Effects of anti-CD4 antibody treatment on calcium ions inf lux in peanut-sensitized C3H/HeJ mice
Junjun Wng, Cui Zhou, Shiwen Hn, Zinu Mjid, N Sun, Huilin Che,*
a Key Laboratory of Precision Nutrition and Food Quality, Key Laboratory of Functional Dairy, Ministry of Education,College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
b School of Public Health, Capital Medical University, Beijing 100069, China
c National Engineering Research Center of Seafood, School of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, China
Keywords:Calcium ions Anti-CD4 C3H/HeJ mice Peanut Allergy
A B S T R A C T The precise mechanism underlying the effects of anti-CD4 antibody and calcium ions (Ca2+) in peanut allergy remains unknown. C3H/HeJ mice sensitized with peanut protein extract (PPE) were injected with anti-CD4 antibodies for 4 weeks. Stimulation with PPE increased the specific immunoglobulin E (IgE), cytokine,histamine, and mMcp-1 levels, upregulated decorin (Dcn) expression, induced Ca2+ inflow in the spleen,and augmented the expression of the transcription factors GATA-3 and Foxp3, which resulted in Th2 and Treg cell activation. Notably, the Ca2+ levels were positively correlated with the histamine, interleukin (IL)-4, IL-5,and IL-13 levels, and negatively correlated with IL-10 levels. However, administration of anti-CD4 antibodies markedly alleviated allergic symptoms, activated T cells, and reduced Ca2+ infl ow, cytokine, histamine, mMcp-1,and the IgHG3, CXCL12, MMP2 and FABP4 gene. Our results indicated that anti-CD4 antibodies can
1. Introduction
Peanut allergy is one of the most common and severe food allergic reactions, affecting 1% to 2% of people in the developed world [1].Peanut allergy is attributed to immunoglobulin E (IgE)-mediated type I hypersensitivity where antigens recognized by antigen-presenting cells (APCs) lead to an adverse Th2-cell skewed response, with symptoms of urticaria, angioedema and even allergic asthma [2,3].There is currently no effective treatment for peanut allergy, and its causation and pathobiology remain unclear.
Calcium ions ( Ca2+) act as secondary messengers governing the cellular fate and play a key role in lymphocyte function and immunity [4]. Upon antigen stimulation, an allergen is recognized by APCs. T-cell receptor (TCR) and CD4 co-receptor recognition of its ligand induce activation of protein tyrosine kinases, which initiate phosphorylation of adaptor proteins. This leads to the activation of downstream signaling pathways. Among them, inositol 1,4,5 triphosphate (InsP3) binds to and alters the configuration of receptors in the endoplasmic reticulum membrane, resulting in the release of Ca2+from intracellular Ca2+stores [5-7]. Yarova et al. [8]found an increase in Ca2+in airway smooth muscle from asthmatic patients, which drove the remodeling and production of a range of inf lammatory cytokines and other mediators in asthma. Subsequently,a systematic review and meta-analysis found that intervention with calcium channel blockers could decrease forced expiratory volume and improve lung function in asthma, especially in exercise-induced asthma [9]. Moreover, calcium channels are related to Th2-mediated diseases, such as allergic asthma, because Th2-cells up-regulate calcium voltage-gated channel subunitα1C mRNA, which encodes calcium channels [10,11]. However, the clear molecular role of Ca2+in peanut allergy remains unreported.
There is increasing evidence implicating the use of non-depleting CD4 monoclonal antibodies targeting T cell CD4 molecules to treat immune diseases [12,13]. Non-depleting CD4 antibodies were able to ameliorate allergic airway disease in mice sensitized with ovalbumin and house dust mite allergens by decreasing eosinophilia, goblet cell hyperplasia, and production of histamine and specific IgE [14].Strikingly, non-depleting anti-CD4 antibody could inhibit ovalbumininduced Th2 responses and allergic lung inflammation, with reduced infiltration of eosinophils in tissue and the levels of histamine and Th2 cytokines interleukin (IL)-4 and IL-5 [15]. In addition, histamine is able to induce a transient Ca2+release in dendritic cells (DCs)through histamine 1 receptor and histamine 4 receptor, and storeoperated calcium channel entry is involved in the histamine-induced maturation and Th2 response of DCs [16]. However, few studies have investigated the role of Ca2+in anti-CD4 modulation of the immune response during peanut allergy.
In this study, we focused on investigating the molecular roles of anti-CD4 antibodies and Ca2+in regulating peanut allergy. The potential association between anti-CD4 treatment and Ca2+was explored. The findings may provide new targets for alleviating peanut allergy.
2. Materials and methods
2.1 Preparation of peanut protein extract (PPE)
PPE was obtained as previously described with some modifications [17]. Defatted peanut flour was stirred in Tris buffer(20 mmol/L, pH 7.2) for 2 h at room temperature and centrifuged at 3 000 ×gfor 30 min at 4 °C. The supernatant was collected and centrifuged again at 10 000 ×gfor 30 min at 4 °C. Protein concentration in the supernatants of extracts was determined using the bicinchoninic acid (BCA) method. PPE was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1).

Fig. 1 Coomassie-stained SDS-PAGE of PPE.
2.2 Animals and experimental design
Six-week-old female C3H/HeJ mice purchased from the Animal Experiment Center of Wuhan University (Wuhan, China) were maintained at a temperature of (22 ± 1) °C, relative humidity of(55 ± 5)%, and a 12 h/12 h light-dark cycle. Mice were provided sterile distilled water and food ad libitum. After adaptive feeding for one week, mice were randomly divided into 4 groups: PBS, PPE,PPE+isotype, and PPE+anti-CD4 groups. Mice in the PPE group were intragastrically administered 0.2 mL of 5 mg/mL PPE dissolved in PBS on days 0, 7, 14, 21 and 28. Based on the PPE group, mice were injected with isotype antibody (YKIX302, Abcam, Cambridge, MA,USA) or non-depleting anti-CD4 (YTS177.9, Abcam) monoclonal antibody via the tail vein in the PPE+isotype and PPE+anti-CD4 group, respectively. The PBS group was treated with sterile PBS.All mice were orally challenged with PPE (10 mg/mouse) on day 42,except for the PBS group. Rectal temperature was measured 10 min before and 50 min after the challenge with PPE using a WI88375 probe(Beijing Science and Technology, Beijing, China). The clinical allergic symptom score was determined as previously described [18]. Abdominal albumin was determined using a BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China). The experiments were conducted according to animal care guidelines and were approved by the China Agricultural University.
2.3 Evaluation of allergic inflammation
Blood samples were collected from the orbital venous plexus of the mice on days 7, 14, 21, 28 and 35. After overnight storage at 4 °C,the samples were centrifuged for 15 min at 4 000 ×g, 4 °C.The serum samples were collected and stored at -80 °C. Serum PPE-specific antibodies, including IgE, IgG1, and IgG2a, were detected as previously described [18].
Blood was collected again from the orbital venous plexus of the mice 45 min after challenge. Blood samples were centrifuged for 10 min at 3 000 ×g, 4 °C to obtain the plasma and serum. The serum concentrations of histamine in plasma and mouse mast cell protease-1(mMCP-1) were detected using an ELISA kit (Tiangen, Beijing,China) in accordance with the manufacturer’s instructions.
Spleen cells were extracted from the spleens of each group of mice by pressing the tissue through a cell strainer. The spleen lymphocyte subpopulation was analyzed by flow cytometry using monoclonal antibodies against the different lymphocyte subpopulations antigens.The splenocytes were stained with phycoerythrin (PE) anti-mouse CD11c, fluorescein isothiocyanate (FITC) anti-mouse CD4 and PE anti-mouse CD25, PE anti-CD69, anti-mouse CD183/CXCR3, FITC anti-mouse CD69, and PE anti-mouse T1/ST2 (eBioscience Inc., San Diego, CA, USA) for 30 min. DCs as well as Treg, Th1, and Th2 cells were analyzed by flow cytometry.
Grampy held my hand tightly. Together we looked up the street and down, and back up again. He stepped off the curb and told me it was safe to cross. He let go of my hand and I ran. I ran faster than I had ever run before. The street seemed wide. I wondered if I would make it to the other side. Reaching the other side, I turned to find Grampy. There he was, standing12 exactly where I had left him, smiling proudly. I waved.
In addition, the spleen cells were seeded in 96-well cell culture plates (2 × 105cells/100 μL/well) and incubated in RPMI1640 medium containing 200 μL/mL of PPE for 72 h at 37 °C in a 5%CO2incubator. Cytokines were quantified using a commercial mouse ELISA kit (eBioscience, Inc.).
2.4 Real-time quantitative PCR
Total RNA from spleen samples was extracted using Trizol. The RNA (2 μg) was synthesized cDNA using FastQuant cDNA (Tiangen).The mRNA expressions of T-box transcription factor TBX21 (T-bet),GATA binding protein 3 (GATA3), and Forkhead box P3 (Foxp3)were measured with an ABI 7500 Fast ReaL-Time PCR device (Life technologies, Carlsbad, CA, USA) using RealMasterMix (SYBR Green, Tiangen). The sequences of T-bet, GATA3, and Foxp3 were determined as previously described [19].
2.5 Western blotting
Splenocytes stimulated with PPE for 72 h were lysed with 200 μL of lysate buffer for 10 min on ice. The mixture was collected,vortexed for 10 min, and centrifuged for 10 min at 14 000 ×g, 4 °C.The protein concentration of the supernatants was determined using a BCA Protein Assay Kit (Beyotime Biotechnology). Protein samples(30 μg) were separated by 10% SDS-PAGE. The separated proteins were transferred to polyvinylidene difluoride membranes, blocked in Tris buffered saline-Tween containing 5% nonfat dried milk, and probed with antibodies to T-bet, GATA3, and Foxp3 (sc-67302,Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4 °C.Immunoreactive bands were detected by chemiluminescence using horseradish peroxidase-conjugated secondary antibodies (#7074S,Cell Signaling Technology Inc., Beverly, MA, USA) and enhanced chemiluminescence (ECL) detection reagents, followed by exposure to X-ray film using a MiniChemi II system (Sage Creation Science Co., Beijing, China). Quantitative changes in band intensities were evaluated using ImageJ software (NIH, Bethesda, MD, USA).
2.6 Determination of intracellular Ca2+ levels
Splenocytes were seeded into 96-well black culture plates and treated with PPE for 72 h. The PPE-sensitized cells were incubated with Fluo-3 acetoxymethyl (Fluo-3 AM, 30 nmol/L) for 30 min.The fluorescence intensity (FI) was detected using an excitation wavelength of 488 nm and an emission wavelength of 525 nm with a full-wavelength scanner (Thermo Fisher Scientific, Waltham, MA,USA). Data were calculated using FI/FI0, where FI0is the fluorescence intensity in the PBS group.
2.7 β-Hexosaminidase, histamine and cytokine from Ca2+-stimulated RBL-2H3 cells
The rat basophilic leukemia cells (RBL-2H3) were cultured in 96-well culture plates in MEM medium containing Ca2+(0, 1,2, 3 mmol/L) for 24 h and stimulated for 1 h in the presence of C48/80 (200 μg/mL). 1% Triton X-100 was used as a positive control. Supernatant (30 μL) was transferred to a fresh 96-well plate and incubated with 50 μL ofp-nitrophenyl-N-acetylβ-D-glucosaminide (1.3 mg/mL in 0.1 mol/L citric acid buffer,pH 4.5) for 1 h at 37 °C. The reaction was terminated by adding 200 μL of stop solution (0.1 mol/L Na2CO3/NaHCO3, pH 10.0). The absorbance at 405 nm was measured. The formula of the release ofβ-hexosaminidase calculation is:

The RBL-2H3 cells were incubated with anti-DNP IgE (1 μg/mL)for 2 h at 37 °C and stimulated with DNP-HSA (100 ng/mL) for 45 min at 37 °C in the presence of Ca2+(0, 1, 2, 3 mmol/L). Cytokine and histamine were quantified using a commercial ELISA kit(eBioscience, Inc.).
2.8 RNA isolation and library construction
Total RNA samples were extracted from the spleen using a Total RNA kit (Tiangen). Total RNA purity and degradation were checked by agarose gel electrophoresis, and RNA integrity and concentration were analyzed using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara,CA, USA) in accordance with an RNA integrity number (RIN) >7.0.
The mRNA was isolated by oligo (dT) and fragmented using fragmentation buffer. First- and second-strand cDNA were synthesized using fragments template and random hexamers. The PCR products were purified using the AMPure XP system (Beckman Coulter, Palo Alto, CA, USA) and then assessed using the 2100 system (Agilent Technologies) to establish a cDNA library.
2.9 Identification and annotation of differentially expressed genes (DEGs)
DEGs between the PPE and anti-CD4 groups were identified as a log2fold change > 1 andP-value < 0.05 compared to the PBS group.DEGs were mapped to terms in the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases to analyze GO functions and protein-protein interaction networks.
2.10 Statistical analysis
All results are expressed as mean ± SD. Statistical analysis was performed using SPSS 19.0 statistical software (SPSS Inc., Chicago,IL, USA). All variables were compared between the groups using the ANOVA-LSD post hoc test. Values ofP< 0.05 were considered statistically significant.
3. Results
3.1 Anti-CD4 treatment relieved allergic symptoms in PPEsensitized mice
The PPE allergen-induced allergic murine model was employed to evaluate the role of anti-CD4 treatment in peanut allergy (Fig. 2A).Upon intragastric administration of PPE, an increase in the anaphylactic score was observed (Fig. 2B). In contrast, anti-CD4 treatment had a significant inhibitory effect on PPE-sensitized murine allergic symptom scores. Further analyses showed that body temperature was reduced in the PPE allergic group (Fig. 2C).Additionally, by measuring the albumin level in murine peritoneal lavage fluid, the vascular permeability was quantitatively analyzed to evaluate the symptoms of systemic allergic reactions. The albumin level was significantly increased in the PPE-sensitized mice compared to the level in the PBS group (P< 0.05). The albumin level in the PPE+anti-CD4 group was close to that in the PBS group, with no significant difference (P> 0.05, Fig. 2D). Compared with the PBS group, stimulation with PPE led to a significant increase in the level of PPE-specific IgG1 and IgG2a (Fig. 2E). In contrast, treatment with anti-CD4 reduced PPE-specific antibodies. In particular, the level of PPE-specific IgE was also significantly reduced after the intervention with anti-CD4 antibody (P< 0.05). Moreover, the level of PPEspecific IgE was not significant in the PPE+isotype and PPE groups(P >0.05). In general, PPE-induced food allergy was relieved after treatment with anti-CD4.

Fig. 2 Modulation of allergic symptoms by anti-CD4 treatment of PPE-sensitized mice. (A) Timeline of PPE-induced food-allergic model. Following anti-CD4 treatment of PPE-sensitized mice, (B) the decline in body temperature, (C) allergic symptoms score, (D) albumin level, and (E) PPE-specific IgE, IgG1 and IgG2a production in serum were measured. The results represent the mean ± SD of three independent experiments (n = 7 mice in each group). Different lowercase letters in the figure represent significant differences between the groups (P < 0.05, ANOVA-LSD post hoc test). Abbreviations are i.g.: intragastric and i.v.: intravenous.
3.2 Anti-CD4 treatment reduces Ca2+ in PPE-sensitized mice
Ca2+influx was investigated to assess the role of Ca2+in anti-CD4 treatment of PPE-sensitized mice. Based on the fluorescent intensity results, the level of Ca2+increased in response to PPE+isotype stimulation. As expected, anti-CD4 treatment significantly decreased Ca2+influx (Fig. 3,P< 0.05).

Fig. 3 Effect of anti-CD4 on Ca2+ inflow in PPE-sensitized C3H/HeJ mice.PPE-sensitized splenocytes in vitro were incubated with Fluo-3 AM (30 nmol/L)for 30 min. Fura-3 AM fluorescence was monitored at an excitation wavelength of 488 nm and an emission wavelength of 525 nm. The results represent the mean ± SD of three independent experiments. Different lowercase letters in the figure represent significant differences between the groups (P < 0.05, ANOVA-LSD post hoc test).
3.3 Effect of anti-CD4 treatment on expression of transcription factors and production of cytokines, histamine,and mMcp-1 in PPE-sensitized mice
Ca2+signaling activates T-cell activities and regulates the release of mediators [20,21]. We further investigated the expression of T cell related transcription factors and the production of cytokines,histamine, and mMcp-1. Based on protein and mRNA results, the expression of T-bet, a transcription factor of Th1, was downregulated by stimulation with PPE+isotype (Fig. 4). The expression of GATA-3,a transcription factor of Th2, was downregulated by anti-CD4 treatment, which was drastically upregulated in the PPE+isotype group. In particular, the expression of Foxp3, a transcription factor of Treg in the PPE+isotype group was upregulated compared to that in the PBS group.
The concentrations of cytokines, including IL-4, IL-5, IL-10, IL-13,and interferon-gamma (IFN-γ), were increased by PPE challenge compared to the PBS group (Fig. 5A). In contrast, anti-CD4 treatment decreased the concentration of cytokines (IL-4, IL-5, IL-13, and IFN-γ), but increased the concentration of IL-10. Sensitizing mice with PPE allergen led to the induction of high concentrations of histamine and mMcp-1. However, the anti-CD4 antibody significantly suppressed the concentrations of histamine and mMcp-1, which were similar to those in the PBS group (Fig. 5B). Notably, the level of Ca2+was positively correlated with the production of histamine, IL-4, IL-5,and IL-13, but was negatively correlated with the production of IL-10(P< 0.05, Fig. 5C). In addition, the results of cell experimentsin vitrohad similar trends (Fig. 6).
3.4 Effect of anti-CD4 treatment on DCs and CD4+ T cell subpopulation

Fig. 4 Effect of anti-CD4 on the expression of transcription factors in PPE-sensitized C3H/HeJ mice. The expression of GATA-3, Foxp3, and T-bet in PPEsensitized splenocytes in vitro was detected by Western blotting (A), and the mRNA expression of transcription factors was detected by real-time PCR (B). The results are the mean ± SD of three independent experiments. Different lowercase letters in the figure represent significant differences between the groups (P < 0.05,ANOVA-LSD post hoc test).

Fig. 5 Effect of anti-CD4 on cytokines, histamine and mMcp-1 production in PPE-sensitized C3H/HeJ mice. C3H/HeJ mice were sensitized with 5 mg/mL PPE and administered anti-CD4 for 4 weeks. The spleen and serum were harvested after euthanasia of mice to measure the concentration of cytokines in the spleen(A), and histamine and mMcp-1 (B). The correlation between the expression of Ca2+ in the splenocytes and the levels of histamine, IL-4, IL-5, IL-13, and IL-10 in the serum of the PPE+isotype group compared to the PPE+anti-CD4 group and the PBS group compared to the PPE+isotype group (C). The results represent the mean± SD of three independent experiments. Different lowercase letters in the figure represent significant differences between values at P < 0.05 in the groups(* P < 0.05, ANOVA-LSD post hoc test).
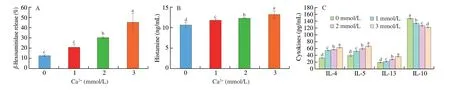
Fig. 6 Effect of extracellular Ca2+ on cytokine, histamine and β-hexosaminidase production in vitro. RBL-2H3 cells were cultured in 96-well culture plates in MEM medium containing Ca2+ (0, 1, 2, 3 mmol/L) in the presence of C48/80 (200 μg/mL). The supernatant was used to measured β-hexosaminidase release (A).RBL-2H3 cells were incubated with anti-DNP IgE (1 μg/mL) and DNP-HSA (100 ng/mL) in the presence of Ca2+ (0, 1, 2, 3 mmol/L). The production of histamine(B) and cytokine (C) were measured in supernatant. The results represent the mean ± SD of three independent experiments. Different lowercase letters in the figure represent significant differences between the groups (P < 0.05, ANOVA-LSD post hoc test).
To better understand T cell activation, the percentages of DCs and subpopulations of CD4+T cells was investigated. As shown in Fig. 7A, the level of differentiation and proliferation of CD4+T cells to Th1 and Th2 cell subsets in the spleen of experimental animals in the PPE group was significantly higher than that in the PBS group, Furthermore, the differentiation level of Treg cells was also increased, suggesting that PPE treatment activated CD4+T cells in the spleen of animals and induced significant Th cell differentiation. The differentiation of the CD4+T cell subsets also depended on the expression of corresponding transcription factors. The activation and proliferation signals of most CD4+cells are generated by the interaction between MHC on DC and TCR. The number of DCs was obviously elevated in the PPE group (Fig. 7B). Anti-CD4 treatment inhibited the percentages of DCs and differentiation levels of Th1 and Th2 cells. However,the differentiation level of Treg cells, which mainly inhibited the immune response, was obviously increased compared to that in the PPE group. This may be due to the body’s protective effect in an allergic state.

Fig. 7 The impact of anti-CD4 treatment on the percentages of DCs and subpopulation of CD4+ T cells in PPE-sensitized C3H/HeJ mice. C3H/HeJ mice were sensitized with 5 mg/mL PPE and administered anti-CD4 for 4 weeks. The spleen cells were extracted after euthanasia of mice to measure the subpopulation of CD4+ T cells (A) and percentage of DCs (B) by flow cytometry. The results represent the mean ± SD of three independent experiments. Different lowercase letters in the figure represent significant differences between the groups (P < 0.05, ANOVA-LSD post hoc test).

Fig. 8 Impact of anti-CD4 treatment on transcript expression pattern in spleens of PPE-sensitized C3H/HeJ mice. (A) Individual genes were plotted by up- or down-regulation when the PPE+isotype group and the PPE+anti-CD4 group were compared with the control group. Differentially expressed genes (DEGs) in both groups were clustered into quadrants. DEGs expressed only in one group were plotted on axes. (B) Hierarchical clustering analysis of DEGs in the PBS,PPE+isotype and PPE+anti-CD4 groups. (C) K-means clustering of DEGs. Gray curves showed the transcription profiles of individual genes. The red curve indicates the overall pattern in each cluster. (D) Interaction network map of STRING protein of differential genes related to anti-CD4 treatment. (E) Gene Ontology analysis of DEGs induced by anti-CD4 treatment showing individual genes induced in the PPE+anti-CD4 group associated with allergic response processes.
3.5 Effect of anti-CD4 treatment on the expression of genes related to asthma in PPE-sensitized mice
To explore the molecular mechanism of the alleviation of peanut allergy by anti-CD4 treatment, the total RNA from spleens of mice in the PBS, PPE+isotype, and PPE+anti-CD4 groups was extracted for digital expression profiling analysis. A total of 279 genes were up or downregulated in the PPE+isotype and PPE+anti-CD4 groups compared with the PBS group (Fig. 8A). Twenty-three genes (quadrant I)were upregulated and 36 genes (quadrant III) were downregulated in both treatment groups. After PPE challenge, 115 genes (xa) and 7 genes (ya) were upregulated only in the PPE+anti-CD4 group and the PPE+isotype group, respectively. Eighty-three genes (xb) and 15 genes (yb) were downregulated in PPE + anti-CD4 and PPE +isotype groups, respectively. These results indicated that there is a class of genes (xa,xb,yaandyb) related to non-exhaustive resistance to blocking CD4 molecules in the spleen.
Hierarchical clustering analysis of all the differential genes in the PBS, PPE+isotype, and PPE+anti-CD4 groups was performed. Anti-CD4 treatment reduced the expression level of one type of gene in the spleen (Fig. 8B, greener color), while the expression level of the other type of differential genes increased (Fig. 8B, from green to white or red),showing a different change compared to the PBS PPE+isotype group.

Fig. 8 (Continued)
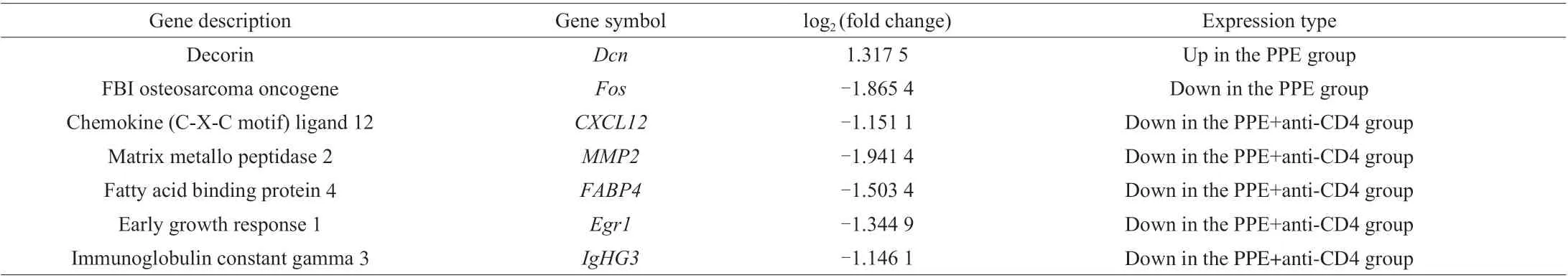
Table 1 Significant differentially expressed genes in the PPE and PPE+anti-CD4 groups, compared to those in the PBS group.
K-means clustering of all DEGs according to similar expression patterns revealed a total of four major DEG subclasses patterns in order from PBS, to PPE+isotype, to PPE+anti-CD4 group. In the first pattern, expression continued to decline significantly (0, -2, -3).In the second pattern, expression continued to decrease (0, -1, -2).These genes were mainly related to anabolic metabolisms, such as nucleosides and amino acids. In the third pattern, expression was initially parallel and then increased (0, 0, 1). These genes were only related to the non-exhaustive blocking of CD4. In the fourth pattern,the expression initially increased and then plateaued (0, 1, 1). These genes were related to the allergic reaction caused by PPE (Fig. 8C).
Additionally, GO analysis revealed that most of the DEGs following anti-CD4 treatment were related to innate and adaptive immune responses (Fig. 8D). Specifically, decorin (Dcn) was upregulated in the PPE group. Whereas early growth response 1(Egr1), chemokine (C-X-C motif) ligand 12 (CXCL12), matrix metallo-peptidase 2 (MMP2), fatty acid-binding protein 4 (FABP4),immunoglobulin constant gamma 3 (IgHG3) and FBI osteosarcoma oncogene (Fos) were downregulated in the PPE+anti-CD4 group and PPE group, respectively (Table 1).
4. Discussion
Peanut allergy has attracted widespread attention because of its high sensitization rate and severity. Moreover, peanut allergy may serve as an early marker of asthma morbidity [22-24]. In this study, a model of peanut allergy was established in C3H/HeJ mice by PPE oral sensitization. The allergic mice exhibited an active systemic allergic reaction and enhanced PPE-specific IgE concentration. Emerging data suggest that Ca2+signals are related to allergic inflammation [25]. For example, Houtman et al. [26]. demonstrated that attenuation of mast cell degranulation by Ca2+-like peptide was due to inhibition of the Ca2+release activated Ca2+current in ovalbumin-induced inflammation.Subsequently, Yang et al. [27] showed that FcεRI-activated Ca2+mobilization was increased by lipopolysaccharide in allergic asthma,and that Ca2+entry through store-operated calcium channels evoked by thapsigargin. Additionally, Ca2+is the meeting point of many signal pathways. The degranulation response, cytokine secretion,and arachidonic acid metabolites of mast cells were all dependent on Ca2+signals [28,29]. Similarly, in the present study, we observed that stimulation with PPE increased Ca2+influx in comparison to the PBS group, and showed increased levels of cytokines, histamine,and mMcp-1 in PPE-sensitized mice. Furthermore, it is worth noting that Ca2+influx was positively correlated with the production of histamine, IL-4, IL-5, and IL-13 in the spleen. It has been reported that intracellular free Ca2+was increased in human airway smooth muscle,and Ca2+influx regulated by STIM1 and Orai1 was responsible for airway remodeling in asthma [30]. Furthermore, Kashiwakura et al. [31]reported that a histamine-releasing factor in na?ve mice can recruit inflammatory immune cells to the lung in a mast cell and Fc receptordependent manner, suggesting that histamine-releasing factor promoted allergic inflammation in the lung. Lee et al. [32] showed that the levels of cytokines, including IL-4, IL-5, and IL-13, may be elevated in acute asthma. Additionally, the higher levels of IL-5 were related to severe airway obstruction. Wang et al. [33] found that histamine could induce Ca2+-activation in airway smooth muscle cells depending on the G protein-coupled receptor-TMEM16A-voltagedependent Ca2+channel signaling axis. This raises the question of whether Ca2+influx could induce allergic asthmatic reaction by this axis in peanut allergy. This will be the subject of planned future studies. Additionally, further research indicated that theDcngene related to asthma in PPE-sensitized mice was upregulated. Dcn has been associated with increased airway inflammation, hyperresponsiveness, and remodeling [34]. Accordingly, we speculate that the influx of Ca2+in peanut allergy causes the release of histamine,IL-4, IL-5, and IL-13, which may cause severe asthma symptoms of peanut allergy.
Several lines of evidence support a potential role for Ca2+influx in T cell development. First, an influx of Ca2+into the cytosol occurs following the engagement of antigen binding to the T cell receptor(TCR) [35]. Second, mice deficient for signaling molecules involved in generating Ca2+signals downstream of the pre-TCR, such as a linker for activation of T cells and IL-2-inducible T-cell kinase, are blocked in T cell development [36,37]. In this case, Ca2+influx induced T cell activation. Presently, stimulation with PPE enhanced the percentages of DCs and activated Th1, Th2, and Treg cells, along with increased expression of transcription factors. Surprisingly, the expression of T-bet was decreased in the PPE group compared to that in the PBS group. Genome-wide expression profiling revealed that Egr1 was downregulated in the PPE group. EGR1 binds to cis-acting elements in the T-bet promoter, which activates T-bet transcription [38].A possible explanation might be that PPE can induce the Th2-type response, and the principal function of GATA-3 in developing Th2 cells may be to negatively regulate T-bet, thereby inhibiting the expression of T-bet in the PPE group.
CD4+T cells are a critical component of the adaptive immune system. Productive TCR signaling can occur through the coordinated interactions within the TCR-CD3-peptides that are present in major histocompatibility molecules (pMHC)-CD4 macro complexes. This signaling informs T cell activation and fate decisions [39]. Therefore,given that CD4/TCR clustering classically promotes T cell functions,reduced CD4 expression may have an additive impact, such as alleviating the progression of immune disease, including remission of dandruff symptoms of autoimmune diseases in mice, inhibition of Th2 response, and allergic pneumonia [15,40]. In the present study,we also found that CD4 blockade prevented a systemic allergic response in PPE-sensitized mice, accompanied by decreased levels of albumin and PPE-specific IgE. To understand the mechanisms by which anti-CD4 inhibited PPE-induced allergic airway inflammation,we tested whether anti-CD4 could modulate Ca2+influx and T cell responses in PPE-sensitized mice. Anti-CD4 treatment significantly decreased the Ca2+influx, together with decreased levels of histamine and mMcp-1, Th1, and Th2 cell responses and the expression of transcription factors (T-bet and GATA-3). We also observed that Treg increased during anti-CD4 treatment similar to what has been described by Duarte et al. [17]. These authors also found that CD4 blockade mitigated anaphylaxis, which was dependent on increased Treg [17]. The expression of Foxp3 was downregulated during the anti-CD4 treatment. A probable explanation is the chemotaxis of Treg cells in other parts of the body to the spleen, which causes an increase in Treg cells. Anti-CD4 treatment also reduced the levels of cytokines, including IL-4, IL-5, IL-13, and IFN-γ, in the spleen and increased the content of IL-10. Additionally, the production of IL-10 was negatively correlated with Ca2+influx. IL-10 regulated tolerance to food, and secretion of IL-10 byLactococcus lactiscould decrease food-induced IgE sensitization [41]. Of note, the administration of anti-CD4 downregulated theIgHG3gene associated with both atopic and non-atopic childhood asthma. Additionally, the expression levels of CXCL12, MMP2 and FABP4, related to asthma symptoms,were downregulated after treatment with anti-CD4. Another study found that CXCL12 could induce neuronal death upstream of p38 mitogen-activated protein kinase (MAPK), which was controlled by Ca2+channels [42]. Furthermore, FABP4 induced the active forms of the nuclear transcription factorsc-junandc-myc, which are regulated by MAPK cascades, and increased the expression of the downstream genescyclin D1,MMP2,CCL2,fibulin 4andfibulin 5,which are involved in vascular smooth muscle cell proliferation [43].Accordingly, MAPK is probably one of the several pathways by which the asthma symptoms in peanut allergy were related to CXCL12, MMP2, and FABP4. Further studies are needed to explore the role of MAPK pathways in peanut allergy.
In general, we demonstrated that anti-CD4 antibody mediates peanut allergy by regulating Ca2+inflow. Augmenting the content of IL-10 and suppression of T cell activation, histamine, IL-4, IL-5,IL-13, and IgHG3, CXCL12, MMP2, and FABP4 might represent a potential mechanism to account for the amelioration of asthma symptoms in peanut allergy. Our present understanding of the role of Ca2+in peanut allergy is still notably limited. However, these findings help to elucidate the mechanism of beneficial anti-CD4 and provide new insight into the relief of peanut allergies. Moreover, the specific pathways of peanut allergy causing asthma symptoms need to be investigated in further studies.
Declaration of interest statement
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31972185).
- 食品科學(xué)與人類健康(英文)的其它文章
- Maternal obesity exacerbates the responsiveness of offspring BALB/c mice to cow’s milk protein-induced food allergy
- Au@Ag-labeled SERS lateral f low assay for highly sensitive detection of allergens in milk
- Impact of three different processing methods on the digestibility and allergenicity of Chinese mitten crab (Eriocheir sinensis) tropomyosin
- Effect of lipid peroxidation on the allergenicity and functional properties of soybean β-conglycinin (7S) and glycinin (11S)
- Comparative acetylome analysis reveals the potential mechanism of high fat diet function in allergic disease
- Therapeutic effects of epigallocatechin and epigallocatechin gallate on the allergic reaction of αs1-casein sensitized mice

