Maternal obesity exacerbates the responsiveness of offspring BALB/c mice to cow’s milk protein-induced food allergy
Jingxin Go, Tinge Li, Dong Ling, Hn Gong, Ling Zho, Xueying Mo,*
a Key Laboratory of Functional Dairy, Ministry of Education, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
b Henan Engineering Technology Research Center of Food Processing and Circulation Safety Control, College of Food Science and Technology,Henan Agricultural University, Zhengzhou 450002, China
c Applied Nutrition I, China National Center for Food Safety Risk Assessment, Beijing 100022, China
Keywords:Cow’s milk allergy Maternal obesity Offspring Intestinal barrier Immune response
A B S T R A C T Food allergy has become a signif icant public health problem affecting a large number of people worldwide.Maternal obesity causes inf lammation and alters the immune system of offspring, which may exacerbate their food allergy. The aim of this study was to determine whether offspring mice born to obese mothers would have more serve reactions to cow’s milk protein-induced food allergy, and further investigate the underlying mechanisms. Female offspring BALB/c mice of mothers with normal and high-fat diets were sensitized with β-lactoglobulin (BLG), respectively. Maternal obesity increased the serum immunoglobulin E and mouse mast cell protease levels, though did not have significant influence on anaphylactic symptom score, core temperature and diarrhea rate of offspring mice after BLG sensitization. Furthermore, maternal obesity led to a lower level of occludin mRNA expression in BLG-sensitized mice. The mice born to obese mothers exhibited increased mRNA expression levels of GATA-3, interleukin (IL)-4 and IL-10 in jejunum after BLG sensitization, indicating maternal obesity intensified Th2-type biased immune responses. In conclusion,maternal obesity exerted exacerbating effects on the responsiveness of their offspring to cow’s milk protein sensitization.
1. Introduction
Food allergy is a serious global public health concern affecting up to 10% of the population worldwide [1]. Cow’s milk is the main ingredient for the production of infant formula milk powder.However, cow’s milk allergy is one of the major allergic diseases in early life, with 0.5%-3.0% of one-year-old children being affected in developed countries [2]. The major allergens in cow’s milk include casein,α-lactalbumin andβ-lactoglobulin (BLG) [3]. Most cow’s milk allergy is associated with BLG which is absent in human milk.Cow’s milk allergy may exhibit a variety of adverse reactions, such as mild itching in the mouth, gastrointestinal disorders, wheezing and even severe life-threatening anaphylaxis [4,5]. The tolerance to food allergens is affected by various factors, including environment,genetics, epigenetics and immune-mediated process [6]. Maternal nutrition changes the risk of immune-mediated diseases (such as asthma) in offspring through exerting epigenetic functions [7].Epidemiological studies have confirmed that a balanced maternal diet can ameliorate the allergy pandemic in both infants and young children, and animal experiment has shown that a high-fiber diet of maternal mice is conducive to the suppression of allergic airway disease in offspring mice [8-10]. However, the studies related to the inf luence of maternal diet on the food allergy of their offspring are rare and need to be further investigated.
Food allergy is the result of immune disorders and oral intolerance to ingested foods, which can be categorized into immunoglobulin E(IgE)-mediated, mixed IgE- and non-IgE-mediated, and non-IgEmediated reactions [6,11]. IgE-mediated food allergy is the most fully characterized type and manifested by acute clinical symptoms, mixed IgE- and non-IgE-mediated food allergy generally appears as atopic dermatitis as well as eosinophilic oesophagitis, and non-IgE-mediated food allergy primarily exhibits as gastrointestinal symptoms [12,13].Cow’s milk protein is the allergen of all these three types of allergies [11].The small intestinal is the main site responsible for food digestion and absorption, and its barrier function plays a crucial role in maintaining the homeostasis in the living body [14]. Tight junction (TJ) proteins are multi-protein complex that seal the space between adjacent epithelial cells and modulate the permeability of intestinal barrier [15].Allergic reactions induced by food allergens are associated with impaired barrier functions. Infants and young children are more likely to develop food allergies because their intestinal barrier is not fully developed. Undigested food protein and large peptide fragments containing antigen epitopes can cross the damaged gut barrier and trigger intestinal immune responses [16]. The response is type-2 helper (Th2) cell-mediated and featured by the differentiation of Th2 cells as well as the release of Th2 cytokines, such as interleukin (IL)-4,IL-5, IL-10 and IL-13 [11]. During immune response in the gut,mast cells play a key role in the accumulation of T cells and B cells and the migration of dendritic cells through releasing inflammatory mediators [17,18]. Although numerous studies have been conducted,the underlying mechanisms by which infants and young children are more susceptible to food allergies are yet to be elucidated, and the maternal factors are rarely considered.
Due to poor dietary habits, obesity is a growing concern.Maternal obesity during pregnancy not only has immediate effects on the fetus, but also has long-term adverse effects on the offspring,such as impaired organ development, decreased insulin sensitivity and cardiovascular disease [19]. Maternal obesity increases insulin resistance and low-grade inflammation in and pups [20], and alters frequency and function of immune cells in umbilical cord blood samples, resulting in dysregulation of human neonatal immune homeostasis [21]. Maternal obesity also induces gut inflammation as well as higher intestinal permeability of the offspring mice [22].High-fat diet (HFD) intake prior to and during pregnancy leads to adverse changes in placental vascularization and impairments in fetal intestinal development [23]. Maternal HFD consumption stimulated inflammatory cytokine expression and neutrophil induction in colonic tissues, further aggravated offspring mice to colitis [24]. Therefore,maternal obesity has detrimental effects on the intestinal health of their offspring. Furthermore, HFD could promote susceptibility to food allergy due to the impaired gut microbiome [25]. Mice born to obese mothers have a higher risk of developing childhood asthma and allergic inflammatory responses [26,27]. A meta-analysis that included 14 studies (108 321 mother-child pairs) showed that maternal obesity during pregnancy and increased maternal gestational weight gain was related to the enhanced risk of childhood asthma [28].However, the effects of maternal obesity on the responsiveness of offspring to food allergens have not been fully investigated. Based on the facts above, we hypothesized that maternal obesity would exacerbate food allergy of their offspring.
This study was designed to investigate whether HFD-induced maternal obesity could affect the responsiveness of offspring BALB/c mice to cow’s milk protein-induced food allergy. The multifaced symptoms after sensitization and challenging with BLG were analyzed in the offspring mice born to obese mothers. The intestinal barrier function and immunity were also evaluated to clarify the underlying mechanisms.
2. Materials and methods
2.1 Animals and experimental design
The procedure of the animal experiment was approved by the Ethics Committee of China Agricultural University (protocol number:AW61110202-4). Female BALB/c mice (aged 3-4 weeks) were provided by Beijing Vital River Laboratory Animal Technology Co.,Ltd. (Beijing, China). The mice were housed in a specific-pathogenfree environment at (22 ± 2) °C under 12 h light/dark cycle and provided food and water ad libitum. After adaptive feeding for a week, the mice were randomly divided into two groups (n= 12 per group). The control group was fed with normal diet (ND, 10% energy from fat, D12450H, Research Diets, New Brunswick, NJ, USA), and the model group was fed with HFD (60% energy from fat, D12492;Research Diets, New Brunswick, NJ, USA) for 10 weeks to induce obesity. The body weight was recorded once a week, and food intake was recorded twice a week. Blood was derived from the orbital vein plexus and centrifugated at 4 000 ×gfor 20 min to obtain serum.The content of total cholesterol (TC), triglyceride (TG), high density liptein cholesterol (HDL-C) and low density liptein cholesterol(LDL-C) in serum were measured using automatic biochemical analyzer (Indiko?, Thermo Fisher Scientific, USA), and the levels of serum inflammatory factors were also analyzed. Then the female mice were mated with 10-week-old male BALB/c mice, and the pregnant female mice were housed individually. After farrowing, the pups were normalized to two females and nursed by maternal mice until weaning at 21 days of age. During lactation, each maternal mouse was housed separately with access to food and water ad libitum, and the offspring mice were kept with their nursing mother. The maternal mice in the control group were fed with ND, in the model group were fed with HFD during pregnancy and lactation. The offspring mice were randomly divided into four groups (n= 10 per group): ND female mice-sham group (ND-Sham), ND female mice-BLG group(ND-BLG), HFD female mice-sham group (HFD-Sham), HFD female mice-BLG group (HFD-BLG). All of the offspring mice were fed with ND.
To induce an allergic response, 4-week-old female offspring mice in ND-BLG and HFD-BLG groups were sensitized by intraperitoneal injection of 0.4 mg bovine BLG (≥ 90% PAGE, L3908, Sigma-Aldrich, St. Louis, MO, USA), together with 100 μL complete Freud’s adjuvant as well as 100 μL normal saline on day 0, and the complete Freud’s adjuvant was replaced by incomplete Freud’s adjuvant on days 7, 14 and 21. For ND-Sham and HFD-Sham groups, the mice were administered with 200 μL of normal saline by intraperitoneal injection. On day 28, mice in all groups were challenged with 50 mg BLG. The core temperature was measured every ten minutes during this period and the allergic symptoms were scored. After BLG challenge for 1 h, mice were sacrificed, and the tissues were weighted and collected. The blood was harvested and then centrifugated at 4 000 ×gfor 20 min to obtain serum. Tissues and serum were stored at -80 °C for further analysis.
2.2 Evaluation of anaphylactic symptoms
One hour after BLG challenge, the anaphylactic symptoms of offspring mice were scored according to the method of Liu et al. [29]with some modifications. In brief, grading was based on the following criteria: 0-no symptoms; 1-scratching and rubbing around the mouth,nose or ears, slightly moist feces; 2-decrease in activity, swelling around the eyes and mouth, shortness of breath, soft and partially formed feces;3-static activity time exceeds 1 min, dyspnea, severe diarrhea, loose and semiliquid feces; 4-muscle twitching, no response to stimulus, watery and mucous-like feces; 5-convulsion or death.
2.3 Measurement of serum biochemical parameters
Serum lipopolysaccharide (LPS), IL-6 of maternal mice and histamine, mouse mast cells protease 1 (mMCP-1) of offspring mice were determined by ELISA with the corresponding kits (Dogesce,China). Serum IgE and IgG1 of offspring mice were also measured using ELISA kits (Immunology Consultants Laboratory, Inc., USA).All procedures were performed as the kit instructions.
2.4 Calculation of immune organ indexes
The spleen and thymus of offspring mice were washed, blotted dry and weighted. Spleen and thymus indexes were calculated using the following formula [29]:
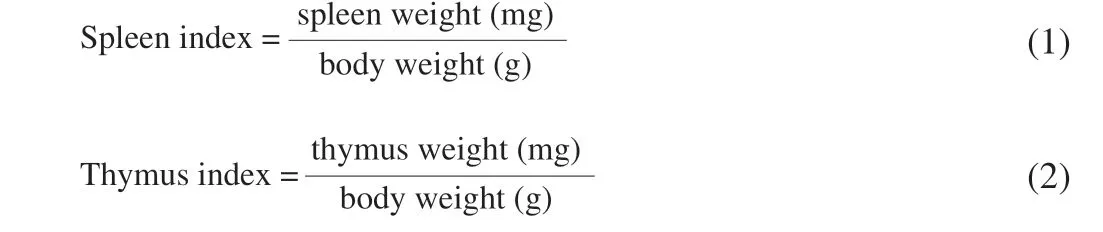
2.5 Histological analysis
For histological analysis, the jejunum of offspring mice was fixed with 4% paraformaldehyde and embedded in paraffin wax. The tissue blocks were cut into 5 μm sections by a rotary microtome, and the layers were pasted onto glass slides, then placed in a 45 °C incubator to dry. The sections were stained with toluidine blue and air-dried.Images were taken with a microscope (Olympus Corporation, Japan)under × 100 magnification.
2.6 Measurement of mRNA levels
The relative mRNA expression levels ofZO-1,occludin,claudin1,T-bet,GATA-3,IL-2, interferon-γ (IFN-γ),IL-4,IL-10in the jejunum of offspring mice were determined by quantitative real-time PCR.The minced jejunum tissue was homogenized in 1 mL cold Trizol(Invitrogen, Carlsbad, CA, USA). After mixing with chloroform, the mixture was centrifuged at 8 000 ×gfor 15 min. Then the supernatant was mixed with an equal volume of prechilled isopropanol. The mixture was allowed to stand for 30 min at room temperature and centrifuge at 8 000 ×gfor 15 min. The precipitate was washed with 75% ethyl alcohol and dissolved with diethylpyrocarbonate-treated water. The cDNA was synthesized with a cDNA synthesis kit (Abm,Richmond, BC, Canada). Real-time PCR analysis was carried out employing SYBR master mix (Takara Bio, Tokyo, Japan) on Techne Quantica real-time PCR detection system (Techne, Staffordshire,UK). Real-time PCR reactions were performed according to the following conditions: 95 °C for 180 s, followed by 45 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s. The mouse primer sequences of various genes are listed in Table 1.β-Actin served as an internal reference gene, and the relative expression of genes was calculated by the 2-ΔΔCtmethod.
2.7 Statistical analysis
Unless otherwise specified, all results were expressed as the mean ±standard error of the mean (SEM) of at least three independent repetitions. One-way analysis of variance (ANOVA) and Tukey’s multiple comparison test were performed using SPSS Version 18.0 (SPSS Inc., Chicago, IL), andP< 0.05 was considered to be statistically different.
3. Results
3.1 Weight and biochemical characteristics of maternal mice
To demonstrate the success of establishing the maternal obesity model, the body weight, the levels of serum lipid indexes (TC, TG,HDL-C, LDL-C) and inflammatory cytokines (LPS, IL-6) of maternal mice were measured. As shown in Fig. 1A, there was no significant difference between the body weight of the HFD group and ND group at week 0, but the body weight of the HFD group ((23.90 ± 0.81) g)was 20.10% higher than that of the ND group ((19.90 ± 0.55) g) at week 10. The body weight of the HFD group was ((23.90 ± 0.81) g)at week 10, which is significantly higher than the body weight((17.16 ± 0.62) g) at week 0 (P< 0.001). Furthermore, the serum levels of TC (Fig. 1B), TG (Fig. 1C), HDL-C (Fig. 1D), LDL-C(Fig. 1E) of the HFD group were significantly higher than that of the ND group at week 10 (P< 0.001). There were also significantly higher serum levels of LPS (Fig. 2A) and IL-6 (Fig. 2B) in the HFD group compared to the ND group (P< 0.05). These results suggested the maternal obesity model was established successfully.

Table 1 Primers used for real-time PCR.
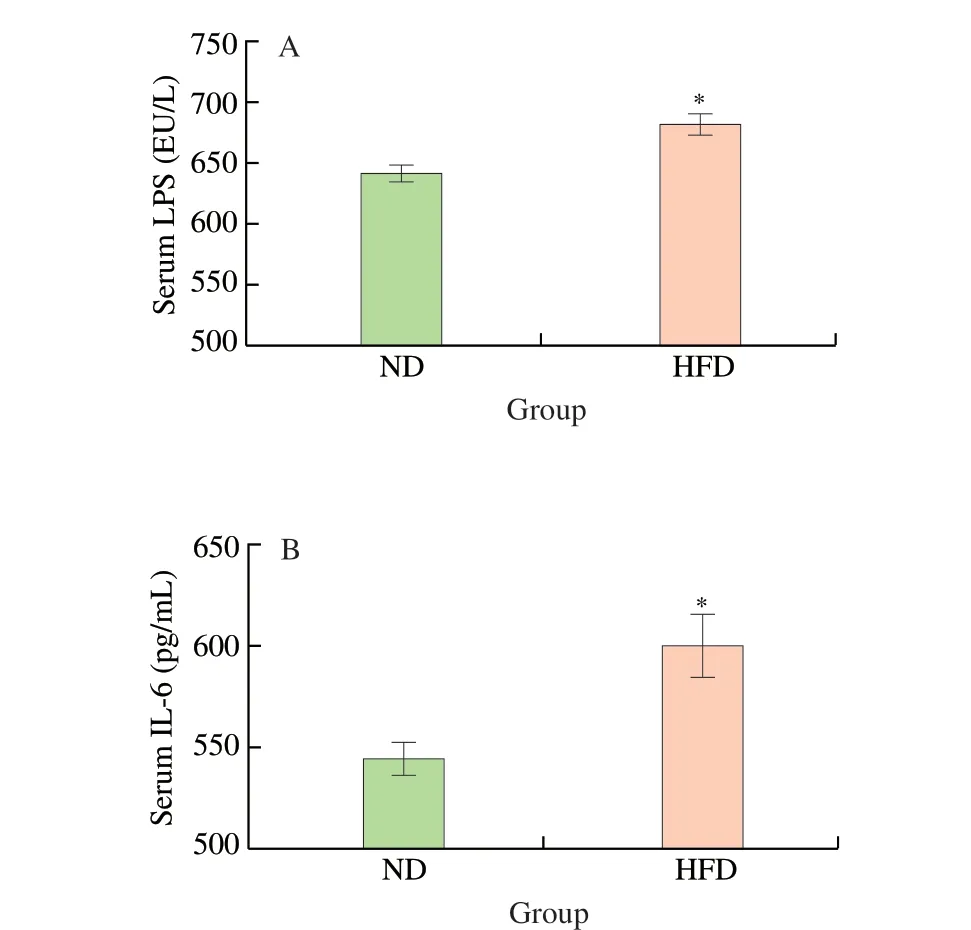
Fig. 2 Serum inflammatory factor levels in maternal mice. (A) Serum LPS;(B) Serum IL-6. Values are expressed as the mean ± SEM of at least three independent determinations. * P < 0.05 vs. ND.
3.2 Anaphylactic symptoms of offspring mice
To investigate the effects of maternal obesity on the response of offspring to cow’s milk protein, the offspring of normal and obesity mice were sensitized with BLG separately. The anaphylactic symptom score (Fig. 3A), core temperature (Fig. 3B), diarrhea rate (Fig. 3C)and spleen index (Fig. 3D) in ND-Sham and HFD-Sham groups were not found to be different. But HFD-Sham group had a higher thymus index compared to the ND-Sham group, with statistically significant differences (P< 0.05) (Fig. 3E). It could be seen that the anaphylactic symptom score (Fig. 3A), core temperature (Fig. 3B) and diarrhea rate (Fig. 3C) in ND-BLG and HFD-BLG groups were significantly higher than ND-Sham group (P< 0.05). However, there was no significant difference between HFD-BLG and ND-BLG groups. BLG sensitization induced a marked increase in the spleen index compared to the ND-Sham group (P< 0.05), but did not cause a significant change in the thymus index (Fig. 3D, E).
3.3 Serological analysis of offspring mice
After the BLG challenge on day 28, the blood serum of offspring mice was collected to determine serological indicators.Though the serum concentrations of IgE (Fig. 4A), IgG1 (Fig. 4B)and histamine (Fig. 4C) in ND-Sham and HFD-Sham group did not yield significant differences, the serum concentrations of mMCP-1 in the HFD-Sham group was significantly higher than that of NDSham group (P< 0.05) (Fig. 4D). BLG sensitization resulted in significant increases in these serological indicators compared to the ND-Sham group (P< 0.05) (Fig. 4). Moreover, the serum IgE and mMCP-1 levels in HFD-BLG group were significantly higher than that of ND-BLG group (P< 0.05) (Fig. 4A, D). However, the serum IgG1 and histamine levels did not significantly differ between ND-BLG and HFD-BLG group (Fig. 4B, C).

Fig. 3 Establishment of sensitized offspring mice model. (A) Anaphylactic symptom score; (B) Core temperature; (C) Diarrhea rate; (D) Spleen index;(E) Thymus index. Values are expressed as the mean ± SEM of at least three independent determinations. *** P < 0.001 vs. ND-Sham,ns means no statistically significant vs. ND-BLG.

Fig. 4 Immunoglobulin, histamine and mMCP-1 level in the serum of offspring mice. (A) IgE; (B) IgG1; (C) histamine; (D) mMCP-1. Values are expressed as the mean ± SEM of at least three independent determinations. ** P < 0.01, *** P < 0.001 vs. ND-Sham, # P < 0.05, ### P < 0.001 vs. ND-BLG, ns means no statistically significant vs. ND-BLG.
3.4 Histological observations of the jejunum of offspring mice
The mast cells in the jejunum of offspring mice were observed using toluidine blue staining. As shown in Fig. 5, the staining indicated that the difference for the number of mast cells in HFDSham and ND-Sham groups failed to show significance. After BLG sensitization, the number of mast cells remarkably increased in comparison with the ND-Sham group (P< 0.05). Furthermore, the staining indicated that the number of mast cells in the HFD-BLG group was more than the ND-BLG group, although there was not a statistically significant difference (Fig. 5).
3.5 Relative mRNA expression of TJ protein genes in the jejunum of offspring mice
The mRNA levels of TJ proteins in the jejunum were examined to investigate the intestinal barrier function of the offspring mice.The mRNA expression ofZO-1(Fig. 6A),occludin(Fig. 6B) andclaudin1(Fig. 6C) were significantly lower in the HFD-Sham group compared with the ND-Sham group (P< 0.05). And as predicted,we found that BLG sensitization evoked a significant decrease in the mRNA expression ofZO-1(Fig. 6A),occludin(Fig. 6B) andclaudin1(Fig. 6C) in comparison with the ND-Sham group (P< 0.05).Additionally, the HFD-BLG group had a remarkably lower occludin mRNA expression than the ND-BLG group (P< 0.05) (Fig. 6B). No significant difference was observed in the mRNA expression of ZO-1 or claudin1 in the HFD-BLG group compared with the ND-BLG group (Fig. 6A, C).

Fig. 5 Toluidine blue staining of the jejunum of offspring mice. Values are expressed as the mean ± SEM of at least three independent determinations. (A)Toluidine blue staining results. (B) Number of mast cells. * P < 0.05, ** P < 0.01 vs. ND-Sham, ns means no statistically significant vs. ND-BLG.
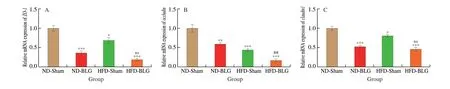
Fig. 6 Relative mRNA expression of tight junction protein in the jejunum of offspring mice. (A) ZO-1; (B) Occludin; (C) Claudin1; Values are expressed as the mean ± SEM of at least three independent determinations. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. ND-Sham, ## P < 0.01 vs. ND-BLG, ns means no statistically significant vs. ND-BLG.
3.6 Th1/Th2 balance in the jejunum of offspring mice
To further explore the gut immune response of offspring mice,the mRNA expression of the transcription factors (T-bet,GATA-3)related to T cell polarization was analyzed. The mRNA expression of Th1-typed transcription factor (T-bet) in HFD-Sham and NDSham groups did not show any significant difference, but that of Th2-typed transcription factor (GATA-3) in the HFD-Sham group was remarkably higher than the ND-Sham group (P< 0.05) (Fig. 7A, B).Both ND-BLG and HFD-BLG groups had significantly lowerT-betmRNA expression (P< 0.05), while significantly higher mRNA expression ofGATA-3(P< 0.05) (Fig. 7A, B). TheGATA-3mRNA expression in the HFD-BLG group was significantly more abundant than that of the ND-BLG group (P< 0.05) (Fig. 7B).
The mRNA expression of Th1-related cytokines (IL-2,IFN-γ) and Th2-related cytokines (IL-4,IL-10) in the jejunum of offspring mice was also evaluated. As presented in Fig. 7C, there were no significant differences inIL-2mRNA expression in ND-BLG and HFD-Sham groups compared with the ND-Sham group, while the HFD-BLG group had a significantly lowerIL-2mRNA expression than the ND-Sham group (P< 0.05). The mRNA expression ofIFN-γin ND-BLG, HFD-Sham and HFD-BLG groups was significantly lower than ND-Sham group (P< 0.05), but no significant difference between HFD-BLG and ND-BLG was observed (Fig. 7D). Fig. 7E showed that there were theIL-4mRNA expression in ND-BLG and HFD-Sham groups is significantly higher than ND-Sham group(P< 0.05), and the HFD-BLG group had a significantly higher IL-4 mRNA expression than ND-BLG group (P< 0.05). The mRNA expression ofIL-10between HFD-Sham and ND-Sham groups did not differ significantly, but the value of the HFD-BLG group is significantly higher than the ND-BLG group (P< 0.05) (Fig. 7F).
4. Discussion
Children of obese mothers have been shown to be more likely to suffer from allergic diseases [26,28], and the candidate mechanisms related to allergic airway disease have been investigated [27].However, there are few studies available on food allergies of offspring born to obese mothers, and the clear mechanism still awaits being elucidated. To the best of our knowledge, relatively few attempts have been made to investigate the effects of maternal obesity on the responsiveness of offspring to food allergen-induced allergy. In the present study, we investigated the anaphylactic symptoms, blood indices (such as IgE, IgG1, histamine, mMCP-1),jejunum TB staining, jejunum mRNA expressions of TJ proteins,transcription factors and related cytokines of mice born to normal and obese mothers after BLG sensitization. Our results demonstrated that maternal obesity did not have significant influence on the anaphylactic symptoms of offspring mice, but resulted in an increase in serum IgE and mMCP-1 levels. Besides, the offspring mice of obese mothers exhibited higher levels of compromised intestinal barrier function and more potent Th2 type immune response polarization than mice born to normal mothers. Our findings suggest that maternal obesity would exacerbate the responsiveness of offspring mice to cow’s milk protein-induced food allergy to a degree.

Fig. 7 Relative mRNA expression of Th1, Th2 transcription factors and cytokines in the jejunum of offspring mice. (A) T-bet; (B) GATA-3; (C) IL-2; (D) IFN-γ;(E) IL-4; (F) IL-10. Values are expressed as the mean ± SEM of at least three independent determinations. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. ND-Sham,# P < 0.05, ## P < 0.01 vs. ND-BLG, ns means no statistically significant vs. ND-BLG.
It is generally accepted that IgE induces anaphylactic reactions,and the high-affinity receptor for IgE (FcεRI)-mediated mast cell activation has been confirmed to play a crucial role during the process of allergic inflammation [30]. Upon re-exposure to the allergen, the IgE binding to mast cells and basophils would crosslink with the allergen, and made mast cells become active [31]. The activated mast cells would degranulate and release mediators, pro-inflammatory cytokines and protease that lead to allergic responses [31]. We observed higher serum IgE levels in the HFD-BLG group than the ND-BLG group, which suggested that maternal obesity induces more severe food allergies. Nevertheless, we found that the mice in the HFD-BLG group had a significantly higher level of serum mMCP-1 than the ND-BLG group (P< 0.05) and observed promoted mast cell accumulation in the jejunum of HFD-BLG group mice. Obesity has been recognized as a state of chronic low-grade inflammation,and maternal HFD promotes inflammation in the offspring [22,32].LPS is the main component of the outer membrane of gram-negative bacteria. HFD alters gut microbiota composition and makes LPSproducing bacteria more abundant, resulting in increased maternal LPS circulation and ultimately damaging the epigenetic state of the offspring [23,33]. Meanwhile, excessive content of LPS in the blood would facilitate the production of pro-inflammatory cytokines, such as IL-1β and IL-6 [34]. IL-6 has been shown to cross the placental barrier and lead to low-grade inflammation in the offspring [35,36]. In this study, the mice in the HFD group had significantly higher serum LPS and IL-6 contents than the ND group (P< 0.05). Inflammatory stimulus including mMCP-1 can induce and expand mast cells, mast cells in turn promote systematic inflammatory response [37,38].mMCP-1 is one of the major proteases expressed by mucosal mast cells that exists in the epithelium of the gastrointestinal tract [39].Consequently, maternal obesity exacerbates the allergic responses of offspring mice may be implemented by promoting FcεRI-mediated mast cell activation.
The epithelial barrier plays a pivotal role in maintaining intestinal homeostasis by preventing the absorption of macromolecular food antigens and other harmful substances [14,40]. Epithelial cells are connected together by TJs, such as claudins and occludins [15].Enhancing the integrity of the intestinal barrier and improving the expression of TJ proteins can repair the intestinal barrier function,and thereby alleviating food allergy [41]. We observed significantly lower mRNA expression ofZO-1,occludinandclaudin1in the HFDSham group compared with the ND-Sham group (P< 0.05), as well as significantly loweroccludinmRNA expression in the HFD-BLG group compared with ND-BLG group (P< 0.05). These are consistent with previous studies that mice born to obese mothers exhibited decreased TJ protein gene expression [42]. BLG-induced allergic mice have decreased mRNA expression ofoccludinandclaudin1[43].Maternal obesity has been revealed to impair the gut barrier function of offspring by enhancing inflammatory response and oxidative stress,which may lead to the development of gut permeability-associated diseases in offspring [22]. Maternal obesity also accelerated offspring colitis symptoms by stimulating inflammatory cytokines expression and neutrophil infiltration in the colon [24]. Maternal HFD could change intestinal development and disrupt mucosal barrier function in offspring, which might be mediated by diversity changes of intestinal microbiota [44]. The alteration of gut microbiota affected maternal LPS production [23,33], which promoted the occurrence of gut inflammation in offspring through toll-like receptors and the placenta [45].Therefore, maternal obesity led to the compromised gut barrier function and intensified the development of food allergies in offspring.However, a study based on a population experiment found that maternal obesity was not associated with food allergy in early childhood and even inversely associated with IgE [46]. The sample from children born to obese mothers of their study was only 16 cases, therefore, further evaluation including more samples is needed. Furthermore, excessive gestational weight gain was shown to be associated with an increase in childhood food allergy [47].In the present study, an animal model of food allergy with increased serum IgE content was established, and the maternal mice were fed with HFD during pregnancy and lactation, which would lead to more body weight gain. These are probably the main reasons for the inconsistent findings.
Food antigens crossing the intestinal barrier elicit abnormal immune responses characterized by imbalanced Th1/Th2 responses [11].T-bet and GATA-3 are two important transcriptional regulatory factors responsible for na?ve T cells differentiating into Th1 and Th2 cell subsets [48]. T-bet is the Th1-specific transcription factor that plays a central role in regulating the initiation of Th1 cells development [49]. Conversely, GATA-3 is the Th2-specific transcription factor that promotes Th2 cell differentiation [50].HFD-induced obesity could worsen Th2 type immune response in mice with allergic disease [51]. The outcome of this study revealed that the jejunum of mice in the ND-BLG group had decreasedT-betmRNA expression and increased GATA-3 expression compared to the ND-Sham group, indicating Th2-type immune polarization occurred after BLG sensitization. Besides, we found that the offspring of obese mice had stronger Th2-type immune polarization. IL-2 and IFN-γ are secreted by Th1 cells, whereas IL-4 and IL-10 are secreted by Th2 cells [52]. Mice born to obese mothers have higher IL-4 levels in bronchoalveolar lavage fluid than that of ND mothers after ovalbumin sensitization and elevated gut pro-inflammatory cytokine gene expressions [24,27]. The observedIL-4andIL-10mRNA expressions in the jejunum of offspring mice in different groups are consistent with the tendency ofGATA-3gene expression. These results donate that maternal obesity accelerates Th2-type immune response of offspring mice. Furthermore, IL-10 is an important immune regulator produced by Tregs, which are essential to induce oral tolerance.IL-10 could inhibit Th17 response and limit inflammatory responses in the gut [53]. Offspring of mother fed with western diet (containing high concentrations of sugar and fat) had increased IL-10 content in the serum [54]. This is consistent with our study. Therefore,the unobtrusive change in anaphylactic symptoms may be due to increased Treg response and inhibited Th 17 response in mice born to obese mothers.
5. Conclusion
Our study demonstrated that maternal obesity did not induce obvious change in the anaphylactic symptoms of offspring mice, but led to a marked increase in serum IgE and mMCP-1 levels, lower TJ protein mRNA expressions and stronger Th2 type immune responses in the model of BLG-induced food allergy. Overall, these findings suggest that maternal obesity exacerbates the responsiveness of offspring mice to cow’s milk protein-induced food allergy.
Conflict of interest
None.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2019YFC1605000) and the Beijing Dairy Industry Innovation Team (BAIC06-2021).
- 食品科學與人類健康(英文)的其它文章
- Au@Ag-labeled SERS lateral f low assay for highly sensitive detection of allergens in milk
- Impact of three different processing methods on the digestibility and allergenicity of Chinese mitten crab (Eriocheir sinensis) tropomyosin
- Effect of lipid peroxidation on the allergenicity and functional properties of soybean β-conglycinin (7S) and glycinin (11S)
- Comparative acetylome analysis reveals the potential mechanism of high fat diet function in allergic disease
- Therapeutic effects of epigallocatechin and epigallocatechin gallate on the allergic reaction of αs1-casein sensitized mice
- Assessment of immune responses and intestinal f lora in BALB/c mice model of wheat food allergy via different sensitization methods

