Enzymatic hydrolysis of silkworm pupa and its allergenicity evaluation by animal model with different immunization routes
Yan Dai, Meijia Huang, Yujuan Xu, Lixia Mu, Jingyan Gao, Honging Chen,Zhihua Wu, Anshu Yang, Yong Wu, Xin Li,*
a State Key Laboratory of Food Science and Technology, Nanchang University, Nanchang 330047, China
b School of Food Science and Technology, Nanchang University, Nanchang 330047, China
c Sericultural and Agri-Food Research Institute Guangdong Academy of Agricultural Sciences, Key Laboratory of Functional Foods,Ministry of Agriculture and Rural Affairs, Guangdong Key Laboratory of Agricultural Products Processing, Guangzhou 510610, China
d Sino-German Joint Research Institute, Nanchang University, Nanchang 330047, China
Keywords:Silkworm pupa Silkworm pupa peptides Enzymatic hydrolysis Allergenicity Immunization Animal model
A B S T R A C T Silkworm pupa is a nourishing food with high nutritional value, but its consumption has been greatly limited given its allergenicity. Enzyme hydrolytic technique is recognized as an effective method to reduce the allergenicity of protein. In this study, we aimed to investigate the effect of enzymolysis on the allergenicity of silkworm pupa. Crude silkworm pupa protein was extracted through alkali extraction and acid precipitation,which included 5 proteins with the molecular weights ranging from 34 kDa to 76 kDa, and silkworm pupa were then hydrolyzed by alkaline protease. The allergenicity of silkworm pupa protein and its enzymatic hydrolysates was evaluated by establishing BALB/c mice model, and the mice were immunized via intragastric gavage and intraperitoneal injection, respectively. The results indicated that the intraperitoneal injection immunization route induced more by detecting with antibodies, histamine and Th2-related cytokines.Moreover, mice treated with silkworm pupa protein peptide displayed no obvious allergic symptoms,indicating that enzyme hydrolytic technique could signif icantly reduce the allergenicity of silkworm pupa.
1. Introduction
As a type of crustacean insect, the consumption of silkworm pupa dates back thousands of years in China. In 2006, silkworm pupa was approved as a new food resource by the Ministry of Health of the People’s Republic of China [1]. Silkworm pupa is popular given its nutritive value and is considered as a delicacy in some Asian countries, such as China, Japan, Korea, and India. The percentages of total protein, lipid contents and ash of silkworm pupa by dry weight are 71.9%, 20.1% and 4.0%, respectively. Amino acid analysis showed that silkworm contains 18 known amino acids, including all of the essential amino acids, thus meeting the optimal amino acid pattern recommended by the FAO/WHO [2]. However, in a paper reported in 2008, greater than 1 000 patients suffered anaphylactic reactions after consuming silkworm pupa each year in China based on a rough estimate [3]. It has been reported that people allergic to silkworm pupa may experience various clinical symptoms, such as pruritus, dizziness,headache, nausea, vomiting, tremors, disturbance of consciousness,asthma or even severe shock [3]. Thus, human consumption of silkworm pupa has been greatly limited.
Enzymatic hydrolysis technology has been widely applied in food processing and has succeeded in changing the nutritional, bioactive and functional properties of food proteins, including reductions in allergenic compounds [4]. Enzymatic processes reduce allergenicity by breaking down proteins into small fragments/peptides or amino acids and usually do not destroy the nutritional value of the protein source under mild conditions. At present, many studies have reported the effect of enzymatic hydrolysis on protein allergenicity. For example, research demonstrated that the allergenicity of several species of crustaceans was reduced upon treatment with proteases [5].In another study, proteinase digestion could effectively reduce the immunoglobulin E (IgE) binding capacity of tropomyosin (TM) from both shrimp and crab [6,7].
Although silkworm pupa has been listed as an important inducer of food allergies [2], few studies have reported allergies to silkworm pupa until now. The aims of the study were to investigate the potential mechanisms of protein allergy in silkworm pupa and the effect of enzyme hydrolysis on the allergenicity of silkworm pupa.The study may be beneficial for the development of hypoallergenic or nonallergenic silkworm pupa products.
2. Materials and methods
2.1 Reagents
Silkworm pupae were provided by the Sericultural and Agri-Food Research Institute Guangdong Academy of Agricultural Sciences,Guangzhou. Alkaline protease was purchased from PangBo (Nanning,China). Cholera toxin (CT), Freund’s complete adjuvant (FCA),Freund’s incomplete adjuvant (FIA) and Tween-20 were purchased from Sigma (St. Louis, MO, USA). HRP-labeled Goat Anti-Mouse IgG (H+L), HRP-labeled Goat Anti-Mouse IgG1 (H+L), and Biotinlabeled Goat Anti-Mouse IgE were purchased from SouthernBiotech(Washington, USA). Other regents were obtained from Sangon(Shanghai, China).
2.2 Animals
Four-week-old female BALB/c mice ranging in weight from 17 to 20 g were purchased from Hunan SJA Laboratory Animal Co., Ltd.(Hunan, China) (Nanchang, China; Permission number “SYXK (Gan)164 2016-0002”). The mice were housed in stainless steel cages with a temperature of 21-25 °C, a relative humidity of 40%-60% and 12 h/12 h light/dark cycles. Animals were fed a standard feed containing no silkworm pupa and ultrapure water for 1 week for adapting the environment. All experiments were performed in strict accordance with the NCH guidelines from China for the care and use of laboratory animals (NCH Publication No.11, Rev. 2016) and approved by the Animal Care Committee of Nanchang University(Nanchang, China).
2.3 Preparation of silkworm pupa crude protein
Dried silkworm pupae were washed with distilled water and 95%alcohol. Silkworm pupae were then dried in an oven at 55 °C for 24 h and pulverized with an ultramicro pulverizer. The pulverized samples were defatted by petroleum ether andn-hexane ether with agitation at 4 °C and then filtrated in a vacuum. Then, crude silkworm pupa protein was extracted by mixing the defatted powder with distilled water at a ratio of 1:10 (m/V) and adjusting the pH to 10 in ultrasonic device for 1 h. The supernatant was collected by centrifugation at 1 000 ×gfor 15 min. Then, samples were subjected to a desalting process immediately via dialysis for 48 h at 4 °C. The concentration of silkworm pupa protein (SP) was determined using the Coomassie brilliant blue G-250-binding method. The final extract was freezedried and then stored at -20 °C until use.
2.4 Enzymatic hydrolysis of crude protein
Firstly, the defatted powder was mixed with distilled water at a powder/solvent ratio of 1:20 (m/V), and the pH was adjusted to 8.Then, the mixture was heated to 55 °C in a water bath and incubated for 10 min. Subsequently, alkaline protease was added (6%, activity of 20 × 104U/g) and enzymatic hydrolysis was performed for 2 h at 55 °C in a magnetic stirring water bath. Finally, enzyme inactivation was performed at 85 °C for 15 min, and the pH was adjusted to 7. The supernatant was collected by centrifugation at 1 400 ×gfor 15 min and freeze-dried. The powder was stored at -20 °C until use.
2.5 Animal sensitization protocol
The experimental scheme was performed as described in Fig. 1.The sensitization routes included intragastric gavage (i.g.) (Fig. 1A)and intraperitoneal injection (i.p.) (Fig. 1B), and these procedures were performed as previously described with minimal modification [8,9].
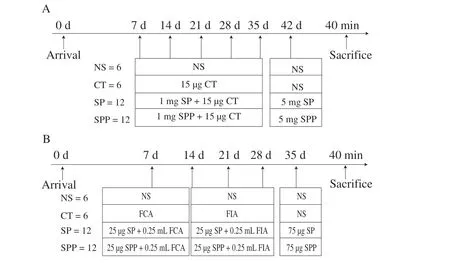
Fig. 1 Immunization protocol for BALB/c mice. (A) Oral immunization of BALB/c mice with SP/SPP. (B) Intraperitoneal immunization of BALB/c mice with SP/SPP.
Mice immunized by i.g. were divided into 4 groups. For the control, 2 groups were sensitized with 0.9% NaCl (NS) and cholera toxin (CT), separately. The other 2 groups were separately sensitized with SP and silkworm pupa protein peptide (SPP). Mice were immunized once a week with 1 mg of SP/SPP together with 15 μg of CT in a final volume of 0.5 mL of NS. On the 42ndday, mice were challenged with 5 mg of protein (SP/SPP).
Mice immunized by i.p. were divided into 4 groups. For the control, 2 groups were sensitized with 0.9% NaCl (NS) and Freund’s adjuvant (CFA), separately. The other 2 sensitization groups were separately sensitized with SP and SPP. Mice in the sensitization group were injected with 0.25 mL of mixture (50 μg of SP/SPP,emulsified with Freund’s adjuvant at 1:1,V/V). On the 35thday, mice were administered the final challenge with 75 μg of protein (SP/SPP).Mice were sacrificed 40 min after stimulation, and the serum samples,spleens and jejunum were collected for detection.
2.6 Allergic phenotype and core temperature after challenge
During the immunization of animal, the net weight of mice was recorded once each week, and their growing status was observed every week. Each mouse’s rectal temperature was assessed 40 min before the last challenge and 40 min after the last challenge using a digital rectal thermometer. The allergic reaction was classified according to the method of Li et al. [10] with slight modification and was shown in Table 1.
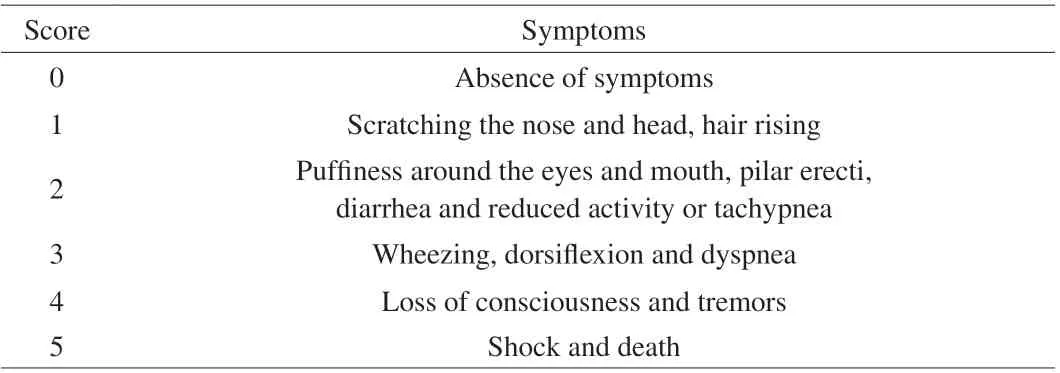
Table 1 Anaphylactic symptom score for allergic mice.
2.7 Serum sampling
Blood samples were collected. The serum was isolated by centrifugation at 700 ×gfor 6 min at 4 °C. Then, the serum was collected and frozen at -80 °C until use in further experiments.
2.8 Assays for specific IgG, IgE and IgG1 antibodies
The titers of specific IgG, IgE and IgG1 levels were measured by indirect ELISA as reported by van Esch et al. [11] with slight modifications.Briefly, each well was coated with 100 μL of SP (1 μg/mL for IgG and IgE; 0.5 μg/mL for IgG1) in 50 mmol/L sodium carbonate buffer(pH 9.6) overnight at 4 °C. The plates were then washed three times in phosphate-buffered saline (10 mmol/L PBS, pH 7.4) (Solarbio Science & Technology Co., Ltd, Beijing, China) containing 0.05%Tween-20 (PBST). Thereafter, all the wells were blocked with 250 μL of 0.01 mol/L PBS containing 3 g/100 mL fish gelatin (Sigma Chemical Co., St. Louis, Mo, USA) for 1.5 h at 37 °C. After washing, diluted serum was added and incubated for 1 h at 37 °C. The plates were then washed, and 100 μL of secondary antibodies was separately incubated at a dilution ratio of 1:5 000 for 1 h at 37 °C. Washing was performed as described above, and color rendering with TMB (Neobioscience,Beijing, China) was subsequently performed for 15 min at 37 °C in the dark. For detection of IgE, 1:60 diluted biotin (Neobioscience,Beijing, China) was added for 1 h at 37 °C before color development.Finally, the reaction was terminated by adding 2 mol/L H2SO4, and then the absorbance was rapidly read at 450 nm using a Varioskan LUX Multimode Microplate Reader (Thermo Fisher, MA, US).
2.9 Assessment of cytokine and plasma histamine levels
Splenocytes at a density of 2 × 106cell per mL were stimulated with 300 μg/mL or 1 500 μg/mL SP/SPP for 72 h and then centrifuged at 240 ×gfor 5 min. Then, supernatants were collected for cytokine detection [12], and IL-4, IL-5, IL-13, and TNF-γ levels were detected using commercially available ELISA assay kits (Neobioscience,Beijing, China) according to the manufacturer’s instructions. Plasma histamine levels were detected using a commercially available histamine ELISA kit (Demeditec, Germany) according to the manufacturer’s instructions. The microporous plates were read at 450 nm using a multifunction reader (Thermo Fisher, MA, US).
2.10 Histological analysis
Jejunum was first extracted under sterile conditions, fixed with 4%paraformaldehyde, embedded in paraffin and stained with hematoxylineosin (HE). Then, slices were observed using phase-contrast microscopy(Carl Zeiss AG, Jena, Germany). Pathology scores were measured as described previously with some modifications, and the allergic reaction severity is presented in Table 2 [13].
2.11 Statistical analysis
Statistical analysis was performed using GraphPad Prism 7.Differences between values were compared using SPSS.P< 0.05 indicated significance, andP< 0.01 indicated extremely significant differences. In contrast, n.s. indicates no significance. The mentioned tests were repeated at least twice.
3. Results
3.1 Characteristics of silkworm protein and silkworm peptide
SP was extracted via a combination of alkali extraction and acid precipitation with the assistance of ultrasonic techniques. SPP were extracted through enzymolysis with alkaline protease. The protein and peptide patterns determined by matrix-assisted laser desorption ionization-time of flight-mass spectrometry (MALDI-TOF-MS) and molecular weight distributions are presented in Fig. 2. MALDI-TOF-MS results showed that SP contained a wide range (80-30 kDa) of proteins with molecular weights of 34, 36, 54, 61 and 76 kDa. As shown in Fig. 2A, SPP were hydrolyzed thoroughly and composed of oligopeptides less than 500 Da in molecular weight.
3.2 Allergic phenotype and body temperature after challenge
The clinical symptom score is an important index to evaluate the occurrence and the severity of food allergy. In our study, the clinical symptoms of the mice were observed within 40 min after the mice were challenged. The scores of hypersensitivity symptoms, rectal temperature and histamine levels of immunized mice were presented in Fig. 3. We observed an obvious difference in the allergenic scores of mice immunized by SP via two different immunized routes, as shown in Fig. 3A and 3C. In our results, all the mice in control groups performed normally with good activity and had no adverse reactions.The only exception was a mouse in the CT group that died due to poor intragastric administration. In the orally immunized groups, all mice exhibited gradually reduced activity in the SP group. Specifically,6 mice exhibited wheezing, 2 mice scratched their noses, and 3 mice exhibited raised hair. One of the SP-treated mice exhibited a state of shock for approximately 30 s after intragastric administration and completely recovered approximately 35 min later. Most of the SP-treated mice had an allergy score ranging 1-2. Similar findings were noted in intraperitoneally immunized groups, and all SP-treated mice exhibited obviously decreased activity as well as wheezing and tachypnea within 2 min after challenge. Specifically, 4 mice scratched their noses, 9 exhibited dorsiflexion and tremors, and 4 exhibited raised hair. However, the average allergic score even reached 3.83,which was obviously increased compared with mice i.g. injected with SPP. Findings based on the two methods of immunization preliminarily indicated that SP could induce allergic reactions in mice,and mice may be more easily sensitized via the i.p. route.
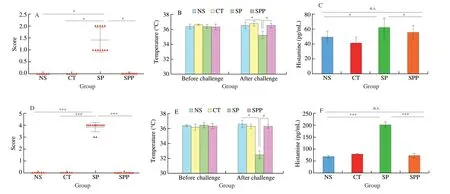
Fig. 3 Score of hypersensitivity symptoms, rectal temperature and histamine level of mice. (A) Clinical symptoms score: mice were measured in 40 min after the last challenge with 5 mg SP immunized via i.g. (n ≥ 5 for each group); (B) Rectal temperature: mice immunized via i.g. were measured 40 min before and 40 min after the last challenge; (C) Histamine level in SP group immunized via i.g. (n = 2 for each group). (D) Clinical symptoms score of mice immunized via i.p. after sensitization with 75 μg SP (n ≥ 6 for each group); (E) Rectal temperature of mice immunized via i.p.; (F) Histamine level in SP group immunized via i.p. (n = 2 for each group).
In addition, the rectal temperature of mice was separately measured 40 min before the last challenge and 40 min after the last challenge.As observed in Fig. 3B and 3D, no apparent changes were noted prior to challenge. However, after challenging, the rectal temperature of SP-treated mice decreased, and the reductions were different for the two immunized routes. When SP-treated mice were immunized via the i.p. route, the rectal temperature of all mice decreased by 3-5 °C,while a 1-2 °C reduction in rectal temperature was noted in the SP group sensitized via the i.g. route. Plasma histamine levels were shown in Fig. 3C and 3F, and the mean histamine level in the SP group was 62 ng/mL. This value was significantly increased compared with control groups. When SP-treated mice were immunized via the i.p. route, the mean histamine level was 202 ng/mL, which was considerably increased compared with that of the control groups. All of these findings indicated that SP could elicit a powerful allergic reaction. The histamine level of SP-treated mice immunized via the i.p. route was approximately triple that in SP groups immunized via the i.g. route, indicating that i.p. immunization was more effective than i.g. immunization. It should be noted that the higher histamine levels were noted in the orally immunized group compared with the intraperitoneally immunized group.
Significantly reduced allergenicity was noted in the SPP groups,and mice immunized to SPP via two different routes did not show any obvious clinical symptoms. No changes in hypersensitivity symptoms,rectal temperature and histamine levels were noted compared to the control groups, suggesting that enzymatic hydrolysis effectively disrupted the allergenicity of SP.
3.3 Specific antibody levels
Specific IgE and IgG antibodies were closely associated with allergic hypersensitivity responses [14-16]. In our study, IgE, IgG and IgG1 titers were detected by indirect ELISA in Fig. 4. In orally immunized groups, 3 types of specific antibodies exhibited no obvious increases in SPP-treated mice compared with levels in the control groups. In SP-immunized mice, IgE, IgG and IgG1 levels increased, and IgG levels were the highest. However, a downward trend in OD values was noted in the SPP group with an IgE dilution of 1:10 as shown in Fig. 4A. Using a different immunization route as depicted in Fig. 4D, IgE antibody titer levels in the SP group were 1:480, which is an approximately 50-fold increase compared with that in Fig. 4A. These results indicate that the intraperitoneal immunization protocol was more effective than the oral immunization protocol. In Fig. 4D, the mean OD450nmof the SPP group was 0.134,which was approximately half that noted in the SP group, suggesting that hydrolysis could greatly reduce the potential allergenicity of SP.However, specific IgG levels in mice immunized via both routes were significantly distinguishable with the dilutions of 1:200 and 1:1.6 × 105in Fig. 4B and Fig. 4E, respectively. IgG titers of the SPP group immunized via the two routes were significantly decreased compared with titers in the SP groups. A similar trend was noted for specific IgG1 antibodies. As depicted in Fig. 4F, IgG1 levels in mice treated with SP intraperitoneally were 1:3.2 × 104, which are increased approximately 640-fold compared with that in the orally immunized SP group. It should be noted that SPP induced reduced IgG1 antibody titers via both immunization routes, and the reduction via the intraperitoneal route was more pronounced. All these data showed that the i.p. immunization protocol was effective and could be more suitable to establish a murine sensitization model for SP. In addition, specific antibodies titers in the SPP groups were obviously reduced, suggesting that enzymatic hydrolysis could reduce the allergenicity of SP.
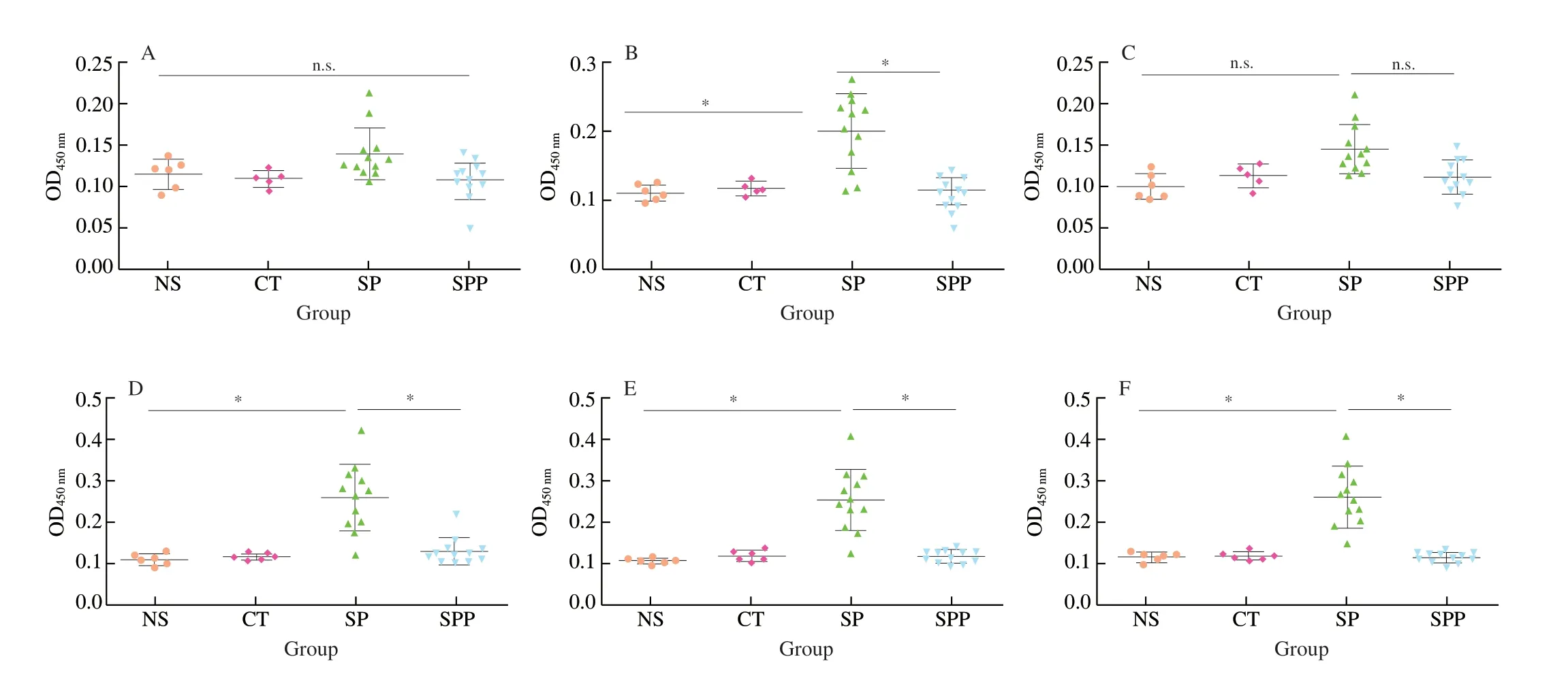
Fig. 4 Specific IgG, IgE and IgG1 antibodies level of mice: mice immunized via i.g. (A-C) and i.p. (D-F). (A, D) IgE; (B, E) IgG; (C, F) IgG1. Data are presented in mean ± SD. *P < 0.05; n.s., not significant.
3.4 Cytokine production in supernatant of splenocyte cultures
As early as 1999, it has been reported that cytokines related to Th2 cells, including IL-4, IL-5 and IL-13, were involved in the production of specific IgE antibodies in food allergens [17]. Levels of IL-4, IL-5, IL-13 and IFN-γ secreted by spleen cells are presented in Fig. 5. In mice sensitized by i.g. (Fig. 5A1-D1), IL-4 levels in Fig. 5A1were obviously increased in SP-treated mice compared with control groups, and IL-13 levels (Fig. 5C1) were extremely high when the concentration of SP was 300 μg/mL. However, the levels of IL-5 in Fig. 5B1and IFN-γ in Fig. 5D1increased slightly compared with the control and SPP groups. However, IL-4, IL-5, IL-13 and IFN-γ levels in the SPP group showed no obvious differences compared with the two control groups.
In i.p. sensitized groups (Fig. 5A2-D2), the levels of IL-4 (Fig. 5A2) and IL-5 (Fig. 5B2) in the SP group were markedly increased with 300 μg/mL of SP. However, IL-4 and IL-5 levels were not further increased when the SP concentration was 1 500 μg/mL.IL-13 levels (Fig. 5B2) notably increased upon stimulation with 300 μg/mL protein. IL-4, IL-5 and IL-13 levels in the SPP group exhibited no significant change compared to NS and CFA groups.The release of IFN-γ, a typical Th1 cytokine that effectively reduces the secretion of Th2 cytokines and IgE [18,19], exhibited minimal differences among the groups (Fig. 5D2).
3.5 Histological examination of jejunum in mice
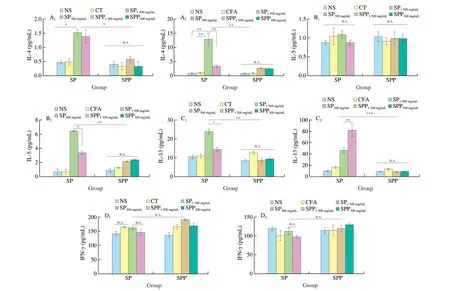
Fig. 5 The levels of cytokines secreted by the cultured spleen cells from i.g. (A1-D1) and i.p. (A2-D2) immunized mice (n = 2 for each group). Data are presented in mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant.
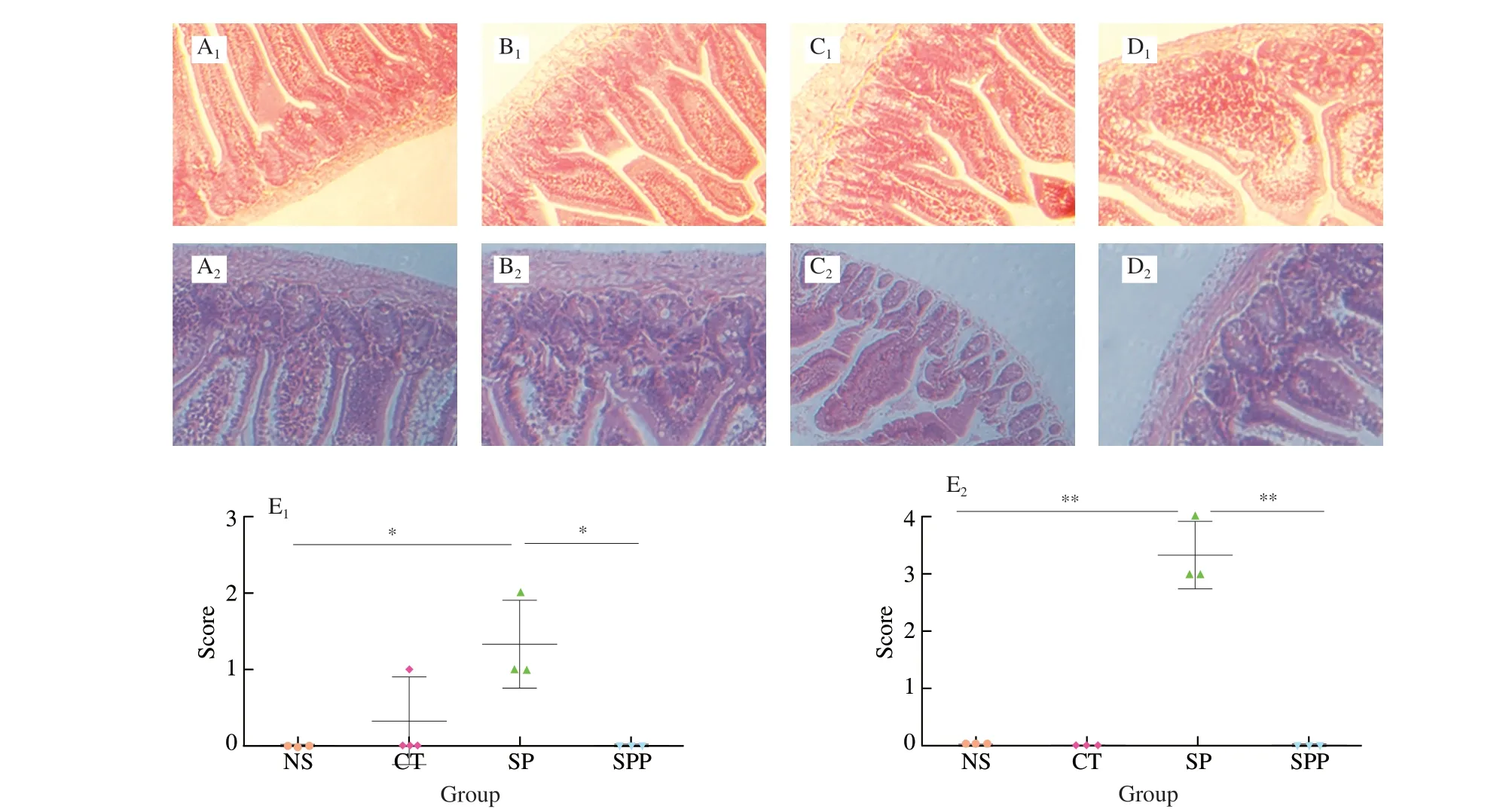
Fig. 6 Images of jejunum and score of jejunal lesions in all groups of i.g. (A1-E1) and i.p. (A2-E2) sensitized mice (n = 3 for each group). (A1) NS; (B1) CT;(C1) SP; (D1) SPP; (E1) Score of jejunum lesions in NS, CT, SP and SPP treated mice. (A2) NS; (B2) CFA; (C2) SP; (D2) SPP; (E2) Score of jejunal lesions in NS,CFA, SP and SPP treated mice. Data are presented in mean ± SD. * P < 0.05, ** P < 0.01 (Observe at 20×).
To explore the allergic effect of silkworm pupa on intestinal injury in jejunum, the tissues of mice immunized via two different routes and the intestinal lesion scores are presented in Fig. 6. The jejunum tissue of mice treated with SP via the i.g. immunized route is shown in Fig. 6C. We found that the tissue exhibited minimal damage. In contrast, the jejunum tissues in the SP group (Fig. 6C)immunized by i.p. showed obvious lesions, large localized shedding of intestinal villi, and broken and irregular villi. These findings indicated that allergic reaction to SP resulted in significant intestinal damage. The jejunum injury score of mice immunization via the i.g.route was shown in Fig. 6E, and the mean intestinal lesion score was 1.33. The mean lesion score in mice treated with SP via the i.p. was 3.33 (Fig. 6E), and this value was greater than that of mice treated with SP via the i.g. route. The data showed that the i.p. immunization route induced a more obvious allergic reaction in BALB/c mice. In mice treated with SPP via two different routes, jejunum tissues were basically free of lesions, and the lesion scores were both 0, indicating that enzymolysis could greatly reduce the allergenicity of SP.
4. Discussion
Enzymatic hydrolysis of silkworm pupa has been studied for years in China since SPP showed its specific function. As early as in 1980s, immunological functions of SPP had been firstly explored. In 2010, Li et al. [20] found that SPP hydrolyzed by protamex showed a strong free radical scavenging ability of DPPH and hydroxyl radicals,which could be used as an antioxidant reagent. Later, SPP also showed an anti-obesity ability in Lee’s study, since it could modulate signal transduction pathways by inhibiting the differentiation of preadipocytes and adipogenesis in a high-fat diet rat model [21].In further, Wu et al. [22] demonstrated an angiotensin converting enzyme (ACE)-inhibitory tripeptide (Ala-Ser-Leu) could assist the treatment of hypertensive, which was isolated from hydrolyzed silkworm pupa by gastrointestinal endopeptidases. Based on these researches, there are some functional products with addition of SPP in China. However, the high allergenicity of silkworm pupa limits its utilization. It has been reported that the antigenicity of several proteins decreased by enzymatic hydrolysis with protease [23], but it did not disappear totally. It should pay more attention to allergenicity of SPP. In our mouse models, we immunized mice via two ways, and we found that almost all allergy-related indicators in mice treated with SPP via both two sensitization methods did not differ from those in the control groups. In general, SPP might serve as a hypoallergenic and functional resource for food and drug material.
Silkworm is an insect that undergoes complete metamorphosis with four developmental stages, including eggs, larvae, pupa and adult (moth), and several of these stages can cause allergic reactions.An investigation revealed that 75.6% of sericulture workers exhibited allergic respiratory symptoms, and silkworm urine was considered an important allergen in these patients [24]. Jeong et al. [25] identified and characterized an arginine kinase as a main allergen in silkworm larva. Virgin silk is a potent antigen that causes an allergic response in some cases [26,27]. Silkworm pupa is an important byproduct in sericulture, and a skin prick test revealed that silkworm pupa was the most frequently sensitized allergen among the 62 common food allergens in Korea [28]. When silkworm pupae turn into silkworm moths via metamorphosis, the allergenicity remains. Reports showed that sensitized patients exhibit a high prevalence of cosensitization between silkworm moth and 5 common inhalant allergens, includingDermatophagoides pteronyssinus,Dermatophagoides farinae,Blomiatropicalis,Blattella germanica, andPeriplaneta americana[29].Another report suggested that silkworm moth allergen has the third highest sensitization rate in patients with severe symptoms [30].Taken together, we deduced that silkworm is a special and severe allergen. Studies on silkworm pupa allergies are therefore necessary.Silkworm pupa is invertebrate with a brown carapace that has some common features with other invertebrates and some crustaceans.Several articles have reported cross-reactivity between silkworm and other allergens. The immunoCAP (Thermo Fisher Scientific,Inc., Waltham, MA, USA) assay showed that most patients with silkworm allergy exhibit IgE reactivity to shrimp and crab, but they have no clinical symptoms of food allergy, they are only allergic to allergens of silkworm pupa [31]. Interestingly, 84.2% of individuals with multiple food allergies in Table S1 were allergic to seafood,suggesting possible cross-reactivity between silkworm pupa and seafood. In another study, 5 patients allergic to vegetable worm(caterpillar fungus,Cordyceps sinensis) exhibited cross-reactivity with silkworm pupa as demonstrated bycompetitive ELISA inhibition tests [32]. These studies suggest that people allergic to silkworm pupa should pay more attention to other invertebrates and crustaceans.
Research on food allergies usually relies on allergic animal models. BALB/c mice have been widely used to establish allergic animal models. Some reports describe intraperitoneal injection in allergic mouse models [33,34]. Oral and intraperitoneal administration are the two most common routes of sensitization [35]. In this study,we compared two administration routes used for the sensitization of mice and found that intraperitoneal injection induced a more apparent allergic reaction. Mice treated with SP via i.p. exhibited severe diarrhea during the immunization process compared to control group mice, while mice orally immunized exhibited a better growth status and recovered faster than mice intraperitoneally immunized.Regarding specific antibodies, IgG, IgE and IgG1antibodies in the SP group of mice immunized by i.p. were 800-fold, 640-fold and 9.6-fold increased, respectively, compared with mice immunized by the i.g. route. We observed that mice immunized by i.p.produced more histamine, activated more allergen-specific Th2 cells demonstrated by IL-4, IL-5 and IL-13 secretion from splenocytes and showed more serious intestinal damage. We deduced that intraperitoneal injection induced a more serious allergic reaction.
In addition, the oral administration of food allergens should mimic the real conditions of human exposure, and CT was proven to be an adjuvant in the immunization of BALB/c mice [36]. In the SP group immunized by i.g., mice produced minimal antibodies, and this effect may be related to antigen dose given that allergic response kinetics exhibit a dependence on the antigen dose in BALB/c mice [37].
SP exhibited strong allergenicity in our allergic mouse model.Firstly, mice displayed severe clinical symptoms, such as wheezing,tachypnea, nose scratching, dorsiflexion and tremors, after the last challenge. Moreover, SP-allergic mice exhibited obvious intestinal lesions. During the process of immunization, mice sensitized to SP via the i.p. route exhibited severe diarrhea and decreased appetite and activity. We conducted a small-scale survey on 50 patients suffering from silkworm pupa allergies, including 28 females, 21 males and one infant. Almost all patients suffered from skin problems, and some exhibited gastrointestinal discomfort and dyspnea, which is consistent with previous reports [2]. The infant in our study did not eat silkworm pupa directly. His mother ate silkworm pupa, and he had an allergy.The infant is still breastfeeding. The result is consist with the finding in Frazier’s study on the potential role of exposure to peanuts through breast milk [38]. Another intriguing paradox here is that the clinical anaphylactic symptoms in mice treated with SP via the i.p. route are not exactly the same as those in humans. Respiratory problems existed in both models. We observed that mice treated with SP via the i.p. route showed limb discomfort via convulsion and twisting, which is consistent with reports of symptoms in animal models [39]. However, no similar symptoms were noted in patients.We deduced that the pain may be induced by FCA as reported in Walker’s study [40]. Both mice and humans experience discomfort for a short period of time. Regarding attack time and allergic symptoms in mice and humans after consuming silkworm pupa,we deduced that silkworm pupa allergy is an immediate systemic hypersensitivity. Furthermore, when exposed to antigens, blood cells of sensitized animals released some of the histamine into plasma.These findings indicated that the amount of histamine released is related to anaphylactic shock in animals [41].
This study explored the potential mechanisms of silkworm pupa allergy by sensitization of animal models and allergenicity changes to peptides from silkworm pupa generated by enzymatic hydrolysis.Our results on silkworm pupa protein showed increased allergenicity,and enzymatic hydrolysis technology could significantly reduce the antigenicity of silkworm pupa given that only peptides less than 500 Da remained. Therefore, enzymatic hydrolysis technology is a robust method to reduce the allergenicity of silkworm pupa.
Conflicts of interest
All authors declare no conflict of interest.
Acknowledgment
The work was supported by Special Project on the Integration of Industry, Education and Research of Guangdong Provine(2013B090600060), National Key R&D Program of China(2018YFC1604205) and National Natural Science Foundation of China (31760431).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.09.011.
- 食品科學與人類健康(英文)的其它文章
- Maternal obesity exacerbates the responsiveness of offspring BALB/c mice to cow’s milk protein-induced food allergy
- Au@Ag-labeled SERS lateral f low assay for highly sensitive detection of allergens in milk
- Impact of three different processing methods on the digestibility and allergenicity of Chinese mitten crab (Eriocheir sinensis) tropomyosin
- Effect of lipid peroxidation on the allergenicity and functional properties of soybean β-conglycinin (7S) and glycinin (11S)
- Comparative acetylome analysis reveals the potential mechanism of high fat diet function in allergic disease
- Therapeutic effects of epigallocatechin and epigallocatechin gallate on the allergic reaction of αs1-casein sensitized mice

