NMR and EPR Studies of Partially Reduced TiO2
Yuhong Li , Xin-Ping Wu , Cong Liu , Meng Wang , Benteng Song , Guiyun Yu ,Gang Yang , Wenhua Hou , Xue-Qing Gong , Luming Peng ,*Suzhou Key Laboratory of Functional Ceramic Materials, School of Chemistry and Material Engineering, Changshu Institute of Technology, Changshu 5500, Jiangsu Province, P. R. China.
2 Key Laboratory of Mesoscopic Chemistry of Ministry of Education, School of Chemistry and Chemical Engineering,Nanjing University, Nanjing 210023, P. R. China.
3 Key Laboratory for Advanced Materials, Centre for Computational Chemistry, Research Institute of Industrial Catalysis,East China University of Science and Technology, Shanghai 200237, P. R. China.
4 School of Chemical and Biological Engineering, Yancheng Institute of Technology, Yancheng 224051, Jiangsu Province,P. R. China.
Abstract:Partially reduced TiO2 nanomaterials have attracted significant interest because of their visible-light activity for catalysis and photodegradation. Herein, we prepared a partially reduced anatase TiO2(Re-A-TiO2) nanoparticle material using a fast combustion method,demonstrating good activity toward decomposing methyl orange under visible light irradiation. The surface structure of the prepared material, after being surface-selectively 17O-labeled with H217O (17O-enriched water), was studied via 17O and 1H solid-state magic angle spinning nuclear magnetic resonance (MAS NMR) spectroscopy and electron paramagnetic resonance (EPR) spectroscopy, and the obtained results were compared to those of non-reduced anatase TiO2 (A-TiO2). The EPR results showed that the concentrations of paramagnetic species (i.e., oxygen vacancies (OV) and Ti3+) in Re-A-TiO2 were much higher than that in A-TiO2, while the former was associated with a higher OV/Ti3+ ratio. The intensities of the EPR signals were significantly affected by the adsorbed water, and this phenomenon was explored in combination with 1H NMR spectroscopy.The 1H species on Re-A-TiO2 appeared at larger chemical shifts, denoting the increased acidity of the sample, and these 1H species on Re-A-TiO2 were more difficult to remove than those on A-TiO2. On the other hand, different features were observed for the signals arising from the two-coordinated oxygen atoms (μ2-O) in 17O NMR, suggesting a typical anatase TiO2(101) surface on A-TiO2, but a more complex surface environment for Re-A-TiO2. Furthermore, a larger amount of hydroxyl groups (OH) were observed on Re-A-TiO2 compared to that on A-TiO2, indicating a larger proportion of exposed(001) facets on Re-A-TiO2. However, the μ2-O signals broadened and became similar when the drying temperature was increased to 100 °C, indicating a non-faceted anatase TiO2 surface in such conditions. Based on the EPR and NMR results,a significant fraction of the OH species is believed to be formed from the reaction of the paramagnetic centers and adsorbed water molecules. The 1H→17O cross polarization (CP) MAS and two-dimensional heteronuclear correlation (2D HETCOR)NMR spectra were used to verify the spatial proximity of the hydrogen and oxygen species, confirming the spectral assignments of a strongly adsorbed water and one type of surface OH species. In particular, the 1H NMR signals at approximately 11 ppm were ascribed to the hydrogen species in the intramolecular hydrogen bond. In summary, this study investigated the paramagnetic species and surface structure of anatase TiO2 materials by combining EPR along with 1H and 17O solid-state NMR spectroscopy. The differences in the surface structures of Re-A-TiO2 and A-TiO2 should be closely related to their different properties toward the photodegradation of methyl orange.
Key Words:Partially reduced TiO2;Solid-state NMR;17O NMR spectrum;1H NMR spectrum;EPR;Hydroxyl group;Hydrogen bond;Visible light degradation
1 Introduction
Recently, partially reduced TiO2has received a vast amount of research attention for its effectiveness in absorbing visible and infrared radiation of the sunlight, which is expected to significantly increase the utilization rate of the solar light energy in photocatalytic reactions1. The structural origin of this phenomenon has been ascribed to Ti3+doping2, disorder in the surface layers of nanophase TiO23, H doping4, the presence of oxygen vacancies5,6, as well as the synergistic effect between Ti3+and doped species7. Although a few investigations using EPR spectroscopy5,8or density functional theory (DFT)calculation3were conducted, there is little detailed information on the local surface structure of this material, which is critical for catalytic reactions. That’s probably one of the reasons why none of the abovementioned explanations has been commonly accepted.
Solid-state NMR spectroscopy is powerful in studying the local structure of a wide range of functional materials on atomic scale.1H,17O and47,49Ti solid-state NMR investigations have been conducted to monitor the local environments of these nuclei in TiO29–21. Since47Ti (I = 5/2) and49Ti (I = 7/2) possess relatively large quadrupole moments and the resonances of47Ti and49Ti usually superimpose with each other, it is very difficult to analyze47,49Ti NMR spectra9–11. With respect to the1H NMR studies on TiO2 materials, some researchers considered that larger1H chemical shifts (6.8/6.7/5.1 ppm) correspond to higher acidity, and these resonances were assigned to bridging hydroxyl groups where the O atoms are attached to two Ti atoms12–14.Therefore,1H signals with smaller chemical shifts (2.1/2.3/3.7 ppm) were assigned to terminal hydroxyl groups which are considered to be alkaline12–14. Other studies considered that1H signals of different chemical shifts can be assigned to water in different adsorption layers15,16or water adsorbed at surface sites with different acidity14,17. In general, the assignment of1H NMR signals of TiO2is remarkably influenced by the water coverage,and becomes even more complex due to the proton exchange between the adsorbed water and TiO2 surface sites.
17O solid-state NMR studies on TiO2can be more informative,because17O has a very large chemical shift range (> 1000 ppm,compared to a range of 12 ppm of1H), and17O NMR spectroscopy has been proved to be highly sensitive to the structural changes of oxide materials22–26. For the case of TiO2,Scolan et al.18successfully observed and assigned four different17O NMR signals in titanium-oxo clusters and nanosized titania particles (2–3 nm), while Blanchard and coworkers19observed the17O resonances of Ti―OH. Sun et al.20used17O MAS NMR to quantitatively investigate the16/17O isotope exchange kinetics on pure-anatase TiO2and mixed-phase TiO2with anatase and rutile. More recently,17O NMR spectroscopy was demonstrated to be able to distinguish faceted anatase titania nanocrystals and provide detailed chemical and structural information on the surface of titania nanostructures21.
Despite these efforts, the structure of hydroxyl groups of TiO2,especially the correlation of17O NMR signals with the1H resonances, has not been carefully studied. It was reported that the hydroxyl groups could largely influence the photocatalytic activity of TiO227–29. Extensive IR investigations have shown that there are terminal, bridging and maybe hydrogen bonded hydroxyl groups on TiO2. However, some of these results are in contradiction with each other30–32. Similar circumstances were encountered in interpreting1H NMR data, as stated above. In addition, the17O NMR signals arising from terminal or bridging OHs, if they exist, have not been distinguished yet.
With respect to solid-state NMR study on partially reduced TiO2, a big challenge is the existence of paramagnetic centers,for example, oxygen vacancy (OV) and Ti3+, which may broaden the spectrum33. Such influences depend on the concentrations and electron relaxation times of the paramagnetic centers. On the other hand, there may be some positive effects, such as significantly shortened relaxation time in NMR. In addition, OV can be active sites for water dissociation, leading to more hydroxyl groups upon water adsorption34, which may facilitate the17O and1H solid-state NMR study, especially for the correlation of the17O and1H NMR signals.
In this work,17O and1H solid-state NMR spectroscopy are employed to investigate the surface structure of a partially reduced anatase TiO2nanoparticle material. In combination with EPR results, the behavior of the1H and17O NMR spectra of this material upon thermal treatment under vacuum are interpreted.1H→17O cross polarization (CP) MAS and two-dimensional heteronuclear correlation (2D HETCOR) are applied to explore the correlation of the hydrogen and oxygen species. The results provide new insight into the local structure of this important material.
2 Experimental
2.1 Materials preparation
All chemical reagents are of analytical grade, unless otherwise stated, and are used without further purification. The dark brown powder of the partially reduced TiO2 nanoparticles was prepared with a combustion method. Typically, an n-propanol solution containing 2.5 g n-propanol, 1 g tertrabutyl titanate, 0.45 g 2-ethylimidazole, 1.25 g 37.1% hydrochloric acid, and 0.5 g distilled water was combusted in a preheated muffle furnace at 350 °C in air, followed by annealing at the same temperature for 0.5 h before it was taken out for fast cooling. This sample is denoted as Re-A-TiO2. For comparison, the obtained Re-A-TiO2 was annealed in a muffle furnace at 400 °C for 1 h with an air flux of 400 cm3·min?1. The resulting powder is light grey in color and denoted as A-TiO2.
2.2 Materials characterization
A Philips X’Pro X-ray diffractometer was applied to collect the samples’ powder XRD patterns, between 10°–80° (2θ),using Cu Kα irradiation (λ = 1.54184 ?, 1 ? = 0.1 nm), operated at 40 kV and 40 mA. A JEOL JEM-2010 transmission electron microscope (TEM) was used to obtain the high-resolution TEM images of the samples. Room-temperature EPR spectra were acquired on both samples with the same masses (40 mg) using a Bruker EMX-10/12 EPR spectrometer. The Brunauer-Emmett-Teller (BET) specific surface areas of both samples were analyzed by nitrogen adsorption at 77 K with a Micromeritics tristar ASAP 2020 surface area analyzer.
2.3 Visible-light photoactivity measurement
The visible light-driven photocatalytic activity of the samples was examined by applying them in photodegradation of methyl orange solutions. A 300 W Xe arc lamp (PLS-SXE300, Beijing Perfectlight Co., Ltd.), equipped with an ultraviolet cutoff filter,was used to provide visible light (λ ≥ 400 nm). The degradation of methyl orange was monitored by UV/Vis spectroscopy (UV-1800, Shimadzu, Japan). In a typical experiment, 50 mg of the sample was added to 100 mL of methyl orange solution at about 1 × 10?4mol?L?1. The solution was stirred and kept in dark for 4 h to reach an adsorption equilibrium. After lighting on the lamp,4 mL solution was taken out every half an hour for analyzing the concentration of the methyl orange.
2.4 17O labeling
Both samples were17O-labeled through a vacuum line(Swagelok Inc., USA) using 90%17O-enriched H2O (H217O,Cambridge Isotope Laboratories, Inc., USA). The sample(typically 150 mg) was first heated from room temperature to 400 °C at a rate of 5 °C?min?1and kept at 400 °C for 1.5 h, in a glass tube exposed to vacuum. After the sample was cooled down to room temperature, H217O vapor was introduced through the vacuum line. A vacuometer (DZA1, Shanghai Yunjie Vacuum Instrument Inc.) was used to measure the pressure to ensure that the sample is saturated with H217O vapor. The sample was then sealed in the glass tube, heated to 40 °C and kept at this temperature for 5 h to make a16/17O exchange between the surface of the sample and H217O.
2.5 Solid-state NMR measurement
All of the MAS NMR experiments were performed on a 9.4 T Bruker Avance III 400 spectrometer (Germany).1H and17O MAS NMR spectra were collected with a rotor-synchronized spin echo sequence, on a 3.2 mm doubly tuned MAS probe (54.2 MHz for17O and 400.0 MHz for1H), with a MAS spinning rate of 20 kHz. Recycle delays were optimized on each sample. After collecting the spectra of the H217O vapor-saturated samples, they were dried under vacuum at room temperature, and the1H and17O MAS NMR spectra were measured once again. This procedure was repeated at different thermal treatment temperatures.1H→17O CP-MAS and 2D1H-17O HETCOR NMR spectra of the samples, which were dried under vacuum at 100 °C, were recorded on a 4.0 mm doubly-tuned MAS probe,with a MAS spinning rate of 14 kHz.1H and17O shifts were referenced to external standards of adamantane at 1.79 ppm and H2O at 0.00 ppm, respectively.
2.6 DFT calculations
DFT calculations have been done and described in a previous work21.
3 Results and discussion
3.1 Morphology and visible-light photocatalytic activity
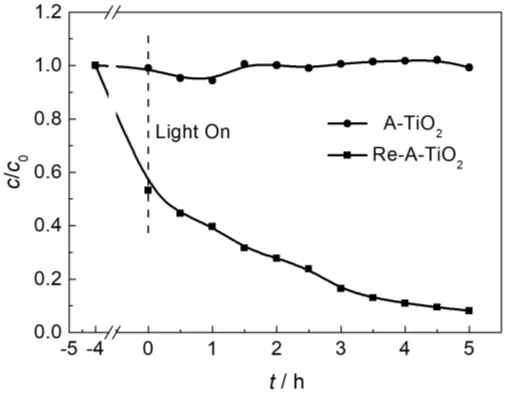
Fig. 1 Photodegradation of methyl orange over Re-A-TiO2 and A-TiO2 under visible-light irradiation.
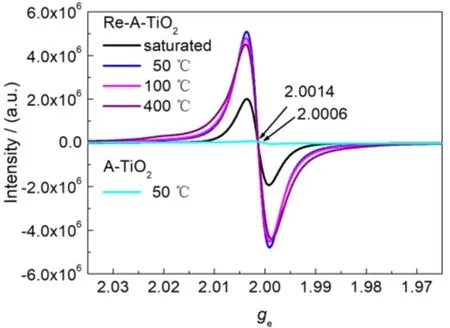
Fig. 2 Room-temperature EPR spectra of Re-A-TiO2 and A-TiO2, saturated with water vapor or dried under vacuum at different temperatures.
The XRD patterns show that both TiO2samples are anatase with a small amount of rutile impurities (~5% weight) (Fig. S1,Supporting Information (SI)). The particle sizes of anatase and rutile phases in both Re-A-TiO2and A-TiO2samples are around 11 nm and 25 nm, respectively, by analyzing the full width at half maximum (FWHM) of XRD reflection peaks according to Scherrer equation. This is in accordance with their high resolution TEM images (Fig. S2 (SI)). A lattice spacing of 0.35 nm can be observed, corresponding to the {101} planes of anatase TiO2phase. Both samples have similar BET surface areas (101.4 m2?g?1for Re-A-TiO2and 104.6 m2?g?1for A-TiO2,respectively), associated with similar pore dimeters of 3.5–3.6 nm (Fig. S3 (SI)).
Visible light-driven photocatalytic activity of the samples is shown in Fig. 1 and Fig. S4 (SI). Re-A-TiO2 absorbs almost half of the methyl orange in 4 h, and then 92% of the methyl orange is gradually photo-decomposed in 5 h. On the contrary, the ATiO2sample does not show any visible light photocatalytic activity.
The visible-light photoactivity of reduced TiO2was considered to be originated from Ti3+, OV, or surface defects,usually connected with the paramagnetic centers of TiO22,3,5,6,which are usually characterized by EPR spectroscopy. Fig. 2 shows the room-temperature EPR spectra of the samples, where geof Re-A-TiO2is 2.0014 and geof A-TiO2is 2.0006, both between the reported gevalue of OV (2.004) and that of Ti3+(1.94–1.99)8,35. Therefore, the observed broad EPR signal of both samples can be described by a superposition of the signals from OV and Ti3+. The larger gevalue of Re-A-TiO2, which is closer to the gevalue of OV than that of A-TiO2, showing that the OV/Ti3+ratio in Re-A-TiO2is larger than in A-TiO2.Furthermore, the EPR signal intensity reflects the concentration of OV and Ti3+. Therefore, the larger EPR intensity of Re-ATiO2, 20–70 times larger than that of A-TiO2, suggesting that Re-A-TiO2has larger concentration of OV and Ti3+. An interesting phenomenon is that the EPR intensity of both samples,especially Re-A-TiO2, is remarkably influenced by water adsorbed on the surface (Fig. 2 and Fig. S5 (SI)). With the removal of the adsorbed water by drying under vacuum, even at a very moderate temperature (50 °C), the EPR intensity increases a lot, indicating that the paramagnetic centers combine adsorbed water molecules through weak interactions. However, further removal of water by drying under vacuum at higher temperatures slightly decreases the EPR intensity. This may indicate a subtle structural change on the surface of Re-A-TiO2, and may be illustrated with solid-state NMR spectroscopy, which is sensitive to the local structure.
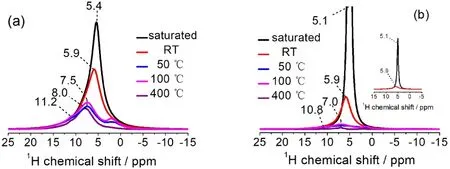
Fig. 3 Normalized 1H spin-echo NMR spectra of Re-A-TiO2 (a) and A-TiO2 (b), saturated with water vapor or dried under vacuum at different temperatures. Pulse length of 2.0 μs corresponding to π/2 pulse was applied. Optimized recycle delays from 0.5 to 10 s were used to ensure quantitative observations of all resonances.
3.2 1H solid-state NMR spectra
Fig. 3 shows the1H solid-state NMR spectra of both samples,where the samples absorb H217O and are then heated under vacuum at room temperature (RT) and elevated temperatures.An obvious difference of the two samples is that the peaks of Re-A-TiO2 appear at higher chemical shifts in general, which may denote higher acidity corresponding to different photocatalytic activity36. This is possibly related to the presence of more oxygen vacancies on the surface, which tend to combine OH?or give H+. These acidic1H species of Re-A-TiO2can hardly be removed even by heating under vacuum at 400 °C (Fig. 3a),which may influence the activity of Re-A-TiO2in hightemperature catalytic processes. Another difference is that the NMR signals of Re-A-TiO2are broader, implying a more complex chemical environment and a lower mobility of the surface water37.
Furthermore, when the samples are dried under vacuum at elevated temperatures, the original single1H signal becomes more complicated. This implies that some of the adsorbed water molecules changes to other type of1H species, such as hydroxyl groups, due to the heating processes. When the drying temperature increases from 50 to 100 °C, the intensity of the1H signals of both samples surprisingly increases slightly. This may be interpreted as that further removal of surface water creates a simpler local environment for these1H species, for example,weaker1H-1H coupling effect from the adsorbed water, thus these signals become sharper. Another possible reason is related to the paramagnetic property of the samples. According to Bakhmutov33, unpaired electrons in paramagnetic centers generate strong local magnetic fields, which will mask original spectroscopic properties of the nuclei investigated. Therefore,the atoms that are bonded to or near the paramagnetic centers may experience such interactions, and it can be difficult to detect these species. In this work, changing the drying temperature from 50 to 100 °C decreases the EPR intensity (see Fig. 2 and Fig. S5 (SI)), therefore the1H species near Ti3+or OV experience less paramagnetic shielding, and the1H NMR signal intensities increases.
The assignment of the different1H sites will be discussed afterward in Section 3.4 with the help from1H-17O 2D HETCOR NMR spectroscopy.

Fig. 4 Normalized 17O spin-echo NMR spectra of Re-A-TiO2 (a) and A-TiO2 (b) saturated with H217O vapor or dried under vacuum at different temperatures. Pulse lengths of 1.2 μs corresponding to π/6 for liquid H217O and optimized recycle delays from 0.3 to 10 s were applied. Detailed experimental parameters are summarized in Table S1. Asterisks and hashtags denote sidebands of the μ3-O and μ2-O signal, respectively.
3.3 17O solid-state NMR spectra
Fig. 4 gives the17O MAS NMR spectra of both the samples,which are saturated with H217O vapor and then dried under vacuum at different temperatures. Both samples have17O signals covering four frequency regions.
The17O signal at the frequency range with the smallest chemical shifts (around ?50 ppm) in both samples can be assigned to adsorbed water, since it can be readily removed by drying under vacuum. For the saturated samples, a sharp17O signal, similar as liquid water, centered at 1.5 ppm for Re-ATiO2and 4.0 ppm for A-TiO2, respectively, can be observed,indicating a large amount of free water on the surface. With the increase of the thermal treatment temperature, the sharp component in this signal disappears, and a broad resonance can be observed at similar frequencies (top lines in Fig. 4, rightmost signal). This signal may correspond to strongly adsorbed water species, in which the linewidth may be associated with a relatively large quadrupolar interaction due to lack of motion.
The17O signal centered at around 160 ppm is assigned to hydroxyl groups (OH) on the surface of anatase TiO219. The saturated samples show lowest OH intensity, presumably due to the hydrogen bonding effects between the OH groups and the adsorbed water, which change the environment of the OH groups.By removing the surface water, the intensities of the OH signals of both samples increase. Furthermore, the influence of oxygen vacancies and Ti3+ions should also be considered. As has been stated in the interpretation for Fig. 2, water can be combined by paramagnetic center through weak interactions. During the thermal treatment, some of the adsorbed water molecules interact with the paramagnetic centers, leading to OH groups. On the other hand, according to previous DFT calculation studies,water tends to dissociate on the (001) surface of TiO238, while molecularly adsorb on its (101) surface39,40. The higher intensity of OH signal of Re-A-TiO2 than that of A-TiO2 may also indicate a higher proportion of exposed (001) facet on Re-A-TiO2.
The other two types of signals, covering the range of 460–600 ppm and 600–850 ppm, are assigned to three-coordinated oxygen (OTi3, denoted as μ3-O hereafter)41and two-coordinated oxygen (OTi2, denoted as μ2-O hereafter)18,41,42, respectively.The μ2-O is coordinatively unsaturated and only exists on surface of the exposed facets. The area of the μ3-O signal in each sample is close to that of the μ2-O one, indicating that the μ3-O signal also originates from the surface or subsurface of the TiO2nanocrystals. Comparing to bulk OTi3 signal21,41, the μ3-O signal in this work is broader, which corresponds to a diversity of the local surface environment. It should be noted that, neither the intensity nor the width of the μ3-O signal changes with the removal of the surface water by heating under vacuum,indicating that surface water does not interact with the μ3-O.
On the contrary, the μ2-O signal broadens and is associated with a larger chemical shift when surface water is removed by drying under vacuum. This can partly be ascribed to the removal of the electron-donating effect (usually causes NMR signals shifting to low frequencies) from the adsorbed water molecule to the μ2-O through a hydrogen bond.
The two μ2-O signals of saturated A-TiO2(bottom line of Fig.4b, marked with a red ellipse) arise from steps on (101) surface of anatase TiO221. The17O resonance at approximately 640 ppm corresponds to μ2-O at flat terraces below adjacent step edge which is hydrogen bonded to adsorbed water. However, this signal becomes much weaker after the sample is exposed to vacuum at room temperature, as compared to the nano octahedra TiO2prepared by the hydrothermal method21. The other μ2-O signal at around 730 ppm, corresponding to one of the step-edge oxygen ions21, shifts to higher frequencies upon thermal treatment under vacuum. The lower content of OH species than Re-A-TiO2 also confirms that A-TiO2 mainly contains anatase TiO2nanocrystals with exposed (101) surface, because water tends to be molecularly adsorbed on (101) surface of TiO2, as stated above.
Different from A-TiO2, the spectrum of saturated Re-A-TiO2shows only one μ2-O signal, covering the whole frequency range of the two μ2-O signals of saturated A-TiO2. This can be attributed to the complex surface environment introduced by the incomplete combustion preparation. This signal also shifts to higher frequencies and broadens remarkably when the thermal treatment temperature is increased. Furthermore, the complexity of the surface also arises from the existence of a larger amount of OH, which indicates a larger proportion of exposed (001)surface38.
When the drying temperature is further increased to 100 °C,μ2-O signals of both samples further broaden and cover the same frequency range of 600–850 ppm. These signals are different from μ2-O signal of either (001) or (101) anatase TiO2surfaces,but close to that of non-faceted surface of anatase TiO221,implying that both samples may adopt a different surface structure when they are exposed to a very dry environment.
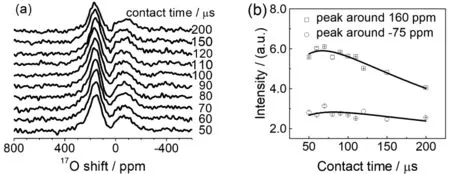
Fig. 5 (a) 1H→17O CP-MAS NMR spectra of Re-A-TiO2, which has been dried under vacuum at 100 °C, as a function of contact time.(b) Peak areas of the two 17O NMR signals as a function of contact time, where the lines are guides for eyes.
3.4 1H→17O CP-MAS and 2D HETCOR NMR spectra
In order to correlate the oxygen and hydrogen species,1H→17O CP-MAS NMR spectra were first acquired. Two17O NMR signals, centered at around 160 and ?75 ppm, were observed for both samples (Fig. S6 (SI)). Obviously, the partially reduced sample (Re-A-TiO2) has a larger amount of hydrogencorrelated oxygen species.
The CP build-up curves (Fig. 5) of Re-A-TiO2, dried under vacuum at 100 °C, show that the contact times for the maximum intensities of both signals are around 70 μs (Fig. 5b), which is the same as the one observed for hydroxyl group on (001)surface of anatase TiO221, and close to those of hydroxyl groups of layered double hydroxides43, CeO223, and ZrO225. Based on their shifts, the peaks at around 160 ppm and ?75 ppm are assigned to surface hydroxyl group and strongly adsorbed water species, respectively.
A 2D1H-17O HETCOR NMR spectrum of Re-A-TiO2was measured (Fig. 6), to explore the detailed correlation of1H and17O species and help spectral assignment of the1H signals obtained in Fig. 3. Two cross-peaks centered at (5.9, ?75) and(7.6, 160) were observed, corresponding to strongly adsorbed water and hydroxyl group, respectively. Therefore, the1H resonance at 5.9 ppm, also observed in the room-temperature dried samples (see Fig. 3), is assigned to1H of strongly adsorbed water. The1H resonance at 7.6 ppm, close to the one found for Re-A-TiO2dried under vacuum at elevated temperatures (7.5–8 ppm, Fig. 3a), is assigned to1H of hydroxyl group. This resonance of Re-A-TiO2shifts from 7.5 to 8.0 ppm when the sample is heated to 400 °C, indicating that it is influenced by the content of surface water. Furthermore, the1H resonance at 7.0 ppm found in A-TiO2dried under vacuum at the same temperatures (Fig. 3b), can also be assigned to1H in the hydroxyl groups.
In addition to the1H resonances at 7.6 and 5.9 ppm, the projection of the 2D HETCOR NMR spectrum on1H dimension shows another resonance at 11.2 ppm, indicating that this1H environment also correlates with oxygen atoms. Previous two independent NMR studies have attributed this signal to different impurities13,32. However, the frequent appearance of this signal in different studies on TiO2indicates that this signal may be related to the surface of TiO2and is important for this material.According to previous studies on other materials, such large1H NMR shift can be assigned to intramolecular hydrogen bond,which does not involve adsorbed water44–46. The following correlation between the1H chemical shift and hydrogen bond length was given in silicate glasses, which are very similar to our sample45:
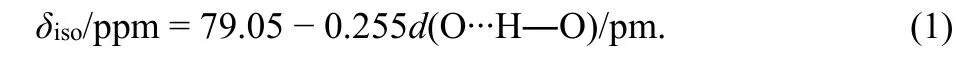

Fig. 6 2D 1H-17O HETCOR NMR spectrum of Re-A-TiO2 (dried under vacuum at 100 °C) obtained at 9.4 T. Contact time, 70 μs;spinning speed, 14 kHz; recycle delay, 0.5 s; A total of 128 and 4096 points were acquired in the F1 and F2 dimensions, respectively, with 9600 scans per time increment. The full 2D spectrum took 1 d and 15 h to acquire.

Fig. 7 Model of the exposed (001) facet of anatase TiO2, with water dissociated on the surface. Grey and white spheres represent Ti and H species, respectively. O species are classified into three categories: two types of hydroxyl group (blue and yellow spheres) and oxygen in the Ti―O―Ti skeleton (red spheres). Experimental 1H and calculated isotropic 17O chemical shifts of both types of hydroxyl groups are shown. The DFT calculated distance between the two oxygen atoms in the hydrogen bond (O??H―O) is 250 pm.
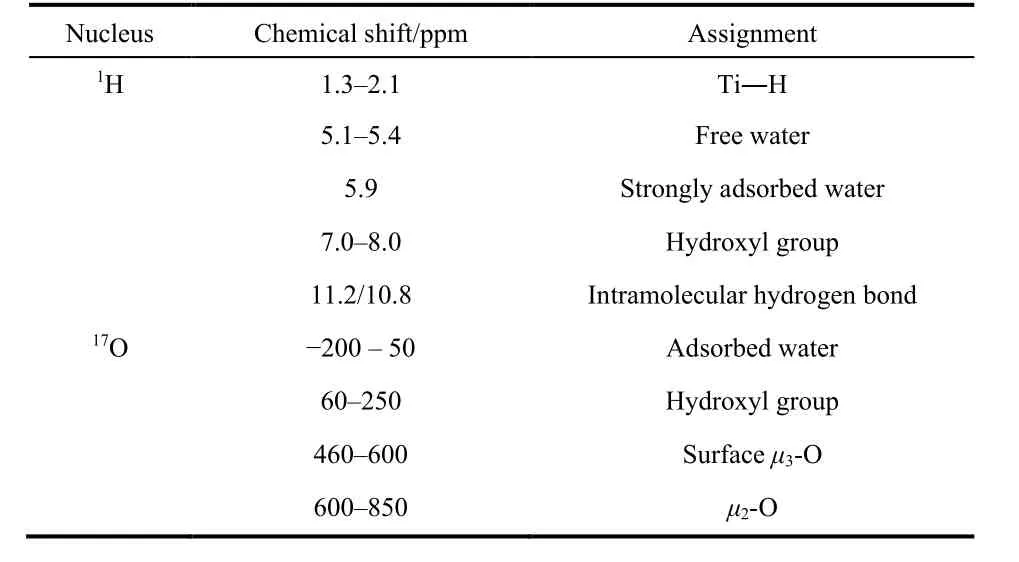
Table 1 Assignment of the 1H and 17O signals of Re-A-TiO2 and A-TiO2.
Based on this relationship, a chemical shift value of 11.2 ppm(or 10.8 ppm in the A-TiO2which is less influenced by paramagnetic centers) corresponds to an O??H―O distance of 266 pm (or 267 pm for 10.8 ppm). In addition, these two resonances (11.2/10.8 ppm) found in the 1D1H NMR spectra of Re-A-TiO2(Fig. 3) does not shift with the removal the surface water, proving that this hydrogen bond is an intramolecular one.
Our previous DFT calculations gave an optimized structure where water molecules dissociate on (001) exposed facet of anatase TiO2(Fig. 7)21. The calculation shows that the distance between the two oxygen atoms in the hydrogen bond (O??H―O)is 250 pm (or 253 pm by adding up the O??H and H―O distances), which is close to the one derived from Equation 1,considering the difference between the systems studied. This hydrogen bond is formed by the oxygen ion (with a calculated isotropic chemical shift of 235 ppm and a center of gravity of 131 ppm21) in one OH group with the hydrogen ion in another OH group (Fig. 7). The isotropic17O chemical shift and its center of gravity, of the latter hydroxyl group, are calculated as 430 and 403 ppm21, which are not observed in this study (Figs. 4–6),possibly because this oxygen ion originates from the non-labeled oxygen of TiO2itself. Therefore, our NMR and calculation data suggest no bridging hydroxyl group but two types of terminal hydroxyl sites, which is different from the conclusions that there are terminal and bridging hydroxyl groups on TiO2based on1H NMR12–14and IR30–32investigations.
In addition to the three1H NMR resonances that have been assigned according to their correlation with the17O NMR signals,there are other two1H NMR signals in Fig. 3. The resonances at 5.4 ppm for Re-A-TiO2and 5.1 ppm for A-TiO2are assigned to free water on the samples, since the samples are saturated with water vapor. Beyond this, the1H resonances at 1.8 to 2.1 ppm for Re-A-TiO2and 1.3 ppm for A-TiO2are assigned to Ti―H species, since they are not obviously correlated to the17O signals(Fig. 6), and their smaller chemical shifts, compared to the other’s, correspond to hydrogen species with alkalinity.
As a summary, the assignment of all1H and17O signals are listed in Table 1.
4 Conclusions
In this work, the surface structure of a partially reduced anatase TiO2 (Re-A-TiO2) material, which shows visible-light photodegradation activity, is investigated by17O and1H solidstate NMR spectroscopy, as well as EPR measurements, and compared to a non-reduced TiO2sample.
Re-A-TiO2 has intensive EPR signals, which is assigned to the superimposition of oxygen vacancy and Ti3+.1H chemical shifts of Re-A-TiO2are larger than those of its non-reduced counterpart, corresponding to stronger acidity. These hydrogen species cannot be removed by drying under vacuum even at 400 °C.17O NMR spectra shows that Re-A-TiO2has a larger amount of OH groups, indicating a larger proportion of exposed(001) surface. A fraction of the OH groups originate from reaction of the paramagnetic centers of Re-A-TiO2and the combined water molecules, implying that water or humid environment can alter the property of this type of materials and may impair their long-term application performances.
Furthermore, by examining the correlation of hydrogen and oxygen species with1H→17O CP-MAS and 2D HETCOR NMR spectra, combined with the DFT calculations, two important issues are raised. First, no bridging hydroxyl group, but two types of terminal hydroxyl groups exist on the surface of anatase TiO2, usually on (001) surface. The two17O NMR signals obtained in1H→17O CP-MAS NMR are assigned to strongly adsorbed water and one terminal hydroxyl group, respectively.The other terminal hydroxyl group does not appear in the17O NMR spectrum, possibly due to its origination from the skeleton oxygen. Second,1H signals of anatase TiO2material are assigned.In particular, the1H resonance at ~11 ppm, which was ambiguous in literatures, is assigned to hydrogen species in intramolecular hydrogen bonds.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化學(xué)學(xué)報(bào)的其它文章
- 固體NMR研究PAA/PEO共混物中氫鍵相互作用與結(jié)構(gòu)演化
- Hydrogen-Bond Induced Crystallization of Silicalite-1 Zeolite as Revealed by Solid-State NMR Spectroscopy
- Zero-Quantum Homonuclear Recoupling Symmetry Sequences in Solid-State Fast MAS NMR Spectroscopy
- DFT計(jì)算結(jié)合固體NMR研究富鋁SSZ-13的鋁分布和Br?nsted 酸性
- Structural Investigation of Alkaline-Earth Phosphosilicate Glasses Containing Six-Coordinated Silicon by Solid-State NMR
- 超極化核磁共振方法的原理和應(yīng)用

