Neutrophil extracellular traps mediate neuroimmunothrombosis
Jianbo Lou, Jianning Zhang,2, Quanjun Deng,*, Xin Chen,2,*
Abstract Neutrophil extracellular traps are primarily composed of DNA and histones and are released by neutrophils to promote inflammation and thrombosis when stimulated by various inflammatory reactions.Neutrophil extracellular trap formation occurs through lytic and non-lytic pathways that can be further classified by formation mechanisms.Histones, von Willebrand factor, fibrin, and many other factors participate in the interplay between inflammation and thrombosis.Neuroimmunothrombosis summarizes the intricate interplay between inflammation and thrombosis during neural development and the pathogenesis of neurological diseases, providing cutting-edge insights into post-neurotrauma thrombotic events.The blood-brain barrier defends the brain and spinal cord against external assaults, and neutrophil extracellular trap involvement in blood-brain barrier disruption and immunothrombosis contributes substantially to secondary injuries in neurological diseases.Further research is needed to understand how neutrophil extracellular traps promote blood-brain barrier disruption and immunothrombosis, but recent studies have demonstrated that neutrophil extracellular traps play a crucial role in immunothrombosis, and identified modulators of neuro-immunothrombosis.However, these neurological diseases occur in blood vessels, and the mechanisms are unclear by which neutrophil extracellular traps penetrate the blood-brain barrier to participate in immunothrombosis in traumatic brain injury.This review discusses the role of neutrophil extracellular traps in neuro-immunothrombosis and explores potential therapeutic interventions to modulate neutrophil extracellular traps that may reduce immunothrombosis and improve traumatic brain injury outcomes.
Key Words: inflammation; neuro-immunothrombosis; neurologic diseases; neurotrauma; neutrophil extracellular traps; platelet; thrombosis; traumatic brain injury
Introduction
Neutrophil extracellular traps (NETs) are net-like extrusions of genetic material generated by neutrophils.Neutrophils, the most common type of leukocytes in the blood, are the essential first line of defense of the innate immune system (Castanheira and Kubes, 2019).Neutrophils deploy NETs as a protective mechanism by expelling their genetic material to form web-like traps that catch pathogens and promote clotting (Silva et al., 2021; Schultz et al., 2022), though NETs can also trigger harmful inflammation in the absence of bacteria (Domer et al., 2021).Recently, in severe cases of coronavirus disease 2019 (COVID-19), improperly regulated NETs were found to generate inflammation and microvascular thrombosis (Zuo et al., 2020).More specifically, Al-Kuraishy et al.(2022) found that excessive NET development can cause acute lung injury, acute respiratory distress syndrome, and immunothrombosis, all of which indicate a poor COVID-19 prognosis.Recent studies of NETs have revealed their roles not only in inflammation and thrombosis but also in many other clinical conditions such as diabetes (Wong et al., 2015),systemic lupus erythematosus (Hakkim et al., 2010), pre-eclampsia (Gupta et al., 2006), and certain types of cancers (Demers et al., 2012), all of which are known to be associated with failures of NET regulation.
The formation of NETs, also known as NETosis, was originally thought to help neutrophils catch and kill bacteria (Brinkmann et al., 2004), fungi (Urban et al., 2006), and viruses (Saitoh et al., 2012), but NETosis may also be induced without infection, such as in autoimmunity, coagulation, acute injuries, and cancer (Jorch and Kubes, 2017).The relationship between inflammation and thrombosis is better understood since the discovery of NETs, which are key mediators of inflammation and thrombosis along with platelets, von Willebrand factor (vWF), and fibrin, and have been widely accepted as a mechanism of deep vein thrombosis.Inflammatory conditions increase the risk for venous thrombosis and may be associated with the formation of NETs, which can combine with and activate platelets during deep venous thrombosis (Fuchs et al., 2010) along with contributions from vWF (Moschonas and Tselepis, 2019).NETosis has been observed in the earliest stages of thrombus formation in patients with venous thromboembolism, suggesting that therapeutic intervention for NET formation at this stage may reverse thrombosis and improve prognosis (Savchenko et al., 2014).Bystrzycka et al.(2017) showed that azithromycin and chloramphenicol can modify neutrophil function without affecting degranulation and apoptosis, and that NET release is regulated by reactive oxygen species (ROS) production.During sepsis,neutrophils and NETs degrade the glycocalyx on the endothelial cell surface and increase endothelial permeability (Zhang et al., 2023), ultimately leading to coagulation dysfunction and thrombosis.In traditional Chinese medicine,forsythiaside B (He et al., 2022) and Liang-Ge (He et al., 2023) decoctions have a therapeutic effect in rats with sepsis and alleviate coagulopathies associated with sepsis; these substances act by inhibiting NETosis through the reduction of protein-arginine deiminase (PAD) 4 expression.Few studies have examined the influence of antibiotics on thrombosis via NET regulation or the role of PAD4 in such regulation.More research is needed to determine the possible link between antibiotics, NETs, and thrombosis.Anticoagulants and procoagulant interferents compromise bacterial capture by NETs (Massberg et al., 2010), and many other factors influence NET formation; interference with other physiological activities should be avoided when NETosis is regulated to prevent thrombosis and inflammation.We review these factors here to identify pathways that mediate inflammation and thrombosis.
The brain and spinal cord are protected from immune responses by the blood-brain barrier (BBB), but a damaged BBB and abnormally activated immune system allow the infiltration of inflammatory mediators that cause neuroinflammation of the central nervous system (CNS).Simultaneously,BBB destruction disrupts the balance between hemostasis and clotting in the CNS; BBB dysfunction after traumatic brain injury (TBI) drives thrombosis and inflammation (Hubbard et al., 2021).Neuroinflammation and thrombosis in the CNS are closely associated with NETosis (Li et al., 2022a; Ansari et al.,2023).The release of NETs is accompanied by reduced neovascularization and increased BBB damage after stroke, which can be remediated via structural disintegration of NETs by deoxyribonuclease (DNase) 1 and the inhibition of NETosis by genetic ablation or pharmacological inhibition of PAD (Kang et al., 2020).Neurological diseases are influenced by NETs, which could be targeted to control thrombosis without affecting hemostasis when managing secondary CNS injury.
This research clearly indicates that NETs are essential for both inflammation and thrombosis (Papayannopoulos, 2018), but more studies are needed on the mechanisms by which NETs mediate both inflammation and thrombosis (i.e., immunothrombosis).Outcomes in TBI may be related to immunothrombosis (Albert-Weissenberger et al., 2019), and the inhibition of immunothrombosis via NET regulation may improve TBI outcomes.
Retrieval Strategy
A computer-based online search of the PubMed database was performed to retrieve articles published through July 2023.A combination of the following text words (MeSH terms) was used to maximize search specificity and sensitivity: “neutrophil extracellular traps”; “NETs”; “inflammation”;“neuroinflammation”; “thrombosis”; “immunothrombosis”; “neurologic diseases”; “neurotrauma”; “traumatic brain injury”.The results were further screened by title and abstract, and only those studies exploring the relationship between NETs, immunothrombosis, and neurologic diseases were included; studies that discussed only immunothrombosis and neurologic disease (but not NETs) were excluded.No language or study type restrictions were applied.
The Origin of Neutrophil Extracellular Traps
NETs are released by neutrophils, the most abundant circulating leukocytes in humans.The release of NETs was first reported by Volker Brinkmann in 2004 (Brinkmann et al., 2004) and is now known as NETosis (Remijsen et al., 2011).NETosis can be triggered by bacteria, sterile activators (Branzk et al., 2014), or through the chain reaction of specific receptors including complement, antibodies, cytokines, and toll-like receptors (TLRs) (Clark et al., 2007).PAD4 is an arginine-citrullinated enzyme that enables histone citrullination, reduces interactions between histones and DNA, and promotes chromatin decondensation (Wang et al., 2009).Neutrophil elastase and other granule proteases can detach histones from DNA (Papayannopoulos et al.,2010).Patteson et al.(2019) found that loss of PAD4 delays the disassembly of lamins and vimentin, both of which are believed to maintain nuclear mechanical integrity.PAD4 might promote chromatin decondensation by ensuring lamin and vimentin disassembly to destabilize chromatin.Proteases and PAD4 are related to the cellular stimuli and species that induce NETosis,which is activated by innate immune receptors and their downstream intracellular mediators, including ROS produced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or mitochondria, which activate myeloperoxidase, neutrophil elastase, and PAD4 to promote chromatin decondensation (Papayannopoulos, 2018; Mutua and Gershwin, 2021).In acute ischemic stroke, Li et al.(2022a) concluded that NETosis may also be affected by the acid-base environment, oxygen concentration, and iron ions around the infarct.Neutrophil elastase and myeloperoxidase are proteases that contribute to the degradation of histones and the further development of chromatin, and are released by neutrophil granules and transported into the nucleus (Papayannopoulos et al., 2010).Overall, neutrophil elastase,PAD4, and myeloperoxidase are crucial for NETosis.
Changes in intracellular calcium levels are thought to play a role in NETosis,which triggers intracellular calcium influx (Alpizar et al., 2017; Gupta et al.,2018) that promotes the disassembly of microtubules, actin, and vimentin filaments, suggesting an important role for calcium in disrupting the nuclear and plasma membrane.Actin disassembly is essential for the plasma membrane rupture that enables NET release, so the induction of actin disassembly with small molecules could be therapeutically useful for NETosis inhibition (Thiam et al., 2020).Elevated cytosolic calcium levels activate both PAD4 and calpain in neutrophils, leading to nuclear decoagulation in classical NETosis (Gosswein et al., 2019).Therefore, further studies are required to determine whether regulating calcium concentrations and related proteins is an effective way to mediate NETosis.
NETosis is considered to occur through either lytic or non-lytic pathways(Table 1).Lytic, or suicidal, NETosis is a type of cell death characterized by chromatin decondensation and disintegration of the nuclear envelope, which is preceded by the loss of cellular polarization prior to plasma membrane rapture.This process differs from necrosis and apoptosis (Fuchs et al., 2007),though it is a form of programmed cell death (Jorgensen et al., 2017).These NETosis pathways can be further classified as NADPH oxidase-independent or NADPH oxidase-dependent (Rosazza et al., 2021), based on different requirements for ROS production via NADPH oxidase.During lytic NETosis,the plasma membrane undergoes significant changes, including microvesicle formation and shedding, and regulation of permeability.Microvesicles are important systemic messengers for conveying pressure signals (Hugel et al., 2005), contributing to disease (Genschmer et al., 2019), and promoting thrombosis (Hrachovinová et al., 2003).NETosis proceeds through a step-bystep sequence of intercellular interactions.Thiama et al.(2020) demonstrated that nuclear envelope rupture is critical for NETosis which begins with the rapid decomposition of the actin cytoskeleton, followed by the shedding of particles and cytoplasm via plasma membrane microvesicles, and chromatin decondensation and nuclear rounding.Finally, progressive plasma membrane and neutrophil elastase permeabilization allows the release of chromatin into the cytoplasm prior to discharge as extracellular chromatin (Thiam et al.,2020).
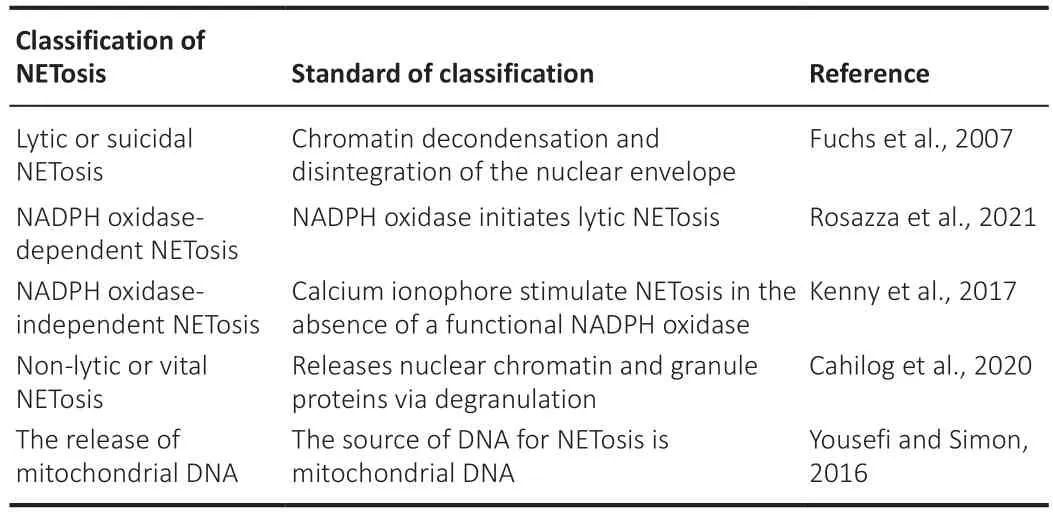
Table 1 |Classification of neutrophil extracellular traps (NETs)
Non-lytic, or vital, NETosis works independent of cellular death and releases nuclear chromatin and granule proteins via degranulation (Cahilog et al.,2020).Non-lytic NETosis contradicts known mechanisms and postulates that neutrophils can release NETs without plasma membrane destruction (Clark et al., 2007).After non-lytic NETosis, neutrophils continue to participate in chemotaxis, phagocytosis, and bacterial killing (Yipp et al., 2012).Konig and Andrade (2016) suggested that vital NETosis could be a side effect of abnormal calcium influx caused by toxins such as those produced byStaphylococcus aureus, but more research is required to determine whether there are more specific toxins that can cause vital NETosis.Pieterse et al.(2016)found that NETs released through vital NETosis have lower neutrophil elastase activity, indicating that they are less effective than suicidal NETs in killing bacteria.The release of mitochondrial DNA (mtDNA) may offer an alternative source of DNA for NETosis (Yousefi and Simon, 2016) that is independent of cellular death.mtDNA release is NADPH-dependent but does not require neutrophil lysis (Yousefi et al., 2009).However, Itagaki et al.(2015) observed that mtDNA can induce suicidal NETosis, indicating that mtDNA release does indeed disrupt phagocytosis by neutrophils.Therefore, identifying the source and specific functions of mtDNA is crucial.
The Components of Neutrophil Extracellular Traps and Their Roles in Immunothrombosis
NETs primarily comprise DNA, histones, and various enzymes; DNA is the essential structure that supports the other components.Purified DNA and RNA can bind to and activate proteins of the contact system, which work together to enhance thrombin generation and clot formation in plateletpoor plasma (Bhagirath et al., 2015; Vu et al., 2016).Other NET components,including histones and granule proteins, have also been implicated as activators of coagulation (McDonald et al., 2017).Purified histones can enhance thrombin generation in plasma via platelet-dependent (Semeraro et al., 2011) and platelet-independent mechanisms (Ammollo et al., 2011).NETs are increasingly recognized as procoagulant surfaces with the ability to promote thrombosis bothin vitroand in animal models of deep vein and arterial thrombosis (Martinod et al., 2013; Abdol Razak et al., 2017).
DNA as the bridge between inflammation and thrombosis
The DNA of NETs mainly originates from nuclear DNA; mtDNA has a different origin.Purified DNA impairs fibrinolysis by inhibiting plasmin-mediated fibrin degradation through the formation of complexes with plasmin and fibrin(Gould et al., 2015).Levels of cell-free DNA in plasma are an alternative marker for NETs and an elevated risk of thrombosis (Lopez et al., 2022).Cellfree DNA has also been shown to activate thrombin through the intrinsic coagulation pathway (Swystun et al., 2011), and NETs may modify this pathwayin vivo.More importantly, DNA serves as the reaction medium for several other processes.
In addition, DNase reduces NET release by degrading DNA (Li et al., 2022a).NET production during infection can promote cancer metastasis, which can be blocked by inhibiting NETosis or digesting NETs with DNase I (Park et al., 2016).Targeting NETs with DNase I may affect thrombus stability in the treatment of acute ischemic stroke, and thrombolysis in patients is more successfulin vitrowith DNase I added to standard tissue plasminogen activator (t-PA) (Laridan et al., 2017).Targeting NETs using recombinant human DNase reduces NET production, which has important therapeutic implications for COVID-19(Fisher et al., 2021).DNA digestion by DNase I and DNase1L3 is the ratelimiting factor for NET accumulation (Angeletti et al., 2021), so DNase can slow NETosis.However, Früh et al.(2021) reported that intravenous RNase significantly abrogates the NET burden in the brain parenchyma, and other nucleases may also contribute to NET production.
Histones and NETs
Histones, the major protein components of NETs, are considered a new class of damage-associated molecular patterns that damage organs via TLRs or direct epithelial and endothelial cell death after being released into the extracellular space (Huang et al., 2011; Allam et al., 2012).Histones are important forin vitroandin vivoinduction of NETosis (Shrestha et al., 2019),and recombinant thrombomodulin suppresses histone-induced NET release(Shimomura et al., 2016; Hayase et al., 2019).NET histones, particularly H3 and H4, send feedback to platelets to promote their recruitment and activation (Xu et al., 2009; Fuchs et al., 2010).Histones are primarily cytotoxic to nucleated cells because they promote platelet activation, resulting in thrombosis and thrombocytopenia (Kordbacheh et al., 2017), and H3 and H4 can directly induce the aggregation of human platelets (Fuchs et al., 2010).In conclusion, histones activate platelets, which promotes coagulation,and histones produce platelet-rich microthrombi, which can lead to thrombocytopenia.
PAD4 is a critical enzyme involved in NETosis (Wang et al., 2021) that catalyzes the conversion of histone-associated arginine residues to the non-canonical amino acid citrulline, and this histone citrullination is the centralin vivolink in NETosis (Sorensen and Borregaard, 2016).Targeting PAD4 and NADPH oxidases limits pathological H3cit+neutrophil release of NETs, which explains the mechanism of attenuation of cerebral thrombosis (Ansari et al., 2023)and informs the role of PAD4 in neuro-immunothrombosis.PAD4-knockout mice have undetectable levels of histone hypercitrullination, do not produce neutrophil NETs (Li et al., 2010), and generate fewer thrombi than wildtype mice after the induction of inferior vena cava stenosis (Martinod et al.,2013).PAD4 from bone marrow-derived cells and NETs contributes to acute thrombotic complications of intimal lesions, and PAD4 deficiency abrogates NETosis in experimental atheromata (Franck et al., 2018).PAD4 inhibitors are potential drug targets for deep vein thrombosis (Martinod et al., 2013) and represent a highly attractive strategy for preventing immunothrombosis.
Histone-activated platelets have a procoagulant phenotype that could drive plasma thrombin generation, suggesting that this TLR2- and TLR4-mediated activation ultimately damages tissues (Semeraro et al., 2011; Kumar et al.,2015).Zinc can downregulate NET release by inhibiting H3 citrullination(Ku?micka et al., 2020), and histones inhibit thrombomodulin (TM)-dependent protein C activation (Ammollo et al., 2011), meaning that anticoagulant pathways can be modulated through histones, which are modulated by neutrophilic granular content (Massberg et al., 2010; Ammollo et al., 2011).
Negatively-charged surface in the activation of coagulation factor
Factor XII (FXII) initiates the intrinsic coagulation pathway and is assisted by NETs in coagulation, which provide negatively charged surfaces for FXII activation (von Bruhl et al., 2012).This procoagulant polyanionic surface is a combination of NETs and polyphosphates (Rangaswamy et al., 2021).Scanning electron microscopy of NETs produced by platelet-activated neutrophils reveals a DNA skeleton bound by FXII and factor XI (FXI), substrates of the coagulation pathway (Delabranche et al., 2017).Soluble DNA purified from neutrophils, as well as NETs induced by glucose oxide or interleukin-8 stimulation, can assemble and activate FXIIin vitro(Weidmann et al., 2017).Taken together, both purified DNA and NETs can activate FXII to participate in the intrinsic coagulation pathway.
Furthermore, H4 on NETs can perforate platelets and cause the release of procoagulant polyphosphates (Bender et al., 2017) that may also activate FXII.In addition to contact-activated FXII, the DNA provides a reaction surface for thrombin-dependent FXI activation (Labberton et al., 2016).In a TBI mouse model, both FXII genetic deficiency and FXII inhibition diminish bradykinin release from the contact kinin system, minimizing brain lesion size,BBB leakage, brain edema formation, and inflammation (Hopp et al., 2017).Therefore, the inhibition of NETosis may improve TBI outcomes by reducing FXII activation.
Facilitators of Neutrophil Extracellular Traps in Blood for Immunothrombosis
Thrombosis requires many blood factors beyond NETs (Figure 1), which promote the accumulation of prothrombotic molecules such as vWF and fibrinogen (Martinod et al., 2013) that bind and activate platelets (Fuchs et al., 2010; Martinod and Wagner, 2014).NET-stimulated fibrin deposition can cause thrombosis, and NETs are a platform for many agonists.
NETs bind vWF to promote thrombosis
NETs and vWF are essential for inflammation and thrombosis.vWF binds collagen and platelet receptors to mediate platelet adherence to damaged vessel walls, and NETs act as scaffolds for vWF to promote thrombosis and support platelet recruitment (Engelmann and Massberg, 2013).Slc44a2, a receptor for vWF expressed by neutrophils, mediates NETosis on vWF at the venous shear, which explains the reduced risk of venous thrombosis (Zirka et al., 2021).All of these events result in vessel thrombosis.Our mechanistic understanding of vWF stems from TBI studies (Zeineddin et al., 2021), which reveal that vWF, a binding ligand synthesized and released by endothelial cells, plays a paradoxical role in promoting local hemostasis at the primary site of injury and systemically propagates TBI-induced epitheliopathy and coagulopathy (Xu et al., 2020).
Furthermore, a disintegrin and metalloprotease with thrombospondin type 1 repeat 13 (ADAMTS-13), an enzyme that cleaves prothrombotic and proinflammatory vWF, appears to be an interacting factor between vWF and NETs.Serine protease inhibitors against antithrombin, fibrinogen, and ADAMTS-13 inhibit PAD4-mediated citrullination (Tilvawala et al., 2018),and injection of PAD4 induced the citrullination of plasma proteins such as ADAMTS-13, reducing cleavage activity of ADAMTS-13 against vWF, and producing a vWF-platelet substance prior to thrombosis (Sorvillo et al., 2019).Recombinant ADAMTS-13 prevents TBI-induced coagulation dysfunction in mice by enhancing vWF cleavage while protecting the integrity of the endothelial cell barrier (Wu et al., 2018).Therefore, PAD4 inhibits thrombosis by reducing cleavage of vWF by ADAMTS-13 without compromising hemostasis.
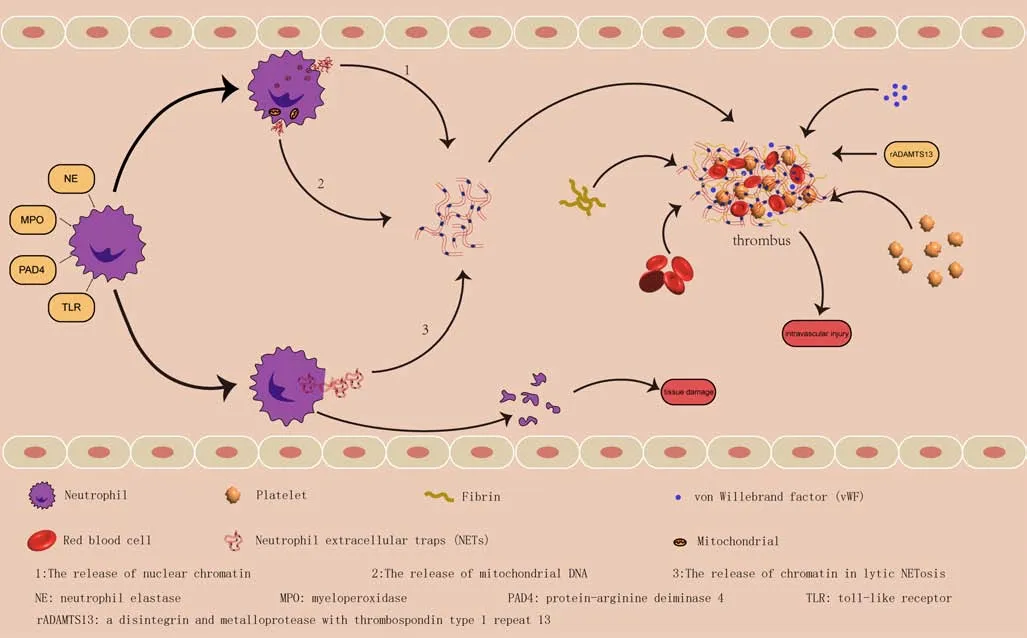
Figure 1 |Neutrophil extracellular traps (NETs) mediate neuro-immunothrombosis.
NETs stimulate fibrin deposition that results in thrombosis
The discovery of fibrin in extravascular locations established the link between inflammation and thrombosis (Zinsser and Pryde, 1952).Conventionally,fibrin is required for thrombosis; without fibrin, the NET scaffold supports clot formation (Fuchs et al., 2010).Moreover, with the emergence of histone-DNA complexes, fibrinogen clotting results in thicker fibrin fibers, higher fibrin clot stability and rigidity, and a significantly prolonged clot lysis time (Longstaffet al., 2013).
In the cardiovascular system, NETs promote thrombosis by stimulating fibrin deposition and increasing NET levels, which increases the risk of a large infarct or major adverse cardiovascular events (Bonaventura et al., 2020).In diabetes mellitus, NETs are formed during the acute phase of the disease and NETosis occurs within the fibrin matrix and influences clot properties (de Vries et al.,2020).Qualitative brain examinations of patients who died of spontaneous intracerebral hemorrhage revealed that neutrophils and NETs were mainly distributed around dense fibrin in the hematoma (Puy et al., 2021).These studies demonstrate the role of NETs in early hemostasis and the surrounding neuroinflammatory response within hematomas.Hence, we investigated the association between inflammation and thrombosis mediated by fibrin and NETs in patients with brain injuries.
Platelets and NETs
Platelets are an important blood component that is critical for controlling hemorrhage in primary hemostasis.Single-stranded DNA can bind to platelets (Dorsch, 1981), whereas double-stranded DNA can induce platelet aggregation.NETs promote thrombosis by serving as scaffolds that activate platelets and coagulation (Laridan et al., 2017), and contribute to the pathogenesis of deep vein thrombosis, myocardial infarction, and stroke(Constantinescu-Bercu et al., 2020).Digestion of NETs by DNase reduces platelet aggregates under flow (Fuchs et al., 2010) and platelet adhesion to NETs under static conditions (Abdol Razak et al., 2017).Histones are platelet agonists that trigger a series of platelet responses via specific surface receptors and signaling pathways (Semeraro et al., 2011).Heparin binds histones, while platelets cannot be bound by heparin (Fuchs et al., 2010).NET-induced platelet activation or aggregation is not reduced by pretreatment of NETs with DNase or heparin, and Elaskalani et al.(2018) inferred that targeting certain platelet activation pathways could more significantly reduce NET-induced platelet aggregation.
Platelet activation and aggregation can be triggered by H3 and H4, both directly via TLR2 and TLR4 and indirectly via fibrinogen (Semeraro et al.,2011).Activated platelets (Brinkmann et al., 2004) directly bind neutrophils and trigger NETosis in response to bacterial products (Clark et al., 2007;Massberg et al., 2010).In patients with gastric cancer, NETs upregulate cell-surface expression of phosphatidylserine and P-selectin to induce a hypercoagulable state in platelets (Li et al., 2022b).In addition, activated platelets can induce NETosis through mechanisms involving TLR4 (Clark et al.,2007), high-mobility group box 1 (HMGB1; Maugeri et al., 2014), and P-selectin(Etulain et al., 2015), indicating complex interactions between platelets and neutrophils during NETosis.Activated platelets release defensins that induce robust NET formation (Kraemer et al., 2011), though platelet depletion does not necessarily prevent NETosis (Brinkmann et al., 2004).
Platelets drive HMGB1 release and NETosis, which exacerbates stroke outcomes (Denorme et al., 2022).In septic mice, a dynamic NET-plateletthrombin axis promotes intravascular coagulation and microvascular dysfunction (McDonald et al., 2017).Lysophosphatidic acid, a bioactive phospholipid released by activated platelets, induces a PAD4-dependent release of NETs from human neutrophils that reactivates platelets in a positive feedback mechanism (Li et al., 2020).Following acute local inflammation,platelets activated by NETs can cause a systemic procoagulant state,resulting in injury to remote organs by immunothrombosis (Zhang et al.,2020).However, further studies are needed to understand the complex and interacting inflammatory and thrombotic mechanisms of NET-mediated platelet activation and coagulation.
Neutrophil Extracellular Traps and Neuro-Immunothrombosis
To survive an infection, the body mounts a complex inflammatory response that includes innate and secondary immune responses.Inflammation is a natural barrier that protects cells from external invasion.Thrombosis is the formation of blood clots in blood vessels, which cause partial or complete vessel occlusion and organ damage.Immunothrombosis is intravascular thrombosis in small or larger vessels, with activated neutrophils and monocytes interacting with platelets (Bonaventura et al., 2021).This concept was first coined in 2013 by Engelmann and Massberg (2013).The main factor in human immunothrombosis is the intravascular proteolytic cascade systems, which include the complement, coagulation, contact, and fibrinolysis systems, as well as blood cells (Anitua et al., 2022), all of which directly drive the progression of venous thromboembolism (Heestermans et al., 2022).In clinical practice, C-reactive protein levels indicate the severity of infection and inflammation, and destabilized isoforms of C-reactive protein possess proinflammatory and pro-thrombotic properties (Dix et al., 2022), which may help assess immunothrombosis in noninfectious diseases.
Immunothrombosis is an innate immune-related intravascular thrombosis that is accompanied by neutrophil activation and subsequent NETosis (Kimball et al., 2016).A study from the COVID-19 pandemic have revealed that NETs may also be involved in COVID-19-associated thromboinflammation and severe lung injury (Middleton et al., 2020).In the lung tissue of COVID-19 patients, FXII is increased and activated, possibly because of defective NET clearance (Englert et al., 2021).Correspondingly, NETs have been found in the pulmonary parenchyma and alveolar space of COVID-19 patients(Radermecker et al., 2020), and NET levels correlate within vivocoagulation,fibrinolysis, and endothelial damage; circulating NETosis markers may guide interventions in COVID-19 treatment (Ng et al., 2021).Therefore, both thrombosis and inflammation occur during COVID-19 infection, and the connection between them requires further study.
Increasing evidence supports the link between microvascular thrombosis and inflammation (De Meyer et al., 2016).The healthy brain is only free of inflammation and thrombosis if neutrophils cannot easily cross the BBB.As components of NETs, neutrophil proteases can degrade basement membrane laminins (Heck et al., 1990) to promote BBB destruction via multiple mechanisms that ultimately increase BBB permeability.Neutrophil elastase is released by activated neutrophils and may be wrapped in NETs, which disrupts the adherent junction proteins VE-cadherin and catenin, thereby increasing BBB permeability (Johnson-Léger et al., 2000).NET-associated matrix metalloproteinase-9 in cerebral microvessels can degrade type-IV collagen in the basal lamina, disrupting BBB integrity (Rosell et al., 2008).Histones also increase BBB permeability by disrupting adherent tight junctions (Villalba et al., 2020).Overall, NETs mediate BBB disruption, which initiates secondary injuries to the brain and spinal cord via trauma, inflammation, ischemia, and degeneration as the CNS is leftvulnerable to attack by many substances.
Neutrophils and NETs have been observed in the CNS after various types of damage including traumatic, infectious, vascular, autoimmune,neurodegenerative, and neoplastic damage (Shafqat et al., 2023), and prevention of NETosis and neutrophil trafficking in the CNS in these diseases may improve brain pathology and neurocognitive outcomes.Neuro-immunothrombosis connects neuroinflammation and thrombosis.Neuroinflammation is crucial in neural development and neurological disease pathology for Alzheimer’s disease, multiple sclerosis, and ischemic stroke(Sun and Langer, 2022).NETosis is also associated with rare diseases such as cerebral venous sinus thrombosis (CVST; Jin et al., 2022) and cerebral hemorrhagic diseases such as intracerebral hemorrhage and subarachnoid hemorrhage (Table 2).
Ischemic stroke is a leading cause of death and disability with complex pathophysiological features; thrombosis and inflammation are highly correlated factors leading to cerebral vessel occlusion, inflammatory responses, and severe neuronal damage following an ischemic event (De Meyer et al., 2022; Zhang et al., 2022a; Ugidos et al., 2023).Despite the success of recanalization of blocked large arteries, most strokes still evolve into persistent ischemic brain damage with neurological deficits (Coutts and Goyal, 2009) resulting from microvascular thrombosis and inflammation that block microvascular re-opening; suppression of neuroinflammation improves ischemic stroke outcomes.Agaphelin, a mosquito salivary protein that inhibits the catalytic activity of neutrophil elastase (Waisberg et al., 2014), prevents acute ischemic stroke, BBB damage, and inflammation in mice by reducing thrombosis (Leinweber et al., 2021).Under normal physiological conditions,the BBB prevents immune cells from entering the CNS (Qin et al., 2023),but BBB destruction during stroke permits the migration of immune cells,especially neutrophils, into the CNS (Tang et al., 2014; Petrovic-Djergovic etal., 2016).T cells participate in ischemia-reperfusion injury in an antigenindependent manner and interact with platelets to facilitate further infarct development (Stoll and Nieswandt, 2019).CD147 is a key activator of splenic inflammation caused by cerebral ischemia (Jin et al., 2019), and inhibition of CD147 improves acute ischemic stroke outcomes by reducing inflammatory responses (Jin et al., 2017).Imperatorin is a potential stroke treatment that exerts an anti-inflammatory effect by downregulating the MAPK and NF-κB signaling pathways (Ge et al., 2022).The contact-kinin pathway is a critical activator of pro-coagulant and pro-inflammatory processes; inhibition of the contact-kinin pathway by sylvestin improves ischemic stroke outcomes(Zhang et al., 2022b).Neuroinflammation and thrombosis occur extensively in the CNS after an ischemic stroke, and these changes are linked to NETosis;HMGB1-induced NETosis following middle cerebral artery occlusion causes a reciprocal feedback loop between neuronal cell death and NETosis (Kim et al., 2019).Therefore, ischemic brain damage outcomes might be improved by targeting NETosis via regulation of HMGB1 expression.Adenosine triphosphate (ATP) contributes to NETosis (Sofoluwe et al., 2019), and ATP accumulates and induces NETosis in the ischemic brain (Kim et al., 2020).
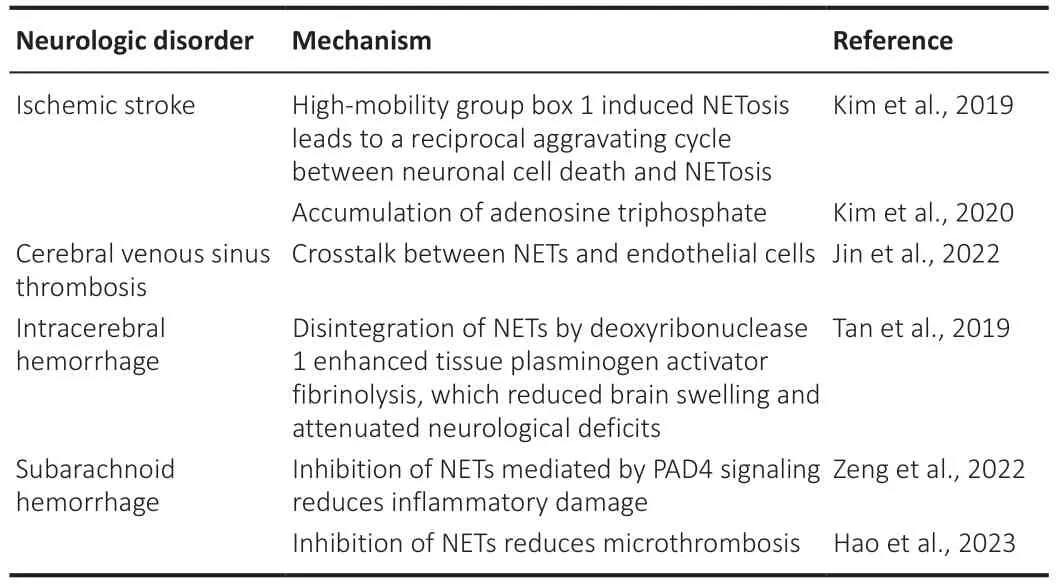
Table 2 |Neutrophil extracellular traps (NETs) and neuro-immunothrombosis
CVST is a cerebrovascular disease with a low morbidity and high mortality rate (Capecchi et al., 2018).NETs have been found in patients with CVST who received the COVID-19 vaccine (Mehta et al., 2021), and NETs are involved in hypercoagulation and thrombus composition in patients with CVST (Jin et al., 2022).The crosstalk between NETs and endothelial cells exacerbates thrombosis, and targeting NETs may reduce CVST-related mortality.
In a study on experimental intracerebral hemorrhage in rats, NETs were present, and their disintegration by DNase 1 enhanced t-PA fibrinolysis, which reduced brain swelling and attenuated neurological deficits (Tan et al., 2019).Microthrombus formation in the brain following subarachnoid hemorrhage is a critical indicator of poor prognosis.NETs promote neuroinflammation after subarachnoid hemorrhage, and inhibition of PAD4-mediated NETosis may reduce inflammatory damage (Zeng et al., 2022).NETosis contributes to microthrombosis after subarachnoid hemorrhage, and inhibition of NETs reduces microthrombosis and improves outcomes.These findings indicate that the inhibition of NETosis or degradation of NETs may relieve thrombosis and improve outcomes in relevant CNS diseases.
Discussion
The discovery of NETs has opened new avenues for understanding the principles of inflammation and thrombosis.Inhibition of NETosis is a promising therapeutic option for thrombosis treatment (Leung et al., 2021; Alsabani et al., 2022), but NETosis may be essential for responding to bacterial infections(Monteith et al., 2021; Schultz et al., 2022), which must be considered when managing thrombosis in the context of sepsis.Future research efforts may address the need to inhibit NETosis without compromising antibacterial activity.
Some novel theories postulate that neutrophils may release NETs via different methods.Suicidal or lytic NETosis occurs in response to need (Leung et al.,2021), while non-lytic or vital NETosis involves the expulsion of DNA-wrapped vesicles into the extracellular space without neutrophil rupture.The release of mtDNA is considered a type of non-lytic NETosis.Anucleated neutrophils can still catch and kill bacteria even after NET expulsion, but lytic NETosis releases NETs via permeabilization of the PM, releasing cell fragments and contents that cause secondary damage to the extracellular compartment.Future studies are needed to differentiate between these two types of NETosis to maintain anti-bacterial functions, inhibit thrombosis, and reduce the side effects of NETs.
Many studies have established the role of NETs in neurological diseases of the CNS (Denorme et al., 2022; Sun and Langer, 2022; Shafqat et al., 2023).NETrelated proteases and citrullinated histones are found in the blood, which suggest that NETs may be involved in the pathogenesis of ischemic stroke.Neutrophil elastase degrades the BBB, causing secondary CNS damage in ischemic stroke that identifies the neuroinflammatory mechanisms associated with NETs (Long et al., 2023).Furthermore, BBB destruction via NETosis may be prevalent in Alzheimer’s disease, and NET levels are significantly higher in high-grade glioma tissues than in low-grade glioma tissues; this phenomenon requires further investigation.The healthy CNS is protected from neutrophils by the BBB; neutrophil numbers increase rapidly in the CNS during pathological changes such as bacterial infections.Neutrophils disrupt the BBB, which enables their participation in the pathogenic CNS disease mechanisms.NETs may also contribute to BBB disruption, though further research is required to understand the mechanisms involved.
SThe inhibition of NET formation can improve disease outcomes, and increasing evidence suggests that microvascular thrombosis and inflammation are linked, leading to thromboinflammation (De Meyer et al., 2016).Here we have reviewed the role of NETs in immunothrombosis and the latest research on neuro-immunothrombosis.NETs can be detectedin vivoafter an ischemic stroke and affect prognosis through several known pathways.NETs are crucial in the course of disease evolution in CVST, intracerebral hemorrhage, and subarachnoid hemorrhage, but further research is needed to understand the role of NETs in neuro-immunothrombosis.Hemostasis is rapidly activated following vascular injury, leading to thrombosis that is essential for maintaining vessel integrity, but excessive hemostasis can also lead to secondary damage and should be avoided.BBB integrity should also be protected from NETs to prevent secondary injury in CNS diseases, including neuro-immunothrombosis.The detection of NETs in cerebrospinal fluid after TBI indicates that targeting NETs might reduce secondary CNS damage.Further research is needed on the role of NETs and their clinical applications in TBI.
Author contributions:JL wrote the manuscript.JZ provided ideas and guided for the writing of the manuscript.QD and XC helped with the critical reading and editing of the manuscript.All the authors have read and approved the final manuscript.
Conflicts of interest:The authors claim no relevant conflict of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- The big data challenge – and how polypharmacology supports the translation from pre-clinical research into clinical use against neurodegenerative diseases and beyond
- P-aminobenzoic acid promotes retinal regeneration through activation of Ascl1a in zebrafish
- Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway
- Two-photon live imaging of direct glia-to-neuron conversion in the mouse cortex
- Ferroptosis mechanism and Alzheimer’s disease
- Impact of increasing one-carbon metabolites on traumatic brain injury outcome using pre-clinical models

