagents for further reactions. Epoxy-activated intermediates are commonly used, which are obtained by reacting with epichlorohydrin [53, 54] or bis-epoxide [55]. The epoxy-ring opens under acidic or alkaline conditions, so that the ligands are coupled with the matrices. Allyl-activated matrix is another significant intermediate for the preparation of functionalized adsorbents, which is reacted with allyl bromide or allyl glycidyl ether under alkaline condition. The allyl group is comparatively inert in alkaline solution but activated by aqueous bromination to form bromo-hydroxypropyl groups to be attached to nucleophilic ligands [56-58]. The third bifunctional reagent often used is divinylsulfone (DVS). DVS-activated matrix is prone to react with functional ligands, and more significantly, it provides a sulfone structure responsible for the thiophilic interactions [59, 60], showing important implications for making affinity adsorbents.
The activating reactions mentioned above are described in Fig. 3.
4.2 Ion-exchange expanded bed adsorbents
Ion-exchange adsorbents are the traditional separation medium widely used in protein separation and purification. They are generally prepared from the epoxy-activated [61], allyl-activated [56] or DVS-activated[8] intermediates, such as Cell-Ti DEAHP adsorbent [61] and Celbeads-EDA [8]. Another method to produce ion-exchange adsorbents is that polyelectrolyte is coated on the surface of the matrix with electrostatic interaction, and crosslinked to form a charged layer, like poly(acrylic acid) (PAA) coated StreamlineTMDEAE adsorbent reported by Dainiak[62].
A special method suggested by Voute and Boschetti [7] was surface polymerization. Zirconia beads were contacted with an acrylic monomer mixture composed of a functionalized monomer and a bifunctional acrylic monomer as crosslinking reagent. Polymerization was started using a radical initiator, and the resulting beads formed. Different monomers could be selected for the preparation of hydrophilic anion exchangers (.. quaternary ammonium salts) or cation exchangers (.. sulfonic acids).
4.3 Affinity expanded bed adsorbents
Affinity adsorbent is one of the most effective and powerful materials for purification of proteins [63, 64]. Various affinity ligands have been developed and widely used. The dyes, such as Cibacron blue (CB) 3GA [8, 65], Procion Yellow H-E3G and Procion Red H-E7B [15], are used as ligands covalently coupled to activated matrices. Additionally, protein A coated expanded bed adsorbents were successfully developed as StreamlineTMProtein A by Pharmacia BioTech (Uppsala, Sweden) and, evaluated by Th?mmes[66]. The dynamic capacity of IgG reached 9 to 16 mg·ml-1depending on the concentration of antibody MAb in the feed and the linear flow-rate employed, and the antibody was concentrated 30 fold in the eluate by employing a standard mode of expanded bed elution.

Figure 3 Schematic diagram of activating reactions
Figure 4 Some mixed mode adsorbents with different ligands [68-70]
Another unique affinity adsorbent for EBA was designed by Nayak[67]. Macroporous crosslinked hydroxyethyl methacrylate/ethylene dimethacrylate copolymeric beads (HEG beads) were synthesized by suspension polymerization. These beads were coupled toa-cyclodextrin using 2,4-tolylene diisocyanate (TDI) as a spacer for purification ofa-CGTase. The adsorbed enzyme in this adsorbent from 5-fold diluted unclarified fermented broth was eluted with the CaCl2/Tris/HCl buffer at a flow rate of 4.4 ml·min-1, yielding 94.7-fold increase in enzyme purification with 37.92% recovery.
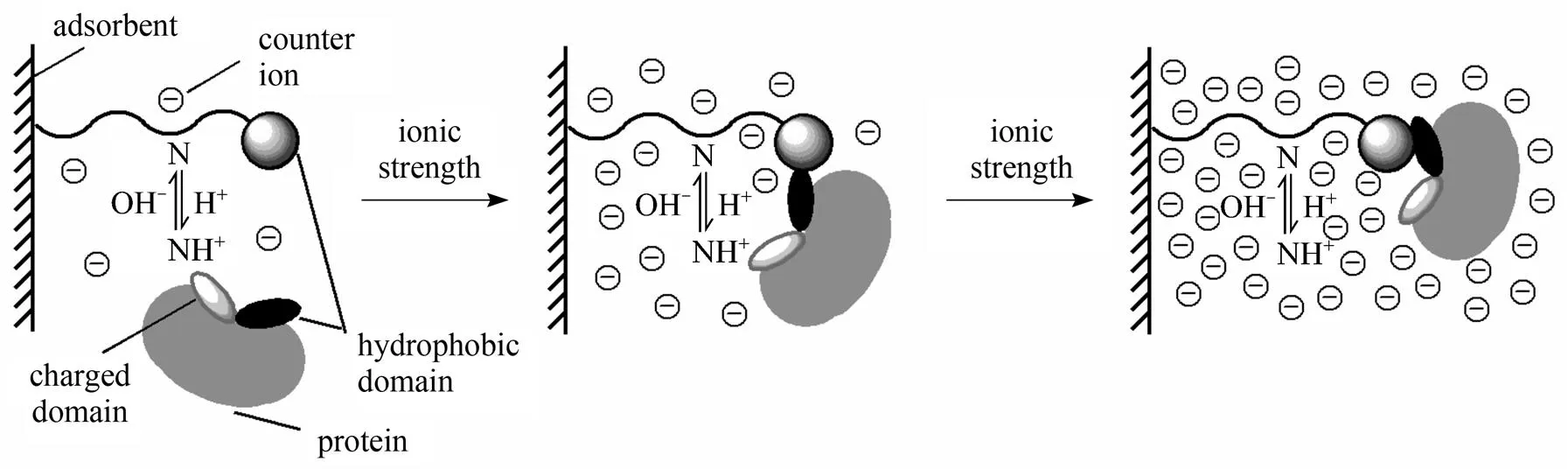
Figure 5 Mixed mode adsorption: features of interaction. Schematic representation (not to scale) of the adsorption characteristics of a globular protein, with both charged and hydrophobic regions, to a typically bifunctional mixed mode matrix in response to environmental conditions of increasing ionic strength (depicted by anions only) [68]
4.4 Mixed-mode adsorbents for EBA
Mixed mode adsorbents comprise low molecular weight ligands that have both hydrophobic (.. aromatic structure) and hydrophilic domains (.. amino group) within the same molecule [68-70] (seen Fig. 4). The charged domain of ligand implements the ion-exchange adsorption of target protein at low ionic strength, and as ionic strength increases the hydrophobic domain of ligand allows hydrophobic interaction to occur in salt-tolerant adsorption [68] (shown in Fig. 5). In contrast to ion-exchangers, the binding strength is strongly pH dependent and largely independent of ionic strength in the raw material. Mixed-mode chromatography is especially appropriate for expanded bed adsorption (EBA) applications to direct isolation of extracellular products from culture broth [69, 71, 72].
A series of mixed mode Sepharose and Perloza bead cellulose adsorbents were prepared by Burton. [73] with various phenylalkylamine ligands attached to epoxy-activated Sepharose. Chymosin was adsorbed at high and low ionic strength with the average adsorption capacity of 60-80 mg·ml-1, and recovered by a small pH change. The methods provided good resolution of chymosin from a crude recombinant source. Gao. [74] prepared a novel mixed-mode expanded bed adsorbent by coupling benzylamine ligand to allyl-activated cellulose/stainless steel matrix. The adsorbents showed the salt-tolerant property that the BSA adsorption capacity just slightly decreased from 85 to 70 mg·ml-1as the NaCl concentration increased from 0 to 0.25 mol·L-1. Furthermore, the adsorption process was patch controlled, and the retention factor showed the typical “U” shape curve as a function of salt concentration, revealing a comprehensive effect on both electrostatic and hydrophobic interactions, which was typical for mixed-mode chromatography [75]. Theb-glucosidase was recovered with StreamlineTMDirect HST in the expanded bed operation [76], and the yield ofb-glucosidase was 74%, significantly higher than that on StreamlineTMSP (48%). Moreover,b-glucosidase was purified with a factor of 4.1 and concentrated with a factor of 17 on Streamline Direct HST1, nearly twice as much as those for StreamlineTMSP.
4.5 Adsorbents for hydrophobic charge induction chromatography
Hydrophobic charge induction chromatography (HCIC) is a novel chromatographic technique for bio-macromolecule separation in expanded bed. It has been proven to be an efficient technique for antibody purification in the fixed bed mode [77, 78]. The ligands of HCIC have multiform functions, commonly combining hydrophobic, electrostatic, dipole and affinity interactions, and the target protein can be adsorbed on the HCIC ligand by affinity and hydrophobic effects and desorbed under the electrostatic repulsion between protein and the charged groups [79]. Xia. [80] designed several HCIC adsorbents for EBA with binding mercapto heterocyclic ligands to the allyl- or DVS-activated macroporous cellulose/tungsten carbide matrix (Fig. 6). These thiophilic ligands showed high adsorption capacities of IgY and the adsorption/desorption could be controlled by a pH change [81] (shown in Fig. 7). For example, the adsorption capacity reached the maximum values of 137.6 mg·ml-1for cell-TuC-DVS-MMI at pH 7, and 101.9 mg·ml-1for cell-TuC-DVS-MBI at pH 4. Furthermore, these HCIC adsorbents showed better selectivity for IgY than BSA (the maximum value of 34.8 mg·ml-1at pH 7), indicating a potential application for immunoglobulin purification in expanded bed mode.

Figure 6 Preparation routes of HCIC adsorbents from the activated matrices [80]
5 PROSPECTS OF ADSORBENTS DEVELOPED FOR EBA
In these two decades, different types of supporting matrices have been developed for EBA, and their preparation methods are being consummated gradually. Researches are being focused on the production of matrices with small particle size, high density, pellicular layer, large pores, and high specific surface area. Furthermore, new materials may be applied to construct novel matrices with improved or unique functions, which will be conveniently modified to produce functionalized adsorbents.
The design and production of adsorbents with functionalized ligand for EBA has always been focused. It is a challenging work greatly concerned about the interactions between biomolecules and ligands, sometimes requiring a large amount of tedious calculation and molecular simulation. Nowadays, ion-exchange adsorbents as the traditional product have been commercialized and widely used in the separating processes. However, ion-exchangers usually show high sensitivity to ionic strength and less selectivity to the target molecules. Therefore, novel mechanisms have been proposed, and various new types of adsorbents have been designed to improve the adsorbing specificity. Affinity, HCIC, and mixed mode adsorbents are developed to increase specific adsorption or to reduce non-specific adsorption. Especially, HCIC with multi-modal interactions combining dipole interaction, hydrophobic, and electrostatic interactions shows a favorable practice in the purification of antibodies.
Expanded bed adsorption, a promising and efficient separation technique, has been paid much attention by the global researchers. However, the application of EBA in industries is rarely reported, due to some problems in process scale-up. Flow distributor and adsorbent are the two main factors influencing the EBA operation processes. A universal large-scale flow distributor developed for EBA is a prerequisite for its industrial application, but compared with the fixed-bed, the flow distributor is more complicated and difficult to design. On the other hand, large amounts of low-cost adsorbents are needed to reduce the production cost. The commercialized adsorbents, however, are so expensive that they are not suitable for industrial use, which it is another bottle-neck for the industrial production. Many efforts are being made in the scale-up of the EBA process. It is hoped that novel flow distributor and cheap adsorbents will be developed in the future and applied to the purification of biomolecules with high efficiency in industrial use.
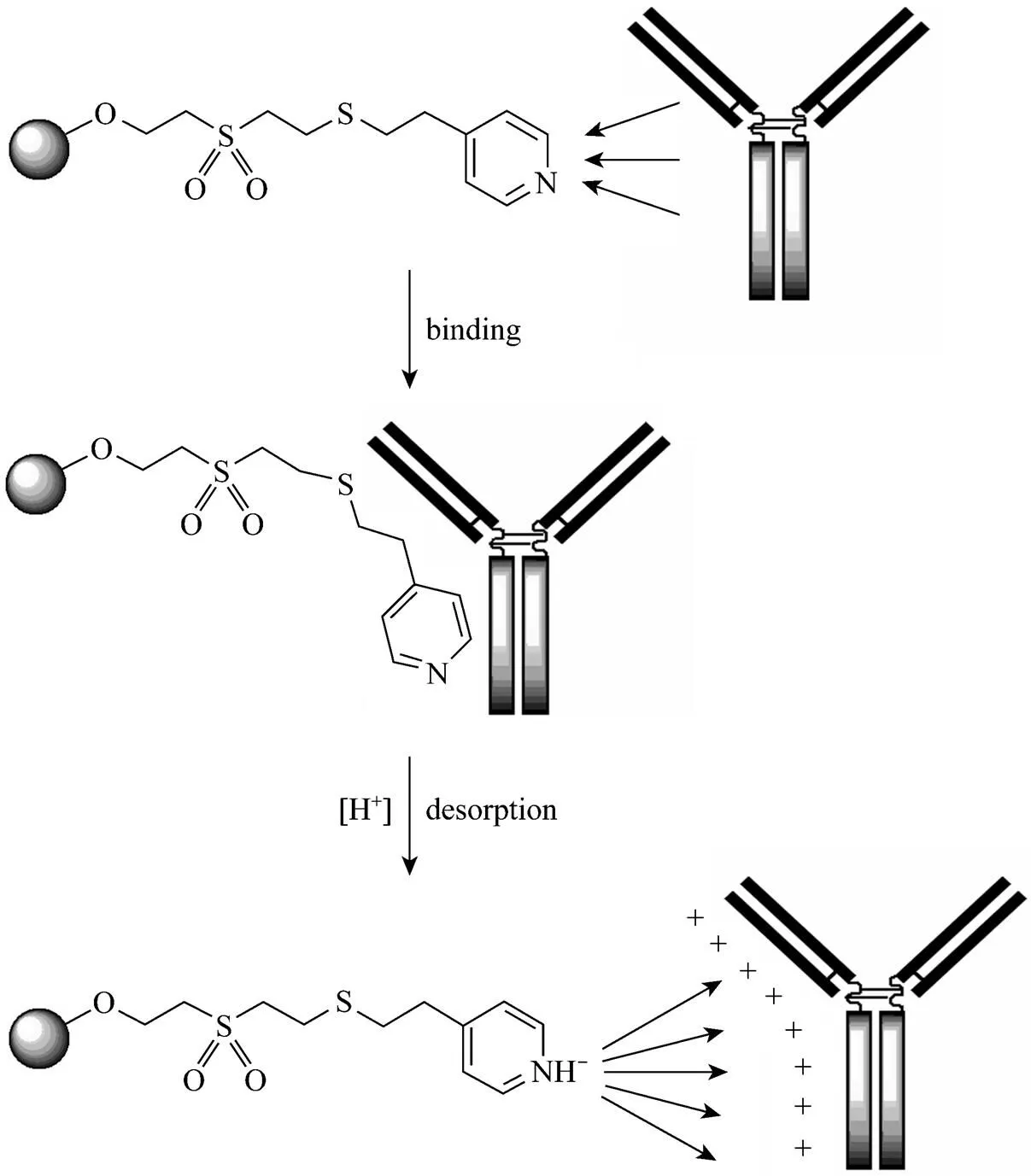
Figure 7 Mechanism of HCIC for antibody separation, showing the interaction between the immunoglobulin molecule and the ligand of 4-mercapto-ethyl-pyridine (MEP) [81]
1 Anspach, F.B., Curbelo, D., Hartmann, R., Garke, G., Deckwer, W.D., “Expanded-bed chromatography in primary protein purification”,.., 865 (1/2), 129-144 (1999).
2 Chase, H.A., “Purification of proteins by adsorption chromatography in expanded beds”,., 12 (8), 296-303 (1994).
3 Hjorth, R., “Expanded-bed adsorption in industrial bioprocessing: Recent developments”,., 15 (6), 230-235 (1997).
4 Th?mmes, J., “Fluidized bed adsorption as a primary recovery step in protein purification”,...., 58, 185-230 (1997).
5 P?lsson, E., Axelsson, A., Larsson, P.O., “Theories of chromatographic efficiency applied to expanded beds”,.., 912, 235-248 (2001).
6 Hubbuch, J., Th?mmes, J., Kula, M.R., “Biochemical engineering aspects of expanded bed adsorption”,...., 92, 101-123 (2005).
7 Voute, N., Boschetti, E., “Highly dense beaded sorbents suitable for fluidized bed applications”,, 8, 115-120 (1999).
8 Pai, A., Gondkar, S., Lali, A., “Enhanced performance of expanded bed chromatography on rigid superporous adsorbent matrix”,.., 867, 113-130 (2000).
9 P?lsson, E., Gustavsson, P.E., Larsson, P.O., “Pellicular expanded bed matrix suitable for high flow rates”,.., 878, 17-25 (2000).
10 Al-Dibouni, M.R., Garside, J., “Particle mixing and classification in liquid fluidized beds”,...., 57, 94-103 (1979).
11 Lei, Y.L., Yao, S.J., Liu, Z.Z., Zhu, Z.Q., “Advances in matrices for expanded bed adsorption”,, 15 (2), 219-224 (2002). (in Chinese)
12 Xia, H.F., Lin, D.Q., Yao, S.J., “Evaluation of new high-density ion exchange adsorbents for expanded bed adsorption chromatography”,.., 1145, 58-66 (2007).
13 Miao, Z.J., Lin, D.Q., Yao, S.J., “Preparation and characterization of cellulose-stainless steel powder composite particles customized for expanded bed application”,...., 44 (22), 8218-8224 (2005).
14 Jahanshahi, M., Partida-Martinezb, L., Hajizadeha, S., “Preparation and evaluation of polymer-coated adsorbents for the expanded bed recovery of protein products from particulate feedstocks”,.., 1203, 13-20 (2008).
15 McCreath, G.E., Chase, H.A., Owen, R.O., “Expanded bed affinity chromatography of dehydrogenases from Baker’s yeast using dye-ligand perfluoropolymer supports”,.., 48, 341-354 (1995).
16 Lei, Y.L., Lin, D.Q., Yao, S.J., Zhu, Z.Q., “Preparation and characterization of titanium oxide-densified cellulose beads for expanded bed adsorption”,...., 90, 2848-2854 (2003).
17 Xia, H.F., Lin, D.Q., Yao, S.J., “Spherical cellulose-nickel powder composite matrix customized for expanded bed application”,...., 104, 740-747 (2007).
18 Lei, Y.L., “Preparation of spherical cellulose/TiO2composite adsorbent for expanded bed adsorption and its application to purification of proteins”, Ph. D. Thesis, Zhejiang Univ., China (2003). (in Chinese)
19 Rodrigues, A.E., Lu, Z.P., Loureiro, J.M., “Residence time distribution of inert and linearly adsorbed species in a fixed bed containing ‘large-pore’ supports: applications in separation engineering”,..., 46 (11), 2765-2773 (1991).
20 Xia, H.F., Lin, D.Q., Yao, S.J., “Preparation and characterization of macroporous cellulose–tungsten carbide composite beads for expanded bed applications”,.., 1175, 55-62 (2007).
21 Xia, H.F., Lin, D.Q., Yao, S.J., “Chromatographic performance of macroporous cellulose-tungsten carbide composite beads as anion-exchanger for expanded bed adsorption at high fluid velocity”,.., 1195, 60-66 (2008).
22 Zhang, M.L., Sun, Y., “Cooperation of solid granule and solvent as porogenic agents. Novel porogenic mode of biporous media for protein chromatography”,.., 922, 77-86 (2001).
23 Shi, Q.H., Zhou, X., Sun, Y., “A novel superporous agarose medium for high-speed protein chromatography”,.., 92, 643-651 (2005).
24 Wang, D.M., Hao, G., Shi, Q.H., Sun, Y., “Fabrication and characterization of superporous cellulose bead for high-speed protein chromatography”,.., 1146, 32-40 (2007).
25 Th?mmes, J., Halfar, M., Lenz, S., Kula, M.R., “Purification of monoclonal antibodies from whole hybridoma fermentation broth by fluidized bed adsorption”,.., 45, 205-211 (1995).
26 Voute, N., Bataille, D., Girot, P., Boschetti, E., “Characterization of very dense mineral oxide-gel composites for fluidized-bed adsorption of biomolecules”,, 8, 121-129 (1999).
27 Griffith, C.M., Morris, J., Robichaud, M., Annen, M.J., McCormick, A.V., Flickinger, M.C., “Fluidization characteristics of and protein adsorption on fluoride-modified porous zirconium oxide particles”,.., 776, 179-195 (1997).
28 Mullick, A., Griffith, C.M., Flickinger, M.C., “Expanded and packed bed albumin adsorption on fluoride modified zirconia”,.., 60 (3), 333-340 (1998).
29 Lihme, A., Zafirakos, E., Hansen, M., Olander, M., “Simplified and more robust EBA processes by elution in expanded bed mode”,, 8, 93-97 (1999).
30 Pall Corporation, “DEAE & SP Spherodex?LS silica/dextran composite ion exchangers for liquid chromatography of biologicals”, http://www.pall.com/pdf/35753.pdf.
31 Tong, X.D., Sun, Y., “Nd-Fe-B alloy-densified agarose gel for expanded bed adsorption of proteins”,.., 943, 63-75 (2001).
32 Jahanshahi, M., Sun, Y., Santos, E., Pacek, A., Franco, T.T., Nienow, A., Lyddiatt A., “Operational intensification by direct product sequestration from cell disruptates: application of a pellicular adsorbent in a mechanically integrated disruption-fluidised bed adsorption process”,.., 80 (2), 201-212 (2002).
33 Amersham Pharmacia Biotech., “Expanded bed adsorption-principles and methods”, Code No. 18-1124-26, Uppsala, Sweden (1998).
34 Yun, J.X., Lin, D.Q., Lu, M.H., Zhong, L.N., Yao, S.J., “Measurement and modeling of axial distribution of adsorbent particles in expanded bed: taking into account the particle density difference”,..., 59, 5873-5881 (2004).
35 Dowding, P.J., Vincent, B., “Suspension polymerisation to form polymer beads”,.., 161, 259-269 (2000).
36 Dawkins, J.V., “Aqueous suspension polymerizations”, In: Comprehensive Polymer Science, The Synthesis, Characterization and Applications of Polymers, IV, Pergamon, Oxford, 231-241 (1989).
37 Pan, Z.R., Weng, Z.X., Huang, Z.M., Suspension Polymerization, Chemical Industry Press, Beijing, China (1997). (in Chinese)
38 Kumaran, S., Chantaiah, B., Rao, T.S., “Effect of niobium and aluminium additions in TiAl prealloyed powders during high-energy ball milling”,..., 108, 97-101 (2008).
39 Ma, T., Huang, Y., Yang, J.L., He, J.T., Zhao, L., “Preparation of spherical zirconia powder in microemulsion system and its densification behavior”,., 25, 515-519 (2004).
40 Lee, M.H., Tai, C.Y., Lu, C.H., “Synthesis of spherical zirconia by precipitation between two water/oil emulsions”,...., 19, 2593-2603 (1999).
41 Robichaud, M.J., Sathyagal, A.N., Carr, P.W., McCormick, A.V., Flickinger, M.C., “An improved oil emulsion synthesis method for large, porous zirconia particles for packed- or fluidized-bed protein chromatography”,..., 32 (15), 2547-2559 (1997).
42 Morimoto, T., Kittaka, S., “Spherical particles and their surface properties: II. Preparation of spherical particles of titanium dioxide”,..., 78 (2), 356-361 (1980).
43 Li, Y.L., Ishigaki, T., “Synthesis of crystalline micron spheres of titanium dioxide by thermal plasma oxidation of titanium carbide”,.., 13, 1577-1584 (2001).
44 Pai, A., Gondkar, S., Sundaram, S., Lali, A., “Expanded bed adsorption on supermacroporous cross-linked cellulose matrix”,, 8, 131-138 (1999).
45 Zhou, X., Shi, Q.H., Bai, S., Sun, Y., “Dense pellicular agarose-glass beads for expanded bed application: Fabrication and characterization for effective protein adsorption”,..., 18, 81-88 (2004).
46 Jahanshahi, M., Pacek, A.W., Nienow, A.W., Lyddiatt, A., “Fabrication by three-phase emulsification of pellicular adsorbents customised for liquid fluidised bed adsorption of bioproducts”,...., 78, 1111-1120 (2003).
47 Jahanshahi, M., Zhang, Z., Lyddiatt, A., “Subtractive chromatography for purification and recovery of nano-bioproducts”,.., 152 (3), 121-126 (2005).
48 ?tamberg, J., “Bead cellulose”,.., 17 (2), 155-183 (1988).
49 Arthur, J.C.Jr., “Chemical modification of cellulose and its derivatives”, In: Comprehensive Polymer Science, The Synthesis, Characterization and Applications of Polymers, VI, Pergamon, Oxford, 49-80 (1989).
50 Kuga, S., “New cellulose gel for chromatography”,.., 195, 221-230 (1980).
51 Fink, H.P., Weigel, P., Purz, H.J., Ganster, J., “Structure formation of regenerated cellulose materials from NMMO-solutions”,..., 26, 1473-1524 (2001).
52 Dogan, H., Hilmioglu, N.D., “Dissolution of cellulose with NMMO by microware heating”,.., 75, 90-94 (2009).
53 Axén, R., Drevin, H., Carlsson, J., “Preparation of modified agarose gels containing thiol groups”,.., 29, 471-474 (1975).
54 Scoble, J.A., Scopes, R.K., “Assay for determining the number of reactive groups on gels used in affinity chromatography and its application to the optimisation of the epichlorohydrin and divinylsulfone activation reactions”,.., 752, 67-76 (1996).
55 Sundberg, L., Porath, J., “Preparation of adsorbents for biospecific affinity chromatography (I) Attachment of group-containing ligands to insoluble polymers by means of bifunctional oxiranes”,.., 90 (1), 87-98 (1974).
56 Burton, S.C., Harding, D.R.K., “Bifunctional etherification of a bead cellulose for ligand attachment with allyl bromide and allyl glycidyl ether”,.., 775, 29-38 (1997).
57 Burton, S.C., Harding, D.R.K., “High-density ligand attachment to brominated allyl matrices and application to mixed mode chromatography of chymosin”,.., 775, 39-50 (1997).
58 Burton, S.C., Harding, D.R.K., “Preparation of chromatographic matrices by free radical addition ligands attachment to allyl groups”,.., 796, 273-282 (1998).
59 Noel, R.J., O’Hare, W.T., Street, G., “Thiophilic nature of divinylsulphone cross-linked agarose”,.., 734, 241-246 (1996).
60 Scoble, J.A., Scopes, R.K., “Ligand structure of the divinylsulfone-based T-gel”,.., 787, 47-54 (1997).
61 Lei, Y.L., Lin, D.Q., Yao, S.J., Zhu, Z.Q., “Preparation of an anion exchanger based on TiO2-densified cellulose beads for expanded bed adsorption”,..., 62, 169-177 (2005).
62 Dainiak, M.B., Galaev, I.Y., Mattiasson, B., “Polyelectrolyte-coated ion exchangers for cell-resistant expanded bed adsorption”,.., 18, 815-820 (2002).
63 Linhult, M., Gülich, S., Hober, S., “Affinity ligands for industrial protein purification”,., 12, 305-310 (2005).
64 Li, J.H., Feng, J., Dang, Q.Q., Qiao, Y.T., Zhao, J.X., Zhang, S.H., Sun, H.W., Wen, X., Yuan, Z., “Affinity adsorption mechanism studies of adsorbents for oligopeptides using model polymer”,, 50, 1602-1608 (2009).
65 He, L.Z., Gan, Y.R., Sun, Y., “Adsorption-desorption of BSA to highly-substituted dye-ligand adsorbent: quantitative study of the effect of ionic strength”,.., 17, 301-305 (1997).
66 Th?mmes, J., Bader, A., Halfar, M., Karau, A., Kula, M.R., “Isolation of monoclonal antibodies from cell containing hybridoma broth using a protein A coated adsorbent in expanded beds”,.., 752, 111-122 (1996).
67 Nayak, D.P., Ponrathnam, S., Rajan, C.R., “Macroporous copolymer matrix IV. Expanded bed adsorption application”,.., 922, 63-76 (2001).
68 Hamilton, G.E., Luechau, F., Burton, S.C., Lyddiatt, A., “Development of a mixed mode adsorption process for the direct product sequestration of an extracellular protease from microbial batch cultures”,.., 79, 103-115 (2000).
69 Gao, D., Lin, D.Q., Yao, S.J., “Protein adsorption kinetics of mixed-mode adsorbent with benzylamine as functional ligand”,..., 61, 7260-7268 (2006).
70 Gao, D., Lin, D.Q., Yao, S.J., “Mechanistic analysis on the effects of salt concentration and pH on protein adsorption onto a mixed-mode adsorbent with cation ligand”,.., 859, 16-23 (2007).
71 Burton, S.C., Harding, D.R.K., “Salt-independent adsorption chromatography: new broad-spectrum affinity methods for protein capture”,..., 49, 275-287 (2001).
72 Lu, M.H., Lin, D.Q., Wu, Y.C., Yun, J.X., Mei, L.H., Yao, S.J., “Separation of nattokinase from Bacillus subtilis fermentation broth by expanded bed adsorption with mixed-mode adsorbent”,..., 10, 128-135 (2005).
73 Burton, S.C., haggarty, N.W., Harding, D.R.K., “One step purification of chymosin by mixed mode chromatography”,.., 56, 45-55 (1997).
74 Gao, D., Yao, S.J., Lin, D.Q., “Preparation and adsorption behavior of a cellulose-based, mixed-mode adsorbent with a benzylamine ligand for expanded bed applications”,...., 107, 674-682 (2008).
75 Gao, D., Lin, D.Q., Yao, S.J., “Patch controlled protein adsorption in mixed-mode chromatography with benzylamine as functional ligand”,..., 38, 355-361 (2008).
76 Charoenrat, T., Ketudat-Cairns, M., Jahic, M., Enfors, S.O., Veide, A., “Recovery of recombinantb-glucosidase by expanded bed adsorption from Pichia pastoris high-cell-density culture broth”,.., 122, 86-98 (2006).
77 Boschetti, E., “Antibody separation by hydrophobic charge induction chromatography”,., 20 (8), 333-337 (2002).
78 Ghose, S., Hubbard, B., Cramer, S.M., “Protein interactions in hydrophobic charge induction chromatography (HCIC)”,.., 21, 498-508 (2005).
79 Burton, S.C., Harding, D.R.K., “Hydrophobic charge induction chromatography: salt independent protein adsorption and facile elution with aqueous buffers”,.., 814, 71-81 (1998).
80 Xia, H.F., Lin, D.Q., Wang, L.P., Chen, Z.J., Yao, S.J., “Preparation and evaluation of cellulose adsorbents for hydrophobic charge induction chromatography”,...., 47 (23), 9566-9572 (2008).
81 Xia, H.F., “Fundamental research on the functionalization and application of cellulose-based composite matrix for chromatography”, Ph. D. Thesis, Zhejiang Univ., China (2008). (in Chinese)
2009-05-18,
2009-06-08.
the National Natural Science Foundation of China (20876139, 20776129) and the National Basic Research Program of China (2007CB707805).
** To whom correspondence should be addressed. E-mail: yaosj@zju.edu.cn
 Chinese Journal of Chemical Engineering2009年4期
Chinese Journal of Chemical Engineering2009年4期
- Chinese Journal of Chemical Engineering的其它文章
- Removal of Uranium (VI) by Fixed Bed Ion-exchange Column Using Natural Zeolite Coated with Manganese Oxide*
- Phase Equilibrium of Isobutanol in Supercritical CO2
- Conversion of Methane by Steam Reforming Using Dielectric-barrier Discharge*
- Permeability and Selectivity of Sulfur Dioxide and Carbon Dioxide in Supported Ionic Liquid Membranes*
- Hydroxyapatite Coatings on Titanium Prepared by Electrodeposition in a Modified Simulated Body Fluid*
- Model Study on a Submerged Catalysis/Membrane Filtration System for Phenol Hydroxylation Catalyzed by TS-1*
