Structure and allergenicity of α-lactalbumin: effects of ultrasonic prior to glycation and subsequent phosphorylation
Wenmei Chen, Qiongzhen Chen, Houze Zhou, Ynhong Sho,Yng Wng, Jun Liu,*, Zongci Tu,b,*
a National R & D Center for Freshwater Fish Processing, College of Life Science, Jiangxi Normal University, Nanchang 330022, China
b State Key Laboratory of Food Science and Technology, Nanchang University, Nanchang 330047, China
Keywords:Bovine α-lactalbumin Allergenicity Ultrasonic Protein modif ication
A B S T R A C T Bovine α-lactalbumin (BLA) induced severe cow’s milk allergy. In this study, a novel strategy combining ultrasonication, performed before glycation, and phosphorylation was proposed to reduce BLA allergenicity.Result showed that IgE- and IgG-binding capacities and the release rates of histamine and interleukin-6 from RBL-2H3 were reduced. Moreover, intrinsic fluorescence intensity and surface hydrophobicity were decreased, whereas glycated sites (R10, N44, K79, K108, N102 and K114) and phosphorylated sites(Y36 and S112) of BLA were increased. Minimum allergenicity was detected during BLA treatment after ultrasonic prior to glycation and subsequent phosphorylation because of considerable increase in glycated and phosphorylated sites. Therefore, the decrease in allergenicity of BLA, the effect correlated well with the shielding effect of glycated sites combined with phosphorylated sites and the conformational changes. This study provides important theoretical foundations for improving and using the ultrasonic technology combined with protein modif ication in allergenic protein processing.
1. Introduction
Bovineα-lactalbumin (BLA) is the second major whey proteins of bovine milk and the primary whey protein in human milk [1,2].BLA is a 14.2 kDa Ca-binding monomeric globular protein, which has 123 amino acid residues with 4 disulf ide bridges. It consists of anα-helical domain and aβ-sheet domain, which are connected by a calcium binding loop. BLA strongly binds metal ions and widely accepted as a component of infant formulas for its nutritional properties. However, potential allergic reaction to BLA, which causes about 30%-35% of IgE-mediated allergic reactions to cow’s milk, has resulted in the limited use of BLA [3]. Thus, its allergenicity should be eliminated.
Non-thermal processing technologies, chemical modification,and combination therapies, have been used to reduce the allergenicity of allergic proteins. Non-thermal processing technologies (e.g.,ultrasound, dynamic high pressure microfluidization and pulsed electric f ield, etc.) can maintain a protein’s inherent nutritional ingredients and quality, and reduce the allergenicity by disrupting the linear and conformational structures [4,5]. Protein modification processes,such as glycation, phosphorylation, and acylation, have been used in food processing [6,7]. They can effectively improve the functional characteristics of proteins [8]. Glycation causes the shielding effects,leading to reduce the allergenicity of BLA [9],β-lactoglobulin [10],and ovalbumin [11]. Our previous study confirmed that glycation,phosphorylation and acylation could decrease the allergenic reactivity of BLA [12]. Anti-BLA antibody response can be reduced significantly by glycation-assisted phosphorylation [13]. Non-thermal processing technologies combined with protein modification have attracted considerable attention because they can reduce protein allergenicity and have advantages over methods. For example,ultrasonic-assisted with glycated modification [14-16], dynamic high pressure microfluidization-assisted with glycated modification [17,18],and pulsed electric field-assisted with glycated modification [19] can significantly reduce the allergenicity of allergic protein because of considerable increase in shielding effects. Ultrasound-assisted with glycated modification requires no extraneous chemicals. In addition,glycated modification can reduce the allergenicity of allergenic protein, ultrasound pre-treatment can greatly accelerate the glycation reaction and increase the glycation sites, and ultrasound-assisted with glycation alters the linear and conformational structures of allergic proteins, thus effectively reduce the allergenicity. Therefore,this technology provides a broad prospects for the application of ultrasound-assisted with glycated modification in food industry.
Allergenic epitopes of BLA are classified into linear epitopes and conformational epitopes. The peptide 1-16, 7-18, 13-26, 17-58,47-58, 51-56, 93-102, 89-108, 109-123 and (6-10):S-S:(115-123)are probable allergenic epitopes of BLA. In a previous study,we evaluated ultrasound-assisted with glycated modification at different power levels and found that this process affects the IgEand IgG-binding capabilities of BLA [14]. We also compared the effects of different protein modification techniques on allergenicity of BLA [12]. These modification techniques can greatly affect the allergenic epitopes of BLA, leading to the reduction in the allergenicity. To date, little is known about ultrasonic prior to glycation and subsequent phosphorylation and their effects on the allergenicity of BLA. The binding capacity of BLA for allergenspecific antibodies can be weakened through ultrasonic prior to glycation because the process can destroy allergenic epitopes.However, whether ultrasound prior to phosphorylation can affect the binding capacity of BLA and whether this process and subsequent phosphorylation can reduce allergenicity of BLA are unknown. Moreover, the release of allergic mediators is related to the occurrence of type I allergic reactions, and IgE-stimulated mast cells and basophil cells can secret mediator (e.g. interleukin-6 (IL-6)and histamine). Thus, allergic mediators release test for the allergens is an indicator of food allergy, it also can reflect the ability of BLA allergenicity. However, the effect of the treated BLA by ultrasonic prior to glycation and subsequent phosphorylation on the mediator release from basophils is rare.
This study aimed to provide a strategy and analyze changes in the binding capacity of specific antibody and the histamine and IL-6 release rates of modified BLA by ultrasonic prior to glycation and subsequent phosphorylation. Furthermore, multi-structural changes,glycation and phosphorylation sites of BLA were characterized. We hope that this study provides useful information on the mechanism by which ultrasonic prior to glycation and subsequent phosphorylation decrease protein allergenicity.
2. Materials and methods
2.1 Materials
BLA (L6010, Type III, ≥85%), goat anti-human IgE-horseradish peroxidase (HRP) conjugate (A9667) were from Sigma-Aldrich(St. Louis, MO, USA). Sodium pyrophosphate (SP) and goat antirabbit IgG-HRP conjugate (SE131) were obtained from Beijing Solarbio Technology Co., Ltd. (Beijing, China). Human antisera were from Plasma Lab International (Everett, WA, USA). The symptoms of five human antisera were described in the Table S1. Rabbit antisera were prepared using a previously reported protocol [2].
2.2 Sample preparetion
Approximately 10 mL of native BLA (1.0 mg/mL) was treated using a Q700 sonicator equipped with 3 mm microtip probe with a 9 s on and 1 s off pulsation at 150 W/cm2for 10 min. Then, 3.0 mg of galactose (Gal) was added to 3.0 mL of the ultrasonicated BLA solution. After lyophilization, native BLA-Gal poweder and ultrasonicated BLA-Gal complex were incubated at 65% relative humidity (saturated potassium iodide solution) and 55 °C for 3 h.The reaction was stopped in an ice bath, then the solution was removed before it reacted with Gal and salts. Approximately 3.0 mg of SP was dispersed in 3 mL of the glycated BLA solution. The sample was lyophilized to powder. The subsequent procedure was the same as that used for heat treatment. The reaction was stopped,and the salts were removed with the same methods. The samples(2.0 mg/mL) were prepared for future use. Native BLA was named N-BLA. BLA samples were incubated under the conditions mentioned above as the control, then named DH-BLA. Modified samples subjected to phosphorylation, ultrasonic prior to phosphorylation were named SP-BLA and U-SP-BLA, respectively.The modified samples subjected to glycation and subsequent phosphorylation was named Gal-SP-BLA, and those subjected to ultrasonic prior to glycation and subsequent phosphorylation was named U-Gal-SP-BLA. A schematic depiction of the sample preparation was shown in Fig. S1.
2.3 IgE- and IgG-binding capacity
The IgE- and IgG-binding capacity of DH-BLA, SP-BLA, U-SP-BLA,Gal-SP-BLA and U-Gal-SP-BLA was estimated by inhibition ELISA assays [14], which involved mixing the same amounts of sera of five patients to yield a serum pool and rabbit antisera. The absorption was monitored at 450 nm by a microplate reader (BioTek Instruments Co. Ltd., Vermont, USA). Inhibition rate was calculated as follow:

WhereAandA0are the absorbance value of the well with and without native samples, respectively.
2.4 Allergenicity in RBL-2H3 cells
RBL-2H3 from rat mast cells was cultured in RPMI-1640 medium supplemented with FBS in a 5% CO2-95% humidity incubator at 37 °C, then human antisera were immediately added, following with test samples stimulating the cells. Histamine and IL-6 release were estimated according to previously reported methods [12].
2.5 Fluorescence spectroscopy
Fluorescence intensity of DH-BLA, SP-BLA, U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA (1.0 mg/mL) were obtained with a Hitachi F-7000 fluorescence spectrophotometer (Japan). The emission spectra were recorded from 300 to 400 nm (both at a constant slit of 5 nm) with excitation wavelength at 290 nm [20].
2.6 Surface hydrophobicity
Surface hydrophobicity of DH-BLA, SP-BLA, U-SP-BLA, Gal-SPBLA and U-Gal-SP-BLA was determined by 8-anilinonaphthalene-1-sulfonic acid (ANS) assay [12]. The fluorescence intensity of test sample was measured at 390 nm (excitation) and 470 nm (emission) using a Hitachi F-7000 fluorescence spectrophotometer.
2.7 Identification of the modified sites
Test samples of DH-BLA, SP-BLA, U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA were digested [20], and the digested mixture was transferred to RP-C18column of peptide separation. The eluent was given for Thermo Fisher Orbitrap Fusion Mass Spectrometer (USA)to obtain the modified peptide and sites. Them/zof peptides and peptide fragments was detected by the following methods: full scanevery time, and then collected 20 fragmentography (MS2scan). Data analysis was performed with the previous method [20].
2.8 Statistical analysis
The experiments were carried out in triplicate and the results were presented as mean ± SD.
3. Results and discussion
3.1 IgE- and IgG-binding capacity
For the estimation of the effect of ultrasonic prior to glycation and subsequent phosphorylation on the allergenicity of BLA, inhibition ELISA was used in estimating IgG- and IgE-binding capacities for DH-BLA, SP-BLA, U-SP-BLA, Gal-SP-BLA, and U-Gal-SP-BLA. A rabbit antibody and pooled sera from patients with cow’s milk allergy were used. The IC50values (the concentration of inhibitors that causes a 50% inhibition of antibody binding (μg/mL)) of SP-BLA, U-SP-BLA,Gal-SP-BLA, and U-Gal-SP-BLA shifted to 8.482, 9.488, 16.544, and 19.218 μg/mL, respectively, which were much higher than the IC50value of DH-BLA (7.146 μg/mL, Fig. 1A). A similar phenomenon was shown in Fig. 1B. The IC50values of SP-BLA, U-SP-BLA, Gal-SPBLA, and U-Gal-SP-BLA were 1.06-, 1.22-, 1.78- and 1.98-times higher than the IC50value of DH-BLA (3.840 μg/mL), respectively. A high IC50value implies low IgG- and IgE-binding capacities. The IgEand IgG-binding capacities of SP-BLA decreased when BLA was modified through phosphorylation. After ultrasound pretreatment, the IgE- and IgG-binding capacities of U-SP-BLA decreased considerably compared with those for SP-BLA. The result can be attributed to changes in allergenic epitopes after dry heating and modification through phosphorylation [13,21]. When BLA was glycated and phosphorylated, binding capacity was greatly reduced compared with that of U-SP-BLA possibly because glycation combined with phosphorylation changed the epitopes [22]. Interestingly, ultrasonic prior to glycation and subsequent phosphorylation induced the largest decrease in binding capacity. This result showed that ultrasound pretreatment plays a role in changing the structure of BLA, accelerates reaction between Gal and SP, and when combined with protein modification, effectively reduces the allergenicity of BLA.

Fig. 1 The IgE (A) and IgG (B) binding ability of DH-BLA, SP-BLA, U-SPBLA, Gal-SP-BLA and U-Gal-SP-BLA was performed by inhibition ELISA.Anti-BLA rabbit pooled sera or anti-BLA patients’ pooled sera (50 μL/well)were incubated separately with 0.781 25, 3.125, 12.5, 25, 50, 100 μg/mL of the corresponding modified BLA as inhibitors.
3.2 Histamine and IL-6 levels
The mediators release is closely related to the occurrence of type I allergic reactions [23]. To determine whether or not ultrasonic prior to glycation and subsequent phosphorylation affects the allergenicity of DH-BLA, SP-BLA, U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA, cell experiment was used to evaluate the mediators release from RBL-2H3 cells. As shown in Fig. 2, the group with BLA modified through phosphorylation, ultrasonic prior to phosphorylation, glycation combined with phosphorylation, and ultrasonic prior to glycation and subsequent phosphorylation showed significant reduction in mediator release rate compared with the normal group and the group subjected to heated BLA alone (P< 0.05). Histamine and IL-6 release rates was observed in the order DH-BLA > SP-BLA > U-SP-BLA > Gal-SPBLA > U-Gal-SP-BLA, suggesting that glycation and phosphorylation can reduce mediator release rates, which are related to allergic symptoms [12,24]. After ultrasound and glycation pretreatments,histamine and IL-6 release rates decreased dramatically. When BLA was treated by ultrasonic prior to glycation and subsequent phosphorylation, histamine and IL-6 release rates showed the lowest values. This result may be due to the responses of RBL-2H3 to SP-BLA, U-SP-BLA, Gal-SP-BLA, and U-Gal-SP-BLA, which can change IgE epitopes on protein surfaces and hinder the degranulation of basophil, especially Gal-SP-BLA and U-Gal-SP-BLA. As indicated by IgE- and IgG-binding capacities in Fig. 1, the release of histamine and IL-6 (Fig. 2), BLA allergenicity was significantly reduced after ultrasonic prior to glycation and subsequent phosphorylation. The mechanism of reduction in the allergenicity of BLA was related to structural changes. The structural changes were subsequently investigated through spectroscopy and mass spectrometry.

Fig. 2 Effects of DH-BLA, SP-BLA, U-SP-BLA, Gal-SP-BLA, and U-Gal-SP-BLA on the release of histamine (A) and IL-6 (B) from the rat mast cell lines RBL-2H3 cell. Letters (a-f) on the bars mean significantly different (P < 0.05). Normal represents normal RBL-2H3 cells not stimulated by patient serum.
3.3 Intrinsic fluorescence emission spectroscopy
Conformational changes in N-BLA, DH-BLA, SP-BLA,U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA were reflected by the tryptophan fluorescence spectra. When excited at 290 nm,fluorescence intensity of DH-BLA, SP-BLA, U-SP-BLA, Gal-SPBLA and U-Gal-SP-BLA gradually decreased (Fig. 3A), suggesting that the reductions may have been caused by shielding effect and solvent relaxation [25,26]. Phosphorylation can reduce the intrinsic fluorescence intensity of BLA, and ultrasound pretreatment promotes the reduction. The intrinsic fluorescence intensity of U-Gal-SPBLA reached a minimum value compared with the fluorescence intensities of DH-BLA, SP-BLA, U-SP-BLA, and Gal-SP-BLA. The maximum emission wavelength showed a slightly increase in Gal-SPBLA and U-Gal-SP-BLA, suggesting that glycation and subsequent phosphorylation induced the changes of BLA polarity, leading to the exposure of tryptophan residues to the microenvironment.Intrinsic fluorescence emission spectroscopy analysis showed that the conformational structures of BLA exposed to different treatments were changed.

Fig. 3 The intrinsic fluorescence spectra (A) and surface hydrophobicity (B) of N-BLA, DH-BLA, SP-BLA, U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA.Letters (a-d) on the bars mean significantly different (P < 0.05).
3.4 Surface hydrophobicity
As shown in Fig. 3B, surface hydrophobicity of DH-BLA,SP-BLA, U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA was gradually decreased, but the surface hydrophobicity of N-BLA and DH-BLA showed no significant changes (P> 0.05). Phosphorylation and glycation induced the masking of some hydrophobic groups and decreased surface hydrophobicity [27]. Ultrasonic prior to phosphorylation, glycation combined with phosphorylation, ultrasonic prior to glycation and subsequent phosphorylation were expected to show similar results. Moreover, ANS bind with some amino acids of BLA (eg., Arg and Ser residues), decreasing the surface hydrophobicity of BLA [12].
3.5 Modified sites determination
After glycation and phosphorylation, the molecular weights much higher than the mass of the polypeptide backbone of N-BLA,14 221.9 Da(Fig. S2). Our previous study has identified the glycated and phosphorylated sites of the modified BLA [12]. In this study,a similar method was used for determining location and number of the modified sites of U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA.As shown in Fig. 4A, native peptide 1-25 (with a sequence of EQLTKCEVFRELKDLKGYGGVSLPE) of U-Gal-SP-BLA was 725.827 74+, while new peak of the phosphorylated and glycated peptide was 765.338 74+and 805.124 84+, respectively. This result indicated that the peptide 1-25 was modified by one molecule of Gal and two molecules of phosphate groups. Them/zpeaks of the un-glycated peptide 92-102 of U-Gal-SP-BLA was 409.599 03+, while correspondingm/zof modified peptide was 571.615 73+(Fig. 4B),indicating that this peptide has a triglycosylated peptide.

Fig. 4 Mass spectra for the modified peptides. (A) peptide 1-25 at m/z 805.124 84+, (B) peptide 92-102 at m/z 571.651 73+. The determined peptides are labeled by residue numbers. The m/z differences between modified and un-modified peptides are indicated above the arrows. (C) The HCD/ETDMS/MS spectra of peptide 1-25 (EQLTKCEVFRELKDLKGYGGVSLPE)with m/z of 805.124 84+. (D) The HCD/ETD-MS/MS spectra of peptide 92-102 (VKKILDKVGIN) with m/z of 571.651 73+. The sequence of per peptide is depicted on the top of the spectrum. The identified the modified sites are indicated by a line with Gal and phospho. The c and z or b and y ions are shown by the numbers and line.
The glycated and phosphorylated sites of U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA were identified accurately through higher energy collision dissociation/electron transfer dissociation(HCD/ETD)-MS/MS. Modified peptide 1-25 (sequence of EQLTKCEVFRELKDLKGYGGVSLPE) of U-Gal-SP-BLA with a peak atm/z805.124 84+(Fig. 4C). T4, K13 and S22 of peptide 1-25 were identified by the compete matching of b and y fragment ions.Their presence confirmed that T4 and S22 were phosphorylated by one molecule of phosphate group, and K13 was modified by glycation. K93, K94 and K98 of peptide 92-102 (sequence of VKKILDKVGIN) were glycated as showed in Fig. 4D. The sequence and modified sites of all the phosphorylated and glycated peptides are listed in Table 1. The phosphorylated sites T4, Y18, S22, T29,T30, T33, S34, T38, S69, S76 and Y103 were identified in the U-SPBLA. Glycated sites K13, K16, K58, K62, K93, K94, K98, K108,K114 and phosphorylated sites T4, S22, T29, T30, T33, S34, S69 of Gal-SP-BLA were obtained. For U-Gal-SP-BLA, R10, K13, K16,N44, K58, K62, K79, K93, K94, K98, N102, K108 and K114 were glycated by Gal, T4, Y18, S22, T33, S34, Y36, T38, Y103 and S112 were phosphorylated with phosphate group. These results showed that ultrasound pre-treatment promoted the increase in glycation and phosphorylation sites.
3.6 Mechanism of the reduction in the allergenicity of BLA by ultrasonic prior to glycation and subsequent phosphorylation
BLA is a potential allergen of whey protein and can cause severe cow’s milk allergy. To date, effective strategies for preventing protein allergy are still lacking. Non-thermal processing technology combined with protein modification can eliminate whey protein allergy by changing whey protein structure [16,17]. In the current research,we gained insight into ultrasonic prior to glycation and subsequent phosphorylation. The allergenicity of BLA was reduced through this method, and the reduction was reflected by the reductions in IgG- and IgE-binding capacities (Fig. 1), histamine and IL-6 release (Fig. 2).The underlying mechanisms were investigated.
Allergenic protein molecule has the conformational and linear epitopes [28]. The fluorescence intensity (Fig. 3A) and surface hydrophobicity (Fig. 3B) of SP-BLA, U-SP-BLA, Gal-SP-BLA and U-Gal-SP-BLA considerably changed, suggesting that these methods can change the conformational structure of BLA, destroy conformational epitopes, and ultimately reduce the allergenicity of BLA. Ultrasonic prior to glycation and subsequent phosphorylation destroy the conformational structure of BLA, thereby lowering allergenicity to a higher degree than the other modified methods. The peptide 1-16, 7-18, 13-26, 17-58, 47-58, 51-56, 93-102, 89-108,109-123 and (6-10):S-S:(115-123) are probable allergenic epitopes of BLA [29-31]. The peptides contained one or more Lys, Ser,Thr and Tyr, and protein modification results in the destruction of allergenic epitopes and reduction in BLA allergenicity. As shown in the Table 1, 2 tyrosine residues, 4 serine residues and 5 threonine residues of U-SP-BLA (T4, Y18, S22, T29, T30, T33, S34, T38, S69,S76 and Y103) were phosphorylated, and linear epitopes changed.These effects resulted in a lower allergenicity compared with that in DH-BLA and SP-BLA, which had 5 phosphorylated sites [12].BLA reacted with Gal and SP through glycation and subsequent phosphorylation, and 9 glycated sites and 7 phosphorylated sites were confirmed in the Fig. 5. These glycated sites combined with phosphorylated sites can effectively mask the linear epitopes of BLA,and resulting in allergenicity that is lower than that after glycation or phosphorylation alone. The most striking finding of this experiment was the minimum allergenicity of the treated BLA by ultrasonic prior to glycation and subsequent phosphorylation compare to other experimental groups. This finding may be attributed to a large number of modified sites (Fig. 5), and the subsequent increase in effects on the epitopes. This study provides data that the reductionin allergenicity of BLA in connection with the different glycated sites and phosphorylated sites. Similar results have been achieved in Liu et al. [12], who demonstrated similar changes in BLA structure.Overall, ultrasonic prior to glycation and subsequent phosphorylation can significantly reduce allergenicity of BLA by changing the structure, and the reduction is reflected by the shielding effect of glycated sites combined with phosphorylated sites and the conformational changes in BLA structure.
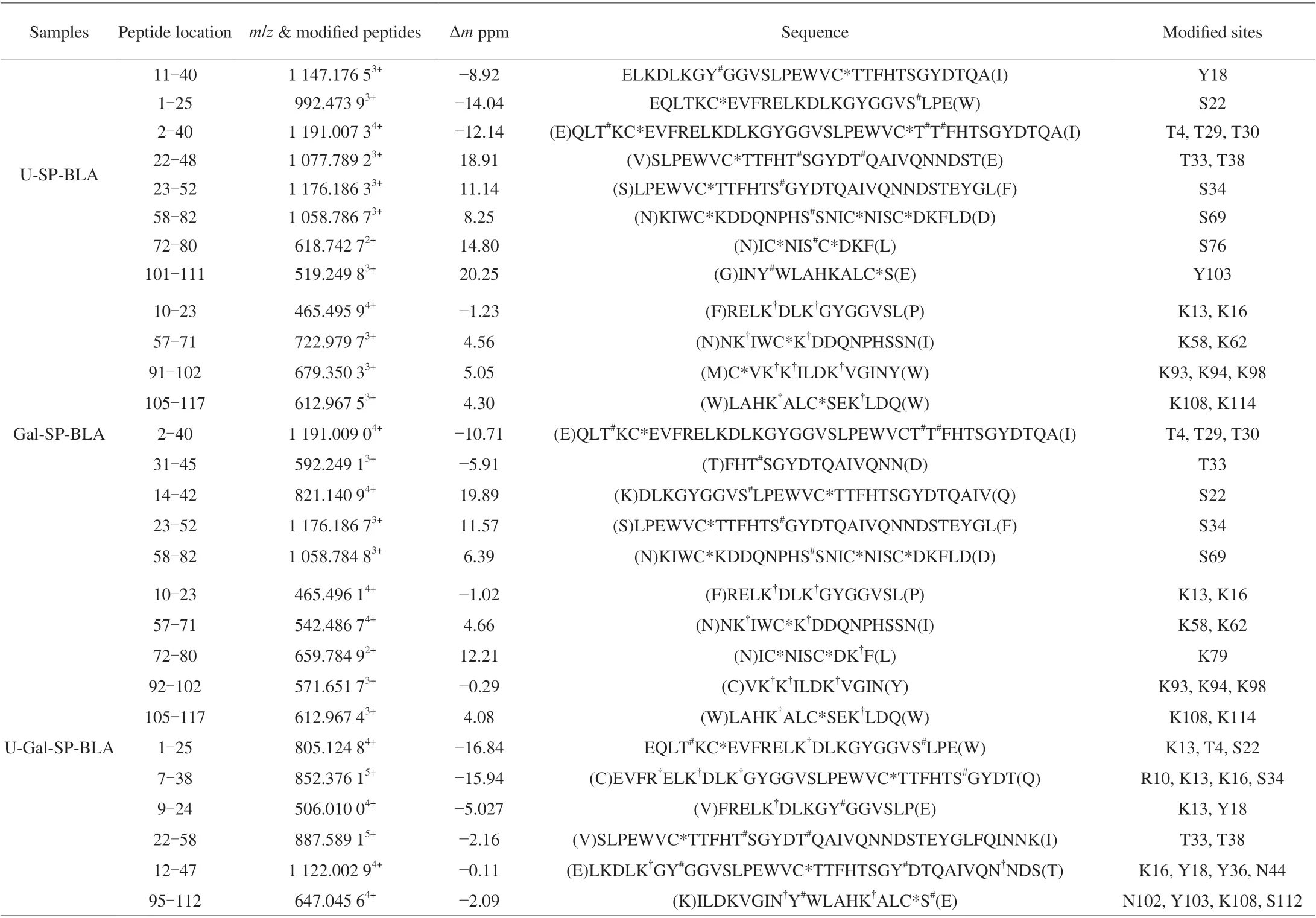
Table 1 Summary of the modified peptides in the U-SP-BLA, Gal-SP-BLA, and U-Gal-SP-BLA.

Fig. 5 Ribbon diagram of U-SP-BLA, Gal-SP-BLA, and U-Gal-SP-BLA (PDB 1F6S). The modified sites are colored as follows: palecyan, framework of BLA;red, phosphorylation sites of BLA; green, glycation sites of BLA.
4. Conclusion
In the present study, ultrasonic prior to glycation and subsequent phosphorylation significantly reduced the allergenicity of BLA by inducing the change of linear and conformational epitopes. The underlying mechanism was revealed. Although this method is a promising food processing technology, it can produce advanced glycation end products (AGEs). Thus, measuring the content of AGEs in the next step is necessary. This procedure ensures that the method is safe and effective. Moreover, the evaluation of protein allergenicity was not comprehensive, and multiple studies are needed to confirm allergenicity reduction.
Conflict of interest
The authors declare no competing financial interest.
Acknowledgments
This work was supported by Science Foundation for Young Scientists of Jiangxi Province (20202BABL215027), and National Natural Science Foundation of China (31960457).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.09.021.
- 食品科學(xué)與人類健康(英文)的其它文章
- Maternal obesity exacerbates the responsiveness of offspring BALB/c mice to cow’s milk protein-induced food allergy
- Au@Ag-labeled SERS lateral f low assay for highly sensitive detection of allergens in milk
- Impact of three different processing methods on the digestibility and allergenicity of Chinese mitten crab (Eriocheir sinensis) tropomyosin
- Effect of lipid peroxidation on the allergenicity and functional properties of soybean β-conglycinin (7S) and glycinin (11S)
- Comparative acetylome analysis reveals the potential mechanism of high fat diet function in allergic disease
- Therapeutic effects of epigallocatechin and epigallocatechin gallate on the allergic reaction of αs1-casein sensitized mice

