Non-thermal atmospheric-pressure positive pulsating corona discharge in degradation of textile dye Reactive Blue 19 enhanced by Bi2O3 catalyst
Milica PETROVI?,Dragan RADIVOJEVI?,Sa?a RAN?EV,Nena VELINOV,Milo? KOSTI?,Danijela BOJI? and Aleksandar BOJI?
1 Department of Chemistry,Faculty of Sciences and Mathematics,University of Ni?,Ni? 18000,Serbia
2 Department of Physics,Faculty of Sciences and Mathematics,University of Ni?,Ni? 18000,Serbia
Abstract In this work,monoclinic Bi2O3 was applied for the first time,to the best of our knowledge,as a catalyst in the process of dye degradation by a non-thermal atmospheric-pressure positive pulsating corona discharge.The research focused on the interaction of the plasma-generated species and the catalyst,as well as the role of the catalyst in the degradation process.Plasma decomposition of the anthraquinone reactive dye Reactive Blue 19 (RB 19) was performed in a selfmade reactor system.Bi2O3 was prepared by electrodeposition followed by thermal treatment,and characterized by x-ray diffraction,scanning electron microscopy and energy-dispersive xray techniques.It was observed that the catalyst promoted decomposition of plasma-generated H2O2 into ·OH radicals,the principal dye-degrading reagent,which further attacked the dye molecules.The catalyst improved the decolorization rate by 2.5 times,the energy yield by 93.4%and total organic carbon removal by 7.1%.Excitation of the catalyst mostly occurred through strikes by plasma-generated reactive ions and radical species from the air,accelerated by the electric field,as well as by fast electrons with an energy of up to 15 eV generated by the streamers reaching the liquid surface.These strikes transferred the energy to the catalyst and created the electrons and holes,which further reacted with H2O2 and water,producing ·OH radicals.This was indentified as the primary role of the catalyst in this process.Decolorization reactions followed pseudo first-order kinetics.Production of H2O2 and the dye degradation rate increased with increase in the input voltage.The optimal catalyst dose was 500 mg·dm-3.The decolorization rate was a little lower in river water compared with that in deionized water due to the side reactions of ·OH radicals with organic matter and inorganic ions dissolved in the river water.
Keywords: corona,RB 19,Bi2O3,catalyst,degradation
1.Introduction
Synthetic dyes constitute a large and important group of organic compounds in various industries.Their production and usage have experienced continuous growth,accompanied by increasing environmental concerns regarding their presence in aquatic media.Thus,procedures for their removal are still being developed and improved today and this represents a very active field of research.Dyes are difficult to degrade due to their high chemical stability in common environmental conditions.Therefore,advanced oxidation processes have been developed for their removal.These processes are based on the production of highly reactive oxygen species such as hydroxyl radicals (·OH) with high oxidation potential that can completely degrade and mineralize the dye molecules.The reactive species are produced by a sufficiently powerful energy source,such as UV radiation or plasma discharge [1-4].The latter has been recently developed and has become a very promising technique for the removal of organics from water due to its high efficiency,low energy and operational requirements and ecofriendly nature [5,6].Several types of plasma discharge have been used for water treatment,such as dielectric barrier discharge (DBD) [6-9],plasma jet [2,10],gliding arc discharge (GAD) [11-13] and atmospheric pressure corona discharge.Corona type plasma is created in a strong nonuniform electric field,on the tips and in the direct vicinity of the corona-carrying electrode,which is usually a system of multiple needles or wires with a small radius of curvature.Its advantages are the ability to operate in open air at atmospheric pressure and ambient temperature and to degrade several pollutants simultaneously,its simplicity of construction and a high energy yield in the dye decolorization processes [14,15].
Although in general highly efficient,the plasma decolorization processes can be further enhanced.Recent literature reports the combination of plasma and Fenton processes[16,17] and the deployment of a catalyst,which is commonly a semiconductive material with the proper band gap.When the energy from a certain power source exceeds the material’s band gap it causes the creation of electron/hole(e-/h+) pairs,which may attack the surrounding water molecules and generate strong oxidants which further degrade the organic molecules.While this phenomenon has been largely examined and used to develop a variety of catalysts for photodegradation processes [18,19],research into plasma-catalyzed organic degradation in water remains limited.The literature data report the use of BiPO4in decolorization of crystal violet [20],g-C3N4/TiO2catalysts for decolorization of Acid Orange 7 [21],Mn-doped cobalt oxyhydroxide catalyst in decolorization of caffeic acid [9]and Bi0.5Fe0.5VO4/honeycomb ceramic plate in decolorization of doxycycline hyclate [7] by DBD plasma,tin containing aluminophosphate molecular sieves in decolorization of Acid Green 25 [13],TiO2in decolorization of textile wastewaters [12] and laterite soil in decolorization of Orange G [11] by GAD plasma,and Cu-TiO2in decolorization of Reactive Red-198 by an atmospheric pressure Ar plasma jet[10].However,there is limited documentation of the use of catalysts in corona plasma degradation.In some previous research,n-type semiconductors such as CeO2,MoO2and MoO3were used as catalysts in corona plasma degradation.Their catalytic mechanism and interactions in the plasma-water-dye system were examined,and it was found that they enhanced the dye degradation rate,total organic carbon (TOC) removal and energy yield of dye degradation[22,23].Thus,the usage of n-type catalysts further improved the process of corona discharge degradation with all its already mentioned advantages,making it even more effective.Nevertheless,to the best of our knowledge,there is currently no documentation on the use of a p-type semiconductor catalyst in these processes.
Bi2O3is a p-type semiconductor,which means that it has holes as the majority charge carriers.Therefore Bi2O3should be able to transfer e-faster towards e-acceptors in the solution [19].Bi2O3has many applications in the field of electronics,sensors,optics and catalysis.The reason for this lies mostly in its structure and characteristics: it appears in several crystallographic polymorphs [α (monoclinic),β(tetragonal),γ (body-centered cubic),δ (face-centered cubic),ε (orthorhombic) and ω (triclinic)],and can be obtained in many forms,both micro- and nanocrystalline,in the form of nanoparticles,nanowires,nanosheets,microrods,microflowers etc,depending on its synthesis routes and conditions [24-31].The most common synthetic methods for Bi2O3are thermal oxidation of Bi [24],hydrothermal [25],thermal decomposition of a precursor [26],precipitation [28]and the grinding method [30].Electrochemical synthesis of Bi2O3has many advantages: it enables excellent parameter control,simple equipment and low-cost materials and procedures [32].
Bi2O3absorbs radiation in the UV-visible range,which makes it a potential photocatalyst [24,30,33].Literature data report the use of Bi2O3as a photocatalyst in UV-visible light-driven degradation of dyes and medicines,for example methylene blue [31,33],rhodamine B [24-26],methyl orange and ciprofloxacin [30],but to the best of our knowledge there are no reports of its use in plasma decolorization processes.
In this work,the application of electrodeposited Bi2O3as a catalyst for corona plasma decolorization of the reactive textile dye Reactive Blue 19 (RB 19) was examined.Bi2O3was chosen as a p-type semiconductor with interesting catalytic properties in certain processes,which can be well controlled by the synthetic route and conditions.Besides,Bi2O3is stable,non-toxic,environmentally friendly compound that can be synthesized relatively easily using simple and low-cost materials.The processes and interactions in the plasma discharge gap,between the positive anode and liquid cathode,as well as in the dye solution in the presence of a catalyst during exposure to a non-thermal atmospheric-pressure positive pulsating corona discharge,were examined.The plasma catalytic mechanism for Bi2O3in the described process is proposed,which is the principal novelty of this work.
2.Experiment
2.1.Materials
Bi(NO3)3·5H2O,RB 19,HNO3,H2SO4and acetone (Sigma Aldrich),all of reagent grade,were used without further purification.All solutions were prepared with deionized water (18 MΩ,Smart2Pure,Thermo Scientific,USA),except in the experiments with the river water (see below).Basic data for RB 19 are given in table 1.
2.2.Preparation and characterization of Bi2O3
Electrodeposition was performed at a constant current density of 70 mA·cm-2for 15 min in 0.50 mol·dm-3Bi(NO3)3·5H2O dissolved in 0.50 mol·dm-3HNO3using an Amel 510 DC potentiostat (Materials Mates,Italy)controlled by VoltaScope software at 25 °C in a two-electrode cell with a Ti sheet (10 mm × 20 mm) as a substrate(cathode) and a Pt sheet (10 mm × 20 mm) as counter electrode (anode).Prior to electrodeposition,the substrate was ultrasonically treated in water and acetone,then electrochemically etched in 2 mol·dm-3H2SO4at 300 mA·cm-2and rinsed with water.After the deposition,the cathodic precipitate was stripped,dried at ambient temperature and thermally treated in furnace at 400 °C for 60 min.This material was characterized and used in decolorization experiments.
Scanning electron microscopy (SEM) analysis was performed by attaching the sample to aluminum stubs with Leit-c conductive carbon cement.Secondary electron images were taken by a Hitachi High Technologies SU8030 (Tokyo,Japan) field emission gun at an accelerating voltage of 2 kV.Energy-dispersive X-ray (EDX) data were taken on the same instrument at 20 kV using a Thermo Scientific System 7 Ultradry EDX detector (Massachusetts,USA) and five random areas of the sample were analyzed.X-ray diffraction (XRD) analysis was done on a Bruker D8 Advance Xray diffractometer (Bruker-AXS,Germany) in theta-theta geometry in reflection mode.The sample was packed into Si low-background holders.Cu Kα radiation was used at 40 mA and 40 kV with a LynxEye silicon strip position-sensitive detector.Data were taken between 2θvalues of 10° and 80° with a step size of 0.02° and a counting time of 0.5 s per step,by DIFFRAC plus XRD Commander 2.6.1 software(Bruker-AXS).Qualitative assessment was done with the aid of EVA 5 (Bruker-AXS) and the PDF-2 2020 database(ICDD).The materials’ band gap was determined by cyclic voltammetry based on the highest occupied molecular orbital/lowest unoccupied molecular orbital levels [34] using the same potentiostat as for the electrosynthesis and a saturated calomel electrode as a reference electrode.
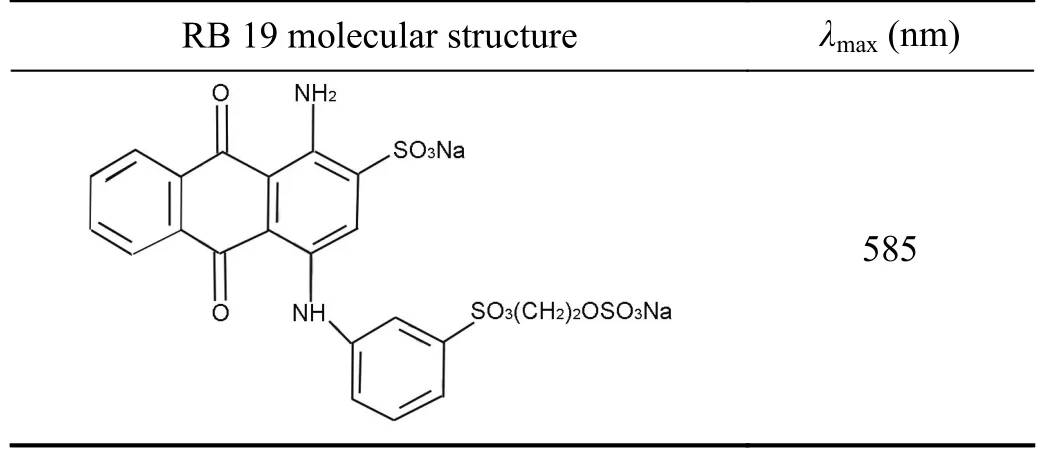
Table 1.Molecular structure and maximum absorption wavelength(λmax) of RB 19.
2.3.Plasma catalytic decolorization experiments
2.3.1.Plasma reactor
All plasma experiments were done using a self-made device,which consisted of a power supply,DC pulse generator,highvoltage output stage and open reactor chamber.The chamber was placed in a fume hood with an AC centrifugal fan R2E225-BD92-09.The frequency of the device was set to 40 kHz.A positive corona discharge was created in the gas phase over the liquid on the tips of the stainless steel needles of mutually equal dimensions,which together formed the multipoint anode.The surface of the liquid (dye solution)acted as the cathode.The reactor cell was a polyvinyl chloride cylinder (with inner diameter and height of 46 and 27 mm,respectively).The round stainless steel contact electrode (6 mm in diameter and 1.5 mm high) was centered at the bottom of the reactor cell and connected to the ground potential.The discharge current was automatically controlled by the electronic circuitry of the device,adjusting the voltage at the multipoint anode.The distance from the anode to the liquid surface was 16 mm in all cases.Current densityj(μA·cm-2) was calculated using the liquid electrode surface area of 16.61 cm2and adjusted from 9.15μA·cm-2to 0.30μA·cm-2.Gases produced by the discharge were removed through the exhaust system with a flow rate of 1 m3·min-1.The average total current of the reactor was measured by an Iskra Unimer 06 multimeter.The average discharge voltageU(V) was measured with a Tektronix P6015A high-voltage probe.The current and voltage signals were recorded using a TDS 2024C digital oscilloscope.The experimental set-up is schematically shown in figure 1.
During the discharge,the voltage never dropped to zero(ground).Each pulse provoked an immediate increase in voltage on the anode and,after that,the voltage would start to decrease until the next pulse; then the cycle repeated.Thus,the corona was sustained at all times with defined oscillations at a given pulse frequency.The experimental conditions were chosen in such way that the corona discharge operated without sparks and with the appearance of low-intensity streamers.Some of the streamers reached the liquid surface and extinguished without transitioning into sparks.Ion wind,generated during the treatment,enabled the constant turbulent movement of the Bi2O3particles,as well as their relatively homogeneous distribution in the solution.

Figure 1.Scheme of the plasma experimental set-up.
2.3.2.Dye decolorization
RB 19 model solutions were prepared by dissolving 1.0 g of powdered dye in 1.0 dm3of deionized water (stock solution),and by dilution of the required amount of this solution to obtain working solutions.The model solutions of river water polluted with RB 19 were prepared by dissolving the proper amount of powdered dye in a flask of water from the Ni?ava River (Republic of Serbia).Before the experiments,the river water was filtered through 0.2μm filter.Plasma treatments of the model RB 19 solutions with river water were done under the same conditions as the model solutions with deionized water.Decolorization experiments were done using the described plasma reactor at ambient temperature of (20 ± 0.5)°C at native pH (5.55).Powdered Bi2O3was added to the dye solutions and they were stirred for 15 min with a magnetic stirrer prior to exposure to the plasma.Dye concentration was measured by a Shimadzu UV-1650 PC(Japan) UV-visible spectrophotometer at 592 nm.Hydrogen peroxide (H2O2) formed during the treatments was determined spectrophotometrically by an iodometric method [35].
Energy yield was calculated using equation (1)
whereG50(g·kWh-1) is the energy yield,C0(mg·dm-3) is the concentration of the dye,V0(dm3) is the volume of treated solution,P(W) is the discharge power of the reactor [calculated by the formulaP=IU,whereIis the measured discharge current (A),andU(V) is the measured discharge voltage] andt50(s) is the time required for 50% decolorization of the dye in the treated solution.TheG50value was chosen as the most convenient value for comparison with the literature.
3.Results and discussion
3.1.Characteristics of the material
The obtained material was highly crystalline and composed of monoclinic (α)-Bi2O3(space group P21/c) with the most intense characteristic peaks in its XRD pattern at 27.4°,33.3°and 46.3°,which corresponded to (120),(200) and (041)reflections,respectively (figure 2).The minor peaks positioned at 21.51°,25.56°,26.66°,27.75°,33.01°,34.88°,35.22°,35.75°,36.82°,37.46°,39.91°,41.29°,41.76°,42.19°,45.03°,46.80°,48.43° and 49.28° corresponding to 020,002,111,012,122,212,031,102,130,112,222,131,213,122,023,140,104 and 133,respectively [30],also originated from α-Bi2O3(some other low-intensity peaks may originate from the traces of some other Bi2O3phase or some impurity).The corresponding lattice constants werea= 5.85 ?,b= 8.16 ? andc= 7.51 ?,with the angleβ= 112.977°.
The material surface appeared to be irregular,of indeterminate shape and form,foam-like,and composed of many oval particles of various shape and size,mutually well sintered,which formed bigger monoliths in places.Many pores and holes of different shapes and sizes could be spotted,with many of them being smaller than 1μm,but there were also larger ones (figure 3).
EDX analysis showed that the material surface contained Bi and O atoms.Their average atomic ratio on the examined surface was Bi : O = 0.385 : 0.615,which was close enough to their theoretical value in Bi2O3(0.40 : 0.60).The element distribution on the surface was relatively uniform and varied within ±3.50%.A slightly lower content of O on the surface compared with the theoretical one implied that the obtained compound might be oxygen deficient,since traces of metallic bismuth were not detected in the XRD pattern [36].The possible presence of oxygen vacancies represented structural defects that might react as potential catalytic sites,as will be presented in the discussion section.The band gap of the obtained Bi2O3was found to be 3.17 eV.
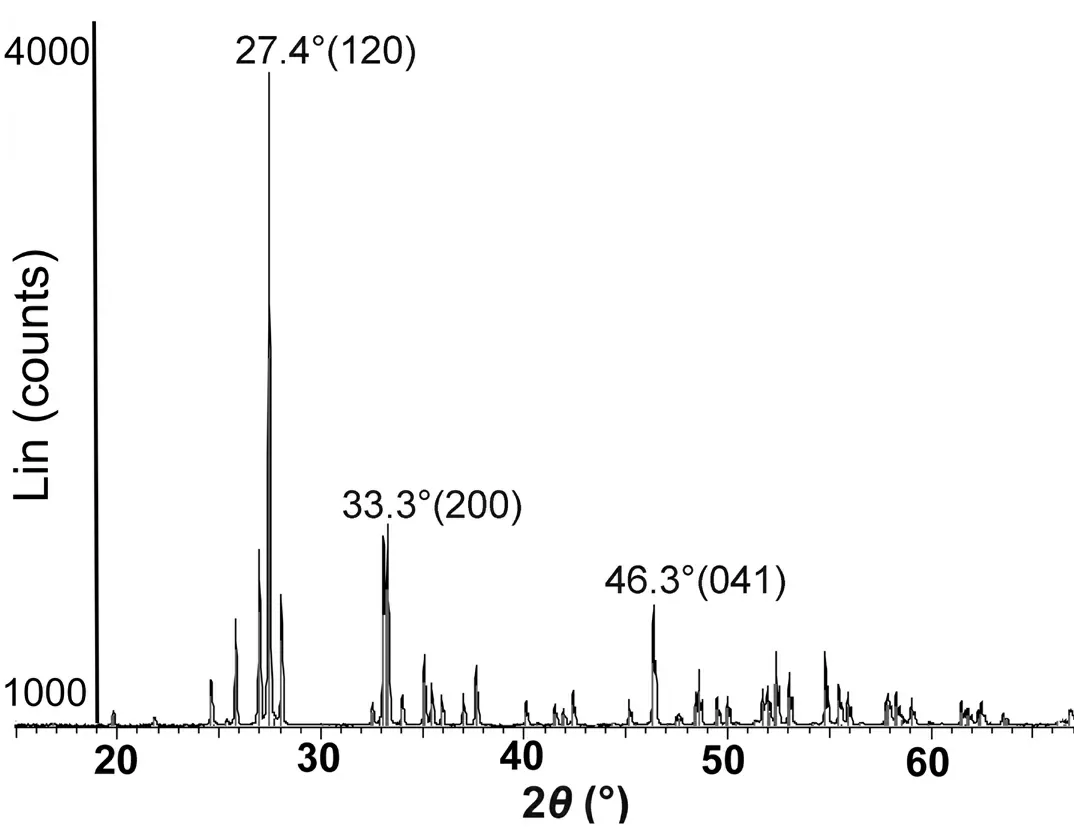
Figure 2.XRD pattern of the obtained α-Bi2O3.
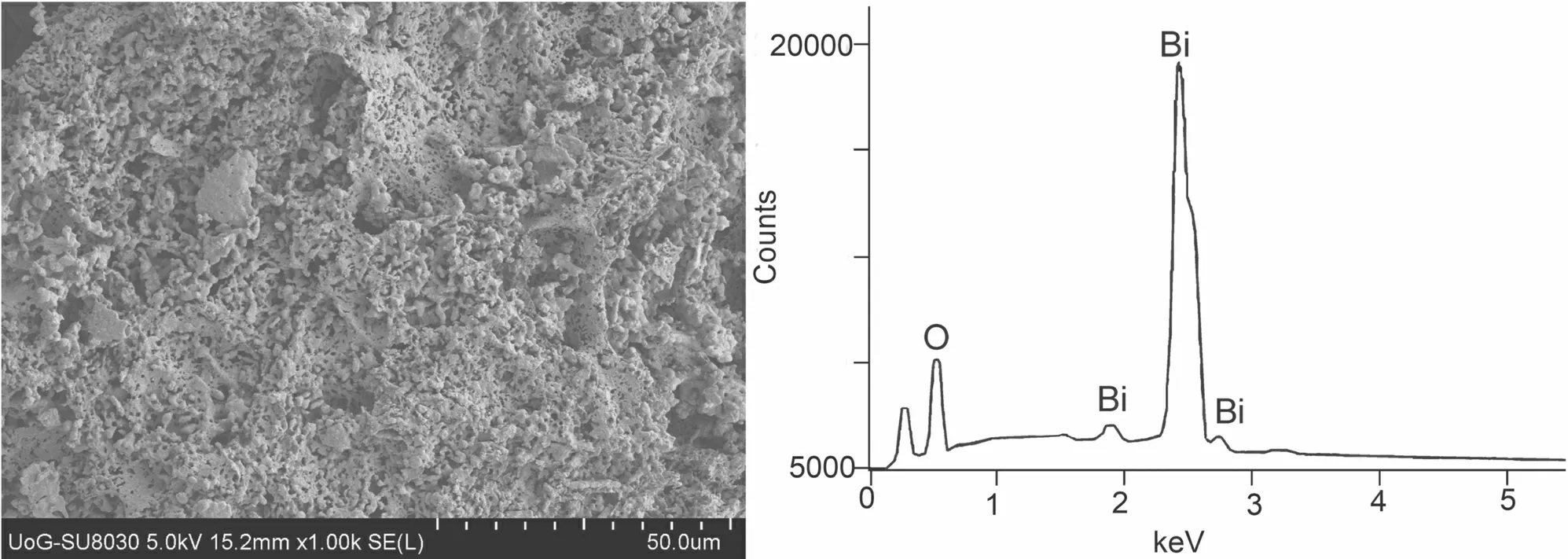
Figure 3.SEM micrograph and EDX spectrum of the electrodeposited α-Bi2O3.
3.2.Dye decolorization
3.2.1.Role of the plasma - formed and decomposed H2O2
RB 19 is stable under visible and UV light alone,as well as in H2O2solution.The corona discharge caused fast decolorization of RB 19 aqueous solutions.The rate of RB 19 decolorization by plasma was about 2.5 times higher in the presence of Bi2O3than by plasma alone,meaning that Bi2O3enhanced the dye decolorization process (figure figure 4(a)).
A high-voltage electrical corona discharge between the multi-needle anode and liquid surface-cathode caused ionization of the air between them (plasma discharge gap).Fast electrons with an energy of up to 15 eV [40] struck the bulk gas molecules,creating many reactive radicals and ion species,such as ·OH,·O,·NO,O+,O2+,etc.These reactions could occur in the gas and bulk liquid phase as well as at the gas-liquid interface [37-40].In this work,the majority of charge carriers were positive ions.Charged particles,accelerated by the strong electric field,were transferred from gas into the liquid phase where they reacted with more stable species such as H2O molecules.They could also transfer their momentum to neutral species and radicals,creating an ion wind.Some of these species were also capable of reacting with dye molecules and degrading them.
In order to check which species among the most common active species degraded RB 19,the characteristic radical scavengers were added to the system.The decrease of the reaction rate caused by isopropanol (·OH scavenger) was almost 95%,while it was negligible with the addition of Na2-EDTA (h+scavenger) and benzene quinone (O2·-scavenger).Therefore,it was assumed that the ·OH radical was the major dye-degrading reagent,although minor contributions of the others,as well as direct oxidation of the dye,were not excluded.
The ·OH radical can be formed in numerous processes,which occur when the high-energy electrons or other reactive species strike neutral molecules such as O2,H2O and N2present in the air and O3generated by plasma [10,12-14,38]:
The ·OH radical can participate in many reactions.One of them is the formation of H2O2[12].
H2O2was detected in all three examined systems: water,aqueous dye solution and aqueous dye solution with dispersed Bi2O3.The concentration of H2O2increased during the plasma treatment in all three cases,but in a different manner (figure 4(b)).In water it increased approximately linearly.In the dye solution it also increased linearly,but the rate of that increase was lower than in water.In the presence of Bi2O3,the rate of increase of H2O2content was significantly lower than in its absence.This,together with the decrease in the dye concentration,implied that H2O2participated in dye decolorization and that Bi2O3had a significant role in the process.
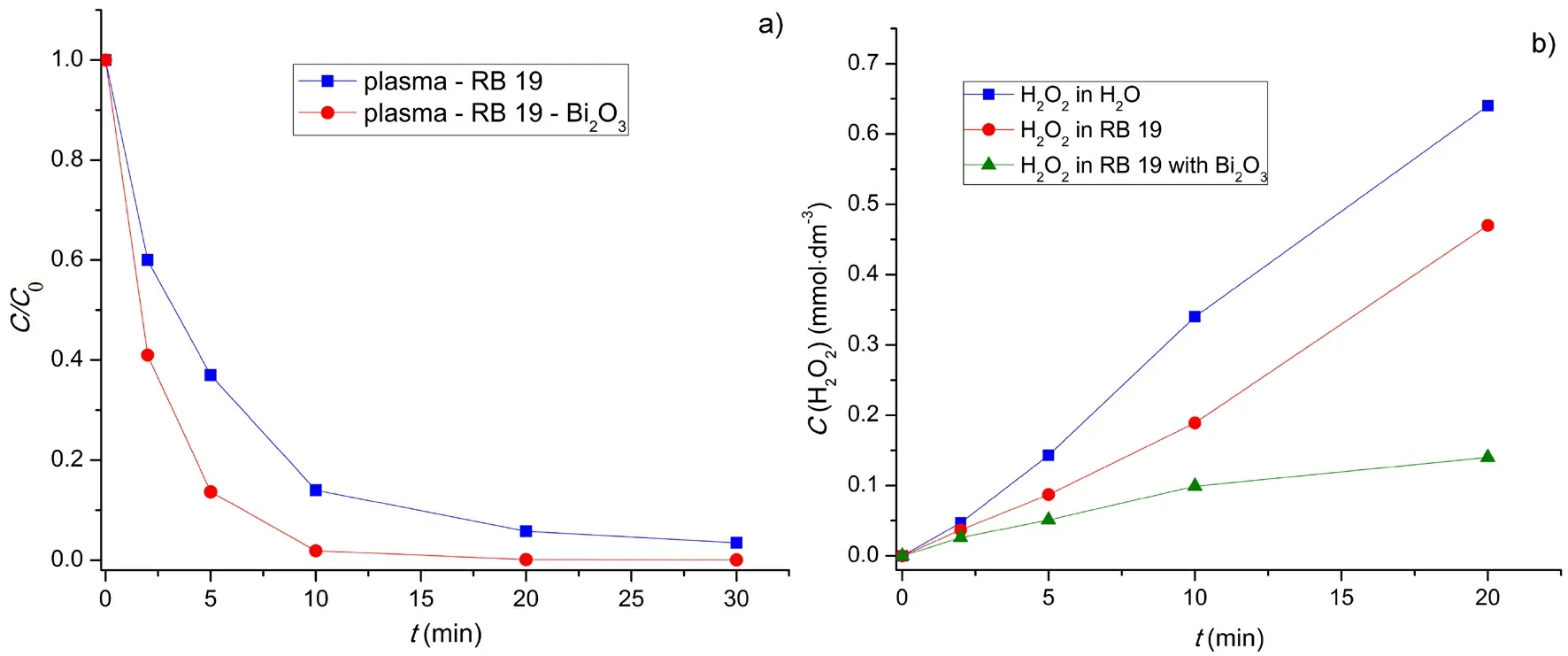
Figure 4.(a) Decolorization of RB 19 by plasma with and without Bi2O3.(b) Formation of H2O2 by plasma [C0 (RB 19) = 50 mg·dm-3,U =13.3 kV,C (Bi2O3) = 500 mg·dm-3].
During the plasma treatment,H2O2was decomposed by corona discharge,leading to the formation of unstable,shortlived ·OH radicals which practically only existed during the treatment [10,12]:
The lower content of H2O2in the plasma-treated dye solution compared with its content in water strongly indicated that H2O2was consumed in the dye decolorization reactions.Based on this,and on the findings obtained from experiments with the radical scavengers,it was assumed that the dye was degraded mostly by the ·OH radicals generated by plasma decomposition of previously plasma-generated H2O2.The participation of ·OH radicals generated in other processes during the discharge was also possible:
3.2.2.Role of the catalyst and the proposed catalytic mechanism
The effect of characteristic radical scavengers on Bi2O3-catalyzed plasma decolorization was also examined.In the presence of Bi2O3catalyst,isopropanol caused the decolorization rate to decrease by about 88%,but a slight decrease was also detected with the addition of Na2-EDTA.Benzene quinone practically did not affect the reaction rate.Thus,it was concluded that the dye was degraded mostly by the action of ·OH radicals in the presence of Bi2O3(as was the case with the non-catalyzed decolorization).It also seems that h+had certain role in the catalytic process,either via participation in the generation of ·OH or by direct reaction with the dye molecules.
As mentioned above,the H2O2content slowly increased during the plasma catalytic decolorization,and it was significantly lower than that without the catalyst.It could be concluded that the catalyst apparently reduced the rate of production of H2O2.However,at the same time,dye decolorization proceeded considerably faster in the presence of catalyst (figure 4).These two facts,as well as the finding that the ·OH radical (formed by H2O2decomposition) was the major decolorization reagent,led to the conclusion that the catalyst actually promoted the consumption of H2O2during the plasma discharge.
The experiment showed that Bi2O3did not react with H2O2spontaneously.Their interaction was caused by plasma discharge and only took place during the discharge.The pulsating positive corona discharge used in these experiments was generated at relatively high frequency,with the occurrence of relatively low-intensity streamers.This provided fast electrons and radical species as well as UV radiation [15].The literature reports the catalytic decolorization of organics with Bi2O3caused by UV radiation [24-26,29,31],but measurements showed that the intensity of UV radiation in this system was rather low,so it is unlikely that this is the principal method of excitation,although it had a good penetration through the liquid layer.The measured UV radiation parameters (wavelength,relative intensity and energy) of this discharge are given in supplementary table S1.On the other hand,Bi2O3particles on the liquid surface area(where they were constantly present due to the mixing caused by plasma-generated ion wind) were exposed to the strikes of corona-generated reactive species accelerated by the strong electric field.Besides,a certain number of streamers that reached the surface of the solution contained fast electrons of up to 15 eV,which interacted with the suspended catalyst particles.Overall,fast electrons and reactive ions and radicals with an energy higher than the catalyst’s band gap,transferred that energy to the semiconducting catalyst by striking it,causing the creation of e-/h+pairs,i.e.excitation of the catalyst [37,41,42].UV radiation,although of a low intensity,could also contribute the excitation process.Once created,e-from the conductive band attacked the plasma-generated H2O2and decomposed it into ·OH radicals,which further attacked the dye molecules.This process is represented by equations (12) and (13)
It was already shown that ·OH radicals are the major dye degrading agent,so this is the most probable method of decolorization.However,examination of the effect of radical scavengers showed that h+also played a certain role in catalytic plasma decolorization.They could react with the water molecules,also leading to the formation of ·OH radicals,which further attacked the dye as in equation (11) [10]
So,there are at lest two possible ways to generate ·OH radicals,a major decolorization reagent,by the action of catalyst: by decomposition of plasma-generated H2O2by the catalyst’s conductive band electrons (more probable) and by the reaction of water with the catalyst’s valence band holes.Briefly,the positive corona plasma catalytic decolorization of a dye in this case can be represented as follows:
(1) Formation of H2O2by the action of plasma-generated reactive species in water (equations (2)-(9)).
(2) Excitation of the catalyst by the strikes of streamers and high-energy plasma-generated species (equation (12))and (with less probability) by the low-intensity UV radiation.
(3) Decomposition of plasma-generated H2O2(and water)at the catalyst’s active sites to form ·OH radicals (equations(13) and (14)).
(4) Decolorization of the dye via oxidation by ·OH radicals (equation (11)).
Parallel reactions,such as direct oxidation of the dye,or its oxidation by the ·OH radicals generated directly by H2O2plasma decomposition [as is the case in the non-catalyzed process (equation (10)] or some other reactive species,such as plasma-generated O3,are also possible to some minor extent.O3was not detected in the plasma-treated liquid,but in the gas phase it could still be the source of ·OH radicals that reached the liquid,where they could attack the dye molecules (despite the fact that the production of ozone and its role as a source of ·OH radicals for this system in such operational conditions were considered low and inefficient[43],especially taking into account that the gases produced above the liquid are exhausted during the treatment).
A simplified scheme of the catalytic mechanism is proposed in figure 5.
3.2.3.Decolorization kinetics
Plasma catalytic decolorization followed pseudo first-order kinetics (figure 6),which is represented by the equation (15)
wherek1,CtandC0are the reaction rate constant (min-1),dye concentration at the reaction timet(mg·dm-3) and initial dye concentration (mg·dm-3),respectively.
The concentration of ·OH radicals is much higher than the concentration of the dye and therefore,according to the pseudo first-order model,it can be considered constant,i.e.only the dye concentration changes during the reaction and the reaction rate should be independent of the initial dye concentration.However,in this case,the reaction rate constants decreased with increase in the initial dye concentration (table 2).That decrease was relatively low at the lowerC0,i.e.the correspondingC0values were similar.At the higherC0the decrease ofk1was significantly higher,i.e.the initial dye concentration had a greater impact on the reaction rate.The reason for this might be the less favorable ratio between ·OH radicals and the dye molecule concentration at higher dye concentrations.At some point this became significant enough,so that the ·OH concentration could not be considered constant compared with the dye concentration,which led to a certain deviation from the pseudo first-order model.Still,that ratio between ·OH and the dye concentration remained high enough in the entire examined range of dye concentration,so that the kinetics still best fitted pseudo first-order.This was confirmed by the highR2values,which only slightly decreased asC0increased (table 1).For comparison,the same kinetics model was obtained for DBD plasma decolorization of doxycycline hyclate using Bi0.5Fe0.5V O4/honeycomb ceramic plate catalyst [7] and DBD-NPT plasma decolorization of AO 7 using g-C3N4/TiO2catalyst[21].The non-catalyzed decolorization of RB 19 by an atmospheric-pressure pulsating corona discharge also followed pseudo first-order kinetics [15],confirming that the decolorization mostly proceeded via the same agent,i.e.·OH radicals.
3.2.4.Effect of the basic operational parameters on dye decolorization

Figure 5.Schematic representation of catalytic mechanism of RB 19 plasma decolorization using Bi2O3 catalyst.
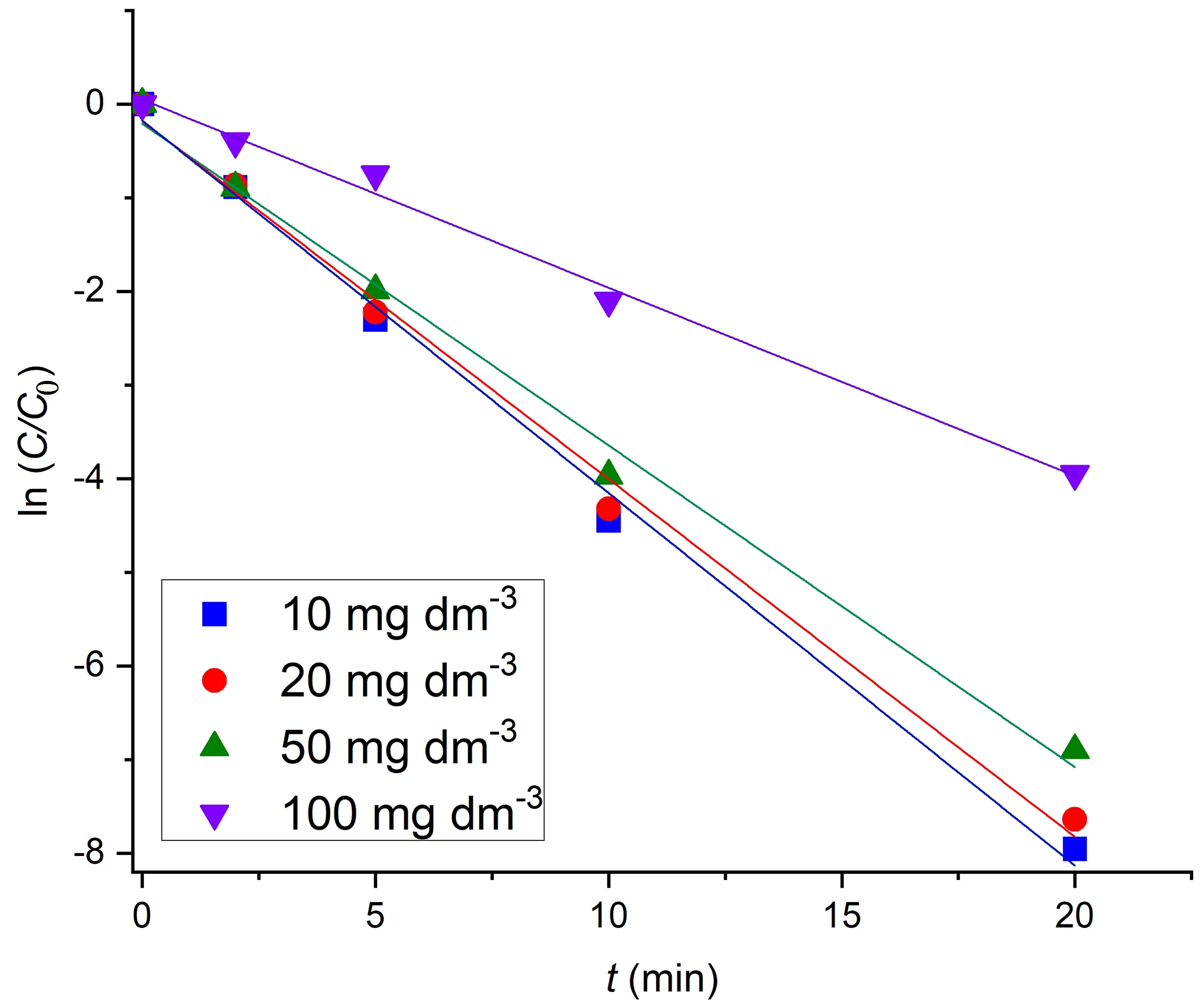
Figure 6.Pseudo first-order kinetics for RB 19 catalytic plasma decolorization [U = 13.3 kV,C (Bi2O3) = 500 mg·dm-3].
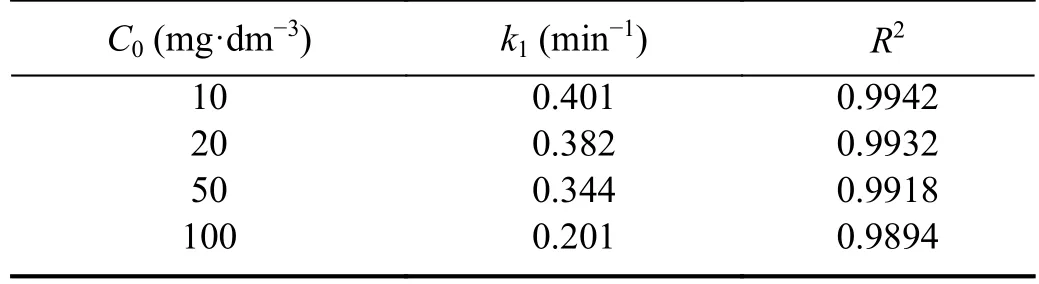
Table 2.Reaction rate constants (k) and R2 values for plasma decolorization of RB 19 catalyzed by Bi2O3.
3.2.4.1.The reactor input voltageThe effect of input voltageUon catalytic plasma decolorization was examined in the range of 7.5 and 13.3 V (corresponding to current densities of 9.15 mA·cm-2and 0.30 mA·cm-2) that provided a stable pulsating corona.The first value was approximately the lowest possible at which the corona visibly disappeared,i.e.the one at which gas ionization and excitation disappeared.The second was the maximum value at which the corona was still stable; at higher voltages,sparks started to appear.
The plasma decolorization rate increased as the reactor input voltage at the anode increased (figure 7).The highest decolorization rate was achieved at 13.3 V.At 7.5 V there was practically no decolorization.At the same time,the concentration of H2O2in the treated solution also increased with increase inUand was highest at 13.3 V (figure 7(b)).At the limitingUof 7.5 V,H2O2was not detected in the treated solution.This once again shows the link between the plasma-produced H2O2concentration and RB 19 decolorization rate.

Figure 7.Effect of the reactor input voltage on (a) decolorization rate and (b) H2O2 concentration [C0 (RB 19) = 50 mg·dm-3,C (Bi2O3) =500 mg·dm-3].
With increase in the input voltage the discharge current density also increased,as well as the electric field and gas ionization.That provided more reactive species for reactions with dye molecules and higher acceleration and energy of those species,and therefore intensified H2O2and ·OH production and thus dye decolorization [40].
In order to trigger plasma-induced dye decolorization,it is necessary to provide gas ionization and other corresponding processes,and that requires a strong enough electric field,i.e.the input voltage must be high enough.
3.2.4.2.The dose of catalystThe decolorization rate significantly increased with increase in the catalyst dose from 250 to 500 mg·dm-3,but a further increase to 750 mg·dm-3did not bring any further improvement (figure 8).
The increase in the catalyst dose meant an increase in the contact surface between liquid and solid (catalyst) and thus more active sites for catalytic reactions at the catalyst’s surface.At the same time,the increase in catalyst dose caused an increased possibility of interactions,i.e.more contact between the catalyst particles and screening of the active sites for catalytic reactions,which consequently reduced the decolorization rate.A similar effect has been reported in the literature,where an increase in catalyst dose even caused the decrease in the reaction rate at some point [12].In our case,a catalyst dose of 500 mg·dm-3was taken as optimal.
3.2.5.Energy yield
Energy yield (G50) increased rapidly at lower initial dye concentrations,and the rate of increase slowed down at higher concentrations (figure 9).
Energy yield reached its highest value of 4.74 g·kWh-1at 1500 mg·dm-3of dye and practically did not change with further increase in initial dye concentration.The energy yield for non-catalyzed decolorization under the same conditions was 2.45 g·kWh-1,meaning that the use of a catalyst improved the energy yield by more than 90%,which is one of the most important reasons for its use.

Figure 8.Effect of catalyst dose on decolorization rate [C0 (RB 19) = 50 mg·dm-3,U = 13.3 kV].
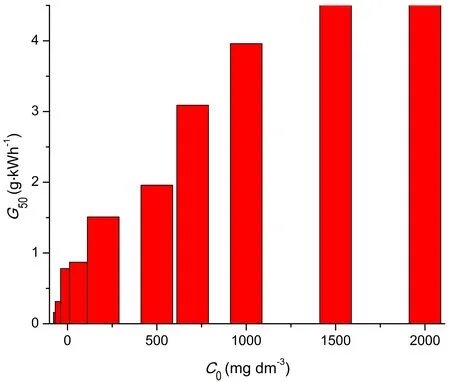
Figure 9.Energy yield of RB 19 decolorization [U = 13.3 kV,C(Bi2O3) = 500 mg·dm-3].
Some recent literature data covering various plasma decolorization systems and compounds,both catalyzed and non-catalyzed,are given in table 3.As can be seen,a very good result is achieved for this system under the optimized conditions.

Table 3.Comparison of energy yield in various plasma decolorization systems.
3.2.6.Total organic carbon
The presence of Bi2O3catalyst improved the system’s ability to remove TOC from 81.8% without Bi2O3to 88.9% with the Bi2O3catalyst after 210 min of treatment (figure 10).
The TOC removal percentage followed a similar pattern in both cases (with and without the catalyst).It rapidly increased during the first 90 min,and after that the rate of increase significantly slowed.It reached a maximum after about 150 min of treatment,and further increase was negligible.A high percentage of TOC removed indicated a high degree of mineralization of the starting compound.So,by prolonging the treatment after the solution was completely decolorized it was possible to completely degrade a high portion of the starting compound to CO2,N2,sulfate and Na+.The presence of SO42-and Na+ions was confirmed in the solution after the treatment.
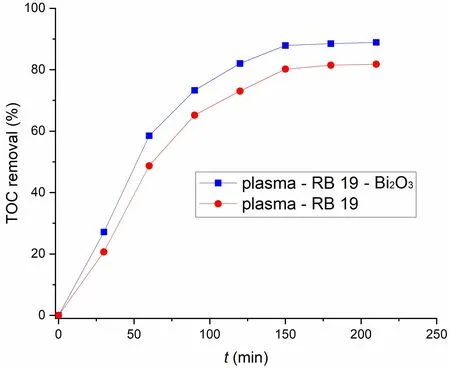
Figure 10.Per cent TOC removal [C0 (RB 19) = 50 mg·dm-3,U = 13.3 kV,C (Bi2O3) = 500 mg·dm-3].
3.2.7.Decolorization of model RB 19 - contaminated river water
The Bi2O3-catalyzed plasma decolorization rate of river water was somewhat lower than that of the model RB 19 solution prepared with deionized water (figure 11).
The river water used in this experiment contained dissolved organic matter as well as ions such as Na+,K+,Ca2+,CO32-,HCO3-,Cl-and SO42-.All these components reacted with the ·OH radicals,thus depleting their concentration in these competitive reactions and making it less accessible to the dye molecules.Reaction of ·OH radicals with some inorganic ions such as HCO3-,Cl-and SO42-also led to the formation of the corresponding radicals (HCO3·,Cl·and SO4-·),which were much less efficient for the oxidation of dye molecules than ·OH radicals [3].Nevertheless,the decolorization rate of the model river water proceeded relatively fast,i.e.side reactions of the ·OH radicals with the substances dissolved in water did not significantly affect the reaction rate compared with that in deionized water.
4.Conclusion
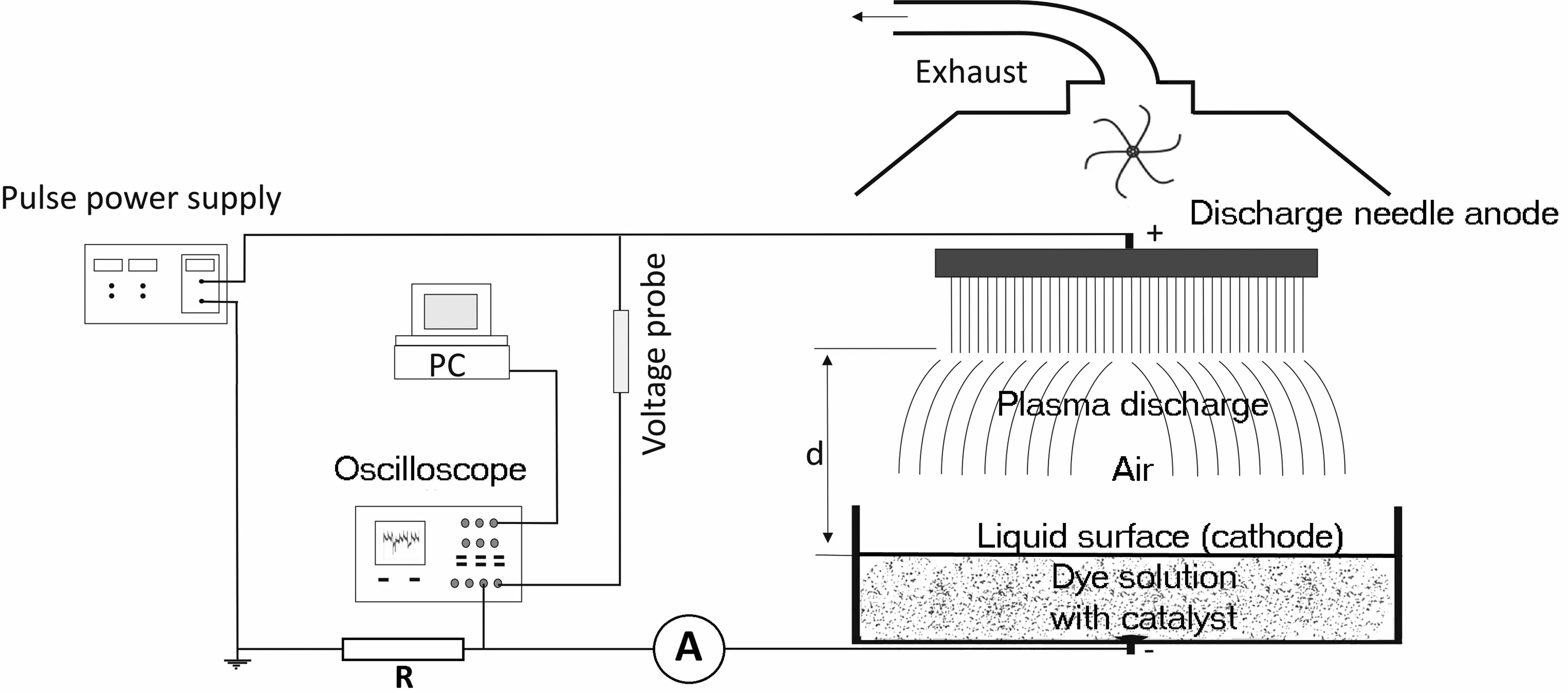
Figure 11.Decolorization rate in model polluted river water [C0 (RB 19) = 50 mg·dm-3,U = 13.3 kV,C (Bi2O3) = 500 mg·dm-3].
Electrodeposited microcrystalline α-Bi2O3catalyst enhanced the degradation of RB 19 reactive dye by a non-thermal atmospheric-pressure positive pulsating corona discharge.The major dye-degrading reagent was ·OH radicals,which were produced by plasma decomposition of previously plasma-generated H2O2.The catalyst promoted decomposition of H2O2into ·OH radicals.The excitation of the catalyst was mostly caused by strikes of high-energy reactive species generated by a corona discharge in air and accelerated by the strong electric field in the discharge gap; the streamers reached the liquid surface and generating fast electrons with an energy of up to 15 eV.Those strikes created e-/h+pairs that further reacted with H2O2,forming ·OH radicals.Decolorization reactions followed pseudo first-order kinetics.Production of H2O2,and consequently the rate of dye degradation,increased with increase in the input voltage.The optimal catalyst dose was 500 mg·dm-3.The energy yield for 50% dye decolorization,as well as the degree of mineralization,was improved by using a catalyst.Decolorization in the model river water proceeded somewhat more slowly than in deionized water due to the presence of other substances dissolved in river water which reacted with the·OH radicals.The findings concerning the interactions in the complex plasma-air-water-catalyst-dye system enable further exploration and improvements of the system’s efficiency.
Data availability statement
Data are available within the article.
Acknowledgment
The authors would like to thank the professor of English,Sandra Vaskovi?,who proofread the paper.The authors would like to acknowledge financial support from the Ministry of Education,Science and Technological Development of the Republic of Serbia (No.451-03-47/2023-01/200124)
 Plasma Science and Technology2024年2期
Plasma Science and Technology2024年2期
- Plasma Science and Technology的其它文章
- Detection of Al,Mg,Ca,and Zn in copper slag by LIBS combined with calibration curve and PLSR methods
- Experimental study on the effect of H2O and O2 on the degradation of SF6 by pulsed dielectric barrier discharge
- Phase field model for electric-thermal coupled discharge breakdown of polyimide nanocomposites under high frequency electrical stress
- Airfoil friction drag reduction based on grid-type and super-dense array plasma actuators
- Characteristics of laser-induced breakdown spectroscopy of liquid slag
- The characteristics of negative corona discharge and radio interference at different altitudes based on coaxial wire-cylinder gap
