Push forward LC-MS-based therapeutic drug monitoring and pharmacometabolomics for anti-tuberculosis precision dosing and comprehensive clinical management
Nguyen Qung Thu , Nguyen Trn Nm Tien , Nguyen Thi Hi Yen ,Thuc-Huy Duong , Nguyen Phuoc Long ,*, Huy Truong Nguyen
a Department of Pharmacology and PharmacoGenomics Research Center, Inje University College of Medicine, Busan, 47392, Republic of Korea
b Department of Chemistry, University of Education, Ho Chi Minh City, 700000, Viet Nam
c Faculty of Pharmacy, Ton Duc Thang University, Ho Chi Minh City, 700000, Viet Nam
Keywords:Tuberculosis Therapeutic drug monitoring LC-MS MIPD Pharmacometabolomics Precision medicine
A B S T R A C T The spread of tuberculosis(TB),especially multidrug-resistant TB and extensively drug-resistant TB,has strongly motivated the research and development of new anti-TB drugs.New strategies to facilitate drug combinations, including pharmacokinetics-guided dose optimization and toxicology studies of first- and second-line anti-TB drugs have also been introduced and recommended.Liquid chromatography-mass spectrometry (LC-MS) has arguably become the gold standard in the analysis of both endo- and exo-genous compounds.This technique has been applied successfully not only for therapeutic drug monitoring (TDM) but also for pharmacometabolomics analysis.TDM improves the effectiveness of treatment, reduces adverse drug reactions, and the likelihood of drug resistance development in TB patients by determining dosage regimens that produce concentrations within the therapeutic target window.Based on TDM, the dose would be optimized individually to achieve favorable outcomes.Pharmacometabolomics is essential in generating and validating hypotheses regarding the metabolism of anti-TB drugs, aiding in the discovery of potential biomarkers for TB diagnostics, treatment monitoring, and outcome evaluation.This article highlighted the current progresses in TDM of anti-TB drugs based on LC-MS bioassay in the last two decades.Besides,we discussed the advantages and disadvantages of this technique in practical use.The pressing need for non-invasive sampling approaches and stability studies of anti-TB drugs was highlighted.Lastly, we provided perspectives on the prospects of combining LC-MS-based TDM and pharmacometabolomics with other advanced strategies (pharmacometrics, drug and vaccine developments, machine learning/artificial intelligence,among others)to encapsulate in an all-inclusive approach to improve treatment outcomes of TB patients.
1.Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis, has consistently held its position as one of the most common causes of communicable disease-related deaths globally.Before the coronavirus disease 2019(COVID-19)pandemic,TB accounted for a higher number of fatalities compared to any other infectious agent,ranked above the acquired immunodeficiency syndrome caused by human immunodeficiency virus (HIV).According to the World Health Organization estimate, 1.6 million people died from TB in 2021 including 1.3-1.5 million and 158,000-218,000 deaths among patients without and with HIV coinfection, respectively [1].Approximately 10 million new cases are reported annually.Southeast Asia, Africa, and the Western Pacific are critical areas with a high TB burden [2].Only about one-third of rifampicinresistant TB cases are detected and treated annually among 399,000-501,000 new cases documented from 2020 to 2021 [1].TB,along with other infectious diseases,has observed a decrease in healthcare services as a result of the resources concentrated against the COVID-19 pandemic.Increased transmission within the community due to less screening and detection of TB patients could set back global TB management by a decade.These data highlight the vulnerability of TB diagnosis and management, emphasizing the need for a more sustainable system for TB prevention, diagnosis,and management [3].
Current endeavors include the adoption of fixed-dose combinations of first-line anti-TB agents (isoniazid (H), rifampicin (R),pyrazinamide (Z), and ethambutol (E)) in clinical practice worldwide [4,5].Unfortunately, not all nations have adopted the standard treatment in its proposed format [2].Recently, second-line anti-TB drugs have been administered due to an increased rate of multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) cases.Candidate analogs are levofloxacin (Lfx), gatifloxacin (Gfx), moxifloxacin (Mfx), p-aminosalicylic acid (PAS),clofazimine (Cfz), linezolid (Lzd), and bedaquiline (Bdq), among others [6].The chemical structures of anti-TB drugs and their metabolites are shown in Figs.S1 and S2.The success of the treatment process is dependent on various factors: weight, age,sex, adherence to treatment, malabsorption, drug-drug interactions (DDIs), and comorbidities, among others [2,7].More than a quarter of Addis Ababa(Ethiopia)TB patients with diabetes mellitus had a higher risk of poor TB treatment outcomes (14.8 times) compared to those without diabetes [7].It was also documented that the area under the curves (AUC) of first-line anti-TB drugs in patients with HIV-TB coinfection was lower than that in TB patients without HIV, which could increase the possibility of treatment failure [8,9].
To overcome the current challenges and strengthen ongoing efforts,successful TB treatment could be achieved by personalized and precision medicine.Therapeutic drug monitoring (TDM) is a crucial strategy for optimizing the dosing within the therapeutic window.TDM might reduce intra- and inter-individual variability in plasma concentrations (Cplasma) via pharmacokinetically guided dose adjustments, and significantly increase efficacy, tolerability,and safety [10].Patients administering an optimal regimen guided by TDM coupled with drug susceptibility testing (DST) had a reduced likelihood of anti-TB drug resistance.However, recent evidence showed that treatment failure could be linked to acquired resistance to H and/or Z during the turnaround time for DST [11].Thus,the contribution of model-informed precision dosing(MIPD)platforms, which leverage individual concentration data, is crucial for suggesting personalized and precise dosage regimens of anti-TB drugs[12].Although routine TDM and/or MIPD are not universally performed in patients receiving anti-TB treatment, they benefit those suffering from high-risk conditions such as HIV, diabetes mellitus, renal and hepatic failure, malnutrition, or infections caused by drug-resistant pathogens [13,14].
To implement MIPD-based TDM at the bedside, an accurate,precise, and practical measurement method is fundamentally required.Herein, liquid chromatography (LC), including highperformance LC and ultra-performance LC (UPLC) coupled with mass spectrometry (MS), has shown advantages over immunoassays [15].Regardless of the type of biospecimens, it includes high sensitivity and selectivity, broad linear ranges, highthroughput capability, and simultaneous analysis (measurement of both parental and active metabolites or multiple comedications) [16].Furthermore, the possibility of dissociating ion(s)into fragments and detecting the mass-to-charge ratio(m/z)of the ion(s) by using LC-tandem mass spectrometry (LC-MS/MS)enhances the selectivity [16,17].Many LC-MS/MS methods have been established to measure concentrations of anti-TB agents and their metabolites in various matrices [18-22].Therefore, it has arguably become the gold standard in research and applications on small molecule analysis, either of exogenous or endogenous origins [17,23].LC-MS is a powerful technique for drug quantification and multi-omics analyses, including proteomics and metabolomics.The ability to distinguish two peaks with approximately m/z, or isobar, could be achieved by high-resolution MS(HRMS).Untargeted metabolic phenotyping is needed to generate hypotheses of metabolism and explore potentially important biomarkers related to a phenotype of interest.These approaches are helpful and have been used extensively to identify metabolic fingerprints of diseases [24,25].In TB, serum metabolic profiling has been shown to have outstanding performance in distinguishing the TB patients from their healthy counterpart.Significant biomarkers include lysophosphatidylcholines, amino acids, bile acids, inosine, cortisol, and kynurenine [26].Meanwhile, targeted or large-scale targeted metabolomics is appropriate for a bioassay based on subsets of endo- and exo-genous compounds to confirm the hypotheses [27].Due to the accurate measurement of the concentrations of the parent substances and/or active metabolites, the characterization of phenotype of patients and drug metabolism could be evaluated simultaneously[28].As a result,a comprehensive and practical approach could be achieved by integrating LC-MS-based TDM and pharmacometabolomics for TB treatment.
This article highlighted the recent advances in the TDM of anti-TB drugs based on the LC-MS/MS techniques.During our discussion, we delved into the potential challenges associated with transitioning LC-MS/MS to the clinical settings and explored innovative avenues for clinical integration(Fig.1).We also touched upon additional critical insights in TB clinical management (pharmacometrics, pharmacometabolomics, multi-modal profiling, machine learning/artificial intelligence (AI), and drug and vaccine development, among others).Finally, we demonstrated our perspectives on integrating these strategies to encapsulate an allinclusive approach toward precision healthcare, thus improving the treatment outcomes of TB patients.
2.TDM of anti-TB drugs based on LC-MS/MS
2.1.Sample collection and preparation
Plasma and serum are commonly used samples for TDM of anti-TB drugs because they are also used for biochemical tests simultaneously.These samples are relatively easy to obtain through minimally invasive procedures, facilitating large-scale studies and routine clinical practice.In addition,these biospecimens have wellestablished collection, storage, and processing protocols that ensure consistent and reliable analysis.They also contain a wide range of circulating molecules.Therefore,not only TDM,the usage of plasma and serum samples may be a practical and scientific way of monitoring and predicting treatment outcomes.Typically, a small volume of plasma/serum ranging from 20 to 200 μL is required, and they are often frozen as soon as possible to avoid degradation (particularly H and ethionamide (Eto)) [29].For firstline drugs, the accurate and precise prediction of 24 h AUC values during treatment initiation for TB patients is based on an optimal sampling strategy at 2,4,and 8 h post-dose[30].Using less invasive and affordable matrices can increase the practicality of TDM for collectors and patients[31].For instance,hair sampling in children avoids the instability of H [32].The acceptability of saliva samples among laboratories could be increased with minimal preprocessing procedures [31].In addition, they offer a reduction in the sampling cost compared to blood samples.

Fig.1.Typical LC-MS process for TDM anti-TB drugs and the prospects.ACN: acetonitrile; Am: amikacin; CO2: carbon dioxide; CSF: cerebrospinal fluid; DBS: dried blood spot; E:ethambutol; HILIC: hydrophylic interaction chromatography; HIV: human immunodeficiency virus; HPLC: high-performance liquid chromatography; ILIS: isotopically labeled internal standard; IS: internal standard; Km: kanamycin; LC: liquid chromatography; LLE: liquid-liquid extraction; MAC: membrane-assisted extraction; MeOH: methanol; MS:mass spectrometry; PK: pharmacokinetic; Pto: prothionamide; QA: quality assurance; QC: quality control; S: streptomycin; SPE: solid-phase extraction; TB: tuberculosis; TCA:trichloroacetic acid; TDM: therapeutic drug monitoring; UPLC: ultra-performance liquid chromatography.
Non-selective sample treatments incentivize high-level recoveries and wide coverage of compounds with different characteristics.However, these preparation methods could complicate matrix effects as potential drawbacks.As a solution, accurate threshold calibration could be employed in a screening setup to correct the differences between cases in ion responses for analytes and reduce identification uncertainties [33,34].To enhance the selectivity of the analysis,there are numerous methods to capture one or some group of analytes.Simultaneously, the interference is removed, and the matrix is cleaned.Liquid-liquid extraction (LLE)or solid-phase extraction (SPE) [35] has been implemented to remove phospholipids, which is a major cause of unacceptable matrix effects for targeted metabolomics [36,37].Protein precipitation can be carried out by methanol (MeOH)/acetonitrile (ACN)/trichloroacetic acid or zinc salt followed by centrifugation [38].In some cases, derivatization may be performed on the analytes to improve quantitative parameters [16,28,39].ACN is a suitable option for extracting a wide range of anti-TB drugs from various samples.However, MeOH may be better for extracting E and prothionamide(Pto)[21].The dilution solvent,for example,diluted an aliquot of serum with MeOH containing sodium hydroxide could lead to an increase in signal intensity [40].
2.2.Calibration internal standards and quality control(QC)sample
The recovery of the internal standard (IS) could be utilized to assess the efficiency of the assay in patient samples.Cost, availability, reliability in forming product ions, solubility, and stability are critical considerations when choosing an IS [41].In previous studies, isotopically labeled IS (especially deuterium isotopelabeled internal standards (DIL-IS) [21,35,39,42,43]) was used to minimize the ionization effect and resolve the isotopic contribution from high levels of an analyte [33,41].In dried blood spot (DBS),there were several options for applying IS,but the application of IS on the spot after spotting is a common choice [44].Besides, QC samples (in two or three concentrations) and standard spiked matrix calibration solutions are also used to quantify and validate these methods [43,45].
2.3.Multi-analysis for anti-TB drugs and their metabolites using LC-MS
Multi-analyte assays are becoming increasingly accessible because of their adequate separation and short run times.Nevertheless,challenges remain in sample preparation and optimization of LC-MS conditions to separate and assay all targeted molecules with different chemical properties [33].This section described methods to quantify anti-TB drugs and their metabolites, focusing on methods established within the past two decades.Furthermore,we also mentioned the validation of these methods in terms of accuracy, precision, linearity, selectivity, carry-over, matrix effects,and drug stability.
2.3.1.First-line drugs and their metabolites
R, H, Z, and E are the four first-line drugs [8] that form the backbone of TB chemotherapy [5].The precision and accuracy of drug quantification are important in MIPD-based TDM strategy.We described and discussed recent advances in the TDM of these drugs and their metabolites.Table 1 [4,19,28,32,35,39,42,43,45-53]summarizes essential information regarding described methods.
Various rapid and selective bioassay methods have been established to quantify first-line drugs in plasma/serum[4,19,28,35,39,42,43,45-48,50,52,53].Most of them applied ACN to precipitate the proteins and used a C18column to separate the analytes.Regarding the mobile phase, binary gradients with solvents made up of water and ACN-containing solvents with additives(formic acid,ammonium acetate,or trifluoroacetic acid)were frequently implemented to shorten the retention time of the analytes and IS without affecting the sensitivity of the analytes [43].Rarely the hydrophilic interaction chromatography(HILIC)column and a high percentage of ACN in the mobile phase were used to determine R, H, and metformin in rat plasma simultaneously [46].
Several studies established a method for quantifying anti-TB drugs and their metabolites in plasma/serum and gaining insightinto the pharmacokinetic (PK) profile, hepatotoxicity, and other phenotypes of patients [27,28].Song et al.[19] demonstrated that serum concentration (Cserum) of H was inversely correlated with acetylisoniazid (Ac-H) (R = -0.739, P <0.001).Other studies applied derivatization to treat H and its metabolites (by p-tolualdehyde [28], and cinnamaldehyde [39]) before being injected into the LC system.Following the population pharmacokinetics(popPK)study of H in Korean patients(Cho et al.[54]),Ky Anh et al.[28]developed an LC-MS method to determine the Cplasmaof H and its metabolites, which has been applied in one-point-based TDM.Furthermore,they also illustrated genotype-phenotype association studies of the H metabolic profile, H-induced hepatotoxicity, and adoption of popPK simulation to predict the initial dose of H [28].For drug stability, Sundell et al.[50] reported that the extracted samples were stable in the autosampler (24 h, 8°C), as well as for up to three freeze-thaw cycles.They also mentioned the interaction between anti-TB and anti-HIV drugs [50].
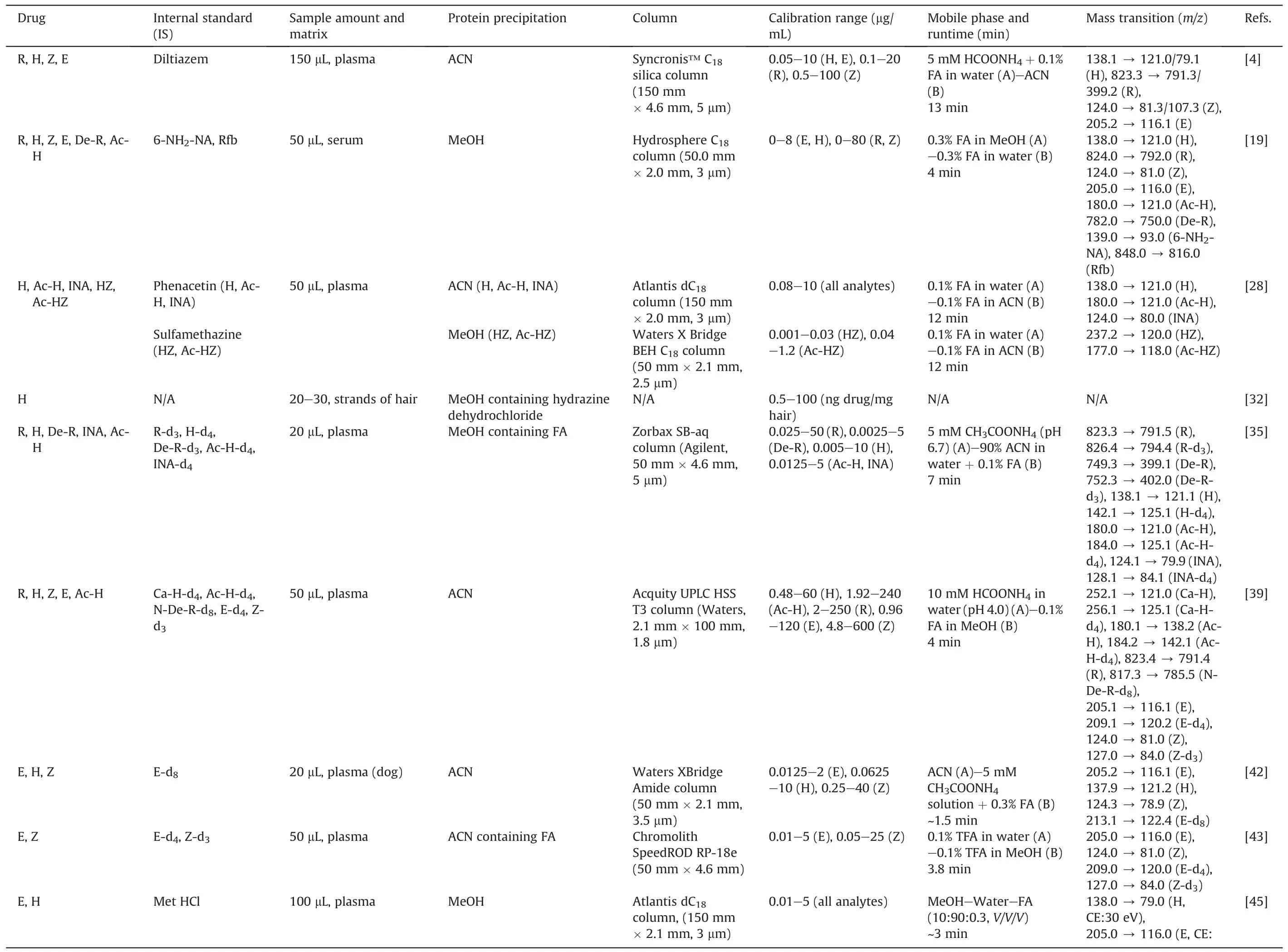
Table 1 LC-MS assays for first-line anti-TB drugs and their metabolites.
Simple sampling technique, convenience in storage, ease of transport, reduced risk of HIV biohazard, small amounts, and avoidance of drug degradation are the strengths of DBS [31].Lee et al.[49]showed a good correlation and proportional bias between Cplasmaof H and its concentration in DBS(CDBS).They also reported that the CDBSfrom venous was slightly lower than that from capillaries[49].Hair is another option for convenient sampling.In the study of Mave et al.[32], concentrations of H were measured over time, which was detectable and showed variability across a dynamic range.H and Ac-H in eccrine sweat could be determined by fingerprint sampling,given a sufficient sample volume,sensitivity,specificity, and accuracy [51].The metabolic profile of urine samples was established to assess the impact of first-line anti-TB drugs on metabolic pathways and predict drug-induced hepatotoxicity in clinical applications [27].To evaluate the potency of both intracellular and extracellular TB, two studies were conducted using peripheral blood mononuclear cell (PBMC) and plasma samples.Hartkoorn et al.[48]first gained insight into the PK profile of R and showed that the R concentration was affected by heat inactivation,but not by freeze-thaw cycles.Another study by Baietto et al.[52]compared drug concentration in intracellular with Cplasma.The results were similar for R, lower for H and Z, and higher for E [52].This method was suitable for both TDM and PK analysis.Utilizing the same approach by Sturkenboom et al.[53],van den Elsen et al.[55] showed that TDM of R via saliva was feasible but not for H.
2.3.2.Second-line drugs and their metabolites
Due to the rise of MDR-TB and the reduction in the efficiency of first-line anti-TB drugs, more second-line anti-TB drugs are being developed and combined therapeutically [6].Therefore, an increasing number of quantitative analyses of second-line anti-TB drugs and their metabolites have been established to optimize the treatment of TB, especially MDR-TB or first-line drugs TB-resistant[56].Table 2 [37,40,57-71] summarizes these LC-MS bioassay methods.
Treatment with Lzd for MDR-TB might be restricted due to its toxicity [29,58].For a long duration of more than 28 days, TDMbased dose adjustment significantly reduced the likelihood of toxicity [72].A retrospective multicenter study in India revealed that peripheral neuropathy was the most prevalent among 42.45%of patients with Lzd's adverse drug reactions (ADRs)[73].Notably,42.22% of permanent Lzd withdrawals from regimens were due to ADRs[73].As a result,TDM methods have been established for this drug.Based on the determination of Lzd in serum [57], two more studies expanded the scope to different convenience matrices:DBS[58], serum, and oral fluid [63].They calculated the correlation factors between drug concentration in the matrices(oral fluid:Coralfluid; DBS: CDBS) to Cplasmaor Cserum.The results were 1.2 (DBS/plasma) and 0.97 (oral fluid/serum).In addition, Souza et al.[67]determined both Lzd and its two primary metabolites in serum and discussed that the accumulation of Lzd and its metabolites could help investigate their relationship with impaired kidney function.
Mfx has the highest in vitro activity against TB (including meningitis TB)compared to other quinolones[74].Using the same LC conditions and IS with Lzd analysis [57], the concentrations of Mfx in plasma and cerebrospinal fluid (CSF) (both bound and unbound/ultrafiltrate fractions) could be determined, and were suitable for monitoring Mfx in these matrices[59].In 2011,Vu et al.[60]analyzed DBS and discussed the influence of the hematocrit value on the analytical result.They also showed that the correlation factor between the concentration of the drug in finger blood and Cplasmawas 1.49.This method can facilitate the PK studies and TDM of this drug in remote rural areas.For Lfx, Ghimire et al.[71,75]conducted two studies.The first was the determination of Lfx and the metabolite desmethyl levofloxacin in plasma [71], followed by an evaluation of Cplasmaand the concentration in saliva (Csaliva) of Lfx, with Csaliva/Cplasma= 0.69 [75].
Several methods for other secondary anti-TB drugs and their metabolites were performed in plasma, oral fluid, lung homogenates,and urine samples.Han et al.[40]quantified nine second-line anti-TB drugs in serum within 3 min of runtime.Bolhuis et al.[63]calculated that the correlation factor between the Coralfluidand Cserumwas 3 for Clr.The conversion of Pto to its active metabolite,prothionamide sulfoxide,was evaluated by Trivedi et al.[65].They also optimized the extraction protocol using ACN and applied it to evaluate the bioequivalence.There were two studies for delamanid(Dlm).The first was in mouse lungs and plasma,which used LLE to extract Dlm to unravel the PK characteristics of this drug in mice[37].Another study by Meng et al.[66] developed another highthroughput method with the aim of determining the concentrations of Dlm and eight metabolites and evaluating their stability in human plasma to assist the clinical development of this drug.In 2015, Alffenaar et al.[61] published a rapid LC-MS/MS method to analyze Bdq and monodesmethyl bedaquiline in the serum using DIL-IS.The accretion of the drug was reported due to the high concentration at the end of the treatment.However,more PK data are warranted to elucidate the association between varied drug concentrations and clinical outcomes or ADRs [61].Metcalfe et al.[62]quantified Bdq in hair samples with full analytical validation of this assay.According to the method proposed by Gray et al.[69],SPE was used to remove interference in plasma samples to quantify rifabutin(Rfb),Bdq,and their metabolites in healthy volunteers.An LC-MS method was established on plasma and urine samples to support a clinical trial (phase I) of macozinone (Mcz), its metabolites, and the dearomatized meisenheimer complex (H2PBTZ).There were two significant findings in this study.First, they observed that H2PBTZ was the most prevalent species circulating in plasma.Second, the low concentration of analytes in urine suggested a high rate of metabolism prior to renal excretion [68].Lee et al.[70] have quantified nine second-line anti-TB drugs via DBS,showing an acceptable correlation between CDBSand the Cplasmaof these drugs.
2.3.3.First-line, second-line drugs and their metabolites
The issue of drug resistance has become increasingly prevalent,necessitating the implementation of more complex treatment regimens.This often involves the combination of first-line and second-line anti-TB drugs.However, the complicated treatment strategies can lead to ADRs and poor patient outcomes,highlighting the need for multiple and intricate approaches to address this challenge [56,76].Therefore, methods for TDM characterizing the PK and interaction of these drugs simultaneously (combined with other agents, such as β-lactams) have been developed.Theseapproaches are summarized in Table 3 [9,18,20-22,64,76-86].We omitted some drugs not typically used in TB, such as ceftazidime,teicoplanin, and tigecycline [77,78].

Table 2 (continued)
A significant number of LC-MS bioassay methods are available to quantify anti-TB drugs and their metabolites (up to more than 10 drugs)[9,22,77,78,83,84].The difference in the chemical properties of the analytes may be a factor that led to multiple processing and analysis strategies being proposed in the same study.For example,three studies used HILIC and reverse-phase LC columns to determine multiple drugs.Besides, the sample preparation could be quite different for each column[18,21,83].For five drugs(R,H,Z,E,and streptomycin) in plasma, the C18and HILIC platforms were successfully applied in TDM [80,81].Of note, streptomycin, a second-line anti-TB drug, is used when amikacin is unavailable or its resistance is verified.
Lei et al.[9]conducted an LC-MS method to quantify 14 anti-TB drugs in plasma, which included an investigation of the concentration ranges and timing of blood sampling.This facilitated the implementation of TDM in 232 patients who received different regimens [9].Zheng et al.[76] validated the LC-MS method using the United States Food and Drug Administration (US FDA) guidelines to assay Mfx, Lfx, Pto, Z, and E.They observed that Pto degraded by over 15%after 3 days at ambient temperature,and the suppression of the Pto signal with hemolysis could be solved by sample dilution[76].Fage et al.[82]not only quantified nine drugs and two metabolites in human plasma but also evaluated the enteric absorption by sampling 2 h and 6 h after oral administration.The results showed that 38% of the patients had low therapeutic levels of both R and H, which could be caused by malabsorption and led to a lower range of the therapeutic interval of R[82].An LC-MS/MS method developed by Wang et al.[85]was successfully applied to TDM with various ranges of 10 anti-TB drugs in plasma and was demonstrated in 34 pulmonary TB patients.Some studies developed quantitative assays for antibiotics,including the ones with anti-TB effects, which were validated according to the European Medicines Agency (EMA) guidelines [78]or 14 drugs that were applied to critically ill pediatric patients[84].
Some studies were performed using various matrices to acquire knowledge of the PK profile of drugs.Wu et al.[21] established a two-dimensional(2D)LC-MS/MS method to quantify E,Z,Pto, and Cfz in plasma, bile, urine, feces, and tissues.It met the validation criteria of the US FDA and could be applied to the PK study in rats after oral administration of the drug combination.Cazorla-Reyes et al.[77] even quantified 21 antibiotics from different groups in serum, urine, CSF, and bronchial aspiration samples within 6 min runtime of LC.They found the highest levels in urine samples of amoxicillin, Lfx, and Lzd.On the contrary, the lowest concentration was detected in bronchial aspirations [77].For hair samples, Gerona et al.[22,79] quantified four anti-MDRTB drugs in 2016, and 11 drugs were measured using this method in 2019(nine of 11 analytes were successfully analyzed in patients with drug-resistant TB, except Pto and Eto) [22].There were two methods for TDM of R,Clr,and their metabolites(De-R,Clr-OH), one in plasma and another in DBS.de Velde et al.[64]reported that plasma components were stable after 3 cycles of freeze-thaw,3 days in the autosampler and refrigerator(5°C),and at room temperature (Clr and Clr-OH: 3 d; R and De-R: 1 d).Vu et al.[20]observed high correlations between CDBSand the Cplasmaof R, Clr and Clr-OH.In particular, the authors demonstrated the role of ethylenediaminetetraacetic acid and deferoxamine in the proper recovery(~100%)of R responses in DBS extraction.Another study was conducted on breast milk matrix using six different sources, and the influence of matrix effects was found insignificant.The method was successfully applied to breast milk samples taken from breastfeeding women treated with H,E,and Z,as well as provided an opportunity to quantify R, Rfb, and their metabolites facilitating clinical practice [86].
3.Six primary prospects in TB clinical management
Due to the transmission of TB,especially MDR-and XDR-TB[6],the adoption of various anti-TB drug combination strategies led to increasing attention and efforts to manage adverse events (AEs)and treatment responses [23].LC-MS/MS bioassay has been established for the TDM of anti-TB drugs to accomplish such a requirement.However, advancing other aspects is paramount to provide comprehensive clinical management for TB patients.They include(1)automated sample management and high-throughputcomparable assay pipeline,(2)pharmacometrics,(3)host-directed therapy (HDT), (4) pharmacometabolomics, (5) drug and vaccine development, and (6) machine learning, AI, and computer algorithms.In this section, we considered our perspectives on challenges and potential of six primary prospects in the clinical implementation of TB.
3.1.Automated sample management and high-throughputcomparable assay development
Automated sampling could be applied for anti-TB drug assays,which provides more convenience than traditional sampling approaches.The proposal is derived partly based on the successful result of automation sampling and measurement by MS of tacrolimus level in kidney transplant patients[87].According to Rao Gajula et al.[88],LC-MS is the best option for green biological approaches,analyzing drugs, and reducing or eliminating hazardous waste.Concerning the degradation of anti-TB drugs,it is one of the severe issues in sampling and storage (especially H in plasma/serum).Therefore, not only to establish stable formulations but also more studies should be expanded on alternative and convenient matrices(urine, hair, fingerprints, DBS, and skin) for specific groups of the population,such as children[89],or DBS to avoid drug degradation in storage and transport(especially in a harsh climate region including Africa).Moreover, Nanthasurasak et al.[90] established an electrokinetic platform to separate the analyte in DBS during transport,which could accelerate the sample preparation process(Fig.1).
The majority of studies used plasma/serum samples, so ACN or MeOH is an appropriate solvent for protein precipitation.Alternatively,96-well plates with SPE sorbent could be applied to remove interferences [36].The ultimate aims are to reduce the separation time and enhance the selectivity, accuracy, and precision of the method.Besides, the polarity of drugs is a variable to take into account when selecting the stationary phase(Am is polar,but H,Z,E, Mfx, Lfx, Bdq, and Dlm are suitable for a less polar assay) [33].Matrix effects are a major issue when conducting a bioassay using MS,especially in using electrospray ionization to analyze extracts of complicated matrices.Nevertheless, optimal sample preparation procedures and control of the LC-MS setup parameters are two ways to minimize or compensate for this issue.As an example,matrix effects could be straightforwardly trivialized by a smaller injection volume and/or diluting the sample.Another way is to alter the LC conditions,for example,by reducing the flow rate and/or the gradient program, splitting post-column, applying a mixed-mode column, using micro or nano-LC, or a 2D-LC system [91].For QC and quality assurance, the validation according to guidelines(especially US FDA[92],and EMA[93])should always be conducted to guarantee the accuracy and reproducibility (inter-laboratory)of the method results[94].
HRMS such as Orbitrap or Fourier transform ion cyclotron resonance provided excellent mass accuracy, acceptable dynamic range, reliable assignments of molecular formulas, and the abilityto analyze trace compounds in complex mixtures [17].Therefore,LC-HRMS could be utilized to conduct untargeted analyzes of anti-TB drugs and endogenous metabolites simultaneously.The use of HRMS in screening forensic drugs could be applied to screening TB drugs [34].In addition, MS-based bioanalysis could be combined with imaging techniques to evaluate drug exposure and PK[95-97], or in the nano-diagnostic of TB [98].Besides, there are portable LC-MS devices, which are suitable for facilitating at the community levels of healthcare to determine the TB drugs and their metabolites [99].

Table 3 LC-MS assays for both first-line, second-line anti-TB drugs and their metabolites.

[64][76][77][78][79]140.0 (Eto),145.0(Eto-d5),79.0(H),82.9(H-d4),220.9 (Lfx),265.1(Lfx-d8),296.0 (Lzd),364.0 (Mfx),154.0 341.2 →297.0(Lzd-d3),125.0 54.0 318.2 384.2 388.2 318.1 368.1 175.0 (Pto),176.0 (OPC-84.0 590.2 (Clr),606.2 (Clr-791.2(R),749.2 (De-R),218.0 (IS)116.1 (E),120.1 (E-d4),81.1(Z),154.0 14714), 181.0→161.1 249.1 163.2 (Am),349.4 (Amx),318.6 (Lfx),384.6 (Mfx),296.5 (Lzd),134.1 (Amx),138.1 (Amx-245.1 296.0 (Lzd),358.1 (Mfx),363.2 (Mfx-167.0 →172.2 →142.2 →362.3 →138.1 →370.2 →338.2 →402.3 →406.2 →(Mfx-13C-d3),360.0 →459.3 →(Ptm), 186.3→(Ptm-d5), 124.1→(Z),128.1 →(Z-15N-d3)OH),823.3 →84.1(Z-15N-748.5 →764.4 →781.4 →306.2 →205.2 →209.2 →124.1 →128.1 →d3), 181.0→(Pto), 188.1→(Pto-d7),362.2→326.2(Lfx), 370.1→(Lfx-d8),402.2→(Mfx), 406.2→(Mfx-d4)586.7 →366.2 →362.3 →402.5 →338.2 →other transitionsrefer[77]366.0 →370.0 →d4), 332.1→(Cfx), 340.1→(Cfx-d8),362.1→322.2(Lfx), 366.1→(Lfx-13C-d3),338.2 →341.2 →297.1(Lzd-d3),402.1 →407.1 →d5), othertransitions refer [78]N/A Water (A)-ACN(B)-aqueousbuffer(10g/L CH3COONH4 +35 mg/L AcOH+ 2mL/L TFA-An inwater) (C)3.6 min 0.1%FA in water(A)-ACN(B)10.5min MeOH(A)-0.01%FA in water (B)6 min 0.1%FA in water(A)-0.1% FA in ACN(B)4-6.5 min(depending onthemethod)1%FA inwater(A)-0.4%FAin MeOH (B)10min 0.1-10(Clr, Clr-OH), 0.2-5(R, De-R)0.1-5 (Mfx), 0.4-40 (Lfx), 0.2-10(Pto),2-100(Z),0.2-10 (E)0.1-5 (μg/mL for urine, serum, CSF;μg/mg forbronchial aspirations)0.05-2.5(Cfx), 0.2-10 (Amx), 0.13-25 (Lfx, Mfx, Lzd)0.1-40(ngdrug/mghair, all analytes)HyPurityAquastar(50 mm ×2.1mm,×50 mm,5 μm)5 μm)AtlantisT3 column(2.1mm ×100mm,3 μm)Acquity UPLC BEHC18 column(100 mm×2.1mm, 1.7μm)Kinetex Polar-C18100?(2.1mm ×100mm,2.6 μm)Synergi Polar-RP-80?(2mm PhenomenexSynergi Polar RP column(2.1mm ×100mm,2.5 μm)MeOHandACN MeOHandACN containingTCA ACN (serum)MeOHcontaining FAor ACN containingFA MeOH 10μL, plasma 100 μL, plasma- 0.5mL, urine- 1mL, serum- 200μL, CSF- 1g, bronchial aspirations 20-25 μL, serum 10-25, strandsof hair Cyanoimipramine Mfx-d4, Lfx-d8, Ptod7, Z-15N-d3, E-d4 Amx, Lfx, Mfx, Am,Lzd andotherISs Amx-d4,Cfx-d8,Lfx-13C-d2, Lzd-d3,Mfx-13C2-d5,other IL-ISs IL-IS (deuterium or 13C)R, Clr, De-R, Clr-OH Mfx, Lfx, Pto, Z, E Amx, Lfx, Mfx, Am,Lzd andother antibiotics Amx, Cfx, Mfx, Lfx,Lzd andother antibiotics Z, Lfx, Mfx, Lzd

Table 3 (continued)
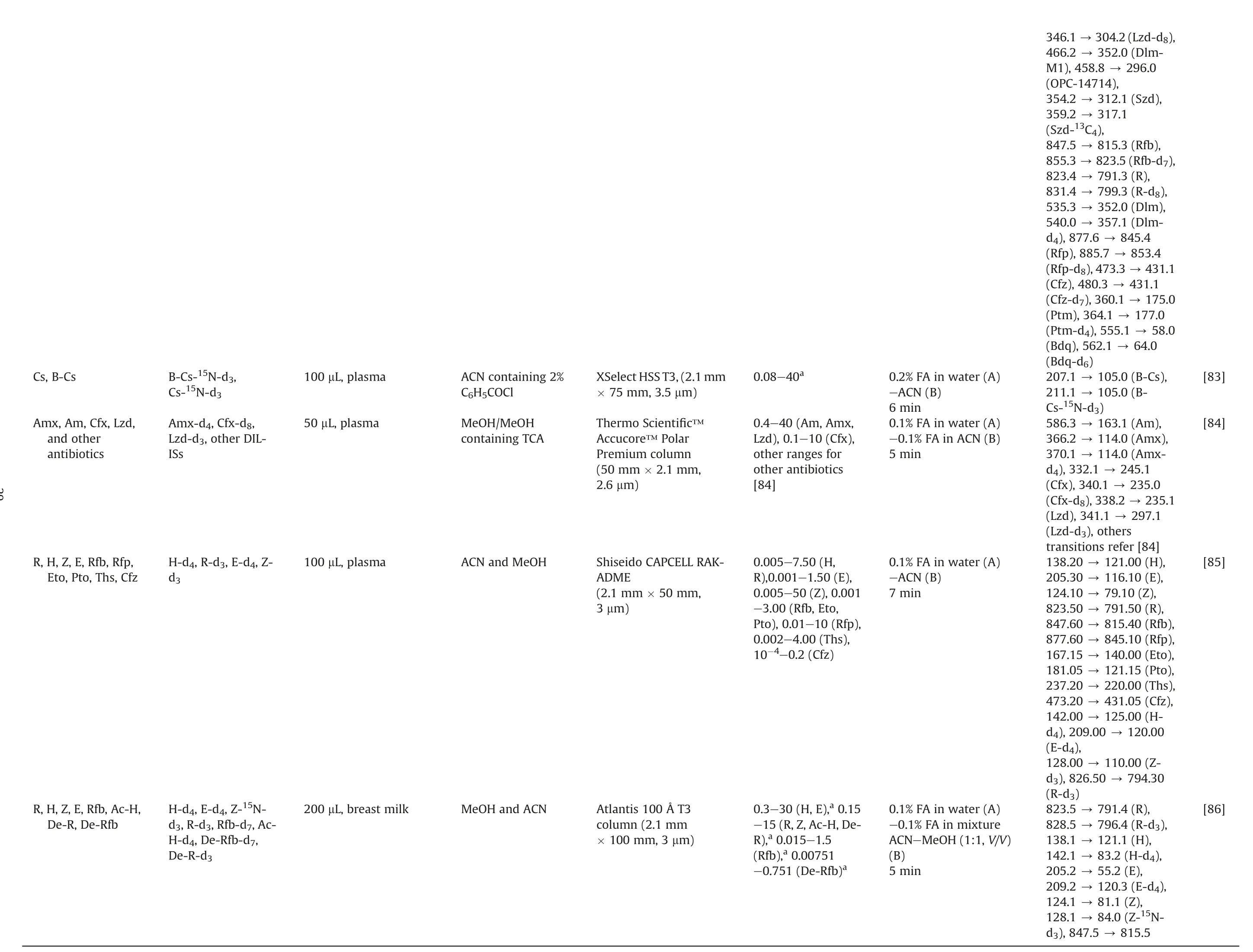
[83][84][85][86]346.1 →304.2(Lzd-d8),352.0 (Dlm-296.0 312.1 (Szd),431.1 845.4 815.3 (Rfb),853.4 823.5(Rfb-d7),175.0 791.3 (R),177.0 799.3 (R-d8),58.0 352.0 (Dlm),235.0 357.1 (Dlm-245.1 105.0 (B-Cs),235.1 105.0 (B-120.00 163.1 (Am),(OPC-14714),794.30 114.0 (Amx),317.1 114.0 (Amx-121.00(H),116.10(E),79.10 (Z),791.50(R),815.40(Rfb),845.10(Rfp),140.00(Eto),121.15(Pto),220.00(Ths),431.05(Cfz),125.00(H-110.00(Z-791.4 (R),796.4 (R-d3),121.1 (H),83.2(H-d4),55.2(E),120.3 (E-d4),81.1(Z),84.0(Z-15N-815.5 466.2 →M1),458.8 →354.2 →359.2 →(Szd-13C4),847.5 →855.3 →823.4 →831.4 →535.3 →540.0 →d4), 877.6→(Rfp), 885.7→(Rfp-d8),473.3→431.1(Cfz), 480.3→(Cfz-d7),360.1→(Ptm), 364.1→(Ptm-d4), 555.1→64.0(Bdq), 562.1→(Bdq-d6)207.1 →211.1 →Cs-15N-d3)586.3 →366.2 →370.1 →d4), 332.1→(Cfx), 340.1→(Cfx-d8),338.2→297.1(Lzd), 341.1→(Lzd-d3), others transitions refer[84]138.20→205.30→124.10→823.50→847.60→877.60→167.15→181.05→237.20→473.20→142.00→d4), 209.00 →(E-d4),128.00→d3), 826.50 →(R-d3)823.5 →828.5 →138.1 →142.1 →205.2 →209.2 →124.1 →128.1 →d3), 847.5→0.08-40a0.2%FA in water(A)-ACN(B)6 min 0.1%FA in water(A)-0.1% FA in ACN(B)5 min 0.1%FA in water(A)-ACN(B)7 min 0.1%FA in water(A)-0.1% FA in mixture ACN-MeOH(1:1, V/V)(B)5 min 0.4-40(Am, Amx,Lzd), 0.1-10 (Cfx),other ranges for other antibiotics[84]0.005-7.50(H,R),0.001-1.50 (E),0.005-50(Z),0.001-3.00 (Rfb, Eto,Pto), 0.01-10(Rfp),0.002-4.00(Ths),10-4-0.2(Cfz)0.3-30(H, E),a 0.15-15(R,Z,Ac-H,De-R),a 0.015-1.5(Rfb),a 0.00751-0.751(De-Rfb)a XSelect HSS T3,(2.1 mm× 75 mm, 3.5μm)ThermoScientific?Accucore?Polar Premium column(50 mm ×2.1mm,2.6 μm)ShiseidoCAPCELLRAKADME(2.1mm ×50 mm,3 μm)Atlantis100?T3 column(2.1 mm× 100mm, 3μm)ACN containing 2%C6H5COCl MeOH/MeOH containingTCA ACN andMeOH MeOHandACN 100 μL, plasma 50μL, plasma 100 μL, plasma 200 μL, breast milk B-Cs-15N-d3,Cs-15N-d3 Amx-d4,Cfx-d8,Lzd-d3, otherDILISs H-d4,R-d3,E-d4, Zd3 H-d4,E-d4, Z-15Nd3,R-d3,Rfb-d7,Ac-H-d4,De-Rfb-d7,De-R-d3 Cs, B-Cs Amx, Am, Cfx, Lzd,and other antibiotics R, H, Z, E, Rfb, Rfp,Eto, Pto, Ths, Cfz R,H,Z,E,Rfb,Ac-H,De-R, De-Rfb

Table 3 (continued)
3.2.Pharmacometrics
A thorough summary of PK parameters and covariates explaining inter-and intra-variability is crucial to optimize anti-TB dosage regimens, especially for at-risk populations.This information can serve as a practical leaflet for clinicians [12,100].Toxicity and PK related to the clinical outcomes of anti-TB drugs are two crucial aspects of TB research, which have been intensively discussed elsewhere [10,27,31,54,74,100-106].In the study on AEs surveillance, Ngoc et al.[104] discussed that the administration of long regimens of injectable anti-TB drugs could result in the increased risk of AEs.They suggested to use the lowest possible injection dose to avoid serious toxicity.There have been challenges in clinical pharmacokinetic/pharmacodynamic(PK/PD)investigations of anti-TB drugs to unravel these characteristics comprehensively.These are(1)the development and integration of bioanalytical techniques to simultaneously measure concentrations of all active metabolites and concomitant drugs; (2) selecting suitable early outcome indicators presenting the therapeutic responses; (3) the unavailability of effective pharmacovigilance systems in underdeveloped countries; (4) clarification of genetic and non-genetic causes of inter-individual PK variability; (5) difficulties in analyzing PK/PD relationships in clinical data; and (6) determination of PK/PD parameters for special populations (children, pregnant women, HIVTB coinfection, TB and diabetes comorbidity, renal and hepatic impairment patients) and at the site of actions [105-107].
Simultaneously with the experimental facet,endeavors from the computational one have been suggested and implemented to fulfill the knowledge gap.Thanks to the pharmacometrics fields including popPK,physiologically-based PK(PBPK),and quantitative systems pharmacology, which have supported the enlightening of the PK parameters at the target sites as well as the source of variation among edge populations, who have been disregarded in TB drug development pipelines [108].Additionally, popPK and PBPK models synergistic with state-of-the-art bioanalytical techniques have been proven to be effective in evaluating DDIs that are widespread among TB patients[108].As a result,this could reduce the ADRs for patients and increase adherence, especially among those infected with MDR-TB requiring long-term and complex regimens[108].Therefore,the measurement of patient adherence,which could be fulfilled by the non-complicated semi-quantification method [109,110], should be considered before implementing optimization approaches [111].
3.3.HDT
In addition to standard antimicrobial treatment, HDT has become a viable adjunct.Regarding TB,HDTs may ameliorate local inflammatory processes, enhance treatment outcomes, shorten treatment duration,and prevent the drug resistance.These include cellular therapy using mesenchymal stromal cells from the bone marrow of the patient.Another HDT is repurposing commonly used drugs for non-communicable diseases (arthritis, diabetes, peptic ulcers, hypercholesterolemia, asthma, and cancer, among others)[112,113].For example, glutathione could be administered as an immunoadjuvant in the treatment of TB [114].It would be extremely challenging to develop a standardized treatment method for HDT, due to the backbone of anti-TB therapy that requires the eradication of M.tuberculosis from patients.Therefore, the genotype-phenotype of the infecting TB strain(s)and the host,the drug susceptibility profile(s), and the status of co-morbidities will be the key factors in considering new drug regimens in the future[115].
TB clinical phenotypes play a significant role in determining the promising strengths of HDT.Beneath the TB clinical phenotypes,‘endotypes’ of TB can be identified.It is described as personalized molecular profiles including specific metabolic, epigenetic, transcriptional, and immune phenotypes [116].To characterize TB endotypes, immunodeficiency or pathologically severe inflammation could be utilized.Additionally, the combination of precise disease phenotyping, in-depth immunological and molecular profiling (including multi-modal omics integration) will be the foundation for the identification of TB endotypes.Precise endotype-specific HDT may significantly improve the outcomes of TB patients [117].
3.4.Pharmacometabolomics and personalized dosing
Metabolomics studies have seen a rise in the analysis of various biospecimens,including urine,tissue,feces,sweat,breast milk,and more.This development could lead to the emergence of personalized medicine,with biomarker discovery and point-of-care testing being made possible[118,119].Establishing a method for detecting and analyzing metabolites as disease biomarkers could also be an important direction for diagnosis and treatment[11].Furthermore,the role of the microbiota has been observed in drug metabolism and toxicity in various in vivo studies[120].Deeper understanding of microbiome provides opportunities for investigating the mechanism and evaluating the effects of drug metabolism and toxicity.Significantly, capturing endogenous metabolites together with anti-TB drugs and their metabolites may expand the use of the developed bioassay for subsequent applications.Typical examples include evaluation of toxicity [27] and individualization of treatment dosage, especially for new analogs [68,121], that could be directed by endogenous compounds[122].
Horizontal (metabolomics and lipidomics) and vertical (genomics,transcriptomics,proteomics,and metabolomics)multi-omics data integration present a powerful approach to capture a holistic view of the molecular landscape, which could provide a comprehensive understanding of an individual's response to anti-TB drugs[123,124].These approaches are described as prospects to improve the better-personalized medicine of TB and allow the discovery of biosignatures for treatment monitoring and clinical outcomes (efficacy and AEs) [123,125-127].Long et al.[124] discovered and validated 10-gene transcriptomic biosignatures representing dynamic responses during TB treatment.The biosignature could translate into an accurate assay and assist clinical decisions in managing TB[124].Additionally,comprehensive lipid profiling was performed in TB patients by Anh et al.[123].They found the alteration of 17 lipid subclasses by pharmacotherapy and identified potential biomarkers, such as phosphatidylcholine (42:6), phosphatidylethanolamine (40:5), cholesterol ester (24:6), and dihexosylceramide (34:2;2O).Besides, the changes in lipid-related metabolic pathways and host-immune responses during treatment were determined.The study showed the potential of using lipids as biomarkers for monitoring TB treatment monitoring [123].Wang et al.[128]conducted metabolic phenotyping in plasma to discover potential biomarkers for the prediction of anti-TB drug-induced liver injuries.Yen et al.[127]established multi-modal data analysis to elucidate the metabolic perturbations in TB patients with concurrent type 2 diabetes.This comprehensive profiling increases the knowledge of TB patients with diabetes comorbidities and contributes to developing more effective and safe treatment [127].Collectively, these multi-modal data will be the foundation for constructing predictive models for personalized dosing, efficacy,ADRs and drug resistance prediction and management, and the estimate of the probability of treatment failure (Fig.2).
Better insights related to treatment response, efficacy, and AEs account for other confounding factors and might provide a qualitative strategy to suggest dosage regimens at the bedside.Precision medicine requires discovering and validating predictive metabolites, which could explain the inter- and intra-sources of variation among individual PK profiles.They could subsequently play as covariates of the popPK model to eventually recommend a personalized and precise dose for clinicians [129].As a result,pharmacometabolomics has been recognized as an opportunity to establish metabolic profiles to elucidate PK and PD characteristics toward drug development and precision medicine [129-132].It could help improve the treatment outcomes of patients with concurrent medical problems [13,14] and be applied for managing TB preventive treatment for the high-risk reactivation of TB [133].Jayanti et al.[134]proposed a straightforward,rapid,and affordable semi-automated TDM procedure associated with MIPD and the clinical setting.After sampling and data collection, the validated popPK and Bayesian forecasting methods were used to estimate PK parameters for the individual patient.This enables clinicians to simulate the PK profile of each drug for each patient in different scenarios,thereby promoting the administration and adjustment of dosage regimens corresponding to the results from the simulation and targeted therapy [134].Incorporating immune profiling into MIPD-based TDM may also be beneficial [135].This platform has provided high throughput in discovering new immune biomarkers for diagnosis, which might be applied in treatment monitoring,dose optimization, and outcome prediction [135].
Regarding drug research and development, pharmaco metabolomics-based biomarker-guided methodology has gained attention [136].The approach played a significant role throughout the discovery phase to clinical one of the drug development pipelines [136], by elucidating the metabolic pathways and processes involved in drug metabolism and responses.This might guide medical chemists in optimizing drug structures to enhance efficacy and minimize AEs.Pharmacometabolomics allows for identifying biomarkers that predict individual variations in drug response, efficacy,toxicity,and therapeutic outcomes.Moreover,by integrating metabolomic data with PK and PD models,a dosage regimen of anti-TB candidates might be proposed more precisely.This also improves efficacy and reduces the risk of toxicity and drug-resistance.Therefore, the development of antimicrobial agents, which were relatively unattractive to pharmaceutical companies[137],could be revolutionized by decreasing the risk of failure(Fig.2).
3.5.Drug and vaccine developments
The burden of MDR-TB and XDR-TB [6] has been a major driving force behind the research and development of new anti-TB medications.Anti-TB candidates and drugs are in clinical trials,such as TBI-166 [138], TBI-223 [139], sutezolid [121], contezolid[140],Mcz[141],BTZ-043[142],OPC-167832[143],and telacebec[144], among others.Enlightening the potential on- and offpharmacological targets, especially multifunctional enzymes simultaneously with discovering the mechanisms underlying the interaction of these drugs, is a favorable direction for developing novel anti-TB drugs [145].Additionally, the DDIs between new and current anti-TB medications should be investigated in order to establish new short and effective regimens.Another aspect of drug development is the ethical barrier to generating evidence at an early stage to optimize treatment in special populations.For instance, the required safety and efficacy studies in children,pregnant women, HIV comorbidity, and diabetes comorbidity patients.In addition, drug development should consider the impact of pharmacogenetics on treatment outcomes before translating it into the clinical setting [107].Furthermore, it is necessary to establish suitable drug formulas, for example, to ensure palatability in children patients, and to ensure treatment effects in this population [146].
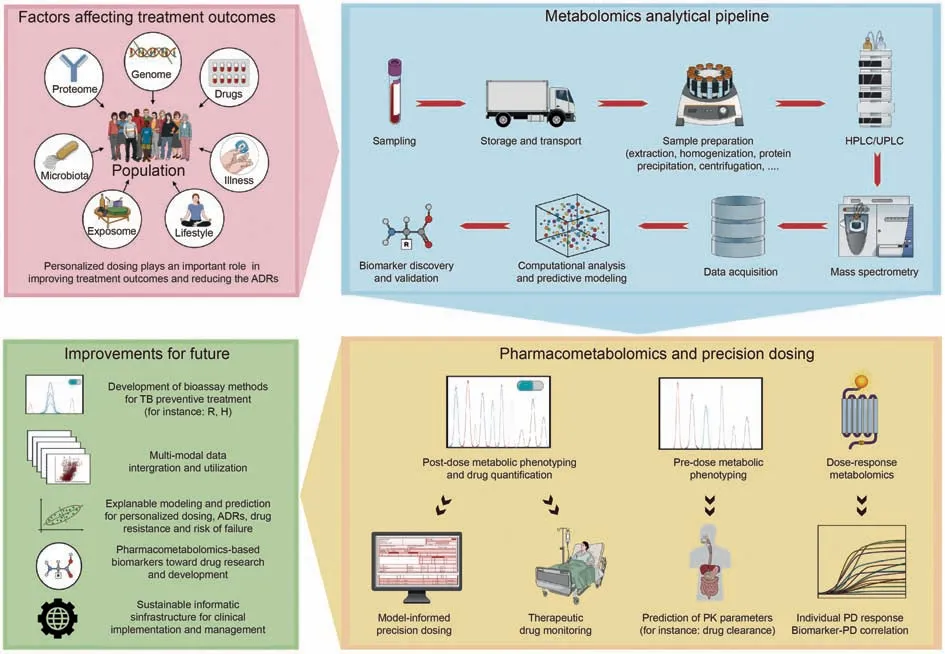
Fig.2.Pharmacometabolomics and personalized dosing-An opportunity for improvement toward better clinical treatment of tuberculosis(TB).ADRs:adverse drug reactions;H:isoniazid; HPLC: high-performance liquid chromatography; PD: pharmacodynamic; PK: pharmacokinetic; R: rifampicin; UPLC: ultra-performance liquid chromatography.
The vaccine is one of the effective ways to combat antimicrobial resistance, including the TB vaccine [147].However, BCG is,yet, the only vaccine approved for global TB prevention [148].During the COVID-19 pandemic, mRNA-based vaccines have demonstrated the possibility of reducing the risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and disease development.This vaccine category is easier to construct,manufacture, and purify than its protein-based subunit counterpart.Larsen [149] mentioned that mRNA could be a hopeful strategy for developing the TB vaccine.They suggested the selection of TB antigens,especially a cluster of differentiation 4 and 8 T-cell epitopes for the novel mRNA vaccine [149].
Regarding pharmacognosy, Chinese medicinal herbs and their ingredients remain diverse sources for drug development[150,151].Combining medicinal herbs with chemotherapy can boost immune system responses and may reduce the ADRs of anti-TB drugs [152].Previous studies have reported and discussed the activity of medicinal herbs and their ingredients in combating TB [150,153].Additionally, LC-MS enables the acquisition of PK parameters for natural anti-TB compounds.These parameters, in turn, facilitate the development and quality control of novel drugs derived from herbal medicine for TB treatment [154].
3.6.Machine learning, AI, and computer algorithms
Developing predictive models using machine learning and AI can greatly aid in TB management.These models can help diagnose TB, forecast disease progression, and even predict treatment responses.These models leverage multi-source data, including demographic information, clinical and para-clinical characteristics,which are continuously updated throughout electronic health records (EHR).The data-driven approach could subsequently generate individualized risk profiles supported by a high level of computing capacity, enabling clinicians to tailor treatment strategies accordingly.For instance, the integration of AI technologies with radiological imaging has shown promise in improving TB detection and diagnosis based on large datasets of chest X-rays and computed tomography scans [155-158].Not limited to the host,but able to expand to the microorganism, these algorithms could provide a framework for identifying and predicting TB drugresistant pathogens[159].
The long duration of TB treatment poses challenges in ensuring patient follow-up and adherence to treatment regimens [160]."Smart" healthcare applications such as mobile health platforms employ machine learning algorithms to deliver personalized reminders,notifications,and educational materials to remind patients to administer their medications regularly [161].By providing consistent reminders, these applications have the potential to decrease the risk of treatment interruption and improve adherence to the prescribed regimen.Furthermore,patient-generated data are analyzed in real-time to monitor the treatment progress and detect potential deviations from the treatment plan.Wearable devices coupled with mobile apps collect data on medication intake,symptom reporting,and physiological parameters,such as heart rate or respiratory function[162-165].This information is subsequently processed to identify patterns or anomalies that indicate potential non-adherence or treatment response issues.Clinicians then be alerted to intervene promptly and provide the necessary support or adjustments to the treatment plan for ensuring optimal outcomes and identifying factors associated with non-adherence or loss of follow-up.
To identify high-risk populations and communities, machine learning algorithms present a practical approach empowered by analyzing epidemiological data, social determinants of health, and environmental factors [166,167].These algorithms could pinpoint geographical areas or demographic groups that are more susceptible to TB outbreaks.Moreover, they could be employed to optimize the allocation of limited healthcare resources for TB management based on historical data on treatment outcomes,resource utilization, and patient characteristics [168,169].Consequently, this information facilitates the guidance of targeted interventions,such as focused screening campaigns,improved access to healthcare resources, and targeted education initiatives, and assists policymakers in designing resource-allocation strategies.
4.Integrative and comprehensive approaches

Fig.3.Schematic illustrating the integration of pharmacometabolomics and PK/PD in order to facilitate the future direction of precision healthcare leveraging from other fields.ADRs: adverse drug reactions; CDSS: clinical decision support systems; EHR: electronic health records; MIPD: model-informed precision dosing; PK: pharmacokinetics; PopPK:population pharmacokinetics; PK/PD: pharmacokinetics/pharmacodynamics; TB: tuberculosis; TDM: therapeutic drug monitoring; TDM: therapeutic drug monitoring.
In order to translate the insights from the bench to the bedside,it is essential to implement easy-to-use and convenient techniques and platforms.Regarding programmatic treatment for TDM,Alffenaar et al.[23] proposed three levels of TDM in the TB management system, including the community level (saliva, urine),regional"level",and central level(plasma,serum).The prospect of simplified LC-MS could lead to the cover of this technique at the community level and gradually replace the traditional LCultraviolet detector-based bioassay with portable LC-MS [99](Fig.3).
It is potential and valuable to explore metabolites predicting alterations in PK parameters and PD responses via the pharmacometabolomics approach [122,129-132].This association could be established and should be subsequently validated when more data are available throughout routine care of TB patients (Fig.3).Validation could be accomplished based on the consistency between predicted PK parameters or PD responses (categorical or continuous, efficacy, or ADRs) and observational counterparts using a ratio threshold of 80%-125% (eg., clearance PK parameters) leveraged from the recommendation of the US FDA guidelines for bioequivalence studies [122].Furthermore, it is possible to obtain information about metabolic response and drug concentration from the same samples and bioanalytical instruments.This offers convenience and reduces the burden on patients, clinical practitioners,and laboratory staff.In addition,owing to the development of technological information and computational capability, MIPD platforms have been developed, implemented, validated, and refined to integrate all available insights into optimizing the dosage regimens of anti-TB drugs [108,134].As a result,these “predictive”metabolites along with other covariates would enhance the feasibility of population-based doses and might predict the efficacy and unexpected ADRs for prospective patients.When more TDM data become available throughout routine TDM practice, those coupled with the above-mentioned metabolites could update the original validated popPK to bolster the predictive performance for suggesting appropriate dosage regimens (Fig.3).Eventually, all workflow and information procedures could be integrated into the EHR to provide comprehensive data application and storage, as well as for labor-saving implementation at the bedside.
The benefits of machine learning and AI in TB management are the potential directions to establish accurate predictive models.For instance, predicting pharmacometrics and optimizing the dosing of Lfx [170],and screening computer-assisted diagnosis in pulmonary imaging[157,158],which could be embedded into the EHR system.The challenges of clinical medicine in general and untargeted metabolomics studies, in particular, could be demolished with the advancement of AI, machine learning, and bioinformatic methods [171,172].With advancements in AI algorithms, particularly self-supervised learning, the focus has shifted towards the development of a comprehensive and universally applicable model capable of improving accuracy and precision in various medical applications.By leveraging both datadriven and knowledge-based approaches, a generalist medical AI(GMAI) model served as a novel and promising paradigm [173].The model was trained based on numerous medical data sources,in which routine data from EHR, and laboratory results, such as medical images or multi-omics information, could be simultaneously used with language data for model building.To better understand the model,it was crucial to rely on medical literature as a key component.This helped not only with validation for use in clinical settings but also in filling knowledge gaps and adjusting the model accordingly [173].
According to Kvarnstr¨om et al.[174],adherence to medication in patients could be improved by better communication and information between physicians and patients.It is worth mentioning that previous studies have utilized digital apps as a remarkable development direction for adherence and personalized TB care,improving self-management of patients [162-165].In 2022, the Center for Personalized Precision Medicine of Tuberculosis: the Smart Research and Development Workstation(cPMTb Smart R&D Workstation) was introduced [175].Comprehensive and diverse databases (such as sample collection, demographics, clinical and biochemical testing results, TDM, ADRs, MIC of TB, and multiomics) provide opportunities to achieve personalized and precision medicine in TB treatment.To bridge the gap between research and clinical practice and implement individualized precision management for TB, the authors aim to develop this cohort for other hospitals from Korea to the world [175].
As a result, the readily available innovations in both analytical and computational aspects should be leveraged with other required advances in screening, diagnostics, and vaccines, as well as drug development, to reach the ultimate destination of precision healthcare toward eradicating TB (Fig.3).
5.Conclusion
Significant progress in LC-MS-based TDM of anti-TB drugs was highlighted over the past two decades,facilitating dose adjustment in clinical settings.These techniques were developed to optimize the analysis process and sampling strategies and to cover both current and new anti-TB drugs, as well as drug combinations.The growth of related areas in TB clinical implementation such as pharmacometrics, multi-modal profiling, drug and vaccine developments, and AI plays a crucial role in TB management.Therefore, the integrative approach of LC-MS-based TDM with pharmacometabolomics and these advancements were proposed for more comprehensive precision healthcare, improving the outcomes of TB patients and effectively eradicating TB in the future.
CRediT author statement
Nguyen Quang Thu: Methodology, Writing - Original draft preparation, Reviewing and Editing;Nguyen Tran Nam Tien:Methodology, Writing - Original draft preparation, Reviewing and Editing;Nguyen Thi Hai Yen: Methodology, Writing - Reviewing and Editing,Thuc-Huy Duong: Conceptualization, Writing -Reviewing and Editing;Nguyen Phuoc Long: Conceptualization,Supervision, Methodology, Writing - Original draft preparation,Reviewing and Editing;Huy Truong Nguyen: Conceptualization,Supervision, Methodology, Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was sponsored by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (MSIT)(Grant No.: 2018R1A5A2021242).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.09.009.
 Journal of Pharmaceutical Analysis2024年1期
Journal of Pharmaceutical Analysis2024年1期
- Journal of Pharmaceutical Analysis的其它文章
- Platycodin D inhibits angiogenic vascular mimicry in NSCLC by regulating the eIF4E-mediated RNA methylome
- Identification of different degrees of processed ginger using GC-IMS combined with machine learning
- Simultaneously quantifying hundreds of acylcarnitines in multiple biological matrices within ten minutes using ultrahigh-performance liquid-chromatography and tandem mass spectrometry
- A proteomic landscape of pharmacologic perturbations for functional relevance
- Licorice-saponin A3 is a broad-spectrum inhibitor for COVID-19 by targeting viral spike and anti-inflammation
- Distinct molecular targets of ProEGCG from EGCG and superior inhibition of angiogenesis signaling pathways for treatment of endometriosis
