Preparation and Thermo-Responsive Properties of Poly(Oligo(Ethylene Glycol) Methacrylate) Copolymers with Hydroxy-Terminated Side Chain
CHEN Yangyi(陳楊軼), SU Tong(蘇 桐), ZHOU Shihang(周仕航), XIE Chendi(謝晨迪), LI Jingzhi(李京芝), QIU Yiping(邱夷平), 2, 3
1 College of Textiles and Apparel, Quanzhou Normal University, Quanzhou 362000, China
2 Institute of Smart &Ecological Textile, Quanzhou Normal University, Quanzhou 362000, China
3 College of Textiles, Donghua University, Shanghai 201620, China
Abstract:Thermo-responsive random copolymers, poly(2-(2-methoxyethoxy) ethoxyethyl methacrylate-co-(ethylene glycol) methyl ether methacrylate) (P(EO2-co-EO4/5)) and poly(2-(2-methoxyethoxy) ethoxyethyl methacrylate-co-ethylene glycol methacrylate (P(EO2-co-EG4/5)) are synthesized via atom transfer radical polymerization (ATRP). The successful synthesis and the narrow polydispersity index (PDI) of two copolymers are indicated by 1H nuclear magnetic resonance (1H-NMR) and gel permeation chromatography (GPC) analyses. The transition behaviors of polymers in the aqueous solution are demonstrated by changes in turbidity and particle sizes. The transition behavior of P(EO2-co-EG4/5) is found to be milder than that of P(EO2-co-EO4/5). Moreover, the presence of hydrogen bonds without thermo-responsive properties established by hydroxyl groups in the end-side chain of P(EO2-co-EG4/5) hinders the dehydration at the transition temperature (TT). Attenuated total reflection Fourier transform infrared spectrometry(ATR-FTIR) analysis along with contact angle measurements reveals that both P(EO2-co-EO4/5) and P(EO2-co-EG4/5) films undergo phase transitions from hydrophilicity to hydrophobicity above TT. By examining the swelling and collapse behaviors of the polymer films during phase transitions, it can be concluded that the end hydroxyl groups may establish hydrogen bonds with neighboring ether groups within the films, which remain intact throughout the phase transition process due to their strong bonding interactions. This leads to an increase in steric hindrance within swollen films thereby impeding dehydration processes and inducing hysteresis during phase transitions.
Key words:thermo-responsive property; poly(oligo(ethylene glycol) methacrylate); polyethylene glycol methacrylate; hydroxy-terminated side chain; contact angle; phase transition
0 Introduction
Thermo-responsive poly(oligo(ethylene glycol) methacrylate) (POEGMA) has gained significant attention in recent years due to its exceptional properties[1-5]. This polymer demonstrates a reversible phase transition in response to temperature fluctuations, which can be attributed to the presence of oligo(ethylene glycol) side chains within its structure. These side chains exhibit a strong affinity for water molecules, leading to polymer hydration at lower temperatures. As the temperature surpasses a specific threshold known as the lower critical solution temperature (LCST), dehydration occurs and the polymer undergoes a phase transition from a hydrated state to an aggregated or collapsed state. LCST can be adjusted by modifying the relative molecular mass and the composition of POEGMA[6-8]. High relative molecular mass typically result in elevated LCST values, while incorporating hydrophobic monomers into the polymer backbone can also enhance LCST. By precisely controlling these parameters, researchers are able to tailor the responsiveness of POEGMA to specific LCST[9-12]. Hence POEGMA and its copolymers have been widely applied in biomedical coatings[13-14], drug delivery systems[5,15-17], tissue engineering[18-20], biosensors[21-23]and so on.
However, POEGMA itself lacks reactive groups, which hinders its effective chemical combination with alternative materials and limits its potential application. Therefore, it has been reported that copolymerization with polyethylene glycol methacrylate (PEGMA) that contains additional hydroxyl groups in the side chains offers a feasible approach to tackle this concern[24-27]. It enhances the reactivity of the copolymer and enables the construction of smart composite materials through combination with different substances. Recently, the copolymers have been successfully integrated with natural textile materials such as cotton[28-29]and silk[30-31]to fabricate smart textiles for various applications including smart cleaning[26,28], comfort control[32-33], oil-water separation[34],etc. Nevertheless, these literature findings indicate that the introduction of hydrophilic hydroxyl groups inevitably influences the polymer properties and subsequently affects the phase transition behavior of the entire smart textiles. However, no specific impacts have been reported as yet. Therefore, with respect to applications of copolymer, it is crucial to comprehend the influence of hydroxyl groups on thermo-responsive properties for design and preparation of related smart composite materials.
The structure of the paper is as follows. Firstly, the synthesis of thermo-responsive random copolymers poly(2-(2-methoxyethoxy) ethoxyethyl methacrylate-co-(ethylene glycol) methyl ether methacrylate) (P(EO2-co-EO4/5)) and poly(2-(2-methoxyethoxy) ethoxyethyl methacrylate-co-ethylene glycol methacrylate (P(EO2-co-EG4/5)) by atom transfer radical polymerization (ATRP), and the characterization of these two polymers, are presented. Subsequently, the transition behavior of thermo-responsive polymers in aqueous solutions is examined and discussed. Following the preparation of the polymer films, an in-depth investigation is conducted on the transition behaviors of two polymer films, including hydration/dehydration, hydrophilicity/hydrophobicity, and swelling/collapse properties, with a particular focus on the impact of the end hydroxy in the side chain.
1 Experiments
1.1 Materials
The monomers 2-(2-methoxyethoxy) ethoxyethyl methacrylate (MEO2MA,M=188.2 g/mol, purity of 95%), (ethylene glycol) methyl ether methacrylate (OEGMA300,M≈300 g/mol, purity of 95%) and ethylene glycol methacrylate (EGMA360,M≈360 g/mol, purity of 95%) were purchased from Sigma-Aldrich, USA, and used as received. Cuprous bromide (CuBr, purity of 98.5%), ethyl 2-bromoisobutyrate (EBiB, purity of 98%) andN,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDETA, purity of 99%) were obtained from Aladdin, China. Silicon wafers (single-side polished, an orientation index of 111) were received from Zhejiang Kaihua Lijing Electronic Co., Ltd., China.
1.2 Methods
1.2.1Synthesisofthermo-responsiverandomcopolymers
Thermo-responsive random copolymers P(EO2-co-EO4/5) and P(EO2-co-EG4/5) were synthesized by ATRP (Fig.1). Firstly, the deoxy monomers MEO2MA (6.28 mL, 24 mmol) and OEGMA300(1.72 mL, 6 mmol) or EGMA360(1.96 mL, 6 mmol) were dissolved in 10 mL anisole in a tube. Then the catalyst CuBr (20 mg, 0.14 mmol) and the ligand PMDETA (50 μL, 0.23 mmol) were added to the mixed solution. After being stirred and dissolved, the initiator EBiB (21 μL, 0.14 mmol) was added and the reaction tube was sealed. Since ATRP is sensitive to oxygen, the above operations were carried out in a glove box (Labstar, M. Braun, Germany). Afterwards, the sealed tube was placed in an oil bath at 90 ℃ for 4 h. The reaction was quenched by immersing the tube in ice water. The mixture was dissolved with tetrahydrofuran (THF), and the catalyst was removed by a filtration column containing neutral alumina. Finally, the collected filtrate was dissolved in THF and precipitated thrice byn-hexane. The obtained copolymer was then dried in a vacuum oven to a constant mass. The product with a yield of 70%-80% was determined by mass.

Fig.1 Schematic presentation of thermo-responsive random copolymers P(EO2-co-EO4/5) and P(EO2-co-EG4/5) with a monomer ratio m∶n = 17∶3
1.2.2Preparationofthinpolymerfilms
The thin thermo-responsive polymer films were prepared by a spin-coater (KW-4A, Institute of Microelectronics of the Chinese Academy of Sciences, China) on silicon wafers. Before spin coating, the silicon wafers were treated with a standard wet chemical cleaning method to remove possible organic traces, as reported in Ref. [35]. Then thin polymer films were prepared by spin coating (3 000 r/min, 30 s) from THF on treated silicon wafers.
1.2.3Spectroscopies
The chemical structures of polymers were measured in deuterated dimethyl sulfoxide (DMSO) by1H nuclear magnetic resonance (1H-NMR) spectroscopy (Avance AV 400 MHz, Bruker, USA). The tetramethylsilane (TMS) served as the internal standard.
The transition behaviors of thermo-responsive polymers in the aqueous solution were probed by a UV-Vis spectroscope (Lambda 35, Perkin Elmer, USA). The test wavelength was 500 nm. The temperature increased from 25 ℃ to 45 ℃ with a heating rate of 0.4 ℃/min.
The attenuated total reflection-Fourier transform infrared spectrometry (ATR-FTIR) (Vertex 70, Bruker, Germany) was used to detect the water bound in polymer films at different temperatures. The scanning range was from 6 000 cm-1to 400 cm-1.
1.2.4Gelpermeationchromatography(GPC)
The relative molecular mass and the distribution of the polymers were determined by GPC (Waters-Breeze, Waters, USA). The mobile phase used in the experiment was THF, while polystyrene served as the standard sample. The flow rate was 1 mL/min, and the test temperature was 30 ℃.
1.2.5Particlesizemeasurement
Z-average of particle sizes in the thermo-responsive polymer aqueous solution was measured by a dynamic light scattering (DLS) nanoparticle size analyzer (Zetasizer Nano S, Malvern, UK). The temperature increased from 25 ℃ to 45 ℃ with a heating rate of 3 ℃/min. During the test, in order to ensure that the polymer particle size reached a constant value, the temperature was thermally stabilized for 5 min.
1.2.6Filmthicknessmeasurement
A white light interferometer (Filmetrics F20, Filmetrics Inc., USA) equipped with a heating and refrigeration circulator (Julabo F12, JULABO, Germany) was used to determine the thickness of thin polymer films. A wavelength range of 400 to 1 100 nm was applied for the measurement. The refractive index of dry P(EO2-co-EO4/5) and P(EO2-co-EG4/5) thin films were 1.49 as reported in Ref. [36].
The obtained polymer film was placed in a customized aluminum chamber to control the temperature and the humidity. In the swelling process, NaCl solution (0.011 g/mL) was injected into the chamber. Meanwhile, the swelling time and the film thickness were recorded. In the collapse process, the temperature in the chamber was raised from 25 ℃ (below TT) to 45 ℃ (above TT) with a rate of 2 ℃ per 15 min, and the film thickness was recorded after a constant thickness was reached.
1.2.7Opticalmicroscopemeasurement
The surface morphologies of the films were observed by an upright metallurgical microscope (DM2700M, Leica, Germany). The films were prepared by spin coating on silicon wafers and then stored in ambient conditions for testing.
1.2.8Contactanglemeasurement
The solubility of P(EO2-co-EO4/5) and P(EO2-co-EG4/5) films in water enables the application of the oil (paraffin oil) contact angle to characterize the hydrophilic/hydrophobic transition of the polymer film. A video tension contact angle tester (DSA-20, Kruss, Germany) was employed to conduct the experiment. In an effort to minimize errors, three distinct points on the surface of the polymer film were selected and examined.
2 Results and Discussion
2.1 Characterization of copolymers P(EO2-co-EO4/5) and P(EO2-co-EG4/5)
The copolymers P(EO2-co-EO4/5) and P(EO2-co-EG4/5) were characterized by1H-NMR in deuterated DMSO (Fig.2). For P(EO2-co-EO4/5) and P(EO2-co-EG4/5), the common chemical shiftsδ×10-6of the protons are attributed to:δ=1.02-0.58 (b,b′, H, —C—H3);δ=1.98-1.59 (a,a′, H, —C—CH2—C);δ=3.38-3.12 (e,e′, H, —O—CH3);δ=4.20-3.91 (d1,d′1,d2,d′2, H, —O—CH2—CH2—O—);δ=4.25-4.11 (c1,c′1,c2,c′2, H, —COO—CH2—). By comparison, it is found that the spectrum of P(EO2-co-EG4/5) has an extra proton peak at the low fieldδ=4.60-4.50, which can be ascribed to —CH2—OH (f). The proton in terminated hydroxy belongs to the side chain of P(EO2-co-EG4/5). This indicates the successful synthesis of copolymers P(EO2-co-EO4/5) and P(EO2-co-EG4/5).
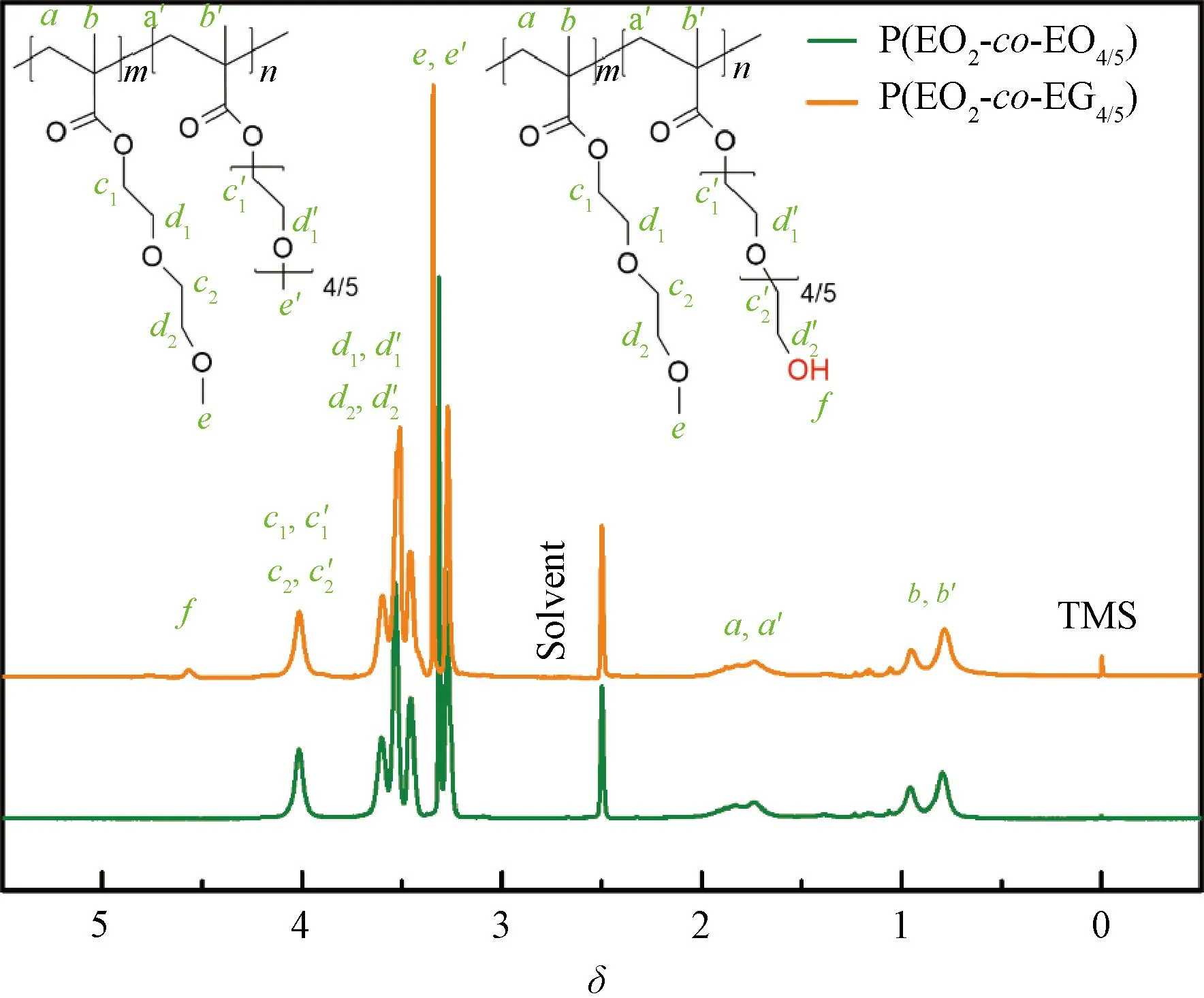
Fig.2 1H-NMR spectra of P(EO2-co-EO4/5) and P(EO2-co-EG4/5)
The relative molecular mass and the distribution were detected by GPC (Table 1). The polymer dispersity index (PDI) of P(EO2-co-EO4/5) and P(EO2-co-EG4/5) is 1.34 and 1.45, respectively, which reveals narrow relative molecular mass distributions. The results confirm the effective control of the relative molecular mass and the distribution of POEGMA copolymers through ATRP.

Table 1 Relative molecular mass and distribution of copolymer
2.2 Thermo-responsive properties of copolymer in aqueous solution
To probe the thermo-responsive properties of copolymers P(EO2-co-EO4/5) and P(EO2-co-EG4/5) in aqueous solutions, the transmittance of the polymer solution (1, 5 and 10 mg/mL) as a function of temperature (25-45 ℃) was measured. It can be seen from Fig.3(a) that the transmittance of the P(EO2-co-EO4/5) solution (1 mg/mL) approaches almost 100% in 25-34 ℃. By increasing the temperature, the transmittance changes sharply and drops almost to 0 at 37 ℃. Further increasing the temperature, the transmittance keeps constant. By calculating the first derivative of the transmittance with respect to temperature (Fig.3(c)), the transition temperature (TT) of 1 mg/mL P(EO2-co-EO4/5) solution can be determined as 35 ℃. When the solution temperature is lower than 35 ℃, the hydrophilic group on the molecular chain of P(EO2-co-EO4/5),i.e.the ethoxy group, binds with the surrounding water molecules by intermolecular hydrogen bonds. Hence the polymer chain stretches in the aqueous solution and dissolves in water, showing a homogeneous phase and resulting in a transmittance of 100%. While increasing the temperature to 35 ℃, the previous intermolecular hydrogen bonds are broken, intramolecular hydrogen bonds are formed, and then the polymer chains repel the water molecules and aggregate together in the solution, resulting in a phase separation. Therefore, the solution becomes turbid and the transmittance decreases to 0%.
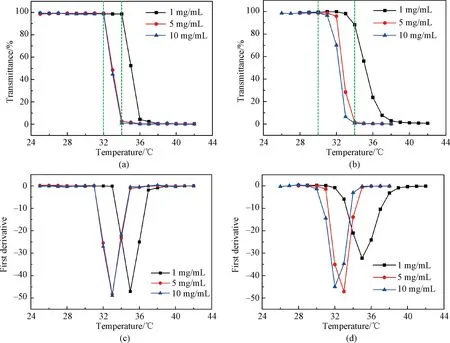
Fig.3 Transmittance of (a) P(EO2-co-EO4/5) and (b) P(EO-co-EG4/5) aqueous solutions as a function of temperature investigated by UV-vis; the first derivatives of transmittances of (c) P(EO2-co-EO4/5) and (d) P(EO2-co-EG4/5) with respect to temperature

Fig.4 Z-average particle sizes of P(EO2-co-EO4/5) and P(EO2-co-EG4/5) in aqueous solution with a concentration of 1 mg/mL as a function of temperature

Fig.5 Film thicknesses of P(EO2-co-EO4/5) and P(EO2-co-EG4/5) films as a function of solution concentrations
When the concentrations of P(EO2-co-EO4/5) solutions increase to 5 and 10 mg/mL, TT drops to 34 ℃. The reason for this phenomenon may be that the collision probability of molecules increases in a high concentration, and the polymer chains are more likely to aggregate together[2]. Hence the transition behavior of polymers occurs at a low temperature, leading to decreased TT. Finally, TT of P(EO2-co-EO4/5) aqueous solutions is fixed to 34 ℃.
For P(EO2-co-EG4/5), TT shows a decreasing trend as the concentration increases, which is similar to that of P(EO2-co-EO4/5). However, it is worth noting that the transition behavior of P(EO2-co-EG4/5) exhibits a milder nature than that of P(EO2-co-EO4/5). This can be attributed to the enhanced ability of hydroxy-terminated ethylene glycol chains in P(EO2-co-EG4/5) to form hydrogen bonds with water molecules. Moreover, the hydrogen bonds without thermo-responsive properties which are established with hydroxyl groups in end-side chains prevent the detachment of water molecules bound to polymer chains at TT. Consequently, this impedes the phase transition process of the polymer solution and leads to a broader transition interval.
In the phase transition process, the polymer gradually precipitates out of the aqueous solution, showing an increasing particle size. Figure 4 illustrates theZ-average particle sizes of P(EO2-co-EO4/5) and P(EO2-co-EG4/5) as a function of temperature. Due to the larger relative molecular mass of the synthesized P(EO2-co-EO4/5), the initial particle size is relatively large. The curve can be divided into two regions. In the first region from 25 ℃ to 35 ℃, the copolymer particle size only shows a slightly increasing trend, and the possible reason is that with the increase in temperature, the thermal movement of the polymer chain in the aqueous solution is also strengthened, resulting in an increase in the probability of collision, and the measured particle diameter also increases. However, in the second region (higher than 35 ℃), the particle size shows a significantly increasing trend. The main reason is that the hydrogen bonds between the polymer chain and water molecules are broken above TT, leading to the phase separation. Consequently, the polymer chains undergo dehydration and aggregate into large particles.
2.3 Characterization of polymer films
In order to investigate the correlation between the film thickness and the concentration, P(EO2-co-EO4/5) and P(EO2-co-EG4/5) solutions with concentrations of 4, 7, 10, 15 and 25 mg/mL were selected. The spin coating speed was maintained at 3 000 r/min for 30 s. Figure 5 illustrates the fitting curve of the film thickness as a function of the solution concentration. It can be concluded that within the range of spin coating concentrations in this work, there is a linear relationship between the concentration of both P(EO2-co-EO4/5) and P(EO2-co-EG4/5) and their respective film thicknesses. However, under identical concentration conditions, the thickness of the P(EO2-co-EG4/5) film is higher than that of the P(EO2-co-EO4/5) film. This discrepancy can be attributed to the presence of terminated hydroxy groups in side chains of P(EO2-co-EG4/5), which enhances its hydrophilicity and binding strength with silicon wafers during the spin coating process. Furthermore, it should be noted from Table 1 thatMwof P(EO2-co-EG4/5) is lower than that of P(EO2-co-EO4/5), yet the thickness of the P(EO2-co-EG4/5) films remains higher than that of the P(EO2-co-EO4/5) films under identical concentration and spin coating conditions. It is evident that the binding strength between the polymer and substrate significantly influences the film thickness during the spin coating.
The homogeneous films of P(EO2-co-EO4/5) and P(EO2-co-EG4/5) were prepared by spin coating on the surface of silicon wafers. To investigate the effect of the molecular structure on film stability, the surface morphology of the films was observed by an optical microscope under atmospheric conditions at room temperature for two days and six days, respectively. The surface morphology was compared with that of as-prepared films. As shown in Fig.6, both P(EO2-co-EO4/5) and P(EO2-co-EG4/5) films exhibit smooth and flat surfaces. However, significant changes are observed in the surface of P(EO2-co-EO4/5) films after being exposed to atmospheric conditions for two days, resulting in numerous tiny holes on their surfaces. The number and the size of these holes significantly increase with an extended time up to six days, indicating a pronounced dewetting phenomenon for the P(EO2-co-EO4/5) film (Fig.6(c)). In contrast, only several holes appear on the surface of the P(EO2-co-EG4/5) film after six day storage, revealing a relatively stable structure. Both polymer films remain intact during the experiment.

Fig.6 Optical micrographs of films as-prepared and stored in atmospheric conditions for several days: (a) as-prepared P(EO2-co-EO4/5); (b) P(EO2-co-EO4/5) after two day storage; (c) P(EO2-co-EO4/5) after six day storage; (d) as-prepared P(EO2-co-EG4/5); (e) P(EO2-co-EG4/5) after two day storage; (f) P(EO2-co-EG4/5) after six day storage (insets are the partial enlarge detail)
During the spin coating process of polymer films, the solution has been spread onto the surface of a silicon wafer under the centrifugal force and the shear force. The film thickness gradually decreases as the solvent evaporates, eventually achieving a uniform film. Due to the presence of flexible ethoxy in side chains of P(EO2-co-EO4/5), its glass transition temperature is below 0 ℃, resulting in high mobility of polymer chains. Consequently, polymer chain segments undergo recombination during the storage period, leading to alterations in surface morphology. Additionally, owing to its hydrophilicity, P(EO2-co-EO4/5) readily absorbs water vapor from the atmosphere, which further accelerates the migration rate of molecular chains. Therefore, the polymer film undergoes dehydration, resulting in the formation of numerous holes on the film surface. In comparison, the P(EO2-co-EG4/5) film shows better stability due to stronger hydrophilicity which enhances the binding strength between the polymer film and the silicon wafer.
2.4 Thermo-responsive properties of copolymer films
The solution transition behavior of POEGMA copolymers was initially understood through the examination of their solution properties in section 2.2. However, during actual application processes, these copolymers predominantly exist in the form of films or hydrogels. Therefore, it is imperative to investigate the transition behavior of copolymer films. In comparison to solutions, thermo-responsive copolymer films also exhibit intermolecular hydrogen bond fracture and intramolecular hydrogen bond formation around TT. This leads to surface hydrophilic-hydrophobic transitions and corresponding changes in film thicknesses.


Fig.7 ATR-FTIR spectra of films at varying relative humidities and temperatures: (a) P(EO2-co-EO4/5); (b) P(EO2-co-EG4/5)
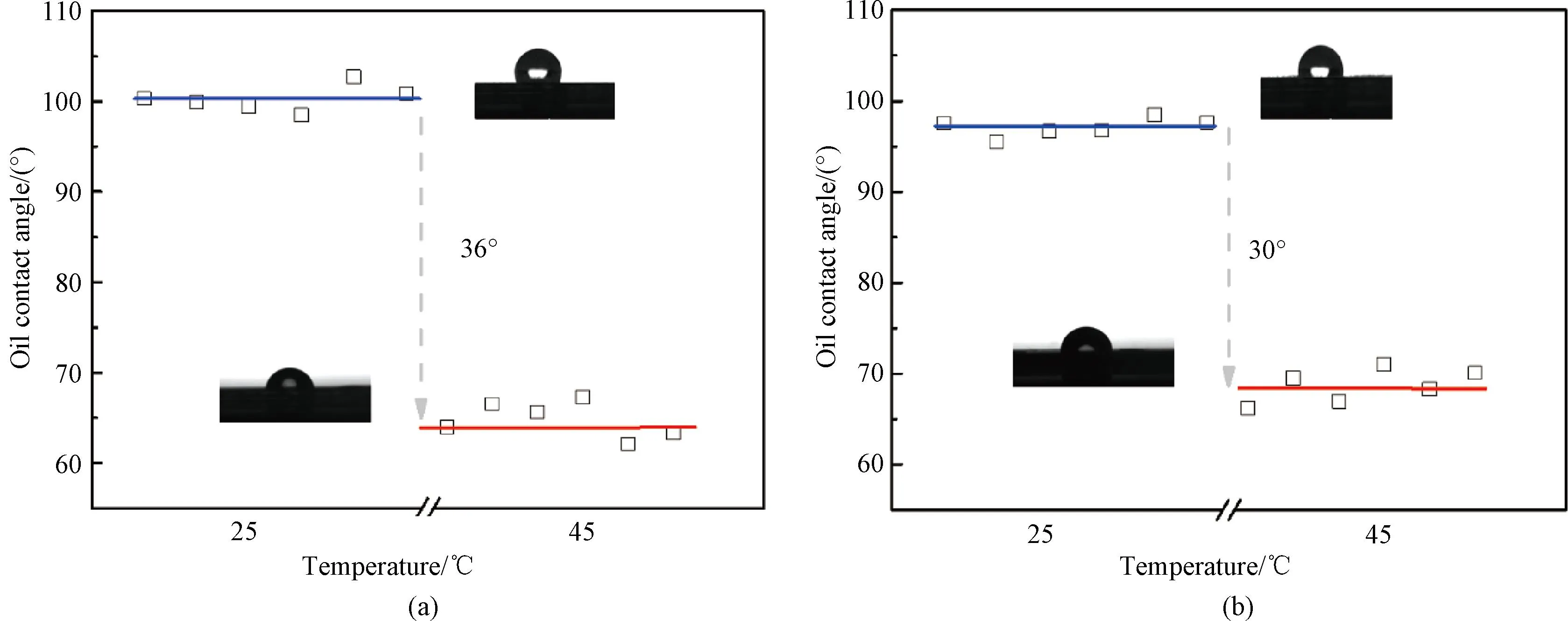
Fig.8 Oil contact angles of thin films at 25 ℃ and 45 ℃: (a) P(EO2-co-EO4/5); (b) P(EO2-co-EG4/5)
The spectra of the P(EO2-co-EG4/5) film in Fig.7(b) basically exhibit identical changes in functional groups as observed for the P(EO2-co-EO4/5) film. However, it is worth noting that the absorption peaks corresponding to O—H and bound water are still observed at 1 650 cm-1and 3 600-3 300 cm-1, respectively. This indicates that even after phase transition, the thin film retains some water molecules, resulting in the formation of hydrogen bonds. Upon comparing the molecular structure formulas of two copolymers, it can be noted that the side chain of P(EO2-co-EG4/5) contains a hydrophilic terminal hydroxy group without thermo-responsive properties. Consequently, the hydrogen bonds are less susceptible to breaking at 45 ℃ (above TT). As a result, the film still retains hydrogen bonds with some water molecules even after the phase transition, leading to observable absorption peaks for both water and hydrogen bonds.
During the phase transition of thermo-responsive copolymers, intermolecular hydrogen bonds are destroyed, causing separation between polymer chains and water. This weakens the hydrophilic effect and promotes the enrichment of hydrophobic chains on the film surface, resulting in a transition from a hydrophilic surface to a hydrophobic surface. Figure 8 illustrates the changes in oil contact angles of two copolymer films. The oil contact angles of P(EO2-co-EO4/5) and P(EO2-co-EG4/5) films at 25℃ are 100° and 97°, respectively. Both films remain in a hydrophilic state with large oil contact angles. When temperatures increase to 45 ℃ (above TT), the oil contact angle of films dramatically drops, attributing to the phase transition. However, by calculating the differences of oil contact angles at 25 ℃ and 45 ℃, it can be found that the P(EO2-co-EG4/5) film shows a less contact angle drop (30o) than that (36o) of the P(EO2-co-EO4/5) film. This phenomenon is also attributed to the additional hydroxyl group in the side chain of P(EO2-co-EG4/5), which preserves its hydrophilicity in the phase transition.
The phase transition behavior of thermo-responsive copolymer films is not only reflected in the transition of surface hydrophobicity and hydrophilicity, but also manifested in the film thickness. Simultaneously, the variation in the film thickness of a thermo-responsive copolymer depending on its TT, is also considered as one of the fundamental characteristics relevant to its practical applications.
The swelling ratio of the P(EO2-co-EO4/5) film is presented in Fig.9(a). The film rapidly absorbs water and expands within the initial 10 min, followed by a gradual rise until reaching an equilibrium after 2 h. This can be attributed to the rapid increase in humidity caused by the addition of saltwater in the aluminum chamber within a few minutes at 25 ℃ (TT below). The hydrophilic groups of the polymer chain establish hydrogen bonds with water vapor in the chamber due to their strong affinity with moisture. Additionally, owing to its good hydrophilicity, water absorbed on the surface of the film continues to penetrate into its interior, resulting in a rapid expansion of the film. Subsequently, as the relative humidity gradually increases in the chamber, there is a corresponding slow increase observed in the film thickness. After 2 h, the saturated vapor pressure in the chamber is reached, and no further changes occur in the film thickness. These results demonstrate that the film swelling ratio synchronizes with the ambient humidity as previously reported in Ref. [35]. Additionally, the longitudinal comparison reveals that films with thicknesses of 32, 40, 67 and 130 nm exhibit the maximum swelling ratio of 1.70, 1.64, 1.53 and 1.38, respectively. This indicates a decrease in the maximum swelling ratio as the film thickness increases. The reason lies in the fact that thinner films possess a higher affinity with water on their surfaces due to the stronger attraction between the silicon wafers and water molecules. Consequently, these thin films can absorb relatively more water, leading to an increase in the swelling raito. As the film thickness continues to increase, this force gradually weakens, leading to a reduction in the moisture content within the film and subsequently decreasing its corresponding swelling ratio.
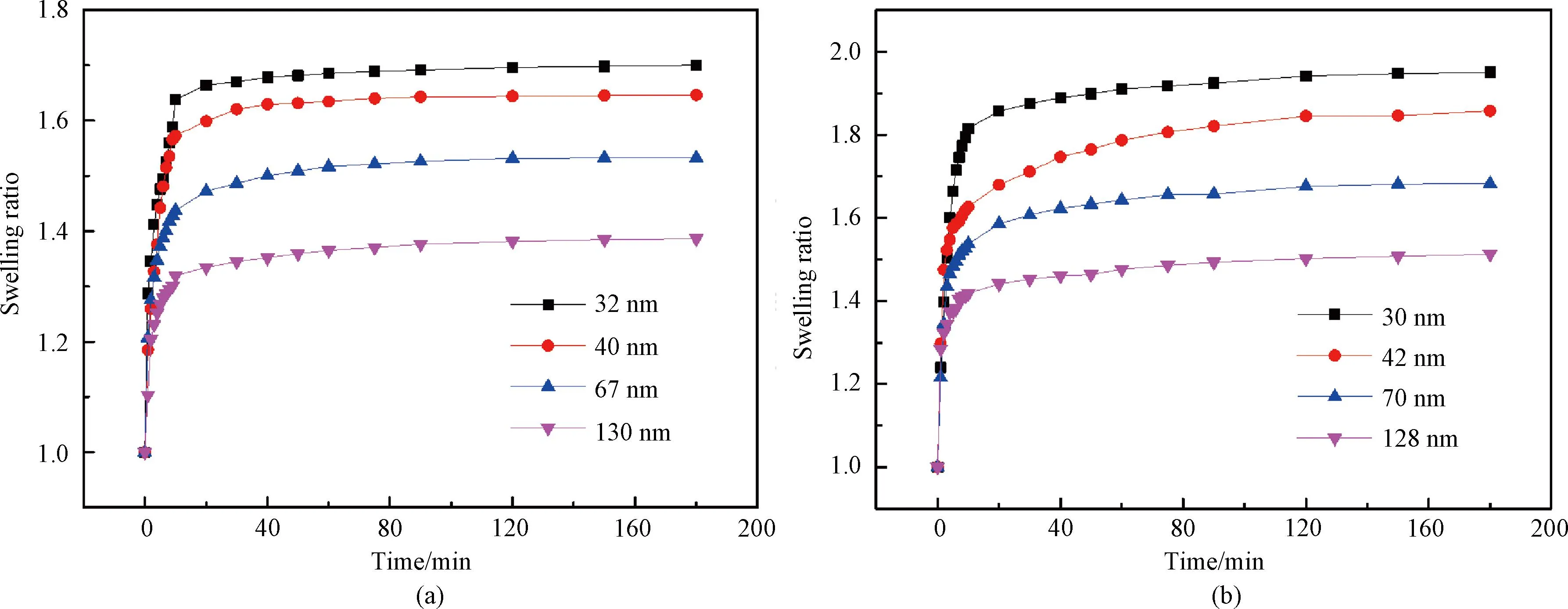
Fig.9 Swelling ratio of films as a function of time: (a) P(EO2-co-EO4/5); (b) P(EO2-co-EG4/5)
In contrast, the P(EO2-co-EG4/5) film also demonstrates a similar swelling process. Figure 9(b) shows that the film initially undergoes rapid expansion, followed by a gradual rise and eventual attainment of swelling equilibrium. The relationship between the film thickness and the swelling ratio aligns with that observed in the P(EO2-co-EO4/5) film. However, due to the presence of additional hydrophilic hydroxyl groups at the end of its side chains, the P(EO2-co-EG4/5) film exhibits enhanced water absorption and a higher swelling ratio under comparable film thickness conditions.
Thermo-responsive copolymer films exhibit water absorption and swelling below TT, while undergoing phase separation, shrinkage, and collapse upon reaching a distinct threshold (TT), resulting in the release of water molecules and reduction in the film thickness. Therefore, the transition process can be characterized by measuring alterations in the film thickness near TT.
After reaching the swelling equilibrium for 3 h, the temperature in the chamber gradually increases, leading to a phase transition of the film. Figure 10(a) illustrates the variation in the film thickness within a temperature range of 25 to 45 ℃. Upon analysis, the change process of P(EO2-co-EO4/5) films can be divided into three stages. In the initial stage at temperatures between 25 ℃ and 33 ℃, there is a slight decrease in the film thickness which may be attributed to enhanced thermal movement of water molecules caused by rising temperature. Some water molecules that fail to form hydrogen bonds with the film separate from the film and migrate towards its surface, resulting in the condensation of water vapor on the upper wall of the chamber and subsequently reducing water content within the film, thereby decreasing its thickness. The second stage is between 33 ℃ and 36 ℃ and represents a phase transition interval for the film. In the second stage, intermolecular hydrogen bonds are disrupted, leading to polymer film shrinkage and collapse, as well as the release of water molecules, and thus the film thickness significantly decreases. In the third stage at temperatures between 36 ℃ and 45 ℃, the temperature continues to increase while the film maintains a constant thickness. A comparison of film shrinkage ratios with varying thicknesses reveals that similar to the swelling ratio, thicker films exhibit lower shrinkage ratios due to their reduced water absorption capacity during the phase transition.
Through comparative analysis, it is found that the phase transition interval (31-37 ℃) of the P(EO2-co-EG4/5) film (Fig.10(b)) is significantly wider than that (33-36 ℃) of the P(EO2-co-EO4/5) film. This result is consistent with their transition behaviors in the aqueous solution. Additionally, thermo-responsive properties of polymer films on silicon wafers can only be demonstrated on a one-dimensional scale,i.e., the change of the film thickness. Therefore, during the phase transition, the dehydration can only occur through diffusion in the direction perpendicular to the substrate, resulting in a more gradual and gentle phase transition than that of the solution.

Fig.10 Shrinkage ratio of films as a function of temperature: (a) P(EO2-co-EO4/5); (b) P(EO2-co-EG4/5)
Moreover, it is observed that the solubility of P(EO2-co-EG4/5) decreases with the increasing monomer ratio of EGMA360in the preliminary experiment. Therefore, in the case of P(EO2-co-EO4/5) films, following water absorption and swelling below TT, it is hypothesized that the terminated hydroxyl group may establish hydrogen bonds with ether groups of neighboring molecules, resulting in the formation of cross-linking sites. This leads to an increase in steric hindrance within the swelling films, impeding the dehydration and causing hysteresis during the phase transition. Ultimately, this phenomenon widens the transition interval. Further experimentation is required to validate this conjecture.
3 Conclusions
Thermo-responsive random copolymers P(EO2-co-EO4/5) and P(EO2-co-EG4/5) were synthesized by ATRP.1H-NMR and GPC results revealed narrow relative molecular mass distributions of two copolymers, and the PDI of P(EO2-co-EO4/5) and P(EO2-co-EG4/5) was 1.34 and 1.45, respectively. Thermo-responsive properties of copolymers in the aqueous solution were characterized by UV-vis and DLS. TT of both P(EO2-co-EO4/5) and P(EO2-co-EG4/5) solutions (10 mg/mL) was found to be 34 ℃. However, the transition behavior of P(EO2-co-EG4/5) was milder than that of P(EO2-co-EO4/5). Moreover, the hydrogen bonds without thermo-responsive properties established with hydroxyl groups in the end-side chain prevented the detachment of water molecules bound to polymer chains at TT. Consequently, this impeded the phase transition process of the polymer solution and led to a broader transition interval. The P(EO2-co-EG4/5) film showed better stability than the P(EO2-co-EO4/5) film due to stronger hydrophilicity which enhanced the binding strength between the polymer film and the silicon wafer. ATR-FTIR and oil contact angle results showed that both P(EO2-co-EO4/5) and P(EO2-co-EG4/5) films underwent the phase transition from hydrophilicity to hydrophobicity above TT. By the measurement of swelling and shrinkage of films, it could be concluded that the end hydroxyl group might establish hydrogen bonds with ether groups of neighboring molecules, and the strong hydrogen bonds might remain intact throughout the phase transition. This led to an increase in steric hindrance within the swelling films, thereby impeding the dehydration and inducing hysteresis during phase transition.
 Journal of Donghua University(English Edition)2023年6期
Journal of Donghua University(English Edition)2023年6期
- Journal of Donghua University(English Edition)的其它文章
- Recent Progress on Fabrication of Thermal Conductive Aluminum Nitride Fibers
- Cleaning of Multi-Source Uncertain Time Series Data Based on PageRank
- Deep Multi-Module Based Language Priors Mitigation Model for Visual Question Answering
- Detection of Residual Yarn in Bobbin Based on Odd Partial Gabor Filter and Multi-Color Space Hierarchical Clustering
- Electromagnetic and Thermal Characteristics of Molybdenite Concentrate in Microwave Field
- Path Planning of UAV by Combing Improved Ant Colony System and Dynamic Window Algorithm
