Buffalo anti-PDC-109 antibodies improve the semen quality profiles and in-vitro zona binding index and minimize the cryoinjury of sperm in cryopreserved buffalo semen
S. S. Ramteke, J. S. Rajoriya, A. M. Shende, S. K. Ghosh, J. K. Prasad, P. Perumal
1MAFSU-College of Veterinary and Animal Sciences, Udgir, Maharashtra, India
2NDVSU-College of Veterinary Science and Animal Husbandry, Rewa, Madhya Pradesh, India
3MAFSU-Nagpur Veterinary College, Nagpur, Maharashtra, India
4ICAR-Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh, India
5ICAR-Central Island Agricultural Research Institute, Port Blair, Andaman and Nicobar Islands, India
ABSTRACT
Objective: To optimize the concentration of PDC-109 protein in semen for higher cryopreservability and fertility by sequestration or neutralization of PDC-109 by its antibodies (anti-PDC-109 antibodies) in bubaline species.
Methods: PDC-109 protein was purified by applying two-step chromatography procedures. Purified protein was injected in rabbits to raise antibodies. These raised anti-PDC-109 antibodies were used in neutralization or sequestration of PDC-109 in in-vitro model. Ejaculates were collected from buffaloes and splited for four groups. Group 1 received egg yolk Tris glycerol extender, without anti-PDC-109 antibodies, while group 2 to 4 received anti-PDC-109 antibodies 266 μg/mL, 80 μg/mL, and 26 μg/mL in Tris-fructosecitrate buffer, respectively. Semen quality parameters viz., forward progressive motility, viability, total morphological abnormality,acrosomal integrity, plasma membrane integrity, cryoinjury and invitro zona binding index were evaluated.
Results: Semen quality parameters of neat semen were within the normal range of bubaline species. Sperm motility, livability,acrosomal integrity, plasma membrane integrity, and cholesterol content of sperm were decreased and total sperm abnormality was increased significantly in post-thaw semen compared to those in pre-freeze and fresh semen (P<0.05). Semen in group 2 had higher sperm motility, livability, acrosomal integrity, plasma membrane integrity, and cholesterol content of sperm and lower total sperm abnormality significantly compared to those in group 1, 3 and 4 at pre-freeze and post-thaw stages (P<0.05).
Conclusions: Sequestration or neutralization of PDC-109 by its antibodies significantly improves pre-freeze, and post-thaw semen quality parameters and in-vitro zona binding index with simultaneously reducing cryoinjury or cryodamage in the sperm of bubaline species.
KEYWORDS: Anti-PDC-109 antibodies; Buffalo; In-vitro capacitation; In-vitro fertilization; Semen
Significance
PDC-109 at higher concentration and prolonged exposure triggers higher damages or injuries on sperm, which in turn significantly reduces semen quality parameters and fertility rate.These adverse effects of PDC-109 can be overcome by anti-PDC-109 antibodies in the semen extender. The present study showed that anti-PDC-109 antibodies (266 μg/mL) prevented sperm cryodamage or injury and improved the semen quality profiles and in-vitro zona binding index by modulating the PDC-109 protein concentration in semen. Anti-PDC-109 antibodies(266 μg/mL) protected sperm from deleterious PDC-109 protein in bubaline species.
1. Introduction
Bovine seminal plasma contains proteins (called as bovine seminal plasma proteins; BSPs) and are secreted by accessory sex glands;these BSPs have beneficial and detrimental effects depending upon their concentration and exposure time on morphology, and functions of sperm and fertility profiles in bovine and bubaline species[1].BSPs originate partly from the blood plasma and are partly synthesized by the testes[2], epididymis[3], vas deferens and seminal vesicles[4]. It was reported that the bovine seminal plasma modulates the fertilizing ability of spermatozoa[5], influences the motility and velocity of spermatozoa, facilitates transport of spermatozoa in the female genital tract[6], and promotes capacitation[7], acrosomal reaction[8] and zona binding interaction[9]. BSPs contain four acidic proteins designated as BSP-A1, BSP-A2, BSP-A3 and BSP-30 kDa[10]and the mixture of BSP-A1 and A2 is called as PDC-109 protein[11].These BSPs represent the major protein fractions in the bull semen[50% -70% of total seminal plasma protein (TSPP) or 35-50 mg/mL][1]. Various studies on these seminal plasma proteins from the different mammalian species demonstrated a similarity in their structure and biological functions[12]. BSPs with molecular weight of 16 kDa could represent the high density lipoprotein (HDL) which is called as “docking protein” and facilitates the lipid exchange between the cell membrane and HDL[13]. Exposure of the sperm to the BSPs for 4 h leads to cholesterol efflux from the sperm at about 25% [14].
PDC-109 protein is considered as a multifunctional protein with at least two different categories of physiologically significant binding interactions[15]. Interaction of sperm plasma membranes with PDC-109 protein in turn induces cholesterol efflux, which is a vital process in the capacitation, a necessary important event before fertilization[16]. Moreover, PDC-109 protein also interacts with fucosylated oligosaccharides available on the epithelium of oviduct in bovine species[17] and it is also responsible for the oviductal sperm reservoir maintenance[18]. Free unbounded floating seminal PDC-109 protein also stimulates the changes in the structure and morphology of sperm plasma membranes by continuously triggering the phospholipid and cholesterol efflux[19]. Bovine PDC-109 protein treatment induces higher level of cryodamage or cryoinjury in the cauda epididymal spermatozoa of buffalo; the degree of cryodamage or cryoinjury depends upon the dosage and exposure time of the protein and higher dosage and prolonged exposure time trigger higher damage or injury in the spermatozoa[20]. Srivastava et al[21] reported that sequestration of PDC-109 protein with anti-PDC-109 antibodies had improved the freezability of spermatozoa in crossbred bull. Harshan[20] also confirmed the presence of PDC-109 like protein in buffalo semen. PDC-109 like protein was purified from buffalo semen with the concentration ranging from 0.5 to 1.5 mg/mL and it was approximately 2% -5% of TSPP[22]. Isolated and purified PDC-109 protein concentration was very low in bubaline (0.5 to 1.5 mg/mL) as compared to bovine(15 to 30 mg/mL) species[21]. Similarly, the PDC-109 protein in bubaline species is 2% -5% of TSPP whereas in bovine species, it is 38% of TSPP[23]. There was no systematic detailed study on effect of buffalo anti-PDC-109 antibodies on cryopreserved spermatozoa in bubaline species. Therefore, this present study was hypothesed that supplementation of anti-PDC-109 antibodies in the Trisfructose-glycerol (TFC) semen extender would enhance the prefreeze and post-thaw semen quality profiles and in-vitro fertility rate and prevent the cryodamage or cryoinjury in bubaline semen.Therefore, the present study was designed to assess the effect of different concentrations of buffalo anti-PDC-109 antibodies on semen quality parameters, in-vitro zona binding index and cryoprotective efficiency in cryopreserved sperm to select an optimum or suitable dose to get maximum benefits in bubaline species.
2. Materials and methods
2.1. Experimental animals and semen collection
Four (n=4) apparently healthy adult Murrah buffalo (Bubalus bubalis) breeding bulls aged 4-6 years with good body condition score (5-6 in score of 10) and weighing 550-650 kg were selected from Germplasm Centre, ICAR-Indian Veterinary Research Institute,Izatnagar, Bareilly, Uttar Pradesh, India for semen collection and isolation, and purification of PDC-109 protein for the present study.These experimental he-buffaloes were maintained under uniform feeding, housing, lighting and managemental practices as per the farm schedule. Semen ejaculates were collected by using standard artificial vagina method during morning hour between 08:00 and 09:00 twice in a week. Tested for routine seminal parameters such as volume, pH, colour, sperm concentration, and mass activity.Ejaculates having a wide pH range, aberrant colour patterns, or a low volume were removed; the remaining ejaculates were evaluated and processed for further analysis. The ejaculates were analysed and accepted for evaluation as tested for routine seminal parameters and accepted for evaluation after meeting the Minimum Standard Protocol (MSP) standards like-wise concentration: >500×106/mL;mass activity: >3+, individual motility: >70% and total sperm abnormality: <10% . The present study consisted of two stages. In the first stage, semen collection, isolation and purification of the PDC-109 protein and raising of antibodies against purified PDC-109 protein (anti-PDC-109 antibodies) were done. In the the second stage, raised antibodies were included in the semen TFC buffer and studied the semen quality parameters, assessed the cryoinjury/cryodamage and in-vitro zona binding ability of the treated and control sperm.
2.2. Isolation and purification of PDC-109 protein
Freshly collected buffalo semen samples were centrifuged at 4 000×g at 5 ℃ for 20 min to separate the seminal plasma and remove the suspended spermatozoa as well as the other particulate matters. The supernatant seminal plasma was separated and collected and the remaining sperm cell pellet was discarded. The seminal plasma was again subsequently centrifuged at 10 000×g at 5 ℃for 60 min to get clear and pure seminal plasma. The clear or pure status of seminal plasma was confirmed by placing a drop under a high power magnification of a microscope, shown as it was free of spermatozoa and was stored in the deep freezer at -80 ℃ until further analysis. Overall concentration of PDC-109 protein in buffalo semen ranged from 0.70 to 0.98 mg/mL with overall mean value of (0.80±0.02) mg/mL in the present study. The percentage of isolated PDC-109 protein from individual ejaculate was approximately 2.50 per cent of TSPP.
Heparin-binding proteins and PDC-109 protein were isolated and purified from buffalo seminal plasma with the method described by Defaus et al[24] with few minor modifications. This method involved progressive purification of seminal plasma proteins by using heparin-sepharose affinity chromatography followed by DEAESephadex ion-exchange chromatography. The isolated protein was identified against protein molecular weight markers on sodium dodecyl-sulphate polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli[25] and confirmed by Western blot technique as per the description was given by Towbin et al[26].
2.3. Raising of hyper immune sera and purification of antibodies
Four adult apparently healthy New Zealand White male rabbits,aged 1-1.5 years, weighing 1.5-2.0 kg were selected and used for raising the anti-sera against purified buffalo PDC-109 protein. Preimmune blood was collected just before immunization from the four rabbits and sera was separated and stored in deep freezer at -80 ℃until for further use. The isolated heparin-binding proteins from heparin sepharose column were identified against protein molecular weight markers on SDS-PAGE. The band position specific to the PDC-109 protein was cut by new Bard Parker blade and cut band was preserved in 100 percent methanol for 36 h. During the period,methanol was changed at 6 h interval. After 36 h, the methanol was replaced by double distilled water for next 48 h with regular double distilled water change at 6 h interval. Washed protein band was triturated and mixed with 0.75 mL Frenaud’s Complete Adjuvant in the pestle and mortar to form emulsion. Emulsification was checked by placing a drop of the mixture on the surface of chilled water. This emulsified mixture was the inoculation dose for the rabbits.
Immediately after blood collection to obtain pre-immune serum,the rabbits were injected with the inoculation dose subcutaneously.The first booster dose was given subcutaneously at week 2 and the second booster dose was given on day 21 followed by the third booster on day 28. Rabbits were bled five days after the third booster dose to assess the level of antibodies produced in the serum. Five mL blood was collected and the serum was separated to assess the presence of antibodies against PDC-109 protein. Checkerboard assay was conducted to assess the presence of antibodies against PDC-109 in the raised hyper immune sera. Enzyme linked transfer blot was done as per the method described by Towbin et al[26] to detect and know the specificity of the raised antisera. These proteins were resolved in the 15% SDS-PAGE. These proteins on the gel were transferred electrophoretically onto nitrocellulose membrane (NCM,Sigma-Aldrich, USA) using blotting apparatus (ATTO, Japan).
2.4. Experimental design
Semen samples were collected from he-buffalo by standard procedure of artificial vagina method in the graduated collection tube. Fresh semen samples were diluted with Tris buffer. Semen samples with mass motility of 3+ or more (0-5 point scale) and individual progressive motility of 70 percent or more were selected.Fresh semen samples were evaluated with the routine semen quality parameters such as mass motility, individual progressive motility,total spermatozoa concentration, viability, total sperm abnormality,acrosomal integrity, plasma membrane integrity and cholesterol content of spermatozoa. In addition to the semen quality parameters,in-vitro zona binding ability assay was performed in anti-PDC-109 antibodies treated post-thaw semen samples. Egg yolk Tris glycerol(EYTG) extender was prepared with the following composition: Tris(hydroxyl methyl) amino methane: 3.028 g; citric acid monohydrate:1.675 g; fructose: 1.250 g; penicillin G sodium: 500-1 000 IU/mL;streptomycin sulphate: 500-1 000 μg/mL; double-glass distilled water up to: 100 mL; glycerol: 7% (v/v) and egg yolk: 10% .
A total of 24 semen samples were collected and aliquoted equally for the four experimental groups. Different concentrations of anti-PDC-109 antibodies (group 1: 0 μg/mL, group 2: 266 μg/mL anti-PDC-109 antibodies, group 3: 80 μg/mL anti-PDC-109 antibodies and group 4: 26 μg/mL anti-PDC-109 antibodies) were included in the semen extender. The extender for the control group (group 1)contained no anti-PDC-109 antibodies. The final pH of extender used in all three groups was adjusted to 6.8-7.0. These experimental groups were incubated at 37 ℃ for a period of 10 min with the purpose of PDC-109 protein-antibody interaction and to allow binding of PDC-109 protein with anti-PDC-109 antibodies, followed by further dilution upto 60×106cells/mL with EYTG extender.Extended semen samples were filled in 0.5 mL straw (IMV, L’Aigle,France).
2.5. Semen freezing and evaluation
Each group of semen samples was extended to get final concentration of 60×106spermatozoa per mL with the semen extender. Extended semen samples of both control and treatment groups were filled in polyvinyl chloride straws (0.5 mL; IMV,L’Aigle, France) with automatic filling and sealing machine (IMV,L’Aigle, France). These filled straws were simultaneously cooled from 37 ℃ to 5 ℃ in a cold cabinet (IMV, L’Aigle, France) at a rate of 0.2 ℃-0.3 ℃ per min and maintained for 4 h at 5 ℃ in a cold cabinet(pre-freeze evaluation). These straws were subsequently wipecleaned with sterile tissue paper, dried and spread over the freezing rack (IMV, L’Aigle, France). The freezing rack with straws was kept in a biological programmable freezer (IMV, L’Aigle, France)for freezing (final temperature maintained for 12 min at -124 ℃)followed by plunging of straws into the liquid nitrogen (-196 ℃)and therein stored. During the time of semen evaluation, the liquid nitrogen stored semen straws were taken out of the cryo-cans and thawed in water bath for 30 s at 37 ℃. Semen quality parameters such as forward progressive motility, viability (Hoechst 33258-PI), total sperm abnormality (eosin-nigrosin staining technique),acrosomal integrity (Giemsa staining), plasma membrane integrity[hypo-osmotic swelling test (HOST)] and cholesterol content of spermatozoa (kit) were measured at both pre-freeze and post-thaw stages for group 1, 2, 3 and 4. Based on the results obtained in the semen quality parameters, group 1 and group 2 were selected and analysed the in-vitro capacitation by chlortetracycline (CTC) assay and homologous in-vitro zona binding ability assay at post-thaw stage of sperm cryopreservation. The details of these experimental procedures were briefly described below.
2.6. Semen evaluation
2.6.1. Mass activity and sperm concentration
Fresh semen samples were evaluated for mass motility, sperm concentration (millions/mL; Sperm Quality Analyzer, SQA-Vb,Medical Electronic Systems), progressive forward motility, viability,total sperm abnormality, plasma membrane integrity, acrosomal integrity and cholesterol content of spermatozoa. The mass activity of the semen sample was determined by assessing the motility of the freshly ejaculated spermatozoa just after semen collection. This was assessed by placing a drop of fresh semen on a clean, grease free glass slide without cover slip mounted on a thermo-stage maintained at 37 ℃ under low power of microscope. It was graded on the scale of 0 to +5, where ‘0’ stands for all dead sperm and +5 indicates ejaculate with 100% motile spermatozoa showing extremely rapid waves. The concentration of spermatozoa (millions/mL) in fresh semen was determined by semen quality analyzer (SQA-Vb,Medical Electronic Systems). The concentration was assessed with the aim of ascertaining the final dilution rate of semen.
2.6.2. Sperm forward progressive motility
Sperm progressive forward motility was measured as the percentage of progressively motile spermatozoa after proper dilution of the semen. Motility was measured by placing a drop of extender diluted semen on a clean, grease-free glass slide mounted on a thermo stage (37 ℃) under high-power objective (Nikon, Eclipse 80i;magnification of 400×) after covering with a cover glass. Individual forward progressive motility was measured as the percentage of the spermatozoa with anterior progressive forward motility.
2.6.3. Spermatozoa viability
Live sperm percentage in the semen aliquots was measured by staining with the fluorochromes Hoechst 33258 (H33258,Sigma Aldrich, India) and propidium iodide (PI) (P4170, Sigma Aldrich, India) as per the method described by de Andrade et al[27]. Spermatozoa were stained simultaneously with PI and Hoechst 33258. A stock of Hoechst 33258 solution was prepared at a concentration of 1 mg/mL in double distilled water and these aliquots were stored frozen at -20 ℃ in the dark. The stock solution was diluted to 1:10 with phosphate buffered saline (PBS) medium,stored at 5 ℃ and shielded from light and was used within a week. A stock solution of 0.50 mg PI/mL was prepared, aliquoted and stored frozen at -20 ℃ in the dark. Spermatozoa were diluted in the saline medium (PBS) for staining and the working Hoechst 33258 solutions were added and the final concentration was 1 μg/mL. Simultaneously,the PI stock solution was added and the final concentration was 5 μg/mL. The sperm suspension was incubated for 5 min at room temperature and spermatozoa were immobilized with formaldehyde[final concentration: 0.003% (w/v)]. Bovine serum albumin (BSA)stock solution (5 μL of 100 mg/mL) was added to avoid spermatozoa sticking to the glass and producing staining artefacts. The stained suspension (10 μL) was placed between a slide and a coverslip with 10 μL of antifade solution of 0.22 M 1, 4-diazo-bicyclo (2, 2, 2)octane in order to preserve fluorescence and sealed with nail varnish.A total of 200 spermatozoa were counted per slide and categorized.
2.6.4. Total sperm abnormality by eosin-nigrosin staining technique
Total sperm abnormalities were assessed by eosin-nigrosin staining technique. The staining procedure was described as follows. Eosin Y yellow (0.67 g) and sodium chloride (0.9 g) were dissolved in 100 mL distilled water under gentle heating. Then, 10 g of nigrosin was added. The solution was brought to the boil and allowed to cool to room temperature (20 ℃) after which it was filtered through filter paper and stored in a dark and sealed glass bottle. Before use, the staining solution was brought to room temperature.Approximately, equal volumes of semen and stain were mixed.One droplet (50 μL) of well mixed semen sample was mixed in a ceramic well with one droplet of the eosin-nigrosin staining solution(50 μL). The suspension was incubated for 30 s at room temperature(20 ℃). Then, a 12 μL droplet was transferred with the pipette to a labelled microscope slide where it was smeared by sliding a cover slip in front of it. Two smears were made from each sample. The smears were air dried and examined directly. At least 200 sperm were assessed at a magnification of 1 000× under oil immersion with a high-resolution 100× bright field objective and Koehler corrected illumination (Nikon, Eclipse 80i; magnification 1000×). Sperm with white (unstained) were classified as live and those showed any pink or red coloration were classified as dead, with the sole exception for sperm with a slight pink or red appearance restricted to the neck region (leaky necks), which were assessed as live. Reliability and repeatability of assessments were supervised through internal quality control where inter-individual variation was <10% .
2.6.5. Acrosomal integrity by fluorescein isothiocynate labelled pisum sativum agglutinin (PSA) lectin
Concentration of spermatozoa (×106/mL) was assessed in fresh,pre-freeze and post-thaw semen samples. The percentage of spermatozoa with intact acrosome was assessed in semen samples using fluorescein-labelled lectin from the peanut plant, Arachis hypogaea (fluorescein isothiocynate labelled pisum sativum agglutinin lectin; FITC-PSA) with slightly modified method[28].Modification was that the PBS buffer was used instead of HEPES(N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer and excess PI was removed by diluting the contents into seven to eight times and centrifugation was done instead of filtration process. Slides were examined within 2 h under the fluorescence microscope (Nikon Microphot FXA EPI-FL3; Nikon, New York, NY, USA) with FITC filter set at 400× magnification. PSA-positive sperm revealed green to yellowish green fluorescence whereas PI-positive spermatozoa revealed red-coloured nuclear material indicated as the sperm membrane has been damaged as intact membrane was impermeable to PI. Sperm cells with retained staining on the equatorial segment were considered as the fully acrosome reacted as these sperm cells were become totally devoid of PSA staining. These PI-positive cells were excluded from the estimation of acrosome-intact and acrosomereacted live spermatozoa. Only PSA-positive and PI-negative spermatozoa were considered as acrosome-intact live. A total of 200 spermatozoa was counted per slide and categorized as follows: PSA positive and PI negative: acrosome-intact live; PSA positive and PI positive: acrosome-intact dead; PSA negative and PI negative:acrosome-reacted live and PSA negative and PI positive: acrosomereacted dead.
2.6.6. Plasma membrane integrity by HOST
Functional plasma membrane integrity of spermatozoa was estimated with HOST. Hypo-osmotic solution is needed to assess the swelling pattern of the sperm. The hypo-osmotic solution was prepared with solution-A (0.734 g sodium citrate) and solution-B(1.351 g fructose) in 100 mL double distilled water. Solution A and B were taken as 0.50 mL each in a clean, pre-warmed(37 ℃) test tube to prepare 1 mL of hypo-osmotic solution of 150 mOsm/L. This solution was always prepared freshly before each test. The prepared hypo-osmotic solution was tested with advanced micro-osmometer (Model No: 3MO, USA)to ensure that osmolality of the solution remains 150 mOsm/L. The hypo-osmotic swelling response was measured as described by Selvaraju et al[29]. A total of 200 spermatozoa were counted per slide and categorized as Pattern A: no swelling and no membrane reaction;Pattern B: swelling of the tip of the tail; Pattern C: different types of hairpin such as swelling pattern or swelling of the mid-piece and Pattern D: complete tail swelling. Spermatozoa displaying pattern B, C or D were considered as HOST positive and maintained higher plasma membrane integrity at fresh, pre-freeze and post-thaw stages.
2.6.7. In-vitro capacitation status by CTC staining
CTC staining was used to determine the capacitation status of spermatozoa[30]. Based on the results obtained in the semen quality parameters, group 1 and group 2 were selected and analysed the in-vitro capacitation by CTC assay at post-thaw stage. The CTC fluorescence was observed with a Nikon microscope equipped with phase-contrast and epifluorescent optics. Post-thaw semen samples were processed in duplicate and 200 spermatozoa per slide were examined and classified according to one of the three staining patterns as described by Rajoriya et al[30]. These patterns were pattern F: uniform bright fluorescence over the whole head,characteristic of non-capacitated cells; pattern B: fluorescence-free band in post-acrosomal region (capacitated cells) and pattern AR:dull fluorescence over the whole head except for a thin punctuate band of fluorescence along the equatorial segment (acrosome-reacted cells). No fluorescence was observed when CTC stain was omitted from the preparation.
2.6.8. In-vitro zona binding assay
This assay was performed to assess the effect of anti-PDC-109 antibodies on zona binding ability of the spermatozoa. Based on the results obtained in the semen quality parameters, group 1 and group 2 were selected and analysed the in-vitro zona binding assay at postthaw stage. Semen samples of the two groups (group 1 and group 2)were subjected to homologous (buffalo oocyte) in-vitro zona-binding assay at post-thaw stage. The zona binding ability of the bubaline spermatozoa was assessed as per the method used by Puglisi et al[31].
2.6.8.1. Collection of ovaries
Ovaries from mature buffaloes were collected from local abattoir immediately after slaughter and transported to the laboratory in a sterile and chilled normal saline solution supplemented with antibiotics. The ovaries were washed several times (6-8 times) with normal saline solution fortified with antibiotics before transportation.
2.6.8.2. Collection of oocytes
Follicular fluid from surface follicle (>2 mm) of buffalo ovaries was collected by aspiration method with 18 G needle attached to a 5 mL syringe containing oocyte collection medium. The collected cumulus oocyte complexes (COCs) were poured in a 50 mL centrifuge tube and kept undisturbed for half an hour in a CO2incubator at 39 ℃, 5% CO2and 95% humidity to allow the COCs to settle. The supernatant was poured off and the sediments containing the COCs were poured into large squared searching petri dish containing oocyte collection medium. Morphologically culturable oocytes i.e. those having compact multilayered COCs and evenly granulated cytoplasm were selected under a stereozoom microscope and transferred to another petri-dish containing oocyte collection medium. The selected COCs were washed six times with oocyte collection medium and finally four washing in oocyte maturation media were carried out.
2.6.8.3. In-vitro maturation of oocytes
The COCs were cultured in 50-100 μL (10-12 oocytes/drop)of maturation media under mineral oil in petridish. They were incubated at 39 ℃, 5% CO2and 95% humidity for 24 h. After 24 h maturation, the ova were assessed under stereo zoom microscope for maturation according to their expanded cumulus cell mass.
2.6.8.4. Sperm capacitation and in-vitro insemination of matured oocytes
Capacitation of spermatozoa was carried out by modified tyrode′s albumin lactate pyruvate (TALP) medium as a capacitation medium covered with mineral oil in 5% CO2and 95% humidity in an Eppendorf tube. Post-thaw semen samples were washed using noncapacitating medium to remove the dead cells. Spermatozoa were then washed twice with the sperm TALP by centrifugation at 170×g for 10 min. Spermatozoa suspension was resuspended in fertilization TALP and concentration was adjusted at 5×107spermatozoa per mL.The aliquots were placed in Eppendorf tube in 5% CO2incubator with 95% humidity at 37 ℃ for 20 min.
After 24 h of maturation, oocytes were removed by gentle pipetting with capillary tube and transferred in disc containing fertilization TALP followed by 6 to 8 times washing in fertilization TALP. After washing, 10 oocytes were placed in 50 μL of fertilization TALP and 20-30 μL of capacitated spermatozoa suspension was added to it and co-incubated at 39 ℃, 5% CO2and 95% humidity for 4 to 6 h in CO2incubator. To remove the free and loosely attached spermatozoa to zona pellucida, fresh fertilization TALP was added to droplets 1 to 2 h later for washing and process was repeated 2 to 3 times. Thereafter, droplets containing oocytes and spermatozoa were again transferred back to CO2incubator for remaining period of incubation.
2.6.8.5. Evaluation of sperm zona binding attachment
After 4 to 6 h of incubation, the penetrated oocytes were washed and processed further for counting the number of spermatozoa bound to zona pellucida (binding index). The oocyte-sperm complex was fixed with 2.5% glutaraldehyde in PBS for 10 min. The fixed oocyte-sperm complex was washed with PBS, stained with 1 μg/mL of Hoechst H33258 stain (Sigma Aldrich, India)for 10 min. The stained oocyte-sperm complex was washed with PBS. The washed oocyte-sperm complex was placed on glass slide,slightly compressed with a coverslip and sealed. The number of spermatozoa bound to zona pellucida of oocyte was counted under Epifluorescent microscope with UV filter. A total of 60 oocytes were used to estimate the zona pellucida binding of each group in postthaw spermatozoa. To minimize the variation, subjective scoring system was followed in the present investigation; each sample was evaluated by two co-authors and their averages were analysed statistically.
2.6.9. Cholesterol content of spermatozoa
Washing of spermatozoa was necessitated to estimate the cholesterol content and capacitation status of sperm (CTC stain) at fresh, pre-freeze and post-thaw stages. Spermatozoa were washed immediately after initial evaluation and selection of ejaculates using Percoll density gradient method to remove the egg yolk (EY)particles, dead cells and debris. One mL layer of 40% Percoll (v/v, Sigma-Aldrich, USA) in non-capacitating medium (KCl:2.7 mM, KH2PO4: 1.5 mM, Na2HPO4: 8.1 mM, NaCl: 137 mM,Glucose: 5.55 mM and Pyruvate: 1 mM) was pipetted carefully over a 1 mL layer of 80% Percoll (v/v in non-capacitating medium) in a disposable 15 mL centrifuge tube. One mL of fresh, pre-freeze and post-thaw semen was gently layered on the top of the two steps Percoll column. This test tube was centrifuged at 400×g for 30 min.After centrifugation, the pellets were washed once again with noncapacitating medium and resuspended in non-capacitating medium to make 100 million spermatozoa per mL. This procedure has to be done immediately after semen collection in fresh semen and immediately after freezing-thawing in the cryopreserved semen.After this procedure, the final content was splited into aliquots of 1 mL in cryovials and stored in deep freezer at -20 ℃ until used for cholesterol estimation.
Cholesterol content of spermatozoa was measured as per the method described by Iverson et al[32] with some minor modifications.A total of 100 million washed spermatozoa were taken into a 10 mL vial. The sperm pellet was extracted with 20 volumes of chloroform: methanol (1:1, v/v) solution and vortexed for 20 s.Thereafter, it was centrifuged at 800×g for 5 min. Spermatozoa were evaporated to dryness under liquid nitrogen gas and kept at -20 ℃.Chloroform 0.5 mL was added at the time of estimation to each vial and cholesterol content was estimated by commercially available cholesterol estimation assay kit (Span Diagnostics Ltd., India, kit No. 001CH010).
2.7. Statistical analysis
The data were statistically analysed as per standard methodologies.Analysis of variance (ANOVA) was done using a generalized liner model (Statistical Analysis System for Windows, SAS Version 9.3;SAS Institute, Inc., Cary, NC, 2001) and treatment means were separated using Student-Newman-Keuls (SNK) multiple range test. The data used in the study were tested for normality before analysis using Shapiro-Wilk statistics. Means were analyzed by one-way ANOVA, followed by Tukey’s post hoc test to determine the significant differences among the control and treatment groups and paired t test was performed between the pre-freeze and post thaw stages for the different semen quality parameters, patterns of capacitation and in-vitro zona binding index using the SAS software/PC computer program. The mean values were expressed as mean±standard deviation (mean±SD). Differences with values of P<0.05 were considered to be statistically significant.
2.8. Ethics statement
The experimental procedure was approved by the Institutional Animal Ethics Committee of ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, Uttar Pradesh, India for research scholar bearing the admission No: 1358 dated 30/10/2014. All animal experiments were performed according to the international guidelines on ethical use of animals.
3. Results
Semen quality parameters of freshly collected buffalo semen were presented in Table 1. Semen quality parameters of neat semen were within the normal range of bubaline species. Sperm motility, livability, acrosomal integrity, plasma membrane integrity,and cholesterol content of sperm were decreased and total sperm abnormality was increased significantly (P<0.05) in post-thaw compared to those in pre-freeze and fresh semen. Group 2 treated semen had higher sperm motility, livability, acrosomal integrity,plasma membrane integrity, and cholesterol content of sperm and lower total sperm abnormality significantly (P<0.05) compared to group 1, 3 and 4 at pre-freeze and post-thaw stages in buffalo.Group 2 treated sperm had higher pattern F (non-capacitated), and lower pattern B (capacitated) and pattern AR (acrosome reacted) and higher zona binding index compared to those in the untreated control group. Thus, the sperm treated with group 2 treatment had significant benefits compared to other treatment and control groups at pre-freeze and post-thaw stages in buffalo. These results indicated that anti-PDC-109 antibodies reduced the cryoinjury or cryodamage in bubaline sperm.
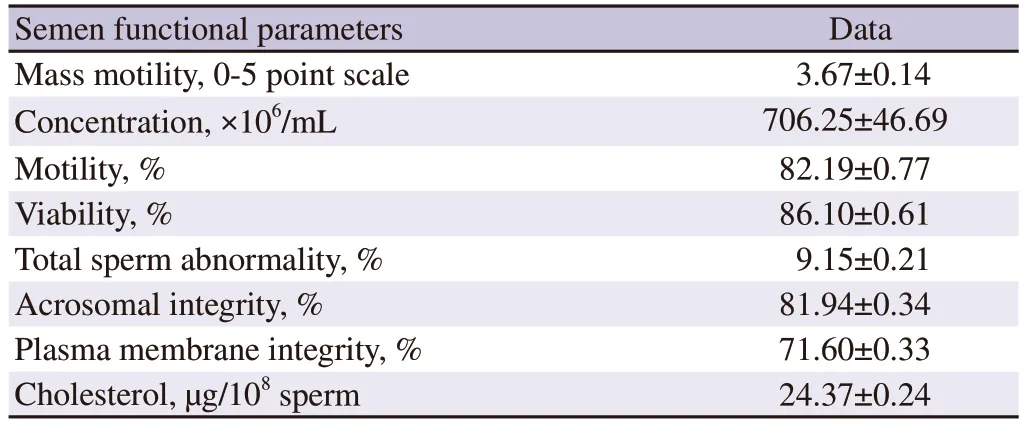
Table 1. Sperm functional parameters of buffalo semen at fresh stage (n=24).
3.1. Forward progressive motility
Forward progressive motility of spermatozoa of different groups was examined at the end of equilibration period for pre-freeze evaluation and after thawing for post-thaw evaluation. Forward progressive motility was differed significantly (P<0.05) among the experimental groups and between the pre-freeze and post-thaw stages. Forward progressive motility was reduced from pre-freeze to the post-thaw at the rate 30% , 12% , 15% and 19% , respectively in group 1, 2, 3 and 4. Forward progressive motility of spermatozoa was significantly higher in group 2 as compared to those in the control and other treatment groups both at pre-freeze and post-thaw stages (P<0.05). Spermatozoa of group 2 had 10% , 5% and 4% higher motility compared to those in the control, group 3 and group 4 at pre-freeze stage of semen preservation. Similarly, spermatozoa of group 2 had 26% , 6% and 11% higher motility compared to those in the control, group 3 and group 4, respectively at post-thaw stage.Group 2 had highest motility followed by group 3, and group 4 and lowest was noted in group 1 (the control group) (Figure 1).
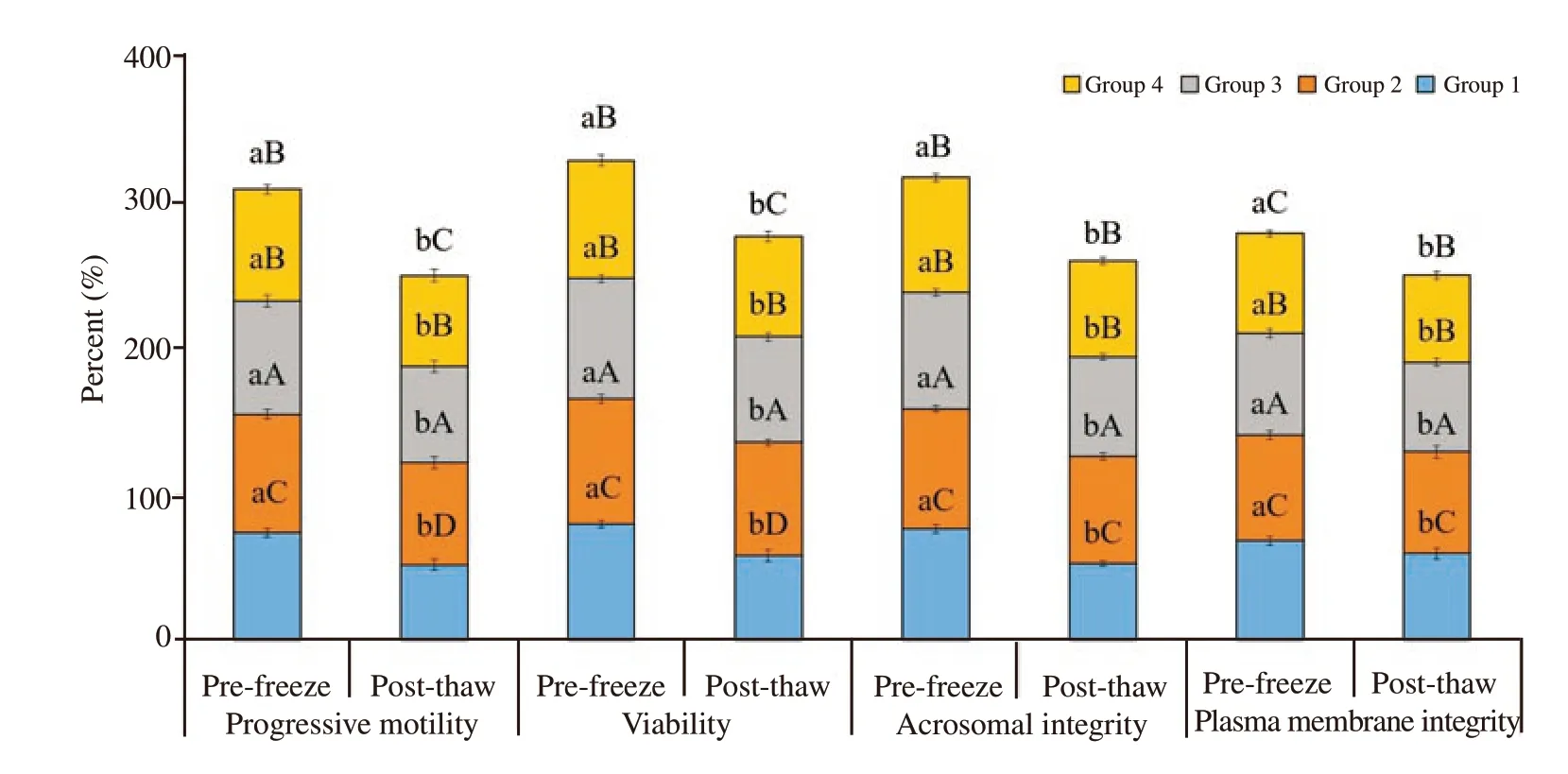
Figure 1. Effect of buffalo anti-PDC-109 antibodies on percentage of progressive motility, viability, acrosomal integrity and plasma membrane integrity of buffalo spermatozoa at pre-freeze and post-thaw stage (mean±SD). Vertical bar on each point represents standard deviation (SD). Different small alphabets(a, b) denote the means are differed significantly between pre-freeze and post-thaw for different semen quality parameters ((P<0.05). Different capital alphabets (A, B, C, D) denote the means are differed significantly between the different experimental groups within pre-freeze and post-thaw for different semen quality parameters (P<0.05). Group 1: the control group (EYTG extender; without anti-PDC-109 antibodies), Group 2: 266 μg/mL of anti-PDC-109 antibodies, Group 3: 80 μg/mL of anti-PDC-109 antibodies and Group 4: 26 μg/mL of anti-PDC-109 antibodies. n=24 samples.
3.2. Viability
Sperm viability percentage showed a significant difference among the experimental groups and between the pre-freeze and post thaw stages (P<0.05). Sperm viability was reduced at the rate of 28% , 8% ,13% and 15% from pre-freeze to post-thaw stage in group 1, 2, 3 and 4, respectively. Viability was significantly higher in group 2 followed by in group 3, group 4 and group 1 in both pre-freeze and post-thaw stages. Spermatozoa of group 2 had 7% , 4% and 6% higher viability compared to those in group 1, 3 and 4, respectively at pre-freeze stage. Similarly, spermatozoa of group 2 had 26% , 7% and 12% higher viability compared to those in group 1, 3 and 4, respectively at post-thaw stage (Figure 1).
3.3. Total sperm abnormality
Total sperm abnormality was estimated at the end of equilibration period for pre-freeze and after freeze-thawing for post-thaw stage.Total sperm abnormality was increased significantly from prefreeze to post-thaw stage at the rate of 19% , 15% , 16% % and 176% respectively in group 1, 2, 3 and 4 (P<0.05). Spermatozoa treated with anti-PDC-109 antibodies had significantly lowered total abnormality compared to those in the untreated control group at prefreeze and post-thaw stages. Spermatozoa treated with group 2 had significantly lower total abnormality compared to those in group 1 (5% ), 3 (2% ) and 4 (3% ) at pre-freeze stage (P<0.05). Similarly,spermatozoa treated with group 2 had significantly lower total abnormality compared to those in group 1 (6% ), 3 (3% ) and 4 (2% )at post-thaw stage (P<0.05) (Figure 2A and Figure 3).
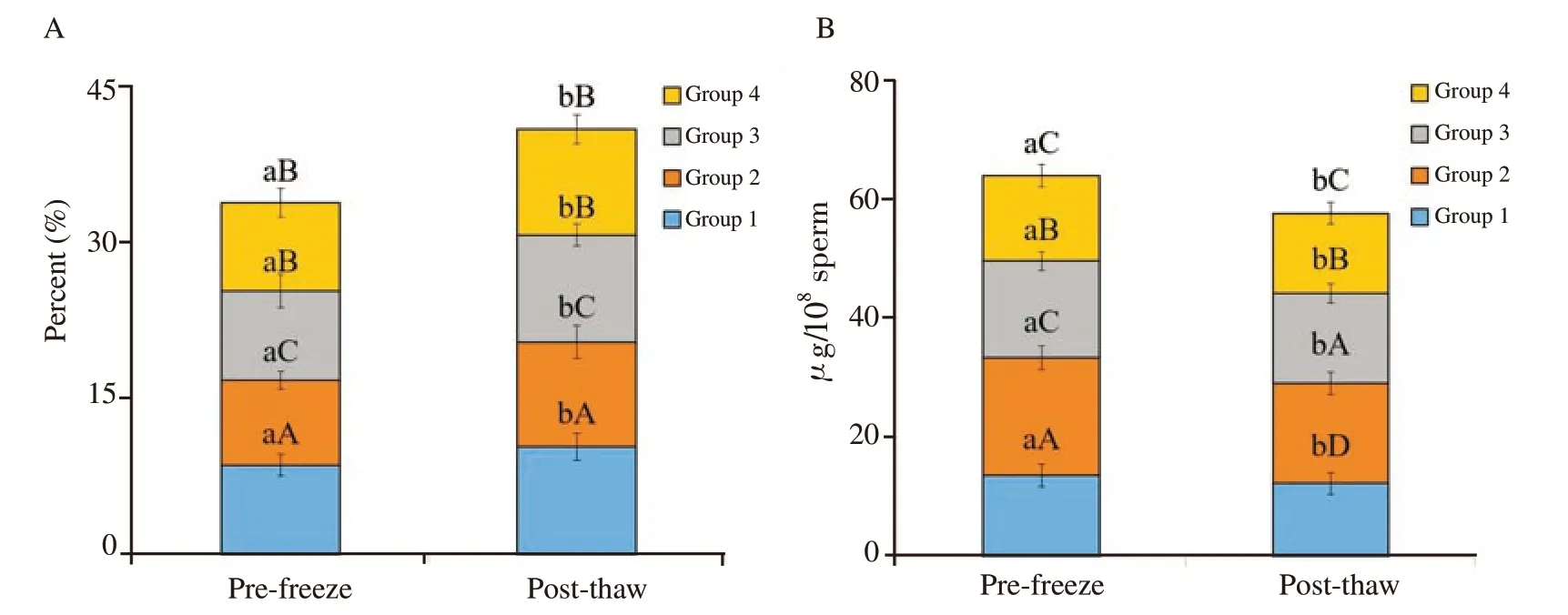
Figure 2. Effect of buffalo anti-PDC-109 antibodies on total sperm abnormality (A) and cholesterol content of buffalo sperm (B) at pre-freeze and postthaw stages (mean±SD). Vertical bar on each point represents standard deviation (SD). Different small alphabets (a, b) denote the means are differed significantly between pre-freeze and post-thaw for different semen quality parameters (P<0.05). Different capital alphabets (A, B, C, D) denote the means are differed significantly between the different experimental groups within pre-freeze and post-thaw for different semen quality parameters (P<0.05). Group 1: the control group (EYTG Dilutor; without anti-PDC-109 antibodies), Group 2: 266 μg/mL anti-PDC-109 antibodies, Group 3: 80 μg/mL anti-PDC-109 antibodies and Group 4: 26 μg/mL anti-PDC-109 antibodies. n=24 samples.
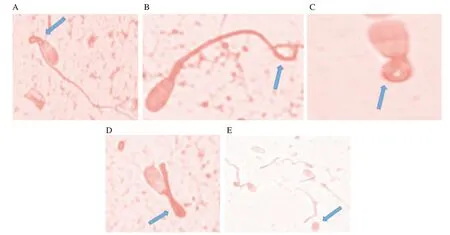
Figure 3. Spermatozoa showing different abnormalities. A: bent midpiece; B: terminally coil tail; C: dag defect; D: bent tail; E: detached head, as shown in the arrow.
3.4. Acrosomal integrity
Acrosomal integrity was analysed at fresh, pre-freeze and postthaw stages. Spermatozoa with acrosomal integrity was significantly decreased from pre-freeze to post-thaw stage at the rate of 31% ,10% , 16% and 17% respectively in group 1, 2, 3 and 4 (P<0.05).Spermatozoa treated with anti-PDC-109 antibodies had significantly higher acrosomal integrity compared to those in the untreated control group at both pre-freeze and post-thaw stages (P<0.05).Spermatozoa treated with group 2 (266 μg/mL) had significantly higher acrosomal integrity compared to those in group 1 (control;8% ), group 3 (3% ) and group 4 (5% ) at pre-freeze stage. Similarly,spermatozoa treated with group 2 (266 μg/mL) had significantly higher acrosomal integrity compared to those in group 1 (control;29% ), group 3 (8% ) and group 4 (11% ) at post-thaw (Figure 1 and Figure 4).
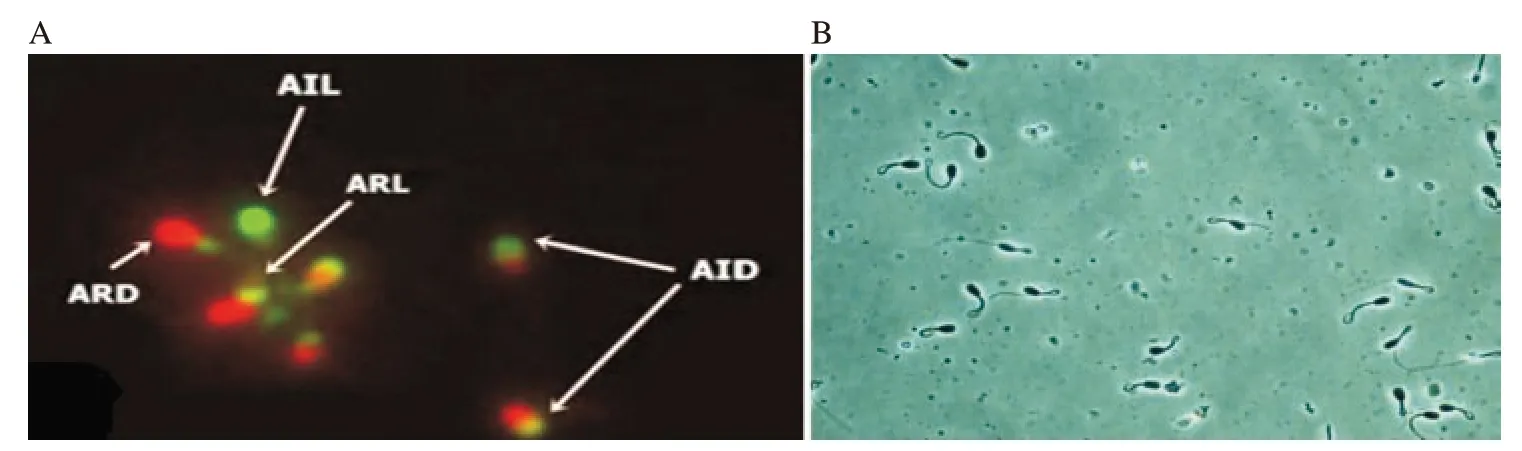
Figure 4. A: Acrosomal status and viability detected with fluorescein isothiocynate (FITC) conjugated with pisum sativum agglutinin (PSA) and propidium iodide (PI) in fresh semen sample. AIL: acrosome intact live spermatozoa (PSA positive and PI negative), AID: acrosome intact dead spermatozoa (PSA positive and PI positive), ARL: acrosome reacted live spermatozoa (PSA negative and PI negative) and ARD: acrosome reacted dead spermatozoa (PSA negative and PI positive). B: Spermatozoa showing hypo-osmotic swelling test (HOST) response.
3.5. Plasma membrane integrity
Plasma membrane integrity was analysed at fresh, pre-freeze and post-thaw stages. Spermatozoa with plasma membrane integrity was significantly decreased from pre-freeze to post-thaw stage at the rate of 14% , 4% , 13% and 15% respectively in group 1, 2, 3 and 4 (P<0.05). Spermatozoa treated with anti-PDC-109 antibodies had significantly higher plasma membrane integrity compared to those in the untreated control group at both pre-freeze and post-thaw stages (P<0.05). Spermatozoa treated with group 2 (266 μg/mL) had significantly higher plasm membrane integrity compared to those in group 1 (control; 6% ), group 3 (3% ) and group 4 (5% ) at prefreeze stage. Spermatozoa treated with group 2 (266 μg/mL) had significantly higher plasma membrane integrity compared to those in group 1 (control; 16% ), group 3 (12% ) and group 4 (15% ) at postthaw (Figure 1 and Figure 4).
3.6. Cholesterol content of spermatozoa
Cholesterol content of sperm was analysed at fresh, pre-freeze and post-thaw stages of cryopreservation. Cholesterol content of spermatozoa was significantly decreased from pre-freeze to postthaw stage at the rate of 14% , 6% , 8% and 11% respectively in group 1, 2, 3 and 4 (P<0.05). Spermatozoa treated with anti-PDC-109 antibodies had significantly higher cholesterol compared to those in the untreated control group at both pre-freeze and postthaw stages (P<0.05). Spermatozoa treated with group 2 (266 μg/mL)had significantly higher cholesterol compared to those in group 1 (control; 31% ), group 3 (19% ) and group 4 (28% ) at pre-freeze stage. Group 2 (266 μg/mL) had significantly higher cholesterol compared to those in group 1 (control; 27% ), group 3 (12% ) and group 4 (21% ) at post-thaw (Figure 2B).
3.7. CTC staining pattern
CTC staining assay was done to assess the effect of anti-PDC-109 antibodies on capacitation status. Based on the results obtained in the semen quality parameters, group 1 and group 2 were selected and analysed the in-vitro capacitation by CTC assay at post-thaw stage and presented the results. Spermatozoa treated with anti-PDC-109 antibodies (group 2; 266 μg/mL) had higher pattern F(non-capacitated; 64.00% vs. 52.33% ) and had lower pattern B(capacitated; 35.17% vs. 26.33% ) and pattern AR (acrosome reacted;12.08% vs. 9.67% ) compared to those in the untreated control group(P<0.05) at post-thaw stage. Ratio of different patterns of CTC staining assay (pattern F, B and AR) was 5.2:3.5:1.3 in group 1 and the corresponding ratio was 6.4:2.5:1.1 in group 2 at post-thaw stage of semen cryopreservation in bubaline species (Figure 5).
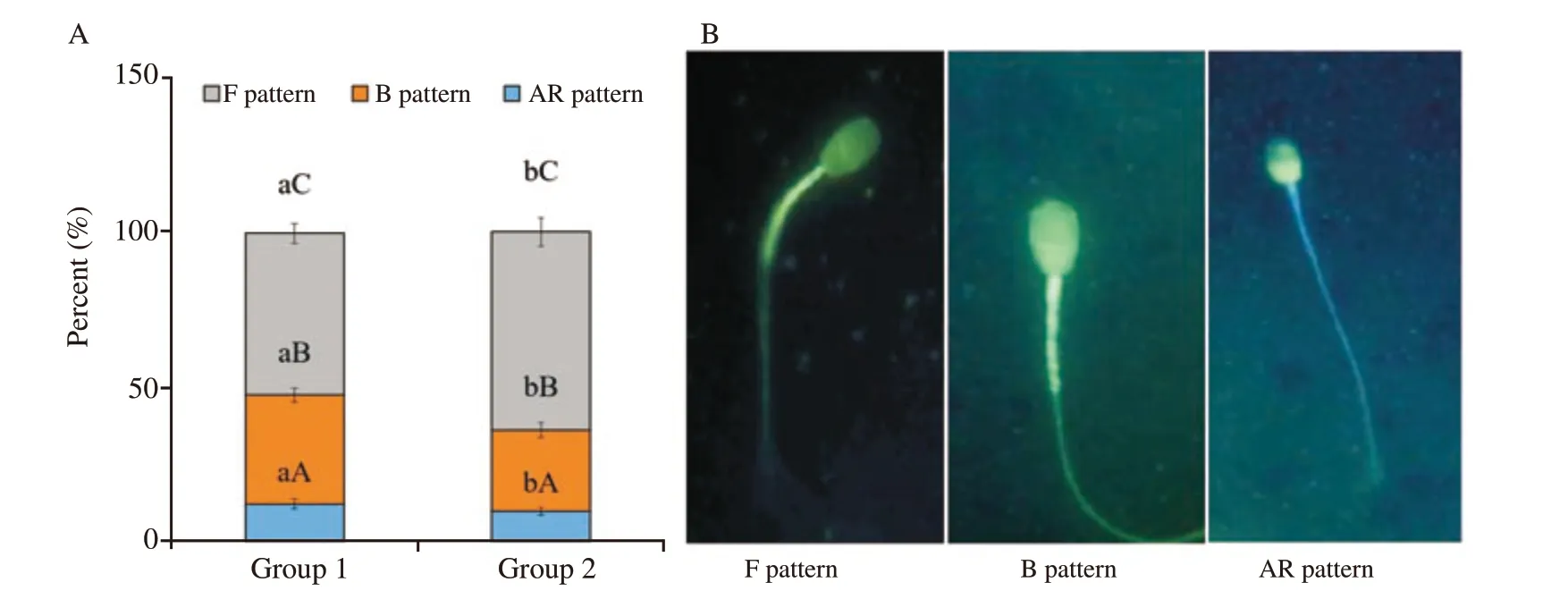
Figure 5. A: Effect of buffalo anti-PDC-109 antibodies on different chlortetracycline (CTC) staining patterns of buffalo sperm at post-thaw stage(mean±SD). Vertical bar on each point represents standard deviation. Different small alphabets (a, b) denote the means are differed significantly between group 1 and group 2 (P<0.05). Different capital alphabets (A, B, C) denote the means are differed significantly between the different CTC patterns within the experimental groups (P<0.05). Group 1: the control group (EYTG extender; without anti-PDC-109 antibodies), Group 2: 266 μg/mL anti-PDC-109 antibodies. n=12 samples. B: Three patterns obtained from CTC stained viable buffalo spermatozoa, i.e. F pattern: non capacitated spermatozoa (uniform bright fluorescence over the whole head), B pattern: capacitated spermatozoa (fluorescence in the acrosomal region and fluorescence free band in the post acrosomal region), AR pattern: acrosomal reacted (dull fluorescence over the whole head except thin punctuate band of fluorescence along the equatorial segment).
3.8. In-vitro zona binding ability assay
In-vitro zona binding ability assay was done to assess the effect of anti-PDC-109 antibodies on fertilization capacity of the sperm.Based on the results obtained in the semen quality parameters, group 1 and group 2 were selected and analysed the in-vitro zona binding ability assay at post-thaw stage and presented the result.Spermatozoa treated with anti-PDC-109 antibodies (group 2;266 μg/mL) had significantly higher zona binding index (55.63 vs.44.37) compared to those in the untreated control group (P<0.05) at post-thaw stage (Figure 6).
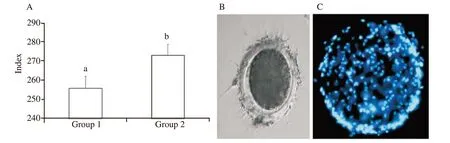
Figure 6. A: Effect of buffalo anti-PDC-109 antibodies on zona binding index of buffalo sperm at post-thaw stage (mean±SD). Vertical bar on each point represents standard deviation (SD). Different small alphabets (a, b) denote the means are differed significantly between group 1 and group 2 (P<0.05).Group 1: the control group (EYTG extender; without anti-PDC-109 antibodies), group 2: 266 μg/mL anti-PDC-109 antibodies. n=12 samples. B: Buffalo spermatozoa bound to zona pellucid of a matured oocyte; C: Buffalo spermatozoa bound to zona pellucid of a matured oocyte. The nucleus of the bound spermatozoa are stained with nucleic acid stain Hoechst 33258.
4. Discussion
This is the first study to assess the effect of purified buffalo anti-PDC-109 antibodies from buffalo semen on semen quality parameters, cryoinjury status, sperm cholesterol content and invitro zona binding ability. Earlier studies reported that free unbound seminal PDC-109 protein has detrimental effect on semen quality parameters and fertility profiles and degree of this detrimental effect was dose and time dependent[20,21]. Important observation was noted in the present experiment that spermatozoa treated with 266 μg/mL anti-PDC-109 antibodies in TFC buffer (group 2) had significantly higher semen quality parameters and zona binding index compared to those in group 3 (80 μg/mL anti-PDC-109 antibodies) and group 4 (26 μg/mL anti-PDC-109 antibodies). This might be due to significant reduction of free-floating PDC-109 protein concentration by anti-PDC-109 antibodies. Anti-PDC-109 antibodies decreased the PDC-109 protein triggered metabolic activity which in turn reduced the phospholipid and cholesterol efflux from the sperm plasma membranes; subsequently, the destabilization of sperm plasma membranes was reduced resulting into higher sperm motility at pre-freeze and post-thaw stages. Srivastava et al[21] reported that sequestration of PDC-109 with anti-PDC-109 antibodies at 1:1 ratio had significantly improved the sperm motility in bovine species.The results of the present study clearly indicated that presence of antibodies in premeditated 1/10th (80 μg/mL anti-PDC-109 antibodies) and 1/30th (26 μg/mL anti-PDC-109 antibodies) doses in group 3 and group 4 were not sufficient to sequestrate the PDC-109 protein completely led to loss of motility in bubaline species.Alternatively, a continuous stimulation of spermatozoa metabolism by remaining free floating PDC-109 protein in the seminal plasma due to incomplete sequestration in group 3 and group 4 might lead to energy loss and lower sperm motility. However, spermatozoa treated with anti-PDC-109 antibodies (266 μg/mL, 80 μg/mL, and 26 μg/mL) had higher benefits as compared to those processing without anti-PDC-109 antibodies for semen quality parameters and fertility profiles. Results of the present study clearly indicated that effect of anti-PDC-109 antibodies on sperm cryopreservation was dose dependent that 266 μg/mL anti-PDC-109 antibodies in TFC buffer was optimum and suitable dose for bubaline semen cryopreservation to get maximum sequestration of PDC-109 protein and higher motility. Lower than the optimum dose of anti-PDC-109 antibodies causes insufficient sequestration of PDC-109 leads to higher availability of PDC-109 protein in the semen. This in turn triggers the cholesterol and phospholipid efflux from sperm plasma membranes resulting in alteration of the capacitation and acrosomal reaction. Excess cholesterol and phospholipid efflux triggers the adverse effect on the fluidity of the plasma membranes resulting in lower sperm motility. Similar result was obtained in group 3 and group 4[33]. However, it is needed to study the effect of anti-PDC-109 antibodies at dose higher than 266 μg/mL on semen quality parameters and fertility profiles to confirm the more suitable/optimal dose of anti-PDC-109 antibodies on sperm cryopreservation in bubaline species.
Spermatozoa treated with group 2 (266 μg/mL anti-PDC-109 antibodies) had higher viability compared to those in other treatment and control groups. Similar result was reported in cattle that inclusion of anti-PDC-109 antibodies in semen extender contained PDC-109 at 1:1 ratio had significantly improved the sperm viability[21]. It could be considered that higher sequestration of PDC-109 protein in the ejaculate by its antibodies in group 2 could have decreased the detrimental effect of the protein vis-à-vis spermatozoa viability. PDC-109 protein concentration was decreased in anti-PDC-109 antibodies treated semen as these antibodies sequestrated the PDC-109 protein. This in turn improved the metabolic activity of sperm and cholesterol and phospholipids effluxes were decreased from the plasma membranes which subsequently reduced the sperm membrane destabilization resulting in higher sperm viability in group 2. However, similar beneficial effects were absent in group 3 and group 4, which might be due to presence of insufficient quantities of anti-PDC-109 antibodies to trigger the desired sequestration resulting in viability loss. Binding of the antibodies to any one type of BSPs prevents the interaction of heparin with the other BSPs possibly by stearic hindrance[34]. Therefore, it could be considered that the unbound free floating PDC-109 protein content of ejaculate was sequestered by antibodies and led to significantly higher sperm viability in group 2, 3 and 4 as compared to the untreated control.The beneficial effect of sequestration of PDC-109 protein had manifested itself at both pre-freeze and post-thaw semen in the present study in bubaline species.
Spermatozoa treated with group 2 had significantly lower total abnormality compared to those in group 3, group 4 and group 1. Literatures on inclusion of premeditated dose of anti-PDC-109 antibodies on total sperm abnormalities in buffalo semen cryopreservation were scanty. Similarly, acrosomal integrity was significantly higher in group 2 compared to other treatment and control groups. Similar result was obtained in bovine species that anti-PDC-109 antibodies and PDC-109 at 1:1 ratio had increased the acrosomal integrity[21]. BSP-30 kDa and PDC-109 proteins are able to trigger the specific phospholipid and cholesterol efflux upto(29.5±4.7)% [35] which in turn stimulates the lipid reorganization in the plasma membranes resulting in higher membrane permeability to Ca2+, HCO3and K+[36]. Higher concentration of these intracellular ions is needed to initiate the acrosome reaction and fusion of sperm with oocyte for fertilization[37]. Inclusion of optimum dose of PDC-109 in the semen extender enhances the protective effect on sperm plasma membranes; on other hand, in normal course of cryopreservation process, the semen samples contain ample quantity of PDC-109 protein which in turn triggers the harmful effects on the stability of plasma membranes. This was the reason in present study that when maximum quantity of PDC-109 was sequestered from spermatozoa by inclusion of antibodies as in group 2, a higher response of acrosome integrity was obtained. It could be safely considered that addition of higher dose of anti-PDC-109 antibodies in the extender would lead to desired protection as in the case of acrosome integrity.
Plasma membrane integrity is an important marker to determine the metabolism and fertilizing ability of the spermatozoa[29]. In the present study, HOST reactive spermatozoa were significantly higher in group 2 compared to those in other three groups. HOS positive spermatozoa were decreased as dose of anti-PDC-109 antibodies decreased from group 3 to group 4 which clearly indicated that the dose dependent variation in the pre-freeze and post-thaw plasma membrane integrity of the sperm was observed. Lower plasma membrane integrity in lower doses of anti-PDC-109 antibodies might be due to deficiency of antibodies in binding with PDC-109 protein led to higher cholesterol efflux which in turn affected the fluidity and stabilization of sperm plasma membranes[21]. On the contrary, non-availability of anti-PDC-109 antibodies in the extender of the control group, excessive binding of PDC-109 protein triggered adverse effect on plasma membranes led to poor plasma membrane integrity and sperm viability[21]. However, optimum dose of anti-PDC-109 antibodies was determined in the cattle species[21]. Similar observation was reported in bubaline species in the present study.
Cholesterol content of spermatozoa was significantly higher in group 2 as compared to in other treatment and control groups.Similarly, in cattle, treatment of PDC-109 with anti-PDC-109 antibodies at 1:1 ratio had reduced the phospholipid and cholesterol efflux with simultaneous enhancement of sperm cholesterol content[21]. Moreover, Manjunath and Therien[38] found that cholesterol efflux from epididymal spermatozoa was dependent upon the PDC-109 protein concentration and its exposure time.Further, they[38] revealed that exposure of sperm to BSPs for 15-30 min triggered the cholesterol efflux at the rate of 7% to 15% from sperm plasma membranes and when the exposure time increased to 4 h led to higher cholesterol efflux for upto 25% .Hence, the cholesterol loss stimulates more stress to the spermatozoa and plasma membrane instability which favours influx of Ca2+which in turn induces the membrane destabilization and fertilization failure or sperm death. Sperm plasma membrane destabilization is also contributed by prolonged exposure of spermatozoa to PDC-109 protein during the cryopreservation process[33]. Sperm plasma membrane permeability was increased which might be due to direct effect of cholesterol efflux induced by presence of higher concentration of PDC-109 protein in the seminal plasma. Gadella et al[39] reported that spermatozoa with high cholesterol content (man and bull) are slow to undergo capacitation whereas spermatozoa with lower cholesterol content (boar and ram) are undergone faster capacitation. Similarly, Muller et al[40] pointed out that PDC-109 protein stimulates strong cholesterol efflux from plasma membranes in presence of phospholipids. However, in our study, PDC-109 protein was sequestered from ejaculated spermatozoa in sufficient quantity by inclusion of ‘a(chǎn)nti-PDC-109 antibodies’ in treatment groups.Therefore, sequestration of PDC-109 protein led to lower cholesterol efflux and subsequently higher cholesterol content in spermatozoa of group 2 followed by group 3 and group 4. Similar beneficial effect of addition of ‘a(chǎn)ntibodies against PDC-109’ in spermatozoa of group 3 and group 4 was less pronounced as these groups contained lower concentration of antibodies. Even then, the cholesterol content of spermatozoa in group 4 was comparable to group 1 (control). Lower concentration of anti-PDC-109 antibodies in group 3 and group 4 led to lower than optimum sequestration of PDC-109 resulting in loss of cholesterol and phospholipids or their ratio in sperm plasma membranes. In the untreated control group, higher level of PDC-109 protein caused excess cholesterol efflux and cholesterol loss in spermatozoa was observed.
Spermatozoa affected with higher acrosomal damage suggest that the spermatozoa are suffered with cryoinjury and cryocapacitation which in turn triggers the lower motility. On the contrary, higher concentration of non-capacitated spermatozoa in the semen indicates that spermatozoa have higher motility. CTC fluoresces assay facilitates to assess the spermatozoa as non-capacitated (pattern F), capacitated (pattern B) and acrosome-reacted (AR) to select the sperm for further processing. In the current study, spermatozoa treated with group 2 (266 μg/mL buffalo anti-PDC-109 antibodies)had shown a significantly higher F pattern and significantly lower B and pattern AR spermatozoa as compared to those in group 3, group 4 and group 1. Higher percentage of spermatozoa with pattern F in group 2 indicated that lower cryocapacitation in this group. The result clearly indicated that anti-PDC-109 antibodies treatment had significant benefit on CTC patterns both at prefreeze and post-thaw stages in bubaline spermatozoa. It is also to be considered that the cryopreservation procedures viz., dilution,cooling, freezing/thawing trigger the capacitation-like changes in the spermatozoa i.e. cryocapacitation[41]. Moreover, freezing process exposes the sperm plasma membranes for prolonged action of PDC-109 protein which in turn alters Ca2+flux in spermatozoa.BSP proteins are involved in the modification of lipid composition of sperm plasma membranes especially during capacitation and acrosome reaction[13]. Lower cholesterol efflux i.e. higher cholesterol content in anti-PDC-109 antibodies treated spermatozoa might be the reason of lower capacitation and acrosome reaction in the antibodies treated compared to the control group. Sequestration of PDC-109 with anti-PDC-109 antibodies had reduced the capacitated(pattern B) spermatozoa in the bovine species[21]. These findings suggested that PDC-109 protein was responsible for capacitation and acrosome reaction of spermatozoa. Sequestration of PDC-109 of spermatozoa by anti-PDC-109 antibodies led to lower cholesterol and phospholipid efflux which in turn improved the stability of the sperm plasma membranes. This might be the reason of getting lower percentage of sperm with patterns B and AR in group 2 as compared to the other treatment and control groups. On the contrary, higher percentage of sperm with F pattern was observed in group 2 as compared to in the other groups in the study in bubaline species.
In the present study, anti-PDC-109 antibodies sequestrated the PDC-109 protein which in turn minimized the cholesterol and phospholipid efflux and improved the membrane stability, and thus lowered incidence of cryoinjury or cryocapacitation in the antibody treated sperm. Cryoprotective effect of anti-PDC-109 antibodies on the semen quality parameters was achieved by binding of antibody with free floating PDC-109 protein in the seminal plasma. This binding of antibody prevented the PDC-109 protein to bind with spermatozoa and lowered the damage/injury in the plasma membranes by continuously decreasing the cholesterol and phospholipid efflux. Significantly higher B and AR patterns in the control group might be due to nil or insufficient sequestration of excess PDC-109 protein by absence of sufficient anti-PDC-109 antibodies. Moreover, inclusion of higher dose of anti-PDC-109 antibodies could have increased the acrosome-intact/non-capacitated spermatozoa in the group 2.
In-vitro zona binding assay was conducted to assess the effect of anti-PDC-109 antibodies on sperm penetration ability/zona binding ability in homologues oocytes. Spermatozoa treated with group 2(266 μg/mL buffalo anti-PDC-109 antibodies) showed significantly higher zona bonded spermatozoa compared to those in the control group (group 1). Srivastava et al[21] reported that sequestration of PDC-109 with anti-PDC-109 antibodies had increased the zona binding index in bovine species. In the present study, PDC-109 protein sequestered spermatozoa in group 2 by antibodies had significantly higher zona binding index. Therefore, sufficient concentration of PDC-109 protein might not be available in the postthaw semen for binding with spermatozoa. This might be the reason for significantly higher binding index in group 2 as compared to in group 1 (control) at post-thaw.
However, the present investigation had some limitations. In the present investigation, we examined the effect of anti-PDC-109 antibodies on in-vitro semen quality parameters, cryoinjury and in-vitro zona binding ability in bubaline species. In this study,we prepared limited experimental groups (26, 80, and 266 μg/mL buffalo anti-PDC-109 antibodies) and revealed that 266 μg per mL was optimum and suitable for bubaline species. Therefore, further studies need to be conducted on effect of anti-PDC-109 antibodies on the in-vivo fertility rate to confirm the present findings of the study. Further studies need to be conducted on higher concentrations of anti-PDC-109 antibodies than 266 μg/mL to optimize the level of antibodies to neutralise the higher PDC-109 protein to get maximum benefits in the buffalo semen cryopreservation.
In conclusion, this is the first report on effect of different concentrations of antibody against the PDC-109 protein isolated from buffalo semen to minimize the sperm cryoinjury and to improve the freezability of spermatozoa in bubaline species. Sequestration or neutralization of PDC-109 by its antibodies significantly improves the pre-freeze, and post-thaw semen quality parameters and in-vitro zona binding index with simultaneously reducing the cryoinjury or cryodamage in the sperm of bubaline species. Although maximum sequestration of PDC-109 proteins is observed at 1/3rd anti-PDC-109 in TFC buffer (266 μg/mL anti-PDC-109 ntibodies) in the present study, further studies need to be conducted on higher concentrations of anti-PDC-109 antibodies than 266 μg/mL to optimize the level of antibodies to neutralise the higher PDC-109 protein to get maximum benefits in the buffalo semen cryopreservation.
Conflict of interest statement
Authors declare that there is no conflict of interest involved in the present work.
Acknowledgements
Authors would like to thank The Director, Joint Director(Academic), Head, Animal Reproduction Division, ICAR-Indian Veterinary Research Institute, Izatnagar, India, for their support and cooperation during the study.
Funding
This study received no extramural funding.
Authors’ contributions
S. S. Ramteke and J. K. Prasad made conceptualization; S. S.Ramteke, J. K Prasad and S. K. Ghosh were responsible for methodology; S. S. Ramteke, J. S. Rajoriya and A. M. Shende performed formal analysis and investigation; P. Perumal executed original draft preparation, writing, review and editing; S. K. Ghosh and J. K. Prasad performed funding acquisition; J. K. Prasad and S. K.Ghosh were responsible for resources; J. K. Prasad and S. K. Ghosh performed supervision.
 Asian Pacific Journal of Reproduction2023年3期
Asian Pacific Journal of Reproduction2023年3期
- Asian Pacific Journal of Reproduction的其它文章
- Do not lose the moon while counting the stars: Conventional IVF versus add-on treatments
- Prevalence and risk factors of infertility in a Mongolian population
- Protein kinase inhibitors affect spermatogenic functions and blood testis barrier remodelling: A scoping review
- Effects of a glyphosate-based herbicide on the oestrous cycle of rats
- Antifertility potential of leaves and seeds of Delonix regia in female rats
