Study on mechanism of increased allergenicity induced by Ara h 3 from roasted peanut using bone marrow-derived dendritic cells
Minji Wng, Shuo Wng, Xiodong Sun, Zhirui Deng*, Bing Niu Qin Chen*
a School of Life Sciences, Shanghai University, Shanghai 200444, China
b Medical School, Shanghai University, Shanghai 200444, China
Keywords:Roasted peanut Ara h 3 Bone marrow-derived dendritic cells (BMDCs)Allergenicity Maillard reactions
A B S T R A C T Little information was so far available about allergenic mechanism of the roasted peanut allergens during initial stages of allergy. The purpose of this study was to determine the inf luence of roasting (150 °C, 20 min)on biochemical and biological properties of Ara h 3, a major peanut allergen. Allergenicity of roasted peanut emulsion to mice, differences in uptakes between Ara h 3 purif ied from raw peanuts (named as Ara h 3-Raw)and that purif ied from roasted peanuts (named as Ara h 3-Roasted) by bone marrow-derived dendritic cells(BMDCs) and the implication of cell surface receptors involving in uptake, and changes in glycosylation and structure of Ara h 3 after roasting were analyzed in this study. This study suggested that roasting increased allergenicity of peanut to BALB/c mice. Maillard reaction and structural changes of Ara h 3 induced by roasting signif icantly altered the uptake of Ara h 3-Roasted by BMDCs, and modif ied Ara h 3 fate in processes involved in immunogenicity and hyper allergenicity, indicating that food processing pattern can change food allergenicity.
1. Introduction
Peanut allergy, as a significant lifelong disease, has a pertinent influence on allergic people. It affects 0.4%-3.0% of the general population and even up to 6%-8% of children worldwide [1,2]. Peanuts are chiefly sourcesof fatal allergic reactions in the US and UK [3],about 59% of all food related deaths have been caused by peanuts allergy. Even for allergy sufferers, only 0.4 mg peanut can cause allergic reactions [4].
Up to now, 17 allergens in peanuts have been labeled and registered officially [5]. Among them, Ara h 3, a 60 kDa protein, as the main allergen was discovered by Eigenmann et al. [6] and Rabjohn at al. [7].Simultaneously, Kleber-Janke et al. [8] def ined an undistinguishable peanut allergen, off icially termed Ara h 4. Ara h 3 and Ara h 4 are deliberated to be the same allergen and are referred to as Ara h 3 in this paper. Ara h 3 belongs to the glycinin family of soy storage proteins which is stored as a trimer in protein storage vacuoles (PSVs) [9].Rabjohn identified four immunoglobulin E (IgE) binding epitopes on the acidic subunit of Ara h 3 by epitope scanning, but none on the basic subunit [10].
Mouse models deliver powerful tools to illuminate the immunologic mechanisms of allergy. Because of the complexity of the anaphylaxis mechanism, multiple indicators of the mouse model were comprehensively evaluate d to determine the severity of allergenicity [11,12]. In the process of Th2 allergic reactions, not only immunoglobulins but also many corresponding cytokines are produced, such as interleukin (IL)-5 and IL-13. They have a certain role in promoting anaphylaxis and the increase of their content indicates the enhancement of anaphylaxis.
In the initial stage of allergenicity, dendritic cells (DCs) as the most important antigen-presenting cells in the immune system, are dedicated for antigen capture, processing, presentation to T cells,and the priming of these cells into CD4+, Th1, Th2 and tolerogenic T-cell subtypes to initiate an adaptive immune response [13]. DCs have several specific pattern-recognition receptors (PRRs) for recognizing glycosylation structures, including scavenger receptors(SRs), mannose receptors (MRs), receptor for advanced glycation end product (RAGE), galactose-3 and CD-36 [14,15]. Previous research has shown that glycosylated allergens can be recognized by DCs via cell receptors, aggrandizing the uptake of allergens by DCs.
To elucidate the vital function of roasted peanut allergen Ara h 3 in anaphylaxis, the changes of structure and physicochemical properties of allergen Ara h 3 after roasting were studied. The result proved that Ara h 3 proteins were recognized and uptake by specific receptors in DCs surface. The uptake of Ara h 3 could be increased by roasting, leading to increased allergic reactions. The study provides a potential for clarifying the mechanism of peanut allergy and for developing new desensitization apprpaches.
2. Materials and methods
2.1 Animals
Female BALB/c mice (Jiesijie, Shanghai, China) were housed under specific pathogen free (SPF) conditions at the animal facility of the Shanghai University. All experiments were approved by the animal ethics committee of the Shanghai University, China.
2.2 Mouse immunization
Fresh peanuts were obtained from the Wal-Mart supermarket(Shanghai). Raw peanuts and roasted peanuts (150 °C, 20 min)were ground into fine powder and peanut powder was blended with phosphate buffered saline (PBS, pH 7.4) to form the emulsion for feeding mice in the immune process. BALB/c mice were randomly divided into three groups according to body weight as follows: control group (n= 4, as control, PBS did not have immunological effects on mice and the preliminary experimental result showed that mice treated only with PBS exhibited normal growth, so only 4 mice were used), raw peanut group (n= 5, as an additional control, raw peanut allergenicity was not vehemence, so 5 mice were used) and roasted peanut group (n= 8, for reliable, accurate and convincing results,eight mice were used). Mice in raw peanut group and roasted peanut group were sensitized by intragastric gavage (i.g) with 0.5 mL (about 10 mg/mL) raw peanut emulsion or roasted peanut emulsion every time on days 1, 2, 3, 7, 14, 21 and 28 [16]. Mice in control group were perfused with equal volume PBS. On day 35, BALB/c mice sensitized with peanut were gavaged with 0.5 mL peanut emulsion (about 50 mg/mL) for restimulation and sacrificed 30 min later.
2.3 Histopathological observation
Jejunum was flushed with PBS before they were inflated with OCT Tissue Tek (Sakura Finetek Europe, Zoeterwoude, the Netherlands) before they were snap frozen in liquid nitrogen. Frozen jejunum sections (6 μm) were stained with Toluidine Blue (Sigma-Aldrich). Frozen lung sections (6 μm) were stained with periodic acid Schiff’s reagent (Sigma-Aldrich). Hank’s liquid (3 mL) was administered to the mice intraperitoneally, and the abdomen was gently rubbed for 1 min before the removal of Hank’s liquid. The intestinal fluid was left to dry and stained with Toluidine Blue.Degranulation of mast cells and basophils in various tissues was observed by a light microscope.
2.4 Enzyme linked immunosorbent assay (ELISA)
The serum of mice was diluted properly. Each experimental condition was analyzed in quadruplicate. The concentrations of IL-5, IL-13, monocyte chemoattractant protein-1 (MCP-1) from serum were determined using commercial ELISA kits (Baoman,Shanghai, China) according to the manufacturer’s instruction.
96-well plates were coated with 100 μL/well raw peanut extracts(100 μg/mL) and incubated overnight at 4 °C. Wells coated with 250 μL blocking solution (PBS-Tween-20 (PBST) 0.1% and 3.0%of non-fat milk), instead of peanut extracts, were used as negative control. After washing with PBST, wells were blocked with 250 μL blocking solution, for 1 h at room temperature. Plates were incubated with 200 μL mixture of serums (expressed as pooled serum, final dilution 1:10) from 8 individual BALB/c mice sensitized with roasted peanuts for 2 h at 37 °C. After wells being washed, 200 μL horseradish peroxidase (HRP)-conjugated goat anti-mouse IgE, IgG1,IgG2a antibody (Abcam, Cambridge, MA, USA, stock 1 mg/mL, used at 1:1 000 dilution in blocking solution) was added and incubated for 1 h at 37 °C. The reaction was developed with 200 μL tetramethylbenzidine(TMB) for 15 min, and stopped with 50 μL 2 mol/L sulfuric acid.The absorbance at 450 nm was read in an EMax Plus single-function optical absorption microplate reader (Molecular Devices Corporation,USA).
2.5 Purification of Ara h 3
Ara h 3-Raw and Ara h 3-Roasted was isolated from peanuts as described previously by Jin et al. [10] and Wang et al. [17]. The protein concentration was assessed by the bicinchoninic acid (BCA) protein assay kit (Pierce, USA).
2.6 Generation of bone marrow-derived dendritic cells(BMDCs)
Bone marrow cells isolated from femurs/tibias of 8 weeks old SPF Kunming mice were cultured in conditioned-complete DCs medium(10% fetal bovine serum, 1% double antibody, 10 mmol/L HEPES and 0.05 mmol/Lβ-mercaptoethanol), with 10 ng/mL cell factor rrGM CSF for induction at 37 °C in a humidified atmosphere of 5% CO2.Medium with new cell factor rrGM-CSF was refreshed every day.Only 6 days old BMDCs were used for the following experiments.
2.7 Ara h 3 uptake by BMDCs
According to the manufacturer’s instruction (Sigma-Aldrich,St. Louis, MO, USA), Ara h 3-Raw and Ara h 3-Roasted were labeled with FITC. FITC-Ara h 3-Raw and FITC-Ara h 3-Roasted were separated from unreacted FITC label using Sephadex G-25 M affinity column (GE Healthcare, Uppsala, Sweden). Absorbances at 280 nm and 495 nm were used to determine labeling efficacy for Ara h 3-Raw and Ara h 3-Roasted. Only FITC-labeled samples with equal fluorescence intensity were used in the experiment below.The concentration of samples was confirmed by BCA assay, and the samples were stored at -20oC in dark until use.
FITC-Ara h 3-Raw and FITC-Ara h 3-Roasted (40 μg/mL) were incubated with 1 × 106BMDCs per mL of cell culture medium for 0, 0.5, 1.0 and 1.5 h at 37 °C, respectively. The uptake rates were recorded using BD Accuri C6 Cytometer (BD Biosciences, San Jose,CA, USA). Mean fluorescence intensity (MFI) was calculated using CytExpert.
2.8 Real-time reverse transcriptase PCR
BMDCs (1 × 106/mL) were incubated with Ara h 3-Raw and Ara h 3-Roasted (40 μg/mL) and lipopolysaccharide (LPS, 1 ng/mL)as described previously for 24 h [18]. Total RNA was isolated from purified pooled BMDCs using the Qiagen RNeasy Mini Kit,according to the manufacturer’s protocol (Qiagen). After reversetranscription of RNA into cDNA, and the expression ofIL-10,IL-12was calculated relative to the housekeeping geneGAPDHas previously described. The following primers were used for analysis:IL-10forward, 5’-CAGAGCCACATGCTCCTAGA-3’,and reverse, 5’-TGTCCAGCTGGTCCTTTGTT-3’;IL-12forward, 5’-AGATGACATCACCTGGACCT-3’, and reverse,5’-GCCATGAGCACGTGAACCGT-3’.
2.9 Route for uptake of Ara h 3-Roasted by BMDCs
To identify the uptake mechanism for Ara h 3-Raw and Ara h 3-Roasted, BMDCs were incubated with 200 μg/mL polyinosine,specific inhibitor for SRs; 20 μg/mL mannan, specific inhibitor for MRs and 0.3 μmol/L FPS-ZM1, specific inhibitor for RAGE, for 30 min at 37 °C, respectively. BMDCs were loaded with 40 μg/mL FITC-Ara h 3-Raw and FITC-Ara h 3-Roasted and incubated 30 min at 37 °C. The medium without protein was set as a blank group. Uptake was recorded with flow cytometry as described above.
2.10 Confocal microscope observation
After being treated with inhibitors, 1 × 105BMDCs were washed and spun onto glass slides by cytospin. Cells were imaged on a Leica TCS SPE-2 confocal laser scanning-microscope on a DMI4000.
2.11 Determination of glycosylation level
For a comprehensive determination on glycosylation level of Ara h 3-Raw and Ara h 3-Roasted, four different methods were used.
1)o-Phthaldialdehyde (OPA) assay
A quota of 50 μL (0.5 mg/mL) samples were mixed with 3 mL OPA reagent. The mixed solutions were incubated for 5 min at room temperature, and the absorbance was measured at 340 nm against a control containing PBS and the OPA reagent. Unreacted amino groups were estimated from a calibration curve established withL-leucine.
The precooled 80 μL protein solutions (0.25 mg/mL) were mixed in 96-well plates (Sigma Aldrich, Germany) with the 200 μL anthrone reagent then incubated for 15 min at 92oC. A glucose standard curve was used to calculate the sugar content.
3)Nitrobluetetrazolium (NBT) assay
The reagent was prepared as described before by Masityama et al. [19]. The reagent and sample were mixed in the ratio 19:1 and incubated at 37 °C. Fructosamine (320 μmol/L) was used as a standard and absorbance was measured in a microplate reader (Asys UVM 340) at 550 nm after incubation for 10 min. The results were calculated using the following formula:

4)Fluorescence determination
A quota of 0.2 mL of Ara h 3 (0.5 mg/mL) sample and free fluorescent compounds in the fluid obtained through ultrafiltration of Ara h 3 by 10 kDa cut-off membrane (ultrafiltrate of Ara h 3)were taken for fluorescence measuring with a fluorescence spectrophotometer (PerkinElmer LS 50B, GB) at excitation wavelength of 374 nm and emission wavelength of 451 nm.
2.12 Determination of the microstructure of Ara h 3
The structure of Ara h 3 was determined by circular dichroism(CD) spectra, ultraviolet (UV) spectroscopy and fluorescence,respectively.
1) CD spectra
Secondary structural changes of Ara h 3-Roasted solution(0.25 mg/mL) were monitored by recording CD spectra on a JASCO J-815 spectropolarimeter (JASCO, Tokyo, Japan). Scanning was set:190-240 nm, 100 nm/min, 0.1 cm light diameter. Each spectrum was acquired 5 times, and the results were averaged. Data was analyzed by CD Pro software to determine the proportion of secondary structures.
2) UV spectroscopy
She thought she could distinguish her father’s castle, and upon it her aged grandmother, with the silver crown on her head, looking through the rushing tide at the keel of the vessel
The absorbance of Ara h 3 (1.0 mg/mL) was determined by UV spectrophotometer within scanning range of 230-450 nm.
3) 8-Anilino-1-naphthalenesulfonic acid (ANS) fluorescence probe emission spectra
Bis-ANS was added to Ara h 3 solution (1.0 mg/mL) to final 80 μmol/L, and mixture stood for 30 min to stabilize at room temperature. For ANS-protein complex fluorescence determination,350 nm was used as exiting light and emission spectra from 400 to 600 nm were collected.
4) Atomic force microscopy (AFM)
Ara h 3 was dissolved in ultrapure H2O to a concentration of 1.0 mg/mL and approximately 5 μL solution was dropped on the surface of a mica sample carrier and dry at room temperature. AFM images of the Ara h 3 were obtained by using a AFM PLUS+with a commercial tapping silicon (Si) tip and the estimated tip radius was < 10 nm. Images were acquired at resolution 256 × 256 points within the range of 5 μm/s scan rate.
2.13 ELISA inhibition assays
Pooled serum was used to analyze differences in IgE/ IgG binding capability by inhibition ELISA method of Sanchiz et al. [20]. 96-well plates were coated with 100 μL/well of extracts (100 μg/mL) from raw peanuts and incubated overnight at 4 °C. In parallel, pooled serum(final dilution 1:10) were pre-incubated with Ara h 3-Raw or Ara h 3-Roasted as inhibitors (final concentrations: 0.05, 0.20, 0.80, 3.20,12.80, 51.20 μg/mL) 1 h at 37 °C with constantsoft stirring. Pooled serum pre-incubated with PBS was also included (non-inhibited serum) as above. Wells were washed then blocked with PBST 3%skimmed milk for 1 h. The well was incubated with 200 μL/well serum pre-incubated with inhibitors or non-inhibited serum at 37 °C for 3 h. After washing, 200 μL/well HRP-conjugated goat anti-mouse IgG antibody (at 1:1 000 dilution) was added and the mixture was incubated for 1 h at 37 °C. After washing, the peroxidase reaction was developed with 100 μL TMB for 15 min, the reaction was stopped with 50 μL 2 mol/L sulfuric acid. The absorbance was read at 450 nm in a plate reader. The percentage of inhibition was calculated with the formula:
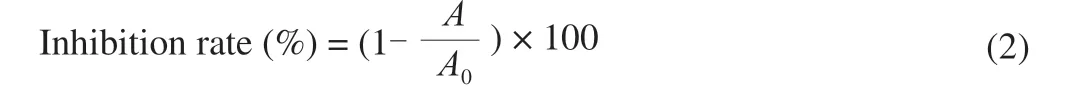
WhereAandA0are absorbance values with and without inhibitors,respectively.
2.14 Statistical analysis
All data were presented as standard error of mean (SEM) and analyzed using GraphPad Prism software (La Jolla, CA). The data were also tested by SPSS (Chicago, IL, USA). WhenP-values were < 0.05,the difference was considered significant.
3. Results
3.1 Observation of jejunum, lung and immune cells in BALB/c mice
The severe intestinal villi rupture, thickening of the intestinal wall and infiltration of blood cells were seen in mice challenged with roasted peanuts compared with PBS-treated mice and raw peanutsensitized mice (Fig. 1A). In the roasted peanut-sensitized mice, lung bronchus was damaged, and inflammatory cell infiltration was more grievous, and bronchoalveolar congestion was more fearful (Fig. 1B).Both mice sensitized with raw peanuts or roasted peanuts showed clear recruitment, disintegration and degranulation of the mast cells and basophils to peritoneal lavage while mice treated with PBS did not show phenomena above (Fig. 1C). The results showed that roasted peanuts caused more somber injury to intestinal mucosa and lung tissue lesion in BALB/c mice.
3.2 Enhanced Th2-type response to roasted peanuts in mice
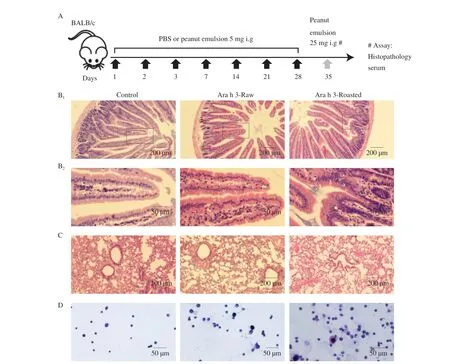
Fig. 1 Effects of peanut allergens on jejunum, lung and immune cells of BALB/c mice. (A): Immunization protocol. The changes of jejunum (B1 × 200, B2 ×400), lung (C × 200) and immune cells in PBS (D ×400), raw peanut or roasted peanut-sensitized BALB/c mice were observed by optical microscopy. Lavender cells are mast cells, dark purple cells are basophils.
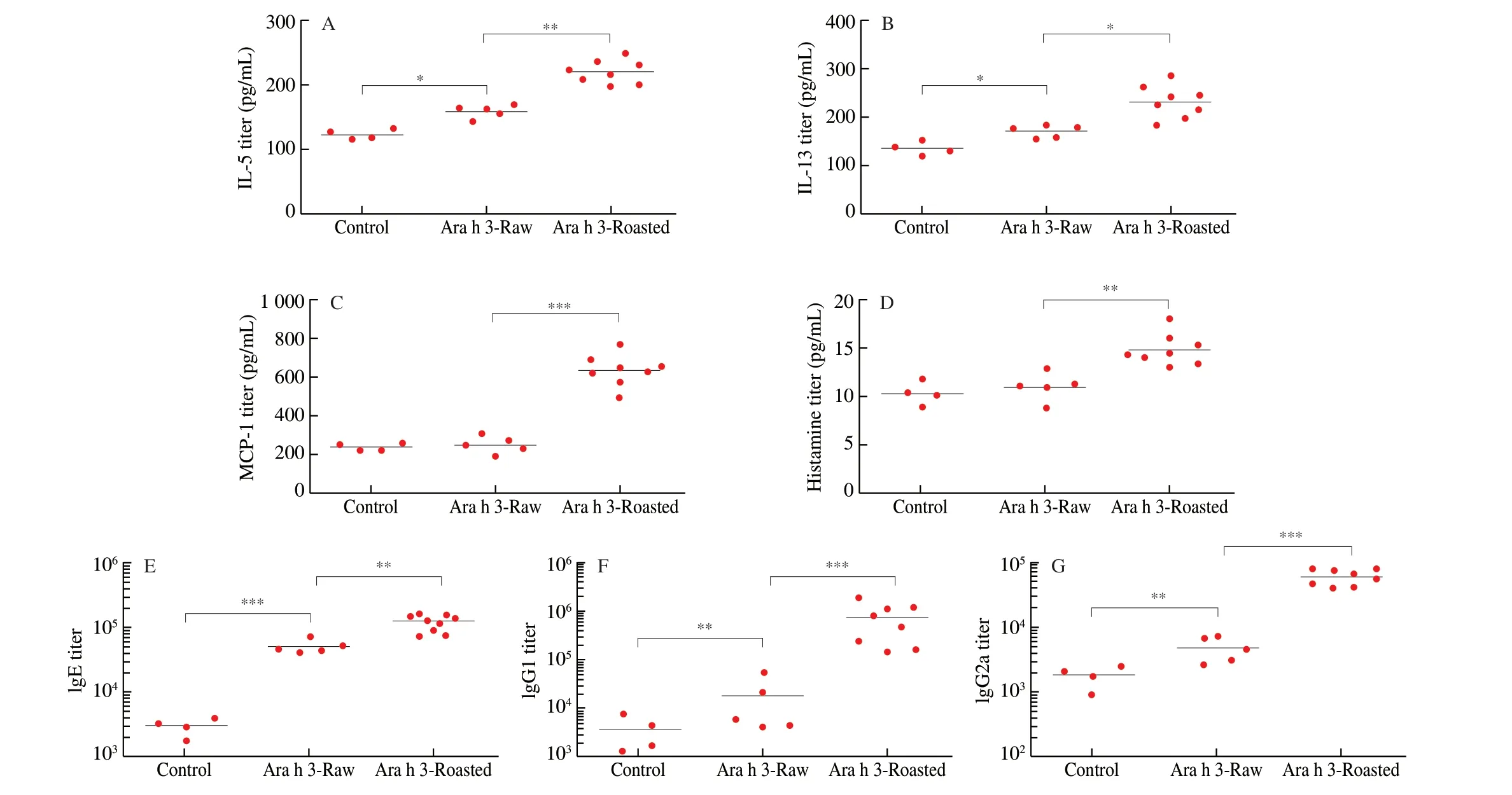
Fig. 2 Effects of peanut allergens on cytokines, histamine and specific antibodies in BALB/c mice serum.
The Th2-type immune response to peanut allergens wasestimated by the levels of cytokines IL-5, IL-13 and MCP-1, inflammatory mediators, histamine, specific antibodies IgE, IgG1 and IgG2a in the serum of mice (Fig. 2). The cytokine production of IL-5, IL-13 and proinflammatory factor MCP-1, inflammatory media histamine all were significantly increased in roasted peanut-sensitized mice when compared with those in mice sensitized with raw peanuts or treated with PBS, indicating roasted peanuts triggered stronger inflammatory response.
To further investigate the effect of roasting on peanut allergenicity,the levels of IgE, IgG1 and IgG2 in the serum of BALB/c mice were monitored. As expected, compared with the control group,the BALB/c mice sensitized with raw peanuts cultivate IgE antibodies peanut allergens, and the serum IgE antibody levels in the BALB/c mice sensitized with roasted peanuts were significantly higher than those of the raw peanut group. The same situation happened to IgG1 and IgG2a, a significant augment in the levels of IgG1/IgG2a was observed in mice sensitized with roasted peanut, as compared with levels of IgG1/IgG2a in mice sensitized with raw peanut. Consequently,roasted peanuts induced higher allergenicity in BALB/c mice.

Fig. 3 Effects of roasting on the uptake of Ara h 3 and cytokine mRNA level of BMDCs. Ara h 3 uptake by BMDCs was analyzed by flow cytometry. Data are expressed as (A) percent of FITC-positive BMDCs and (B) MFI. The level of (C) IL-10 mRNA and (D) IL-12 mRNA was measured in pooled BMDCs by RT-PCR and is presented as relative ratio to the house keeping gene GAPDH. The data are presented as mean ± SD of one representative experiment and analyzed by Student’s t-test. **, *** represent P < 0.01, 0.001, respectively.
3.3 Uptake of Ara h 3 and secretion of cytokines by BMDCs
Further, whether roasting influenced interactions of Ara h 3 with BMDCs were investigated. FITC-labeled Ara h 3-Raw and Ara h 3-Roasted were incubated with BMDCs, and Ara h 3 uptake was trailed duly by flow cytometry. Uptake, expressed as percentage of FITC-cells,was time dependent and attained plateau 1 h later after treatment(Fig. 3A). Ara h 3-Roasted was taken up more efficiently and FITC-BMDCs percentage reached 68.27% while only 47.53% when incubated with FITC-labeled Ara h 3-Raw. Significantly increased uptake of Ara h 3-Roasted was also observed in MFI observation(Fig. 3B). The mRNA level of the Th2-type inflammatory cytokineIL-10was significantly up-regulated and Th2-type cytokineIL-12was markedly down-regulated in Ara h 3-Roasted pretreated BMDCs(Fig. 3C-D), indicating that Ara h 3 from roasted peanuts promoted the occurrence of Th2-type allergic reaction in dendritic cells.
Fig. 3 (Continued)
3.4 Uptake of Ara h 3 by DCs treated with receptor inhibitors
In an attempt to identify receptors involved in the uptake of Ara h 3-Raw and Ara h 3-Roasted, inhibitors for specific endocytic receptors were used. The results showed that all three inhibitors had weak and similar suppression on Ara h 3-Raw uptake. For Ara h 3-Roasted, polyinosine as an inhibitor of SRs, remarkably reduced its uptake. Although slightly weaker than polyinosine in repressing Ara h 3-Roasted uptake, FPS-ZM (RAGE inhibitor), mannan (MRs inhibitor)both exerted certain suppressive effects on uptake of Ara h 3-Roasted by BMDCs (Fig. 4). These findings suggested that roasting promoted Ara h 3 uptake and redistribution of Ara h 3 uptake toward receptor-mediated endocytosis might arise from stronger recognition mainly by SRs due to variations in physicochemical properties.
Confocal micrographs were in agreement with the findings obtained by flow cytometry, BMDCs displayed a weaker fluorescent signal upon inhibition with polyinosine before Ara h 3-Roasted incubation (Fig. 4C).
3.5 Roasting effects on glycosylation, structure and IgE/IgG binding capacity of Ara h 3
To investigate the change in glycosylation of Ara h 3 during roasting, 4 methods were employed to assess glycosylation level.As shown in Fig. 5, Roasting resulted in significant loss of primary amino groups and increased levels of bound sugar in Ara h 3-Roasted.Meaningfully higher amounts of protein-bound glucose were detected in Ara h 3-Roasted when compared with those in Ara h 3-Raw,perhaps owing to structural changes in protein molecules(Fig. 5A1-A2). In addition, fructosamine level in Ara h 3-Roasted group increased to 3.2 times that in Ara h 3-Raw group (Fig. 5A3).The fluorescence intensity from Ara h 3-Roasted was 19.6 times higher than that from Ara h 3-Raw and reached about 1/3 that of ultrafiltrate of Ara h 3-Roasted (Fig. 5A4). Those results suggest that roasting for 20 min under 150oC made maillard reaction reach advanced stages. Combined with the previous experiments, it is speculated that increased uptake of Ara h 3 by DCs was due to its increased glycosylation and products of maillard reaction.
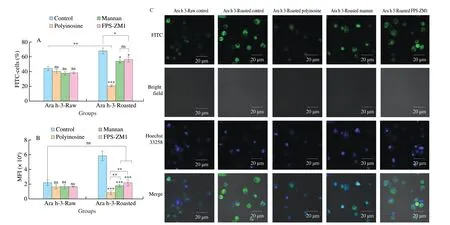
Fig. 4 Uptake of FITC-labeled Ara h 3 by BMDCs when inhibitors were used. Data of flow cytometry are expressed as (A) percent of FITC-positive BMDCs and (B) MFI. (C) Confocal microscopy: uptake of FITC-labeled Ara h 3 by BMDCs in the presence of receptor-specific inhibitors. Control group: without inhibitor. Inhibitor groups: BMDCs were treated with inhibitors for 1 h before being incubated with Ara h 3-Raw or Ara h 3-Roasted for 1 h. Green fluorescence from Ara h 3-Raw or Ara h 3-Roasted; Blue fluorescence from nuclei of dendritic cells. **, ***, and ns, represent P < 0.01, 0.001, and not significant, respectively.
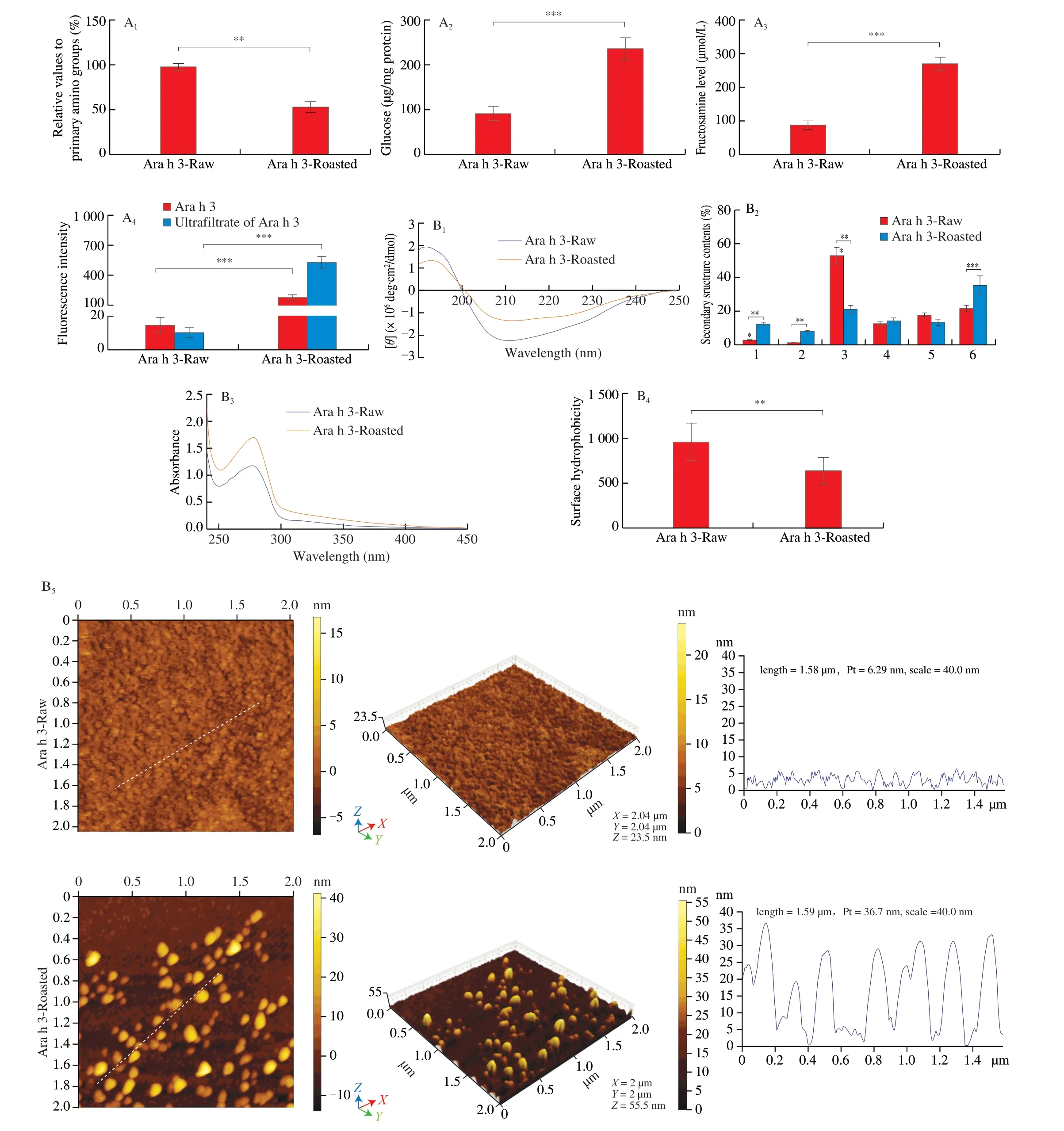
Fig. 5 Effect of roasting on the maillard reaction, structures and IgE/IgG binding capacity of Ara h 3. A1: Percentage of primary amino groups in relation to Ara h 3-Raw(100%) determined with OPA method. A2: Amount of bound sugar determined with anthrone method. A3: The level of fructosamine determined with NBT assay. A4: The fluorescence intensity of Ara h 3 expressed in arbitrary unites. Ultrafiltrate of Ara h 3: fluorescence products obtained via ultrafiltration of Ara h 3-Raw and Ara h 3-Roasted with 10 kDa cut-off membrane. B1: CD spectra of Ara h 3-Raw and Ara h 3-Roasted in UV spectral range. B2: Effects of roasting on the secondary structure content of Ara h 3, 1-6 represent the regular α-helix, irregular α-helix, regular β-strand, irregular β-strand, β-turn, random coil, respectively.B3: Fluorescence emission spectra (295-450 nm) of Ara h 3-Raw and Ara h 3-Roasted. B4: The hydrophobicity on the surface of Ara h 3 detected with ANS fluorescence probe. B5: AFM images of Ara h 3 planar, cubic and protien heights. C1: Inhibition ELISA assay of IgE binding capacity of Ara h 3. C2: Inhibition ELISA assay of IgG binding capacity of Ara h 3. The data were expressed as mean ± SD (** P < 0.01, *** P < 0.001.)
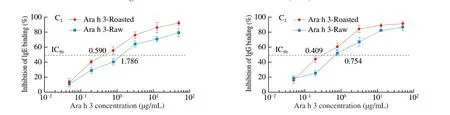
Fig. 5 (Continued)
The effects of roasting on the structure of Ara h 3 are shown in Fig. 5B. The rates of change in the secondary structure of Ara h 3 were followed by Far-UV CD spectra (Fig. 5B1-B2). CD spectra changes are mostly detected in the region between 190 and 240 nm.These changes are consistent with loss ofβ-sheets and increase of random coil fractions. Spectrophotometer showed OD280nmof Ara h 3-Roasted increased to 146% (Fig. 5B3), and ANS fluorescence probe method showed that the hydrophobicity of Ara h 3-Roasted decreased (Fig. 5B4). AFM images showed that Ara h 3-Roasted had a spheroidal or ellipsoidal structural feature (Fig. 5B5). More importantly, the protein average heights of Ara h 3 increased significantly from 1.58 nm to 36.70 nm after roasting, suggesting that Ara h 3 underwent aggregation during roasting.
An inhibition ELISA was performed to evaluate IgE/IgG binding capacity of Ara h 3 for solid determination whether roasting affects its potential allergenicity. Fig. 5C showed that IgE/IgG binding capacity to Ara h 3 was increased by roasting. Compared with the Ara h 3-Raw, the IC50value of IgE binding reduced from 1.786 μg/mL to 0.590 μg/mL,the IC50value of IgG binding reduced from 0.754 μg/mL to 0.409 μg/mL.The results indicated that roasting had obviously promoted IgE/IgG binding capacity of Ara h 3.
4. Discussion
Nowadays, roasted peanuts are increasingly consumed in western diets and augmented allergenicity of several allergens from roasted peanuts has been demonstrated [21]. Roasting can induce a variety of biochemical reactions of peanut ingredients, therein maillard reaction is an important one, which causes the glycosylation of peanut allergens and the generation of advanced glycation end products(AGEs) [15]. The glycosylation of peanut allergens may have a major impact on their allergenicity. The purpose of this study was to investigate the mechanism of increased allergenicity of Ara h 3 after peanuts were roasted. It was attempted to provide a reasonable understanding of how food processes impact food allergy.
For this understanding, BALB/c mice were sensitized with raw peanut and roasted peanut emulsion via gavage and their allergen allergenicity was compared by detecting specific antibodies and cytokines in blood, according to a study of Verma et al. [22]. After being sensitized with roasted peanut via gavage, BALB/c mice developed apparent anaphylaxis, such as increased inflammatory infiltration, hyperemia and peribronchial infiltrates. The degranulation of mast cells and basophils also revealed the causes of jejunum and lung tissue injury and pathological changes [23,24]. Compared with mice sensitized with raw peanuts, mice sensitized with roasted peanuts generated higher levels of Th2-type cytokines (IL-5, IL-13)and increased peanut-specific IgE, IgG1 and IgG2a. And elevated histamine release from mice sensitized with roasted peanut confirmed higher allergenicity of roasted peanut, too. In addition, IgG1/IgG2a was greater than 1, demonstrating that roasted peanut could induce a higher Th2-type of allergic reaction in mice.
For a closer insight, Ara h 3, as one of the main allergens in peanuts [19,25], was purified from raw peanuts and roasted peanuts.To clarify the fortune of Ara h 3-Roasted in downstream processing, its uptake by BMDCs was detected. Compared with the uptake of Ara h 3-Raw, the consequence of uptake of Ara h 3-Roasted by BMDCs continually increased at each measuring time point.
IL-10gene mainly regulates T cells to differentiate into Th2 cells.IL-12gene mainly regulates T cells to differentiate into Th1 cells.The expression ofIL-10gene in BMDCs was significantly increased,accompanied by a noteworthy decrease inIL-12gene expression,suggesting that Th1/Th2 immune imbalance of BMDCs could be the consequence of uptake of Ara h 3-Roasted and the dominant activation of Th2-type immune response.
PRRs is involved in allergens recognition through its carbohydrate recognition domain [26]. Literature suggested that some allergens interacted with PRRs to inspire innate and adaptive responses. In order to make sure if there were some receptors on cell surface that were involved in the increased uptake by BMDCs by enhancing recognition and presentation of Ara h 3-Roasted. Several different pharmacological inhibitors which hinder receptor-mediated endocytosis were utilized, and the result showed that SRs, MRs andRAGAreceptors were involved in the uptake of Ara h 3-Roasted. In addition, compared with uptake of Ara h 3-Raw uptake, polyinosine exhibited the most pronounced inhibition of Ara h 3-Roasted uptake,indicating that Ara h 3-Roasted was mainly recognized and ingested by SRs (Fig. 4). Our results did not completely agree with Novak group’s results. They have shown that uptake of Ara h 3-Roasted mainly depended on MRs [27]. Our experiments showed that SRs was more imperative than MRs in the uptake of Ara h 3-Roasted by BMDCs. The reason may be that different dendritic cell lines were used. Our experiment used BMDCs, while Novak’s study used monocyte-derived dendritic cells (MDDCs). Perusko’s research showed that natural and glycosylatedβ-lactoglobulins participate in the uptake of BMDCs through receptor-mediated endocytosis,and the mainly responsible receptor for the uptake of glycosylatedβ-lactoglobulins was the scavenger receptor [28]. It is worth noting that the ligand of SRs is polyanion, while Ara h 3 is an acidic protein with isoelectric point (pI) value of 5.68, making SRs bind Ara h 3 easier and closer. It can be speculated that roasting preferentially blocks the lysine residues by sugar moieties, thus reducing the pI value of Ara h 3. Acidification of Ara h 3-Roasted enhanced Ara h 3 binding affinity to SRs, promoting the uptake of Ara h 3-Roasted by BMDCs.
Chung’s study showed that maillard reaction took place during peanut roasting, resulting in the formation of AGEs, which led to the increase in peanut allergenicity [29]. In Stanic’s study, DCs were able to absorb more glycosylatedβ-lactoglobulin, resulting in increasedβ-lactoglobulin content in DCs [30]. It is reasonable to speculate that maillard reaction made some contribution to increased uptake of Ara h 3 by BMDCs surface receptors. To verify the hypothesis, the glycosylation level of Ara h 3-Roasted was compared with that of Ara h 3-Raw. During glycosylation, the reduction of sugars needs free amino groups in protein molecules, the higher glycosylation level is,the fewer free amino groups left. Both contents of free amino groups in proteins and contents of bound sugars showed that the reducing sugar was linked to the peptide chain by glycosylation, indicating maillard reaction reached intermediate stage. According to the study of Teodorowicz et al. [31], the formation of small molecules with high fluorescence intensity indicated that the protein browned, that is,maillard reaction reached the end and AGEs were produced (Fig. 5A).The increased glycosylation of Ara h 3-Roasted made Ara h 3 more susceptible to SRs’ recognizing and ingesting. This should be one of important reasons for the increase in peanut allergenicity.
The allergenicity of peanut allergens was significantly affected by other structural changes (except maillard reaction) [32,33].Roasting could make Ara h 3 lose part of secondary structure,increase hydrophobicity, unfold and aggregate (Fig. 5B), and change its biological function. All these changes of Ara h 3 enhanced recognition and uptake of Ara h 3, and markedly stimulated adaptive immune response of BMDCs.
Furthermore, structural changes might expose IgE/IgG binding epitopes of Ara h 3-Roasted, and this also can be used as one of the reasons for the intensification of Ara h 3-Roasted allergenicity(Fig. 5C), being consistent with results of Filep et al. [34] and Kopper et al. [35]. The escalation in IgE/IgG binding capacity may come from cross-linking of Ara h 3-Roasted. During roasting, the modification of amino acid side chains in Ara h 3 may contribute to the increase in previously unexposed allergen-binding sites and enhancement of IgE/IgG binding capacity [36]. Overall, roasting enhanced Ara h 3 allergenicity by altering its physical and chemical properties and promoting its uptake through receptor-mediated endocytosis.
5. Conclusion
BALB/c mice sensitized with roasted peanuts released abundant Th2-type cytokines (IL-5, IL-13, MCP-1), histamine and specific antibodies (IgE and IgG), leading to more grievous degranulation of mast cells and basophils, as well as more obvious inflammatory infiltration and congestion in jejunum and lung tissues. Roasted peanuts triggered more severe allergic reactions in mice than raw peanuts. Escalation in allergenicity of Ara h 3-Roasted was achieved by glycosylation and the disorder of protein structure. The elevated glycosylation and structural changes led to uptake increase of Ara h 3-Roasted by DCs surface receptor SRs, MRs and RAGE.Glycosylation and structural changes also facilitate the possibility of IgE/IgG binding capacity of Ara h 3. It was SRs (as the main responsible receptor among receptors) that enhance most uptake of Ara h 3-Roasted by BMDCs. These are main reasons for the increased allergenicity of Ara h 3-Roasted.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
This study was funded by the National Key Research and Development Program of China (2016YFD0501101), the project of Food Science Discipline Construction of Shanghai University and the National Natural Science Foundation of China (31201306).
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted and ethical approval was obtained from Institutional Review Board (IRB) of Shanghai University for experimental animals and relevant permit number was SYXK(Shanghai) 2019-0020.
- 食品科學(xué)與人類健康(英文)的其它文章
- Maternal obesity exacerbates the responsiveness of offspring BALB/c mice to cow’s milk protein-induced food allergy
- Au@Ag-labeled SERS lateral f low assay for highly sensitive detection of allergens in milk
- Impact of three different processing methods on the digestibility and allergenicity of Chinese mitten crab (Eriocheir sinensis) tropomyosin
- Effect of lipid peroxidation on the allergenicity and functional properties of soybean β-conglycinin (7S) and glycinin (11S)
- Comparative acetylome analysis reveals the potential mechanism of high fat diet function in allergic disease
- Therapeutic effects of epigallocatechin and epigallocatechin gallate on the allergic reaction of αs1-casein sensitized mice

