Experimental clearance rate and intraguild predation of jellyfish Cyanea nozakii*
Pengpeng WANG, Fang ZHANG,**, Song SUN, Shuguo Lü
1 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Sciences, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Jiaozhou Bay Marine Ecosystem Research Station, Chinese Academy of Sciences, Qingdao 266071, China
5 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
6 Institute of Marine Ecology and Environment, Hainan Academy of Environmental Sciences, Haikou 570100, China
Abstract Cyanea nozakii, a common jellyfish distributed in offshore China, has a complex trophic relationship with other zooplankton groups.However, few studies have reported the predation rates and prey selection patterns of C.nozakii medusae on different prey items.Research is also lacking on the intraguild predation of Aurelia coerulea (another common bloom jellyfish in offshore China) by C.nozakii.To address the knowledge gaps, the clearance rates of C.nozakii for different prey items,including copepods (small<1 000 μm and large>1 000 μm), fish larvae, and gelatinous prey (hydromedusae, A.coerulea ephyrae, and chaetognaths), were measured.The influence of predator size on the clearance rate was also determined.Additionally, we examined the intraguild predation of C.nozakii on A.coerulea medusae.The clearance rates of C.nozakii varied widely with prey organisms, being independent of prey concentrations.Gelatinous organisms, except for chaetognaths, were captured with considerably high efficiency, followed by fish larvae and copepods, indicating the preferential prey selection of gelatinous organisms by C.nozakii.The clearance rate increased linearly with the cross-sectional area of C.nozakii.Body size in medusae may, to some extents, underpin their capacity to capture more prey by increasing the encounter rate and capture success through ontogeny.C.nozakii preyed voraciously on A.coerulea in high feeding efficiency, but the clearance rate decreased with increasing A.coerulea (as prey) size.This phenomenon of intraguild predation suggests a speculative hypothesis of potential population regulation of A.coerulea by C.nozakii.The information regarding the feeding ecology of C.nozakii reported in this study is important for understanding plankton dynamics in marine ecosystems with extensive occurrences of this jellyfish.Keyword: Scyphomedusae; predation rate; gelatinous organisms; prey selection; feeding mechanism
1 INTRODUCTION
In recent decades, jellyfish blooms have been reported worldwide; these gelatinous plankton are usually considered as voracious predators with high feeding efficiency (Purcell and Arai, 2001; Dong et al., 2010; Condon et al., 2013; Sun et al., 2015).From the perspective of top-down control, it has been suggested that predation by jellyfish can directly or indirectly decimate populations of other zooplanktonic and ichthyoplanktonic organisms(Olesen, 1995; Purcell, 2003; Uye, 2011; Wang et al.,2020b), and numerous studies have shown that jellyfish blooms are responsible for changes in the structure of marine ecosystems (M?ller, 1980; M?ller and Riisg?rd, 2007; Uye, 2011).
Cyaneanozakiiis a common bloom-forming jellyfish in the coastal waters of China.This jellyfish is distributed mainly in the inshore areas of the East China Sea and Bohai Sea (Xian et al., 2005;Dong et al., 2010, 2013; Zhang et al., 2012) and may have a complex trophic relationship with other zooplankton groups (Wang et al., 2020a).Previous studies have indicated that an increase in the density ofC.nozakiimay cause ecological disasters and imbalances in marine food webs (Yan et al., 2004;Zhong et al., 2004).Therefore, an underlying knowledge of its feeding ecology, such as the predation rates and prey selection patterns ofC.nozakiimedusae, is needed.
Previous studies demonstrated that jellyfish feeding is characterized by selectivity (Ford et al.,1997; Purcell, 1997; Suchman and Sullivan, 2000).Many factors, such as swimming speed and escape ability of prey items, predator size, tentacle length and spacing, swimming behavior, and nematocyst types concerning penetration of prey with different vulnerability to toxins (Ford et al., 1997; Purcell,1997; Sullivan et al., 1997; Suchman and Sullivan,2000; Riisg?rd and Madsen, 2011), affect jellyfish prey selection.The feeding ofCyaneamedusae depends mainly on “random” movements of zooplankton to bring prey into the reach of their tentacles (Fraser, 1969; Madin, 1988); these tentacles can adjust the reach and contraction to capture prey (Wang et al., 2019).Thus, the feeding ofCyaneamedusae depends on prey availability,and the predominance of alternative prey can affect their diets.
Based on laboratory feeding experiments(Fancett, 1988; Suchman and Sullivan, 2000;Titelman et al., 2007; Hosia and Titelman, 2011) and gut content analysis of specimens (Arai, 1997;Purcell, 2003), researchers have proven that copepods,cladocerans, bivalves, and ichthyoplankton all could be captured byCyaneamedusae.In addition, several studies have reported the intraguild predation byCyaneamedusae, indicating that other gelatinous organisms, e.g.,Aureliaspp.,Nemopilemanomurai,and hydromedusae, could be preys ofCyaneapopulations (Purcell, 1991; B?mstedt et al., 1994;Titelman et al., 2007; Wang et al., 2020a).These studies indicated a highly diverse prey spectrum ofCyaneapopulation.However, few studies have reported information about the clearance rates and prey selection patterns ofC.nozakiion different prey items (e.g., copepods, fish, and other gelatinous organisms).Moreover, bloom of another common jellyfish,Aureliacoerulea, has been increasing in coastal waters of China (Wan and Zhang, 2012;Wang et al., 2020b).However, data on the intraguild predation ofC.nozakiion this jellyfish species are lacking.
To understand the prey selectivity patterns and intraguild predation ofC.nozakiiand to enrich knowledge of trophic role ofC.nozakiipopulation in planktonic food web, the clearance rates ofC.nozakiion different prey, including small copepods(<1 000 μm), large copepods (>1 000 μm), fish larvae, hydromedusae,A.coeruleaephyrae, and chaetognaths (gelatinous prey), were measured, and the effect of medusa size onC.nozakiipredation was determined.Finally, the intraguild predation ofC.nozakiion different-sizedA.coerulea(as prey)was analyzed.
2 MATERIAL AND METHOD
2.1 Experimental animal
The experimental animals includedC.nozakii(as predator) and different prey items (small copepods (<1 000 μm), large copepods (>1 000 μm),fish larvae,A.coerulea, hydromedusae, and chaetognaths).A.coeruleaand fish larvae were cultured in the laboratory in the Institute of Oceanology, Chinese Academy of Sciences, Qingdao,China;C.nozakiimedusae, copepods, hydromedusae,and chaetognaths were collected in the Jiaozhou Bay(Fig.1).

Fig.1 Map of the Jiaozhou Bay
2.1.1 Predator
Cyaneanozakiimedusae were collected in Jiaozhou Bay, China, in June 2017 using a longhandled sieve equipped with a large bucket to prevent damaging the jellyfish.Samples were immediately transported to the laboratory, cultivated in 150-L cylindrical glass aquariums (120 cm cross, 15 cm high) with an installed cyclic water system and fed withArtemianauplii twice a day.The temperature and salinity of the filtered seawater were maintained at 18-20 °C and salinity of 31, respectively.To fully adapt to the laboratory environment,C.nozakiiwere acclimated in the laboratory approximately one week before the feeding experiments.
2.1.2 Prey item
Aureliacoeruleaephyrae and medusae were cultivated in the laboratory.After ephyrae were released from polyps, they were transferred to a cylindrical glass aquarium (120 cm cross, 15 cm high) with filtered seawater and offeredArtemianauplii twice a day.Different-sizedA.coeruleamedusae were offered as prey for the feeding experiments.
Zooplankton organisms, including small copepods(<1 000 μm), large copepods (>1 000 μm),hydromedusae, and chaetognaths, were collected using a plankton net (mesh size: 160 μm; mouth:0.08 m2) in Jiaozhou Bay in June 2017.The most abundant species in each taxonomic group are shown in Table 1.The captured zooplankton were immediately transported to the laboratory and then held in plastic buckets containing 15-L filtered seawater (20-μm mesh) with an air pump.To ensure an adequate supply of experimental prey, fishScophthalmusmaximus, which can be artificially propagated, was selected to perform the feeding experiment.S.maximusindividuals used were obtained from Jiaozhou Farm, Qingdao, China.To raise fish larvae, we incubated fertilized fish eggs in filtered seawater at 18-19 °C and salinity of 31.After hatching, fish larvae were incubated in a tankwith 1 000 L of filtered seawater (20-μm mesh)equipped with an air pump; they were offered rotifers (Brachionusplicatilis) daily.Approximately one-third of the tank water was renewed daily, and dead larvae were removed.About one week later,active swimming fish larvae were obtained (Table 2)for the feeding experiments.

Table 1 Zooplankton organisms used as prey in the feeding experiments (showing most abundant species in each taxonomic group)
2.2 Clearance experiment
Four clearance experiments were conducted to investigate (1) the relationships between prey concentrations and clearance rates (small copepods,fish larvae, and chaetognaths were offered as prey)(Table 2, #1-3); (2) the clearance rates of different prey items (small copepods, large copepods, fish larvae,A.coeruleaephyrae, hydromedusae, and chaetognaths) (Table 2, #4-9); (3) the effect of predator size (diameters ofC.nozakiiranged from 2.2±0.2 cm to 14.0±1.1 cm) on the clearance rate;the cross-sectional area ofC.nozakii(A, cm2) was used to represent the predator size, which was defined asπmultiplied by the square of bell diameter because of round medusae body; small copepods, fish larvae, and chaetognaths were offered as prey (Table 2, #10-12); and (4) the clearance rates of different sizedA.coerulea(mean diameter ranging from 2.8±1.0 mm to 26.0±5.5 mm; interrhopalia diameter for ephyrae) were measured to estimate the intraguild predation interactions betweenC.nozakiiandA.coerulea(Table 2, #7 and #13-15).
Cyaneanozakiimedusae that were visually undamaged and actively pulsing were used in the clearance experiments.Selected medusae were starved for 24 h prior to experiments and then transferred to experimental containers (30-cm diameter, 30-cm height) with 20-L filtered seawater(20-μm mesh).OneC.nozakiimedusa was placed in each experimental container.All jellyfish were acclimatized to the experimental containers for at least 1 hour under observation to ensure that their free-swimming behavior was normal.The bell diameter ofC.nozakiimedusae (cm) was measured before the feeding experiments.
To determine the exact number of each prey,copepods were collected using a pipette; large drops of water with prey from cultivating bucket were placed into a Petri disc, and the number of copepods in each drop was counted.Fish larvae and chaetognaths were individually sorted out by a shorthandled sieve (mesh size: 0.5 mm) and transferred into a 2-L bucket.A.coeruleaephyrae and medusae and hydromedusae were individually counted bygently scooping them into a 2-L bucket.

Table 2 Experimental conditions for clearance rates
The clearance rate was obtained by measuring the volume of water cleared of prey items per unit time.The clearance experiments were started by carefully adding the prey items to theC.nozakii(predator) containers.The feeding time should be from the beginning of the experiment until the prey was completely cleared, but the maximum experimental duration was 24 h.Over time, the number of prey gradually declined, and the reduction in the number of prey items as a function of time was followed by removing the jellyfish from one container at periods of time intervals and filtering (20-μm mesh) through all the water (i.e., data for each container represents one time point).The retained prey organisms were counted under a stereomicroscope (Nikon, SMZ745).Because the clearance ofC.nozakiivaried according to prey items, the feeding time of different prey varied between 2 h and 24 h, and sampling frequencies depended on the rate at which prey organisms were removed from the water by the predation ofC.nozakiimedusae.
The concentrations of prey organisms were determined according to the densities of different zooplankton communities in the field.Concentrations (100 small copepods/L,60 fish larvae/L,60 chaetognaths/L, as shown in Table 2) used to study the relationship between prey concentration and clearance rate were higher than the average density of populations in the natural environment to demonstrate thatC.nozakiiwere not saturated at these high prey densities (Wang et al., 2020b).Each clearance experiment was performed in triplicate.For each prey type, an aquarium without jellyfish served as a control.All experiments were conducted at 18-20 °C and salinity of 31 with filtered sea water(20-μm filter).Details of the specific conditions for each experiment are summarized in Table 2.
The individual clearance rate (Cl, L/h) was determined from the exponential reduction in prey concentration from the formula:
whereais the slope of the fitted regression line in a plot of lnCtversus time;Ct(inds./L) is the prey concentration at timet;nis the number of predators in each experimental aquarium, and in the present study,n=1 (oneC.nozakiiin each aquarium); andVis the volume of seawater in an aquarium (20 L).
2.3 Capture success ratio of different sized C.nozakii
The capture success ratio (CSR; %) ofC.nozakiiwith different diameters (ranging from 2.3±0.4 cm to 14.1±0.6 cm) was measured to examine the predation ability of different sizedC.nozakiimedusae.Small copepods, the dominant zooplankton organism in this study area (Wang et al., 2021) were used as the experimental prey.The successfully captured copepods were divided by the total subjects after encounters occurred betweenC.nozakiiand copepods to determine the CSR.To conduct these experiments, 20 small copepods were randomly selected with a dropper (230 mm long, 5 mm cross).Then, we gently kneaded the head of the dropper so that copepods were downstream of the water in the dropper, ensuring that every copepod could touchC.nozakiiindividuals (e.g., oral arms, bell, or tentacles).In this process, the speed of water flow was as slow as possible to reduce the impact of changes in experimenter pressure on the dropper on the number of copepods released.The number of copepods successfully captured byC.nozakiiwas recorded.Before these experiments, theC.nozakiimedusae used for CSR experiments were starved for 24 h, and five medusae with similar diameters were measured in each experiment.
2.4 The relationship between swimming speed of different prey and clearance rate
To better understand the influence of prey swimming speed (mm/s) on predation byC.nozakii,the relationships of cruising speed and bursting speed (escape speed) of different prey items (small copepods, large copepods, fish larvae,Aurelia aurita, hydromedusae, and chaetognaths) with the clearance rate were analyzed.In this study, we did not directly measure the cruising speed and bursting speed of prey but referred to the values determined in previous studies (Table 3).
2.5 Statistical analysis
OriginPro 8.0 was used to arrange and analyze the data.SPSS v16.0 was used for statistical analysis of the processed data.The relationship between clearance rate and prey concentration was analyzed by one-way ANOVA; Kolmogorov-Smirnov and Levene tests were used to analyze normality and equality of variance, respectively, prior to ANOVA.The difference in clearance rates between different experimental groups was analyzed using the nonparametric Kruskal-Wallis test.Spearman correlation analysis was used to analyze the relationship between clearance rate and prey swimming speed.Statistical significance was set atP<0.05.
3 RESULT
3.1 Clearance experiment
3.1.1 Relationship between clearance rate and prey concentration
Small copepods, fish larvae, and chaetognaths were provided as prey at different concentrations to measure the relationship between the clearance rate and prey concentration.One-way ANOVA indicated that clearance rates were not significantly different among the various prey concentrations, regardless of whether copepods, fish or chaetognaths were offered as prey (one-way ANOVA, small copepods:F=1.408, df=4,P=0.305; fish larvae:F=0.632, df=4,P=0.651; chaetognaths:F=0.348, df=4,P=0.840)(Fig.2).
3.1.2 Clearance rates of different prey items
The clearance rates of the different prey items byC.nozakiiindividuals of the same size were measured (Table 2).The results indicate that prey type has a significant effect on the clearance rate.For the prey with no difference in size, the clearance rates ofA.coeruleaephyrae and hydromedusae were significantly higher than those of fish larvae and copepods (H=3.857,P<0.001).The clearance rates of the fish larvae were significantly higher than those of the small and large copepods (H=7.200,P=0.027).However, the clearance rate of the chaetognaths was only 0.31±0.07 L/h, which is significantly lower than that of the other gelatinous prey (A.coeruleaephyrae and hydromedusae) (H=6.253,P=0.012;Fig.3).

Table 3 Cruising speed and bursting speed of different prey items

Fig.2 Clearance rates as a function of prey concentrations
3.1.3 Effect of predator size on clearance rate
Predator size also has a significant effect on the clearance rate.The estimated mean clearance rates of the small copepods increased from 0.20±0.12 L/h for 2.2-cmC.nozakiito 3.80±0.29 L/h for 12-cmC.nozakii.The clearance rates of the fish larvae increased from 0.11±0.02 L/h for 2.2-cmC.nozakiito 5.10±0.48 L/h for 14-cmC.nozakii.The clearance rates of the chaetognaths increased from 0.05±0.02 L/h for 4.5-cmC.nozakiito 2.10±0.48 L/h for 14-cmC.nozakii.Based on the linear regression,the clearance rate linearly increased with increase in the cross-sectional area: Cl=0.032A+0.002 for small copepods (Cl: clearance rate (L/h),A: the crosssectional area ofC.nozakii(cm2);F=240.133, df=4,P<0.001,R2=0.984); Cl=0.038A-0.025 for fish larvae (F=57.109, df=4,P=0.005,R2=0.933); Cl=0.017A-0.211 for chaetognaths (F=78.185, df=3,P=0.013,R2=0.963) (Fig.4).

Fig.3 Clearance rates of C.nozakii feeding on different prey organisms
3.2 Capture success ratio experiments
The CSRs ofC.nozakiimedusae with different sizes were measured, and small copepods were offered as prey.We found that the CSR significantly increased withC.nozakiidiameter (H=17.015, df=3,P=0.001), and the mean CSR for small copepods increased from 10%±8% for 2.2-cmC.nozakiito 62%±8% for 14-cmC.nozakii(Fig.5).
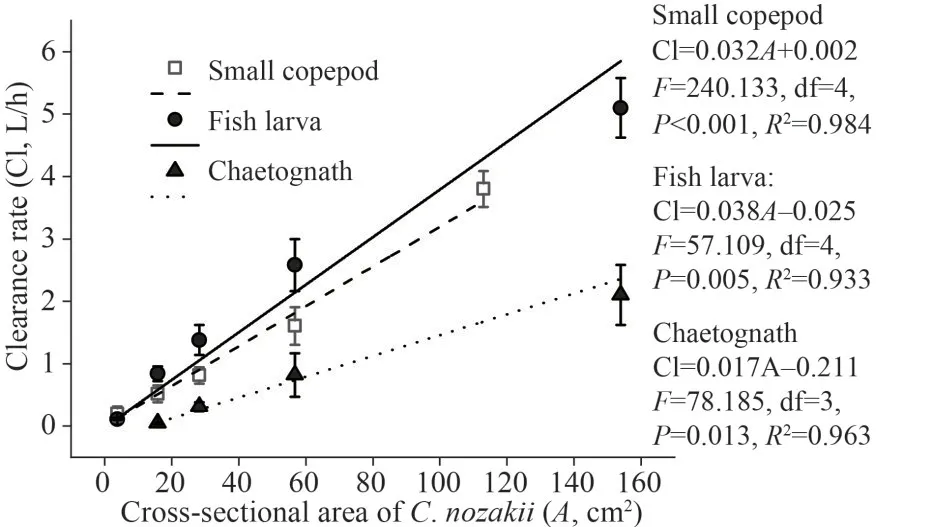
Fig.4 Relationship between the clearance rates (Cl, L/h)and cross-sectional area of C.nozakii (A, cm2) with small copepods, fish larvae, and chaetognaths as prey

Fig.5 Capture success ratio (CSR, %) of different sized C.nozakii with small copepods offered as prey
3.3 The relationship between swimming speed and clearance rate of prey
The relationships between both cruising speed and bursting speed of the different prey items(referring to previous studies; Table 3) and clearance rate (Fig.3) were measured by Spearman analysis.The bursting speed and clearance rate showed a clear inverse association, with the clearance rate ofC.nozakiibeing obviously slow for the prey with a relatively high bursting speed.The results of Spearman correlation analysis indicate that there was a significantly negative correlation between clearance rate and bursting speed (R=-0.898,n=5,P=0.039) (Fig.6).
3.4 Intraguild predation of C.nozakii on A.coerulea
The mean clearance rate ofC.nozakii(diameter:6.1±0.5 cm) varied according toA.coeruleasize.Specifically, the mean clearance rate decreased from 5.81±0.66 L/h for 2.8-mmA.coeruleaephyrae to 0.57±0.46 L/h for 26.0-mm young medusae indicating that the feeding efficiency ofC.nozakiionA.coeruleadecreased with prey size (Fig.7).We found thatC.nozakiicould largely catch and ingest largerA.coeruleamedusae compared to those with their own diameter.In one observation, four to fiveA.coeruleamedusae with a diameter of 9.2±1.0 cm were captured by oneC.nozakiimedusae (diameter: 8.5 cm; Fig.8).Semi-digestedA.coeruleawere often released when the fresh one was encountered.
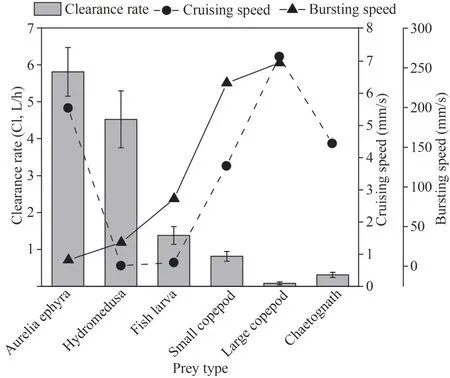
Fig.6 The relationship between both cruising speed (mm/s)and bursting speed (mm/s) of different prey items and clearance rate (L/h)

Fig.7 Clearance rates of C.nozakii for A.coerulea medusae (as prey) of different sizes
4 DISCUSSION
4.1 Predation of C.nozakii on different prey items
Jellyfish are selective feeding predators (Purcell,1992; Ford et al., 1997; Titelman and Hansson,2006; Wang et al., 2020b).Prey selection can be defined as the availability of prey types in the environment (Ivlev, 1961).The relative vulnerability of a particular prey to a predator is considered the product of two separate probabilities: the probability of an encounter between a predator and prey and that of successful prey capture after the encounter occurred (Fulton and Wear, 1985; Greene et al.,1986; Purcell, 1997).Therefore, the features of predator-prey interactions account for different prey vulnerability levels, as indicated by Greene et al.(1986).In this study, copepods, fish larvae, and gelatinous zooplankton (hydromedusae,A.coerulea,and chaetognaths) were provided as foods to determine the clearance rates ofC.nozakiion different prey organisms.Our results indicate thatC.nozakiipredominantly preyed on gelatinous zooplankton, exclusive of chaetognaths, followed by fish larvae and copepods (Fig.3), which might suggest a feeding preference ofC.nozakiimedusae for those gelatinous prey.

Fig.8 Intraguild predation between C.nozakii (as predator) and A.coerulea (as prey)
The variation in clearance rates ofC.nozakiifor different prey items might be determined by both encounters betweenC.nozakiiand prey items and capture success after the encounters (mentioned above).C.nozakii, a species with long trailing tentacles, is considered as an ambush predator based on its prey being selected randomly based on contact with its outstretched tentacles (Madin, 1988;Wang et al., 2019).These jellyfish usually capture their prey by entangling or adhering prey in tentacles; then, the captured prey is transferred to the oral arm and gut (observed in our feeding experiments).However, whenC.nozakiiextended their tentacles in water, the spacing between tentacles was much greater than the diameters of prey items.Hence, the chance of direct interception of prey by a tentacle depends on the swimming characteristics of the prey items (Greene et al.,1986; Madin, 1988; Purcell, 1997; Suchman and Sullivan, 2000).This scenario suggests that the prey with faster cruising speeds would generally be highly selected by tentaculate predators because prey with greater movement would be expected to encounter tentacles more frequently (Purcell, 1997).However, a nonsignificant positive correlation between clearance rate and cruising speed was found in this study.In contrast, there was a significant negative correlation between the clearance rate and prey bursting speed (Fig.6), indicating that the escape ability of prey organisms is important in determining the predation selection ofC.nozakii(Fancett, 1988; Costello and Colin, 1994; Purcell and Arai, 2001).
Copepods have a characteristic escape jump(Hartline et al., 1996); thus, their bursting and vigorous behaviors represent important adaptations for escaping predation and may play an important role in the selective feeding of planktivores(Browman et al., 1989; Buskey et al., 2002; Waggett and Buskey, 2008).This characteristic may be a reasonable explanation for the lower clearance rate ofC.nozakiifor copepods found in this study.Conversely,A.coeruleaephyrae and hydromedusae were measured as being cleared at a relatively high rate byC.nozakii, followed by fish larvae (Fig.3).These results are consistent with those of previous studies (Table 4).For example, Purcell and Arai(2001) indicated that soft-bodied organisms (larvaceans,hydromedusae, and ctenophore) are favorable preyofCyanea, as they are easier to be captured due to lack of the ability to effectively escape (Purcell,1991, 2003).In addition, Fancett (1988) suggested thatCyaneacapillatapreferentially prey upon fish eggs and larvae, whereas copepods were least preferred.For the same sized fish and copepods, the relatively high feeding efficiency on fish mainly resulted from their poor escape ability (Purcell and Arai, 2001).Additionally, we found a lower clearance rate for chaetognath (Fig.3).The “jumping”chaetognath provoked an escape jump away from the predator when an encounter occurred withC.nozakii, as we observed.Therefore, we concluded that even if encounters between copepods, chaetognath(as prey), andC.nozakiioccurred, the stronger escape ability of these prey items, compared to fish larvae,hydromedusae, andA.coerulea, caused the relatively low predation efficiency ofC.nozakiion copepods.
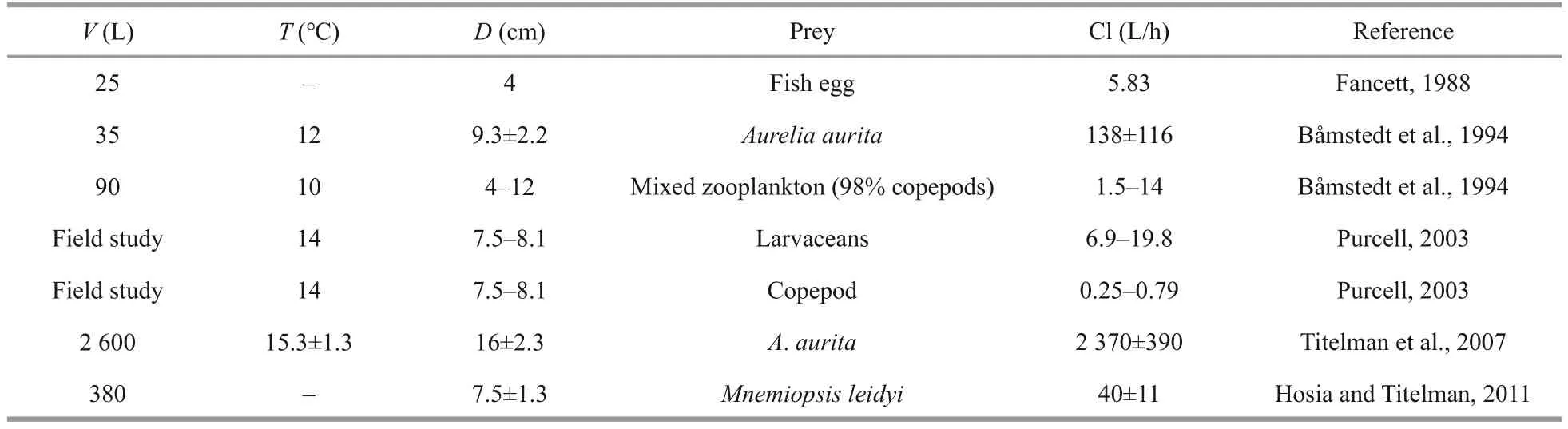
Table 4 Clearance rates (Cl) of Cyanea on different zooplankton organisms reported in previous studies
4.2 The effect of medusae size on predation
Jellyfish predation strongly depends on their size,as indicated by previous studies (Bailey and Battey,1983; Olesen, 1995; Titelman and Hansson, 2006;Wang et al., 2020b).In this study, clearance rates linearly increased with the cross-sectional area ofC.nozakiimedusae with small copepods, fish larvae,and chaetognaths as prey (Fig.4).The stronger predation ability of large medusae may derive from both the relatively high probability of encounters between predator and prey and the great capture success after the encounters occurred.
To study encounters between predator and prey,Madin (1988) proposed the concept of an “encounter zone”—the space around a predator in which the tentacles may be sensed.This concept emphasizes the region into which the predator’s tentacles can extend when medusae conduct capture activities;jellyfish can only capture prey items in this zone.The size of the encounter region is determined by the length, extension, and contraction of jellyfish tentacles (Gerritsen and Strickler, 1977; Madin,1988).C.nozakiigrow more and longer tentacles with increasing diameter, suggesting that largerC.nozakiiindividual generates a greater encounter region, allowing it to scan greater volumes of prey(Suchman and Sullivan, 2000; Titelman et al., 2007;Wang et al., 2019).Moreover, more numerous tentacles in an encounter region would reduce the average distance between adjacent tentacles (Madin,1988), which could also increase the encounter rate of medusae with prey.
Although the probability of prey capture success within an encounter region depends on several morphological and behavioral factors of prey, as indicated by several previous studies (Purcell, 1991;Suchman and Sullivan, 2000; Waggett and Buskey,2008), nematocyst characteristics should also be considered in a conceptual model of prey selection and capture by tentaculate feeders (Madin, 1988;Purcell, 1997), as the structures directly responsible for prey capture are the nematocysts in the tentacles(Purcell, 1984).Previous studies have indicated that in comparison to large specimens, small specimens of jellyfish species have fewer and somewhat smaller nematocysts (Purcell, 1984).There are also size-related differences in the toxicity ofCyanea(Helmholz et al., 2007), and ?stman and Hydman(1997) indicated that asCyaneamedusae increase in size, their nematocysts also increase, implying a stronger ability to adhere to prey with increasing medusa size.Although the nematocyst characteristics ofC.nozakiiwere not addressed in this study, our CSR experiments seemed to confirm this speculation.The CSRs for small copepods increased from 10%±8%for 2.2-cmC.nozakiito 62%±8% for 14-cmC.nozakii(Fig.5), which indicated that more prey could be successfully captured by largeC.nozakiiafter encounters occurred.The stronger ability to adhere to prey allowed larger jellyfish to capture more and larger prey.
In summary, the body size of medusae may, to some extents, underpin their capacity to capture more and larger prey by increasing the encounter rate and capture success through ontogeny.
4.3 Intraguild predation of C.nozakii
Intraguild predation is a general interaction among jellyfish populations.This trophic interaction commonly occurs between two taxa,Cyanea(as predator) andAurelia(as prey), as has been reported based on both laboratory experiments and field observations (B?mstedt et al., 1994; Hansson, 1997;Titelman et al., 2007).However, few studies have reported the intraguild predation interactions betweenC.nozakiiandA.coeruleain offshore China.In the present study, we measured the predation ofC.nozakiionA.coerulea, and the results indicate that the clearance rates ofC.nozakiidecreased with increasingA.coeruleadiameter (Fig.7).A similar result was reported by B?mstedt et al.(1994), who suggested that the capture success ofA.auritabyC.capillataimproved with increasing predator-to-prey size ratios.Moreover,C.nozakiicould catch and ingestA.coeruleamedusae larger than their own diameter.B?mstedt et al.(1994) showed thatC.capillatawith an 11.5-cm diameter could captureA.auritawith a 26-cm diameter in the laboratory, andC.capillatawith an 11.3-cm diameter could catchA.auritaprey with a 20-cm diameter in field studies.Here, we found thatC.nozakiiwith an 8.5-cm diameter could prey on a maximum of four to five individuals ofA.coeruleawith a diameter of 9.2±1.0 cm at once (Fig.8a).This result indicates that oneC.nozakiimedusa could capture an amount ofA.coeruleacorresponding to more than four times its own body weight (according to the relationships between diameter (cm) and wet weight (g) forAureliaandCyaneareported by B?mstedt et al.(1994) and Zhang et al.(2012), respectively).C.nozakiivoraciously preyed onA.coeruleaand captured moreA.coeruleathan needed.Indeed, we observed that semidigestedA.coeruleawere often released when freshA.coeruleawas encountered.SmallerA.coeruleamedusae or ephyrae were swallowed whole byC.nozakii, whereas consumption ofA.coeruleawith a large diameter was first observed where the tissue of the prey was thinnest, i.e., the margin of the umbrella and the tips of the oral arms (Fig.8c-d).
The potential trophic links betweenCyaneaand other gelatinous zooplankton, such as ctenophores,hydromedusae, andN.nomurai(scyphozoan), have also been reported by previous studies (B?mstedt et al., 1997; Liu et al., 2015; Wang et al., 2020a).Gelatinous organisms are considered an essential food source for the growth ofCyanea(B?mstedt et al., 1997; Hansson, 1997; Wang et al., 2020a).For example, based on the results of stable isotope analysis, Wang et al.(2020a) indicated that the mean proportions of gelatinous prey (includingN.nomuraiand small medusae) in theC.nozakiidiet increased significantly with increasing diameter,suggesting that more gelatinous prey were consumed byC.nozakiiduring growth.B?mstedt et al.(1997)reported that the ephyrae ofC.capillatadid not grow when fed only zooplankton dominated byArtemiaor copepods but grew well with a ctenophore as food.B?mstedt et al.(1994) and Hansson (1997) both speculated thatA.auritamight be a key source of food for theC.capillatapopulation.Even though gelatinous organisms are considered a dilute prey source with high water contents and low carbon contents (Schneider, 1992),theCyaneapopulation may need gelatinous prey obtained by intraguild predation to support their growth and development.
In fact, the role of gelatinous zooplankton in the development and growth of theCyaneapopulation has yet to be determined.However, we confirmed thatC.nozakiicapture gelatinous organisms (e.g.,A.coeruleaand hydromedusae) with a relatively high clearance rate based on the results of this study.This finding highlights that gelatinous zooplankton are preferred by the grazingC.nozakiipopulation.Previous studies have indicated that large aggregations ofAureliacan seriously deplete zooplankton and ichthyoplankton populations by direct predation and indirect competition for prey sources (Purcell, 1997;Ishii and Tanaka, 2001; Wang et al., 2020b).Thus,Cyaneapopulation blooms might release predation pressure on zooplankton organisms generated by the capture ofAureliaspecies (B?mstedt et al., 1994;Titelman et al., 2007).In conclusion, the results of intraguild predation presented in this study emphasize the importance of assessing the substantial predation ofC.nozakiionA.coerulea, especially in trophic analyses of food webs where these two populations co-occur.In addition, these trophic relationships betweenCyaneaand other gelatinous zooplankton may be essential in regulating planktonic populations.
5 CONCLUSION
Prey type and predator size both have obvious influences on clearance rate.C.nozakiicaptured gelatinous prey, exclusive of chaetognaths, with high efficiency, followed by fish larvae and copepods.Larger medusae have stronger predation capability,which might originate from both the relatively high probability of encountering prey and the great capture success after the encounter happened.Additionally, the voracious predation ofC.nozakiionA.coerulea, implies the potential population regulation of the latter by the former.
6 DATA AVAILABILITY STATEMENT
The authors declare that the data supporting the findings of this study are available within the article.
7 ACKNOWLEDGMENT
We thank the captain and crew of the R/VHaiou.We are grateful to the anonymous reviewers and the editor for their valuable comments.
 Journal of Oceanology and Limnology2024年1期
Journal of Oceanology and Limnology2024年1期
- Journal of Oceanology and Limnology的其它文章
- Lagrangian coherent structure analysis on transport of Acetes chinensis along coast of Lianyungang, China*
- Transcriptome analysis reveals immune-related genes in tissues of Vibrio anguillarum-infected turbot Scophthalmus maximus*
- Effects of Poria cocos polysaccharide on growth performance, physiological parameters, and lipid metabolism of spotted sea bass Lateolabrax maculatus*
- Production traits and fertility of reciprocal hybrids between Argopecten irradians irradians and A.i.concentricus*
- A new diatom genus Spargeria gen.nov.(Bacillariophyceae)from Xizang, China, and the description of two new species*
- Lineaperpetua gen.nov.: a new diatom genus in the Thalassiosirales supported by morphology and molecular data*
