Protein kinase inhibitors affect spermatogenic functions and blood testis barrier remodelling: A scoping review
Oyovwi Mega Obukohwo, Onome Bright Oghenetega, Falajiki Yewande Faith, Emojevwe Victor,Rotu Arientare Rume, Joseph Gregory Uchechukwu, Oyeleke Abiodun Abioye
1Department of Physiology, Faculty of Basic Medical Sciences, Adeleke University, Ede, Osun State, Nigeria
2Department of Physiology, School of Basic Medical Science, Babcock University, Illisan-Romo, Ogun State, Nigeria
3Department of Physiology, University of Medical Sciences, Ondo State, Nigeria
4Department of Physiology, University of Ibadan, Ibadan, Oyo State, Nigeria
5Department of Medical Laboratory Science, Adeleke University, Ede, Osun State, Nigeria
6Department of Physiology, Federal University Oye-Ekiti, Nigeria
ABSTRACT
Objective: To identify the role of protein kinase in male reproduction in animal models and human spermatogenic function.
Methods: This study assessed the protein kinase of male reproduction in animal models and human using different reviewed paper indexed in PubMed, Science Direct, EBSCO, Scopus,Cochrane Library, Sage Journals, and Google Scholar. Data were charted based on author, year of publication published between 1893 and 2023, country, purpose, data collection, key findings, and research focus/domain.
Results: The MAPK pathway contributed to the growth, maturation,and functionality of male germ cells. We also found out that certain influencing factors categorized into hormonal/non hormonal factors and chemotoxicant, as well as heat stress expressed an inhibitory mechanism on protein kinase, thus affecting spermatogenic functions and maintenance/remodeling of the blood testis barrier, as well as the physiology of the Sertoli cells necessary for nutritional support of spermatogenesis. However, activating protein kinases pathway like the mTOR pathway as well as increased expression of peroxiredoxin-4 and L-carnitine mediated protein kinases may be useful for treating or managing male reproductive dysfunction.
Conclusions: Protein kinase plays an important role in spermatogenic functions and blood testis remodeling in animal and human. Its assessment provides essential information that can guide treatment strategies aimed at improving male reproductive potential.Taken together, these recent advances highlight a future therapeutic intervention in assessing male reproductive potential. It might also be possible to look at potential targets for male contraceptives in the MAPK pathway.
KEYWORDS: Spermatogenesis; Protein kinase; Inhibitors;Sertoli cells; Capacitation; mTOR
1. Introduction
Spermatogenesis is a controlled process in the seminiferous epithelium[1], where germ cells mature into spermatozoa and the testis-specific blood testes barrier (BTB) separates basal and adluminal compartments[2,3]. The BTB controls the passage of ions and molecules from the basal to the adluminal compartment[3], and the apical ectoplasmic specializations (ES) is a special adherens junction between Sertoli cells and spermatids[4]. Kinases are essential for the phosphorylation of important proteins in the BTB and apical ES, and are involved in pre-fertilization events[5]. Protein kinases are essential for spermatogesis and its inhibition can cause parturbation of BTB and spermatogenic disturbances of sperm cellcell interface organization.
The BTB is not a blood-organ barrier, but rather a Sertoli cell barrier that isolates germ cells from the blood. Tight junctions are composed of a branching network of sealing strands, with transmembrane proteins embedded in both plasma membranes and extracellular domains joining one another directly.
Adherens junctions are protein complexes that occur at cell-cell junctions, cell-matrix junctions, and epithelial and endothelial tissues. They can appear as bands or spots of attachment to the extracellular matrix. Adherens junctions are composed of the following proteins: 1) cadherins: The cadherins are a family of transmembrane proteins that form homodimers in a calciumdependent manner with other cadherin molecules on adjacent cells;2) p120 (sometimes called delta catenin) binds the juxtamembrane region of the cadherin; 3) γ-catenin or gamma-catenin (plakoglobin)binds the catenin-binding region of the cadherin; 4) α-catenin or alpha-catenin binds the cadherin indirectly via β-catenin or plakoglob in and links the actin cytoskeleton with cadherin. Significant protein dynamics are thought to be involved.
Gap junctions are specialized intercellular connections that allow molecules, ions and electrical impulses to pass through a regulated gate between cells.
This review examines how inhibiting a particular kinase can affect spermatogenic functions, such as sperm capacitation/acrosome reaction and sperm cell-cell interface organization.
2. Methods
An extensive search of studies published until 2023 was carried out in the databases of PubMed, Science Direct, EBSCO, Scopus,Cochrane Library, Sage Journals, and Google Scholar. The search was conducted with various combinations of the following keywords: ‘spermatogenesis’, ‘protein kinase’, ‘inhibitors’, ‘Sertoli cells’, ‘capacitation’, ‘mTOR’. Articles were evaluated based on their title or abstract, and relevant original research studies and review articles were included in this scoping review. Emphasis was placed on studies addressing the following topics: spermatogenesis,spermatogenic functions, capacitation, protein kinase, physiological role of protein kinase on mammalian sperm capacitation and acrosome reaction, protein kinases: A key regulator of spermatogenic functions, protein kinase and blood testes barrier, importance of protein kinase, protein kinase inhibitors, factors influencing protein kinases during spermatogenic function, therapeutic intervention to protein kinase inhibitor, and prospects for future research. Articles were excluded if they were not written in English. We excluded commentaries and case reports.
3. Spermatogenic functions
3.1. Semen
The ejaculate (semen) is a fluid that contains spermatozoa and secretions of the epididymis, accessory glands, seminal vesicles,prostate and bulbourethral glands. It has a pH between 7.3 and 7.5, a fluid viscosity of 1.028, and an average volume of 2.5 mL to 3.5 mL[6]. It contains more than 90% water and a wide range of compounds, including vitamin C, inositol, trace elements, calcium,zinc, magnesium, copper, and sulfur, and prostaglandins. Spermatozoa move through the female genital system at a speed of 3 nm/min,reaching the uterine tubes 30 to 60 min after vaginal deposition.
Oligozoospermia is caused by low sperm counts and antibodies to sperm antigens, common after vasectomy[1].
3.2. Spermatogenesis
Spermatogenesis is a process that occurs in the seminiferous tubules of the testes and is divided into two main stages:spermatocytogenesis and spermiogenesis[1,7] (Figure 1). It is initiated by the spermatogonial stem cells, which multiply for self-renewal and replicate to give rise to a lineage of developing spermatogenic cells[8]. Secondary spermatocytes are produced when the primary spermatocyte completes meiotic division and two daughter haploid cells are produced[9,10]. Spermiogenesis is the process of condensing the nucleus[11], elongation of the nucleus, formation of the flagellum[12], and shedding of cytoplasm to form mature spermatozoa[1,13].
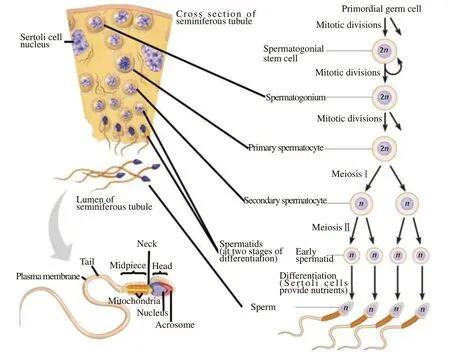
Figure 1. Spermatogenesis processes (adapted from Oyovwi et al[1]).
3.3. Mammalian sperm capacitation and acrosome reaction:Role of protein kinase
Mammalian sperm undergo capacitation and acrosome reaction to merge with an ovum[14]. O2is an essential regulator that supports both hyperactivated motility and the generation of an acrosome response[15-17]. Progesterone, peroxiredoxin-4, and other chemicals released by the oocyte cumulus complex can regulate capacitation[1],and reactive oxygen species can contribute physiologically to the complex process[18,19].
Sperm membrane potential is altered by increasing phosphorylation of tyrosine[1], membrane fluidity, cholesterol efflux, ion fluxes,hyperactivation induction, and acrosome reaction[18]. Protein kinases control intracellular Ca2+and phosphatidylinositol-3-kinase (PI3K)is activated by a protein activated kinase (PKA)-dependent cascade.
Phosphatidylinositol bisphosphate acts as a cofactor for phospholipase D and inhibits actin-severing proteins, leading to actin polymerization and hyperactivated motility.
4. Protein kinases: A key regulator of spermatogenic functions
In order to covalently change proteins, protein kinases attach phosphate groups [from adenosine triphosphate (ATP)] to serine,threonine, and/or tyrosine residues. The substrates of the protein kinase undergo functional changes as a result. Protein kinases transfer phosphate (P) from ATP to a serine (Ser or S), threonine(Thr or T), and tyrosine (Yyr or Y) residue in a protein. A "molecular shift" caused by phosphorylation makes it possible to directly activate or deactivate proteins. On the other hand, protein phosphatases catalyze the removal of the phosphate from the target protein, which suppresses kinase activity and reverses the effects of phosphorylation[20]. More than one-third of all protein phosphorylation events (O-phosphorylation) occur on serine,threonine, and tyrosine residues[20]. Interestingly, proteins kinases belonging to the Ser/Thr family play an important role in controlling signal transduction, cell proliferation, and differentiation processes.These kinases are expressed in abundance at different sperm developmental stages, e.g. (1) p21 protein activated kinase 6 (PAK6):it is expressed in Sertoli cells, Leydig cells and germ cells with a functional role in the phosphorylates nuclear receptors, acrosome reaction and endoplasmic reticulum[21]. (2) mitogen activated protein kinase-4 (MARK4): it is localized in Sertoli cells, germ cells with a functional role in the regulation of polarity in germ cells and BTB[22]. (3) testis specific serine kinase-1 (TSSK1): it is expressed in the late spermatids and sperm, and functions in the reconstruction of the cytoplasm and flagellum during spermatid development[23].(4) testis specific serine kinase-2 (TSSK2): it is expressed in condensed spermatids and mature spermatozoa, and functions in the transformation of a ring-shaped structure around the base of the flagellum originating from the chromatoid body[23]. (5) testis specific serine kinase-3 (TSSK3): it is expressed in round and condensed spermatids, and plays a role in spermiogenesis, sperm function[23].(6) testis specific serine kinase-4 (TSSK4): it is expressed in anterior sperm head and flagellum, and plays a role in spermiogenesis,sperm function[23]. (7) testis specific serine kinase-6 (TSSK6): it is localized in sperm head, and plays a role in DNA condensation during postmeiotic chromatin remodeling[24]. (8) extracellular signal regulated kinase-1 (ERK1): it is localized in Sertoli cells,and helps in the regulation of ES dynamics[25]. (9) extracellular signal regulated kinase-2 (ERK2): it is expressed in Sertoli cells,and helps in the regulation of ES dynamics[25]. (10) extracellular signal regulated kinase-7 (ERK7): it is expressed in rat testes and Sertoli cells, and helps in the regulation of ES dynamics[26].(11) jun N-terminal kinase-1 (JNK1): it is expressed in tetraploid spermatocyte and spermatogonia, and helps in spermatocyte loss through apoptosis[27]. (12) jun N-terminal kinase-2 (JNK2): it is localized in tetraploid spermatocyte and spermatogonia, and helps in spermatocyte loss through apoptosis[28]. (13) (jun N-terminal kinase-3 (JNK3): it is expressed in mammalian testis. Spermatocyte loss through apoptosis[28]. (14) p38: it is expressed in Sertoli cells and elongate spermatids, and helps in cell junction dynamics and apoptosis of germ cells[29]. (15) p38b: it is expressed in Sertoli cells and elongate spermatids, and helps in cell junction dynamics and apoptosis of germ cells[29]. (16) p38b2: it is expressed in Sertoli cells and elongate spermatids, and helps in cell junction dynamics and apoptosis of germ cells[29]. (17) p38d: it is expressed in Sertoli cells and elongate spermatids, and helps in cell junction dynamics and apoptosis of germ cells[29]. (18) LIM kinase 2 transcript (LIMK2t): it is expressed in early stages of spermatogenic cells and somatic cells,and helps in the regulation of cofilin activity localization of germ cells[30]. (19) Polo like kinase 4 (PLK4): it is localized in the testis,and helps in organization and function of mitotic apparatus[31]. (20)mammalian protein kinase (MAK): it is expressed in testis/prostate,and helps in the transcriptional coactivation of acrosome reaction spermatogenesis[32]. (21) c Kit: it is expressed in spermatogonia,acrosomal granules of the round spermatids, and Leydig cells in the adult human testis, and is essential for the migration of primordial germ cells to the genital ridges in the embryo and then functions in the maintenance of primordial germ cells. (22) c-Ret: it is responsible for spermatogonial stem cell self renewal and differentiation as well as establishment of postnatal spermatogenesis[33]. (23) focal adhension kinase (FAK): it usually formed a focal adhesion complex in between multiple tissues and mostly concentrated at the testis.it is localized at the BTB. It has a key component of Sertoli cell junctions; it regulates spermatid transport and spermiation events[34].(24) FerT: it is expressed in spermatocytes at the pachytene stage of meiotic prophase. It is associated with an induction in N-cadherin,b-catenin, and p120ctn at the base of the seminiferous epithelium[35].(25) Fyn: it is expressed in basal and apical ES of Sertoli cells and in sperm head. It helps to regulate the stability and dynamics of ES and regulates sperm head shaping. It is crucial for development of seminiferous tubule[36]. (26) hematopoietic cell kinase (HCK): it is expressed in round and elongating spermatids. It regulates the acrosome formation[37]. (27) Lck/Yes novel tyrosine kinase (Lyn):it is localized in Leydig cells, Sertoli cells, or spermatogonia. It regulates the acrosome formation[38]. (28) sarcoma (Src): it forms a focal adhesion complex and is well expressed in the BTB. It phosphorylates FAK and regulates adhering junction dynamics[39].(29) glycogen synthase kinase 3 (GSK-3a): it is a Serine/threonineprotein kinase which is expressed in spermatozoa motility initiation and stimulation[40].
These kinases have a similar N and C lobe shape with a conserved catalytic domain in the centre[41]. The phosphorylation of conserved Ser/Thr residues in their activation loop activates them after they identify conserved residues in their targets for phosphorylation.Due to their significant roles as regulators of cell growth, division,differentiation, and death, these kinases, particularly family members like MAPK/ERK, have been identified as potential targets in the emergence of a number of cancers.
Sertoli-Sertoli and Sertoli-germ cell interfaces are governed by the dynamics of ES junctions and BTB, which are in turn controlled by MAPKs[42] (Figure 2). They are also essential for the transportation and fertilization of sperm. Contrarily, because members of JNK family are essential for starting the death of germ cells, they have been identified as targets for the development of various male contraceptives[2,41,43]. Well-studied kinase targets in the development and function of sperm include TSSK, MARK, JNK, ERK1/2, PKA,PLK, MAPK1, and MAPK2 (Figure 2). Protein kinases A and C play a key role in sperm capacitation and the acrosome reaction[44-46]. They also serve as major regulators of sperm motility. LIMK2t,a form of LIMK2 that is exclusively found in germ cells and is unique to the testis, is known to be essential for the progression of spermatogenesis[30]. The sperm head shape, sperm motility, and spermatogenesis events are significantly influenced by members of the TSSK family. The MARK kinase isoforms 20, 4, and 20 influence the dynamics of the ES and sperm polarity[22]. We have examined the morphological alterations brought on by the loss of activity of a few important kinases that have been identified as targets, with the aim of using them in the future to develop efficient inhibitors. Additionally, we have emphasized the structural components of their interactions with inhibitors. PAK6 is a newly discovered member of the p21-activated kinase family that binds to androgen and estrogen receptors and alters their activities.The impact of PAK6 on spermatogenesis is paradoxical because precise regulation of PAK6 is required for the normal functioning of androgen receptor-related spermatogenetic processes. Ste20-like Ser/Thr protein kinases (PAK6-PAK6) are group Ⅱ p21-activated kinases that are linked to Rac/Cdc42[47]. The downstream processes that control cell proliferation, motility, and differentiation are modulated by activated PAKs. A conserved p21 binding domain is typically found in PAKs, followed by a kinase domain[47].
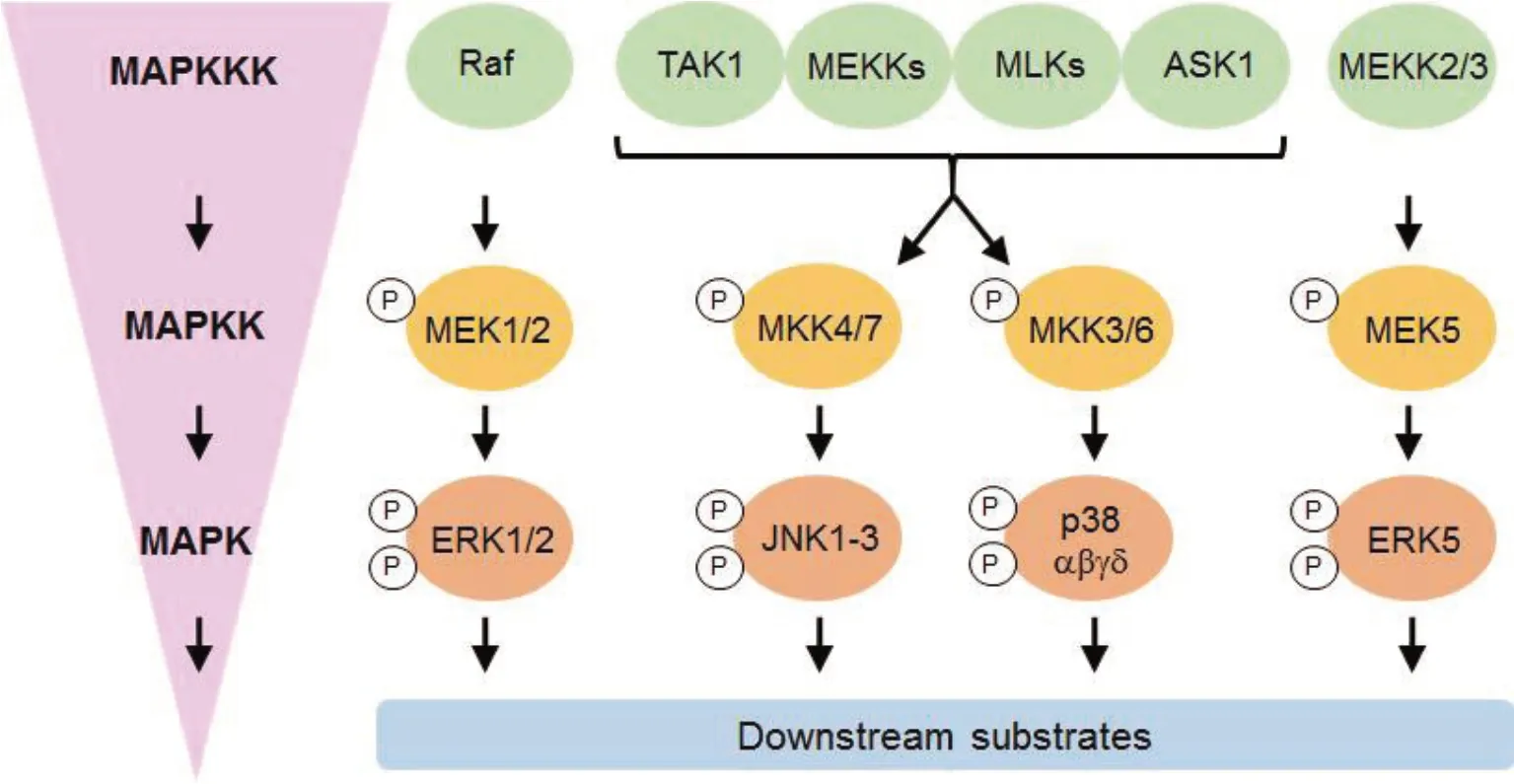
Figure 2. Elements that make up the MAPK signaling pathway′s core (adapted from Gao et al[49]). In order for the right physiological reactions to take place, the MAPK pathway converts signals from external stimuli (such as when testicular cells are exposed to environmental toxins like cadmium or cytokines like TGF-3) to cells (such as Sertoli and/or germ cells). The successive activation of MAP4Ks and MAP3Ks (such as MEKK, TAO, Mos, and Raf) is triggered by external stimuli, as seen in the left-hand box; examples of the MAPK cascade′s components and some frequently used inhibitors are shown in the right-hand box. MAP2Ks are activated by MAP3Ks that have been phosphorylated, including MEK4/7, MEK3/6, MEK1/2, and MEK5.To activate MAPKs like JNK1-3, p38 MAPK-, ERK1/2, and ERK5, dual-specificity MAP2Ks like MEK4/7, MEK3/6, MEK1/2, and MEK5 directly phosphorylate both a threonine and a serine or tyrosine residue when triggered by MAP3Ks. Different stimuli have an impact on various MAPK pathway constituents, and consequently, various MAPK effectors will be engaged for various physiological responses. The inhibitors that are frequently used to block particular MAPKs are displayed. The red-hued inhibitors are primarily employed in fundamental research because the medicinal applications of these compounds are abandoned due to unfavorable side effects.
The testis, prostate, kidney, brain, and placenta all express the new group Ⅱ PAK member PAK6, which has the capacity to bind to nuclear receptors. When produced in prostate cancer cells,the tumor suppressor PAK6 has been found to bind to nuclear acrosome reaction spermatogenesis and disrupt its transcriptional activity, preventing the translocation of nuclear acrosome reaction into the nucleus[47]. Additionally, the ubiquitination of nuclear acrosome reaction is under the control of PAK6, which is activated by androgens. As a result, PAK6 successfully prevents nuclear acrosome reaction from playing a role in the testis by preventing nuclear acrosome reaction from being translocated into the nucleus,where it would otherwise halt its transcriptional activity and be destroyed. Inhibition of PAK6 during spermatogenesis would allow nuclear acrosome reaction in seminiferous epithelium to function normally in stages Ⅶ to Ⅷ of the epithelial cycle. By mediating round spermatids′ attachment to Sertoli cells, nuclear acrosome reaction is in charge of avoiding spermatid death in stages Ⅶ and Ⅷas well as elongating them. This has led to the discovery that specific kinase inhibitors, such staurosporine, PF-3758309, and sunitinib,can lower PAK6 activity[48,49]. One of these drugs, sunitinib, was found by Coburn et al[50] to not have any negative effects on male fertility when used. In that investigation, it was found that sunitinib did not affect the male reproductive system, but at therapeutically effective dosages, it may inhibit some PAK6 forms in the body.The ATP competitive inhibitor sunitinib, which binds in the gap between the lobes of the catalytic domain of active PAK6, helped Gao et al[49] comprehend the structure of PAK6. It completely fills the active site cleft and prevents PAK6 from kinasing by forming hydrogen bonds and hydrophobic interactions with the residues in the kinase linker region. Sunitinib is also a selective PAK6 inhibitor with good PAK6 selectivity, making it a useful therapy for sperm abnormalities brought on by PAK6 activation. Protein kinases, which send messages from the cell membrane to the inside of the cell,are particularly noteworthy. These signals result from interactions between ligands and receptors as well as changes in the environment,including when the membrane is mechanically deformed (i.e.,cell stretch or shear stress). And finally, through the regulation of transcription factors, mRNA stability, or protein translation, the activation of protein kinase-based signaling pathways frequently leads to the reprogramming of gene expression.
5. Protein kinase and blood testes barrier
For spermatogensis to proceed normally, the BTB must be kept intact and germ cells must adhere to Sertoli cells in the seminiferous epithelium[41,50-52]. Premature testicular germ cells′ development and survival would be impacted if they lost touch with Sertoli cells[48].At the Sertoli-Sertoli and Sertoli-germ cell interfaces, there is substantial junction remodeling that occurs as developing germ cells travel through the seminiferous epithelium during spermatogenesis.At the BTB, primary preleptotene and leptotene spermatocytes are in transit at stages Ⅷ and Ⅸ of the seminiferous epithelial cycle[41,53].In order to allow the passage of these primordial spermatocytes,which measure between 8 and 10 micrometers in diameter, and preserve the integrity of the immunological barrier at the same time,the BTB must be "restructured" (or "opened"). Prior to the "old" tight junction-fibrils above the cell in transit being destroyed, tight junction-fibrils were assumed to form below a migrating primary leptotene spermatocyte[41].
Recently, it was proposed that testosterone and cytokines control junction remodeling at the BTB. Transforming growth factor (TGF)-2 and TGF-3 are cytokines that have been shown to cause BTB integrity to be disrupted[54-56]. On the other hand, testosterone has been shown to support BTB integrity[57]. It has been demonstrated that TGF-2 and -3, as well as testosterone, speed up the endocytosis of integral membrane proteins at the BTB, including occludin and junctional adhesion molecule-A[52,58]. A protein that has been endocytosed by cytokines or testosterone is either recycled back to the cell surface or directed to late endosomes for intracellular degradation[52]. Together, TGF-2/TGF-3 and testosterone would make it possible for integral membrane proteins at the BTB to move from the apical to the basal region of the spermatocyte by transcytosis. In order to do this, it would assemble new tight junction-fibrils at its basal area and disassemble old tight junctionfibrils at its apical area as it passed through the BTB. Since their effects on protein endocytosis and recycling occur within 5-30 min, it is likely that testosterone works via the ERK pathway rather than the conventional genomic pathway, according to previous studies[52,54]. A recent study found that the androgen receptor can quickly create physiological effects by activating the ERK pathway with the aid of Src[59].
During spermiogenesis, anchoring junctions like the desmosomelike junction help developing spermatids stay connected to Sertoli cells in the seminiferous epithelium. Spermatids in step 8 form acrosomes above the condensed nucleus, and they are only fixed to the Sertoli cell via the apical ectoplasmic specialization (apical ES, a sort of anchoring junction particular to the testis)[4]. Unlike other adherens junctions, the apical ES controls its remodelling by employing cell-matrix actin-based anchoring junction proteins such as integrin and FAK. These proteins are frequently contained within the focal adhesion complex, also known as the focal contacts,at the cell-matrix interface. One of the primary adhesion protein complexes at the apical ES is actually composed of laminin-333 and 61-integrin, with laminin-333 on the side of the elongating spermatid and 61-integrin on the side of the Sertoli cell[52]. A recent study revealed that the matrix metalloprotease-2 located at the apical ES is activated at late stage Ⅷ of the epithelial cycle during spermiation and is probably employed to cleave the laminin chains[60]. As a result, physiologically active fragments that cause BTB remodelling would be produced, which would then make it easier for primary leptotene spermatocytes to cross the BTB. The tight junctionpermeability barrier of Sertoli cells was shown to be susceptible to disruption by laminin fragments, and ERK1/2 activation was also seen[52]. These findings demonstrate that ERK1/2 is at least partially responsible for the coordination of two biological processes that occur simultaneously at opposite ends of the Sertoli cell epithelium during stageⅧ of the seminiferous epithelial cycle: spermiation and BTB remodelling. These results also imply that altering the seminiferous epithelium′s ERK1/2 activation may be a method for preventing spermatogenesis and lowering fertility.
Meanwhile, understanding each element of the BTB and apical ES, as well as how they function and interact, can help us better understand spermatogenesis and the reasons of some types of male infertility. Through its homophilic and heterophilic interaction, the coxsackievirus and adenovirus receptor (CXADR), a transmembrane component of cell junctions, is essential for testicular activities.According to Zhang et al[61], CXADR receptor was first discovered as a shared receptor for both viruses. It is expressed in a variety of tissues, including the testicles and the heart. Although CXADR is present in both adult and embryonic testes, its expression decreases with aging[61]. Under the influence of MAPK, CXADR is expressed in both Sertoli cells and germ cells. Beta 1 and beta 3 integrins are activated and confined to the site of cell-cell interaction following MAPK activation by CXADR[61]. The main roles of the MAPK signaling pathways during spermatogenesis have been highlighted by evidence amassed over the last ten years, with MAPK signaling controlling germ cell proliferation, meiosis, and Sertoli cell proliferation. When considered as a whole, CXADR is unquestionably a newly discovered signaling molecule that initiates and/or cooperates with other signaling molecules, such as MAPK,to initiate diverse spermatogenic activities and BTB remodeling.Notably, according to Zhang et al[61], the BTB and apical ES of stageⅦ-Ⅷ seminiferous tubules are where the CXADR signal is most concentrated. Studies have also shown that CXADR, a tight junction component, may serve as a regulatory hub for other cell junctions,including as gap junctions, desmosomes, and adherens junctions.Apoptosis of germ cells, early loss of spermatids, and a weakened blood-testis barrier are only a few of the male reproductive activities that have been explicitly shown to be impaired by CXADR knockout in mice Sertoli cells. Recent research has revealed the possible significance of CXADR as a signaling mediator in spermatogenesis in addition to its function as an essential component for cell junctions. This review covers recent developments in the study of CXADR regulation and function in diseased circumstances and spermatogenesis. We anticipate that this assessment will serve as a roadmap for future research on the functions of CXADR. This highlighted the potential that CXADR could act as a signaling mediator and modulator during the stage Ⅷ seminiferous cycle remodeling of the BTB and apical ES.
6. Importance of protein kinase
Protein kinases are involved in almost every aspect of biological activity[62]. Sertoli and germ cell adhesion, the development and differentiation of germ cells throughout their cell cycles, germ cell apoptosis, and germ cell differentiation are all regulated by the intracellular enzymes known as protein kinases. Additionally,it regulates mobility, metabolism, cell motion and division,immunological and neurological system activity, as well as programmed cell death. Protein kinase catalyzes the transfer of phosphate from ATP to its protein substrates[63]. A phosphate is given to other proteins by the high-energy enzyme protein kinase.The process of changing one substance into another is called phosphorylation. In the process below, the high-energy ATP molecule contributes a phosphate group while also receiving a group of phosphates from the substrate. A phosphorylated substrate is produced by trans-esterification; dephosphorylation happens when the phosphorylated substrate donates a phosphate group and is combined with adenosine diphosphate[62]. Because of their crucial function in the signaling systems that drive the features of malignant cells, protein kinases are significant therapeutic targets in cancer.
7. Protein kinase inhibitors
An enzyme inhibitor known as a protein kinase inhibitor can prevent protein kinases from acting[64]. A process known as phosphorylation, in which protein kinases add a phosphate group to a protein, can switch a protein on or off and so influence the amount of activity and function of the protein[65]. According to the amino acid on a protein that they add the phosphate to in order to prevent amino acid from being phosphorylated, such as serine, threonine, or tyrosine, protein kinase inhibitors can be categorized into different categories[66]. Most kinases affect serine and threonine, however some dual-specificity kinases also affect all three of these amino acid residues. Tyrosine kinase, on the other hand, solely affects tyrosine. Histidine kinases, which work on histidine residues, are examples of protein kinases that can also phosphorylate other amino acids. Because phosphorylation is frequently a necessary stage in the development of several malignancies and inflammatory conditions,inhibiting the enzymes that cause phosphorylation offers a potential therapeutic strategy. Examples of drugs used in this manner include chemotherapy drugs that contain tyrosine kinase inhibitors, such as those used to treat cancer and parasites[67]. The anticancer drug dasatinib is used to treat many types of leukemia[68]. PLX5568 is an additional drug that is presently undergoing clinical studies for polycystic kidney disease[69]. Due to the vital function that these highly-affinity cell surface receptors play a role in the development of many malignancies, tyrosine kinase inhibitors are particularly significant drugs. Tyrosine kinases play a role in a number of biological processes in cells, such as cell signaling, cell proliferation,and cell division. These enzymes are overexpressed or present in high concentrations in some types of cancer, and by blocking them,cancer cells cannot multiply. Since they are effective against a variety of malignancies, protein kinase inhibitors are a targeted class of cancer medicines whose use is steadily increasing. However, it is also known to indirectly affect male fertility in the process of finding a cancer cure. Although many protein kinase inhibitor-using men want to have children, little is known about the potential side effects of these drugs on the reproductive system. The mechanism of action of protein kinase inhibitors points to a potential pathway for sperm function or spermatogenesis disruption.
8. Factors influencing protein kinases during spermatogenic function and BTB remodelling
Poor semen quality and/or sexual dysfunction have been linked to male infertility and subfertility. Reduced sperm counts, flaws in spermatozoa′s morphology, genetic makeup, or motility are all examples of abnormal semen quality, whereas impotence or ejaculatory dysfunction are examples of sexual dysfunction[1]. The environment can greatly affect the process of spermatogenesis,especially heat, physical injury to the testicles, radiation, drug and/or alcohol misuse, smoking, environmental contaminants, hormones,and temperatures. The procedure is carried out by interacting with androgen binding protein located in the seminiferous tubules, which calls for a high local concentration of testosterone. The Leydig cells, often referred to as interstitial cells, are situated close to the serminiferous tubule and produce testosterone.
In humans and some other species, the seminiferous epithelium is sensitive to high temperatures and can be adversely affected by temperatures as high as average body temperature. For this reason,the testes are situated outside the body in a skin pouch called the scrotum. For a man or a mouse, the optimal temperature is kept at 20 ℃ or 80 ℃ below body temperature, respectively.
The cremasteric muscle and dartos smooth muscle are oriented toward or away from the body heat to do this[70]. Pathogen illnesses,anabolic steroids, metals (cadmium and lead), X-ray exposure,dioxin, alcohol, drug toxicants, signal transduction disruptions,and other variables can negatively inhibit protein kinases. These elements have an impact on the spermatogenesis rate as well as the capacitation/acrosome reaction necessary for fertilization. The underlying mechanism for comprehending the numerous activities involved in male reproductive biology may be caused by such influencing variables. Therefore, preventing the junction integrity from being damaged by testicular trauma may lessen its negative consequences on male fertility. These influencing factors can be categorized into hormonal/non-hormonal factors and chemotoxicant such as drug and environmental toxicants[47,71-74].
8.1. Hormonal and non-hormonal factors
Male contraceptives, which can be hormonal or non-hormonal,are substances that can disrupt spermatogenic functions including capacitation and acrosome reaction required for the fertilization of sperm and eggs[75]. Contrary to non-hormonal contraceptives, which inhibit proteins essential for controlling spermatogenic function,hormonal contraceptives either limit the release of hormones or block their activities[76,77]. Research is currently heavily concentrated on the creation of potent non-hormonal contraceptives such as adjudin,H2-gamendazole, BMS-189453, and WIN 18,446 since they have several advantages over the development of hormonal contraceptives.In addition to other proteins involved in spermatogenesis, these chemicals hinder integrins and kinases from carrying out their typical roles. The quest for new targets and inhibitors that can irrevocably and specifically cause male sterility must continue, yet all of these medications have significant drawbacks[78].
8.2. Chemotoxicant as inhibitors of protein kinase
The health of the male reproductive system is seriously threatened by exposure to chemotoxicants such fungicides, pesticides, heavy metals, and industrial chemicals, and this could have negative effects on the population as a whole. These chemotoxicants may disrupt the organization of the sperm cell-cell interface and affect spermatogenic processes at different places in the reproductive tract, which can lower male fertility and lead to hormonal function problems[79-81]. For example, neuro-endocrine disrupting substances including polychlorinated bisphenols, endosulfan, phthalate, and cadmium may inhibit protein kinases of signaling transduction pathways like the MAPK/ERK cascade and impede the synthesis and function of hormones in both males and females. Protein kinases and the FAKoccludin-ZO-1 complex, two components of the signaling cascade that restructures the blood-testis barrier, are known to interact with synthetic chemicals such as polychlorinated bisphenol, endosulfan,cadmium, 4-tert-octylphenol, and phthalate. These interactions with the gap junction proteins cause the integrity of BTB to become unstable[82] (Figure 3).
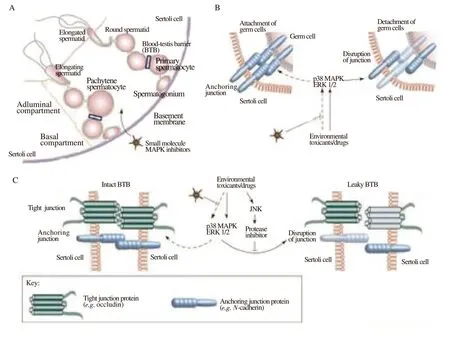
Figure 3. The function of cell adhesion and blood-testis barrier (BTB) dynamics as regulated by MAPK in the testis. (A) A schematic illustration of the seminiferous epithelium in a cross-section of a seminiferous tubule. The seminiferous epithelium is only composed of Sertoli cells and germ cells (such as spermatogonia, spermatocytes, round spermatids, elongating spermatids, and elongated spermatids) in various stages of spermatogensis. The extracellular matrix has been modified to become the basement membrane, which encircles the endothelium. The BTB divides the basal and adluminal compartments of the epithelium (blue-boxed area). Primary preleptotene spermatocytes (diploid, 2n) cross the BTB and develop into leptotene and zygotene spermatocytes at stages Ⅷ and Ⅸ of the epithelial cycle. Once the BTB is past, meiosis occurs in the rat testes at epithelial cycle stage ⅩⅣ. To produce spherical spermatids,tetraploid diplotene spermatocytes go through two cycles of reduction division (meiosisⅠand meiosisⅡ) (haploid, 1n). The BTB acts as an immunological barrier, separating all postmeiotic germ cell development-including the spermatids that undergo spermiogenesis via steps 1 through 19-from the bloodstream. Because of this, the BTB is a unique and significant ultrastructure for preserving spermatogenesis. (B-C) Testicular injuries, such as drug use or exposure to environmental toxins (such as cadmium), may prevent germ cell adhesion via the p38 and ERK MAPK pathways. [Take note that the Sertoli cell and germ cell anchoring junction must be maintained to maintain: (i) proper communication between these cells for germ cell development because germ cells must depend on Sertoli cells for both structural and nutritional support; and (ii) proper orientation because germ cells without an intact anchoring junction either undergo apoptosis or prematurely detach from the epithelium]. Testicular injuries may potentially interfere with the BTB via the p38 and ERK MAPK pathways. (Note that unlike other blood-tissue barriers like the blood-brain barrier, the BTB is made up of both tight and anchoring junctions and is therefore crucial for maintaining the BTB′s immunological barrier function during anchoring junction restructuring through a mechanism that is currently unknown). The breakdown of germ cell adhesion and BTB function may be prevented or controlled by small molecule inhibitors of the kinases in the MAPK cascade (indicated with dashed arrows)[82].
Many of the kinases discussed here, such as MAPK, ERK, JNK,PI3K, PKA, PKC, Src family kinases (SRC), and FAK, have impacts on spermatogenesis in addition to being well-known cellular targets for the creation of anti-cancer drugs[83]. The non-ATP-competitive inhibitor PD184352 reduces MEK/MAPK activity in tumor cells and slows the development of malignancies by precisely binding to and inhibiting MEK (mitogen-activated protein kinase). In addition to these medications, sunitinib, PF562,271, and TAE226 are also known to inhibit FAK and other tyrosine kinases and halt cell proliferation in malignancies of the head and neck, colon,and pancreas. All available evidence shows that these kinases are necessary for the spermatogenic process, and various writers have thoroughly investigated the use of these kinase inhibitors as anticancer medications.
8.3. Heat stress
The quality of sperm in animal and human reproduction can be lowered by an increase in temperature and seasonal climate change.As a result, spermatogenesis is impacted by the death of Sertoli cells under conditions of heat stress, which is linked to decreased blood-testis barrier (BTB) function. According to reports, heat stress decreased Sertoli cell viability and proliferation, as seen by an increase in arachidonic acid release[84]. The fatty acid arachidonic acid, which is present in both the sperm membrane and the testis milieu and is a direct precursor to 20-carbon polyunsaturated fats, is crucial for maintaining cellular function[84]. However, high amounts of arachidonic acid can change the cytomembrane′s structure and function as well as increase its permeability and brittleness, which may cause mitochondria to enlarge, undergo apoptosis or necrosis,among other changes. Poor spermatogenesis results have been linked to disrupted arachidonic acid metabolism[84].
9. Therapeutic intervention to protein kinase inhibitor
Understanding their structural causes at the outset would help in rectifying these effects, as anomalies in male reproductive function have become more prevalent worldwide. Protein kinases are the molecular targets for the majority of anti-cancer and antipsychotic medications as well as chemotoxicants that induce testicular toxicity as well as male contraceptives because they play a significant part in practically all signaling cascades that regulate the proper functioning of the testis. Environmental chemicals, many of which target kinases, can have detrimental effects on the male reproductive system. By physically studying these inhibitory mechanisms, we can reduce non-specific toxicities in drugs and contraceptives while minimizing the effects of toxins. Notably, disruption of the BTB and spermatogenic issues have been connected to the therapeutic strategy of targeting protein kinase in the treatment of cancer.Consequently, we could speculate that treating or managing male reproductive dysfunction may benefit from activating protein kinase pathways like the mammalian target of rapamycin (mTOR)pathway[41,73]. Experimental results in animal models treated with D-Ribose-L-cysteine to improve testicular functioning show that mTOR and Atg7 activation-mediated inhibitions of inflammation,apoptosis, and oxido-nitrergic stress are possible[73]. It is interesting to note that evidence is accumulating indicating mTOR activation occurs in progenitors that are actively engaged in differentiation,and that this activation is stage-dependent. In fact, it seems that the behavior of spermatogenic stem cells is largely determined by the ability of mTOR to flip between its active and inactive states.Retinoic acid administration has also been shown to result in mTOR phosphorylation, which further implies a participation of mTOR in this process. Retinoic acid plays a key role in regulating spermatogenesis and spermatogonia differentiation[85]. All of the evidence points to mTOR′s critical role in regulating the outcome of spermatogenic functions[72,85]. mTOR activity has also been connected to BTB dynamics[85]. Both mTORC1 and mTORC2 act in this barrier in different ways, according to studies using in vitro and in vivo techniques. In contrast to the former, which includes remodeling of the BTB, the former entails making the BTB "tighter".Preleptotene spermatocytes shift to the aluminal compartment when mTORC1 is raised at later stages of the seminiferous epithelial cycle,but mTORC2 is upregulated at earlier stages, as previously stated.This is because mTOR complex expression is stage-dependent. The scientific community is currently focusing on identifying putative signaling pathways governing this complicated interplay, and this effort has already produced some exciting findings, including the prpS6/Akt/Arp3/N-WASP and the p-rps6/Akt/MMP-9 pathways as mediating mTORC1 effects in BTB dynamics. Treatment with taurine and Coenzyme Q10, two essential endogenous molecular regulators of spermatogonia differentiation and spermatocyte formation, also increased the amount of the protein kinase C required for gonadal functions, indicating an involvement of peroxiredoxin-4 and L-carnitine in this process[86]. Together, these investigations support the crucial roles that peroxiredoxin-4 and L-carnitine play in determining the spermatogonial sperm cells′ ultimate fate.
Some targeted MAPK pathway members, like the moderate ERK1/2 and p38 MAPK inhibitor PD98059[87] and the MEK1/2/5 inhibitor PD98059[87], may also be used as therapeutic targets to reverse the decline in semen quality and fertility brought on by sperm function disruption or after testicular injury, like a disruption of the BTB brought on by environmental toxins. It is well known that chemotherapy medicines like cisplatin can harm the testicles in cancer patients[87-89]. These harmful outcomes on fertility could be short-term or long-term. However, the MEK1/2/5 inhibitor PD98059 was unable to counteract the cisplatin-induced increase in interleukins and nitric oxide synthases in Sertoli cells[87]. This implies that these small molecule inhibitors may be used with chemotherapy medications to decrease their toxicity to the testicles.In conclusion, these results suggest the possibility of partially restoring fertility in males with reproductive dysfunction caused by occupational exposure to endocrine disruptors, in patients with poor sperm quality, or in patients with cancer by selectively targeting inhibitors of kinases in the MAPK pathway to Sertoli cells. However,it has been shown that the MAPK pathway contributes to the growth,maturation, and functionality of male germ cells. As a result, it might also be possible to look at potential targets for male contraceptives in the MAPK pathway.
10. Prospects for future research
This review study demonstrates the potential for success in resolving issues related to spermatogenic disruption and parturbation of the BTB caused by various hormonal and non-hormonal agent,chemotoxicants, such as medications and environmental agents. It is anticipated that performing additional experiments to address the anomalies of chemotoxicant would use the success rates of those studies that have overcame the repercussions as a reference point.In order to prevent the parturbation of the BTB and spermatogenic disruption, various supplements that mediate protein kinases will be useful in the future.
11. Conclusions
This review article suggests that to lessen testicular maladaptive responses, small molecule inhibitors could be used with chemotherapeutic drugs like taurine, coenzymes Q10, retinoic acid and D-ribose-L-cysteine. Hence, it has been demonstrated that the MAPK pathway plays a role in the development, maturity, and function of male germ cells. Therefore, it may also be possible to investigate kinases in the MAPK pathway as prospective male contraceptive targets. It also point out that activating protein kinases pathway like the mTOR pathway as well as increased expression of peroxiredoxin-4 and L-carnitine mediated protein kinases may be useful for treating or managing male reproductive dysfunction
Conflict of interest statement
The authors have disclosed no conflicts of interest.
Acknowledgements
We thank everyone for their imput.
Funding
This study received no extramural funding.
Availability of data and materials
All information used in this article can be obtained from the appropriate author upon request.
Authors’ contributions
Oyovwi Mega Obukohwo conducted literature search and wrote rough draft. The study was conceptualized, planned, and conducted by Oyovwi Mega Obukohwo and Onome Bright Oghenetega. He also carried out the literature search and edited and revised the article. Oyovwi Mega Obukohwo, Falajiki Yewande Faith, Emojevwe Victor, Rotu Arientare Rume, Joseph Gregory Uchechukwu, and Oyeleke Abiodun Abioye reviewed and searched the literature. The final manuscript was read and approved by all writers.
 Asian Pacific Journal of Reproduction2023年3期
Asian Pacific Journal of Reproduction2023年3期
- Asian Pacific Journal of Reproduction的其它文章
- Do not lose the moon while counting the stars: Conventional IVF versus add-on treatments
- Prevalence and risk factors of infertility in a Mongolian population
- Buffalo anti-PDC-109 antibodies improve the semen quality profiles and in-vitro zona binding index and minimize the cryoinjury of sperm in cryopreserved buffalo semen
- Effects of a glyphosate-based herbicide on the oestrous cycle of rats
- Antifertility potential of leaves and seeds of Delonix regia in female rats
