Transcriptomics reveals substance biosynthesis and transport on membranes of Listeria monocytogenes affected by antimicrobial lipopeptide brevilaterin B
Yngliu Liu, Ywei Ning, Zhou Chen, Pnpn Hn, Tongxin Zhi,Siting Li, Aijin M,*, Yingmin Ji,*
a School of Food and Health, Beijing Technology and Business University, Beijing 100048, China
b College of Food Science and Biology, Hebei University of Science and Technology, Shijiazhuang 050018, China
Keywords:Antimicrobial lipopeptide Brevilaterin B Antibacterial mechanism Listeria monocytogenes Transcriptomics
A B S T R A C T Listeria monocytogenes is a worrisome food-borne pathogen threatening global food safety. Our previous study proved that lipopeptide brevilaterin B showed eff icient antibacterial activity against L. monocytogenes by interacting with the cell membrane. This research further explored the antibacterial mechanism of brevilaterin B against L. monocytogenes at the sub-minimum inhibition concentration via transcriptomic analysis. Brevilaterin B induced growth inhibition rather than direct membrane lysis in L. monocytogenes at the minimum inhibitory concentration. Transcriptomic analysis showed 1 779 difference expressed genes,including 895 up-regulated and 884 down-regulated genes. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analysis indicated that brevilaterin B influenced multiple pathways of L. monocytogenes,including peptidoglycan biosynthesis, membrane transport (ATP-binding cassette transports, ion transport),cellular metabolism (amino acid and lipid metabolism), ATP synthesis, and activation of the stress response(quorum sensing and bacterial chemotaxis). In conclusion, brevilaterin B affects gene expression related to biosynthesis, transport and stress response pathways on the membrane of L. monocytogenes. The present work provides the f irst transcriptomic assessment of the antibacterial mechanism of lipopeptide brevilaterin B at the gene level.
1. Introduction
Listeria monocytogenesis a concerned food-borne bacteria causing fatal listeriosis diseases. It can grow over a wide temperature range from 2 °C to 45 °C and at a water activity of even 0.90, which makesL. monocytogenesbecome a primary pathogen in ready-to-eat food [1]. Over the last decade, the increasing prevalence of lis teriosis has involved a variety of foods, including dairy products, meat,seafood, and raw vegetables, resulting in food waste and economic loss [2]. In addition, the frequent use of chemical biocides in the food production process has also contributed to the increasing drug resistance inL. monocytogenes,representing a tough challenge for global food and environmental safety [3].Consequently, the eff icient control ofL. monocytogenesis vitally important. And more novel natural antimicrobial agents are urgent to be developed to face the new emerging challenge.
Antimicrobial peptides (AMPs) have received considerable attention for their antimicrobial activity and safety [4]. Recently,
Brevibacillusspp., especiallyBrevibacillus laterosporus, have been
regarded as novelrich sources of AMPs [5]. Many AMPs fromB. Laterosporus, including bogorols, brevilaterins, brevibacillins,brevicidines, relacidines, and laterocidines are cation non-ribosomal lipopeptides [6-8]. The se AMPs exhibit efficient antimicrobial activity against most gram-positive pathogenic bacteria, selective gram-negative bacteria, fungi, and even drug-resistant bacteria methicillin-resistantStaphylococcus aureus(MRSA) [9-12].Therefore, lipopeptides fromB. Laterosporusare regarded as displaying great potential for application in the food industry and agriculture.
Brevilaterins (including brevilaterin A-E) are a group of antimicrobial lipopeptides derived fromB. laterosporusS62-9(CGMCC No. 18629) in our lab. They have a similar structure with bogorols (2-hydroxy-3-methylpentanoicacid-Aba-Val/Met/Leu-Orn-Ile/Val-Val-Val/Ile-Lys-Val-Leu-Lys-Tyr-Leu-valinol) as determined by NMR. Brevilaterins exhibited broad antimicrobial activities against a variety of bacteria and fungiin vitroand were resistant to heat treatment even at 121 °C for 20 min and pH 2-12 [7]. Also, the good antibacterial application in milk, and fungal inhibition on citrus andDendrobium officinalein vivoindicate that brevilaterins has great potential as an alternative antimicrobial agent in the food industry and agriculture [7,13].
Further, to understand the antimicrobial mechanism of brevilaterins, we carried out a serial of physiochemical experimentsin vitroand revealed the membrane interaction of brevilaterin B againstL. monocytogenes[14]. Almost simultaneously, other studies also reported on the antimicrobial mechanism ofB. laterosporusAMPs.For instance, brevibacillin was demonstrated to bind to lipoteichoic acid and then interact with the cytomembrane ofS. aureus[15].Bogorols may create pores in the cell membrane of both gram-positive and gram-negative bacteria [16]. Brevibacillin 2V can bind to Lipid II and increase the cytomembrane permeability of MRSA to exert its bactericidal activity [17]. According to these studies, cytomembrane was proposed to be the primary target ofB. laterosporusAMPs as investigated by biochemical assays. However, the effect ofB. laterosporusAMPs on bacterial physiology has been underexplored. The cyclic lipopeptide relacidine B selectively inhibitedXanthomonas campestrispv.campestrisby binding to the lipopolysaccharides and interfering with the cellular oxidative phosphorylation process and ATP synthesis [16]. Nevertheless, as the primary physiological responses of microorganisms, gene expression at the transcription level of bacteria exposed toB. laterosporusAMPs have not yet been reported. Therefore, it’s necessary to investigate the effects ofB. laterosporusAMPs on bacterial gene expression and elucidate the antimicrobial mechanism underlying the effects.
Transcriptomics is an important approach to explore the bacterial genomic response to antimicrobial agents [18,19]. To obtain deeper insights into the antimicrobial mechanism of brevilaterin B againstL. monocytogenesat the gene level, transcriptomic analysis ofL. monocytogenesin response to brevilaterin B was performed in the present study.
2. Materials and methods
2.1 Antimicrobial peptide preparation
Brevilaterin B was prepared with an HPLC purity of 99.03%using the method described in previous research [7]. The molecule weight was 1 601.004 4 Da determined by MALDI-TOF/TOF MS and the primary structure was determined as 2-hydroxy-3-methylpentanoic acid-Aba-Met-Orn-Ile-Val-Val-Lys-Val-Leu-Lys-Tyr-Leu-valinol by 2D-NMR. The brevilaterin B solutions (10 mmol/L) was prepared in phosphate-buffered saline (PBS, pH 7.4) and stored at -20 °C until use.
2.2 Growth inhibitory assays
Growth inhibitory effect were examined via colony counting assays.L. monocytogenesATCC 19115was incubated in brain heart infusion(BHI) medium at 37 °C for 8 h. Then the bacterial suspension was diluted to OD600nm= 0.3 with medium and inoculated in 50 mL of fresh BHI medium. Brevilaterin B was added at a final concentration of 1 μg/mL (minimum inhibitory concentration (MIC)), which has been determined in the previous research [14]. An equal volume of BHI medium was added as a control. All cultures were incubated at 37 °C and sampled at a specified time. The samples were serially diluted by 10-fold and spread onto BHI agar. Viable cells were counted after 24 h incubation at 37 °C. These experiments were performed 3 times.
2.3 Membrane integrity assay
The membrane integrity ofL. monocytogeneswas determined using a fluorescent dye propidium iodide (PI) as described by Ning et al. with some modifications [20]. In brief,L. monocytogeneswas collected in the logarithmic phase, washed twice, and re-suspended in PBS to a density of approximately 1 × 107CFU/mL.Then, the bacterial suspension was treated with brevilaterin B (final concentration of 1 × MIC and 4 × MIC) for 2 h at 37 °C. The bacterial suspension treated with PBS was used as the negative control. The treated suspension ofL. monocytogeneswas washed once, suspended in PBS and stained with PI (final concentration of 10 μg/mL) in the dark for 20 min. The fluorescence intensity of stained bacterial suspension was finally recorded using a BD Accuri C6-plus flow cytometer(San Jose, CA, USA). The experiments were repeated three times.
2.4 Ultrastructure observation
The ultrastructural changes ofL. monocytogeneswere investigated by transmission electron microscope (TEM). Exponential-phase cells ofL. monocytogenes, was collected, washed twice, and re-suspended in PBS to a density of approximately 1 × 107CFU/mL.Then the bacteria were treated with brevilaterin B at the 1 × MIC and 4 × MIC. A cell suspension treated with PBS was used as the control.All samples were incubated for 2 h at 37 °C. Cells were collected by centrifugation at 8 000 ×gat 4 °C for 5 min. The preparation procedure of TEM samples was the same as our previous research [14].The final bacterial ultrastructure was observed at 80 kV using a JEOL JEM-1400 TEM (Hitachi, Tokyo, Japan).
2.5 ATP level detection
ATP levels were determined using an enhanced ATP assay kit (S0027, Beyotime Biotechnology, Beijing, China) following the manufacturer’s instructions. Exponential-phase cells ofL. monocytogenes, was collected, washed twice, and re-suspended in PBS to a density of approximately 1 × 107CFU/mL. One microlitre of bacterial suspension was exposed to brevilaterin B at 0.5 × MIC,1 × MIC, 2 × MIC, and 4 × MIC at 37 °C for 1 h. Gramicidin and PBS were used as the positive and negative controls, respectively.Treated cells were collected and lysed at 4 °C using 200 μL of ATP lysate. Then, the lysed samples were centrifuged for 5 min at 4 °C.Next, 20 μL of the supernatant were mixed with 100 μL of ATP working fluid in a 96-wells black plate. The relative light unit was detected immediately using a Tecan Spark multi-mode microplate reader (Tecan, M?nnedorf, Switzerland). The experiments were repeated three times.
2.6 Transcriptomic assays
2.6.1 RNA extraction
L. monocytogenes(OD600nm= 0.3) was inoculated to 50 mL of fresh BHI medium by 1% incubated for 4 h at 37 °C. Then,brevilaterin B was added at a final concentration of 0.5 × MIC,whereas an equal volume of PBS was added as the negative control. Each treatment group contained three biological replicates.L. monocytogenescells were then collected via centrifugation at 10 000 ×gfor 10 min after incubation for 60 min at 37 °C. The samples were snap-frozen in liquid nitrogen. The total RNA was extracted using a TRIzol kit (Invitrogen Life Technologies, CA, USA) according to the manufacturer’s instructions. The purity and fragment length of RNA was detected using NanoDrop spectrophotometer (Implen, CA,USA) and Agilent 2100 bioanalyzer (Agilent Technologies, CA,USA), respectively. The integrity of total RNA was determined by gel electrophoresis. Samples with an RNA integrity number exceeding 7.5 were applied in the subsequent analysis.
2.6.2 cDNA library establishment and sequencing
The cDNA library was constructed using NEBNext UltraTMRNA Library Prep Kit (New England Biolabs, MA, USA). Briefly, 3 μg of the qualified mRNA was enriched using a Vazyme ribo-off rRNA depletion kit (Bacteria) (Vazyme Biotech, Nanjing, China), and fragmented using divalent cations. mRNA was reversed-transcribed into first-strand cDNA with random hexamers. Second strand cDNA was synthesized with RNase H, dNTPs and DNA polymerase I. The cDNA was terminal repaired, extended by adding ‘A’ bases, and ligated after purification using AMPure XP beads. The final cDNA library was established after PCR amplification. The library quality was detected by Agilent Bioanalyzer 2100 system, and the effective concentration (> 4 nmol/L) of samples was precisely quantified by quantitative PCR (qPCR). The cDNA library was sequenced on the Illumina Hiseq 4000 high-throughput sequencing platform and 150 bp paired-end reads were generated.
2.6.3 Bioinformatic analysis
Raw reads were firstly filtered through in-house scripts using Trimmomatic v0.33 software [21] to obtain clean reads. These clean reads were mapped to referenced genomes by using Bowtie2 v2.2.6 software [22]. Gene expression was analysed using HTSeq v0.6.0 software (https://pypi.org/project/HTSeq/) and a union model, which calculated fragments per kilobase of per million mapped reads(FPKM > 1). Differential expressed genes (DEGs) was identified using DESeq R package (1.10.1) with the threshold value|log2(Fold change)| > 1 and FDR-adjustedP< 0.05.
Gene Ontology (GO, http://www.geneontology.org/) analysis of DEGs was performed to analyse primary gene and protein function using the GOseq R package. The changes of cellular and metabolism pathways at the transcriptional level were analysed according to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (http://www.kegg.jp). Significant enrichment of KEGG pathways were analysed using KOBAS software.
2.7 Real-time qPCR (RT-qPCR) validation
Several DEGs associated with the key KEGG pathways were verified by RT-qPCR. The methods ofL. monocytogenestreatment and RNA extraction followed those described for transcriptomic assays. cDNA was prepared following the instructions of the PrimeScript? RT reagent Kit with a gDNA Eraser (TaKaRa, Beijing,China). Primers designed using primers 5.0 (Table S1) and SYBR Premix ExTaqII fluorochrome (Tli RNaseH Plus) (TaKaRa, Beijing,China) were applied to quantified gene expression. The final reaction volume of each sample was 20 μL (2× Master Mix, 10 μL; 10 μmol/L of Primer F, 0.5 μL; 10 μmol/L of Primer F 0.5 μL; PCR grade water,7 μL; cDNA, 2 μL). RT-qPCR was performed using the ABI7500 real-time PCR system (Applied Biosystems, CA, USA) using the following protocol: 95 °C for 30 s, 40 cycles of 95 °C for 5 s and 60 °C for 40 s and fluorescence acquisition. After amplification, the melting curve was constructed using the following program: 95 °C for 10 s, 60 °C for 60 s and 95 °C for 15 s, with the temperature ramped from 60 °C to 99 °C at 0.05 °C/s. Both the target genes and internal standard 16S rRNA of each sample were included in PCR. Each sample was tested in 3 replications and the experiment was performed three times. The relative expression of genes was analysed using the 2-ΔΔCtmethod [23].
2.8 Statistical analysis
The results were presented as the mean ± standard deviation (SD).Graphs were constructed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). The significance of differences(P< 0.05) was analysed by one-way analysis of variance using PASW Statistics 18.0 (SPSS Inc., Armonk, NY, USA).
3. Results and discussion
3.1 Inhibition of L. monocytogenes growth by brevilaterin B
Time-dependent kinetic curves were established to study the inhibitory effect of brevilaterin B onL. monocytogenesgrowth using the colony count method.L. monocytogenesuntreated with brevilaterin B showed an ordinary growth during 8 h and kept stable cell density (~109CFU/mL) during 8-24 h. However, brevilaterin B inhibited the growth ofL. monocytogenesin the initial phase and maintained significant growth suppression (P< 0.05) during 4-24 h compared with the control (Fig. 1). Colony counting assays illustrated that brevilaterin B at the 1 × MIC exerted a growth-inhibitory effect rather than a bactericidal effect againstL. monocytogenes.

Fig. 1 Changes in the viability of L. monocytogenes following treatment with brevilaterin B. CK: incubated without brevilaterin B. 1 × MIC: treated with brevilaterin B at the minimum inhibitory concentration. Values are the mean ± SD from three independent repeats.
3.2 Effects of brevilaterin B on membrane integrity
PI can only penetrate the damaged membrane of apoptotic or dead cells and bind to nucleic acid, thus, it was used to detect the membrane integrity via flow cytometry [24]. At a higher brevilaterin B concentration (4 × MIC), the fluorescence intensity of bacteria significantly decreased to 59.13% of the control level (P< 0.05,Fig. 2B), which suggested that brevilaterin B may damage the cell membrane and reduce the number of viable bacteria. However,the fluorescence distribution ofL. monocytogenescells exposed to brevilaterin B (1 × MIC) almost overlapped with that of the control(Fig. 2A) and indicated no significant changes in membrane integrity and the number of live bacteria. It could be concluded that the effects of brevilaterin B on membrane integrity were concentration-dependent.
3.3 Effect of brevilaterin B on the ultrastructure
The cellular ultrastructure ofL. monocytogeneswas observed by TEM, as presented in Fig. 2C. UntreatedL. monocytogenesexhibited a complete cellular structure. The cell walls and membrane ofL. monocytogeneswere distinct, and the cytoplasm was rich and uniform. Following the addition of brevilaterin B (1 × MIC), the cytoplasm ofL. monocytogenesbecame bright, but the cell membrane and wall remained intact. In the presence of 4 × MIC of brevilaterin B,the degree of the damage became more severe, including significant cell shrinkage, broken membrane and light cytoplasm, which indicates that brevilaterin B damaged the cellular membrane and induced the loss of intracellular substance.
Combined with the aforementioned results, it was concluded that the concentration of brevilaterin B affected its antimicrobial performance againstL. monocytogenes. Brevilaterin B exhibited significant membrane-damaged activity at 4 × MIC. The inhibitory action of brevilaterin B at the MIC suggested thatL. monocytogenesitself might activate synthetic and metabolic pathways in response to stress induced by brevilaterin B. Although no membrane lysis was observed via TEM in the presence of brevilaterin B at the 1 × MIC, the obvious reduction of intracellular substances implied the permeability of the membrane was increased (Fig. 2C). It appeared that the induced membrane permeability permitted the leakage of small molecules, such as ions or amino acids. Macromolecular proteins and nucleic acids may not have leaked from cells because PI(M= 668.4 g/mol) could not permeate cell membranes in the presence of brevilaterin B at the 1 × MIC [25,26].
3.4 Transcriptomic analysis
Further, transcriptomic assays were conducted to elucidate the influence of brevilaterin B onL. monocytogenesat the gene level. As illustrated in the volcano plot (Fig. 3A),L. monocytogenestreated with brevilaterin B featured 1 779 DEGs, including 895 up-regulated and 884 down-regulated DEGs. Go analysis revealed that the DEGs were mainly involved in biological processes and molecular function (Fig. 3B). The biological processes included chromosome organization, cellular export, glycosyl compound metabolism, and nucleoside metabolism. The molecular function included anion and small molecules binding, hydrolase activity, nucleotide binding,nuclease activity, ATP/GTP binding and ATPase/GTPase activity,and carbohydrate derivative binding.

Fig. 2 Membrane integrity (A, B) and ultrastructure changes (C) of L. monocytogenes. (A, B) L. monocytogenes treated with brevilaterin B at the 1 × MIC and 4 × MIC. Untreated bacteria severed as the control (CK). The horizontal line separated the whole zone into two sections. Live denotes L. monocytogenes cells that were not stained with PI. Dead denotes L. monocytogenes cells stained with PI. Values are the mean ± SD from 3 trials. **, significant difference (P < 0.01).ns, no significant difference. (C) Solid black arrows denote decreases of cytoplasmic contents. Dotted black arrows denote broken cell walls. Solid red arrows denote plasmolysis. Dotted red arrows denote the mesosome-like structures.
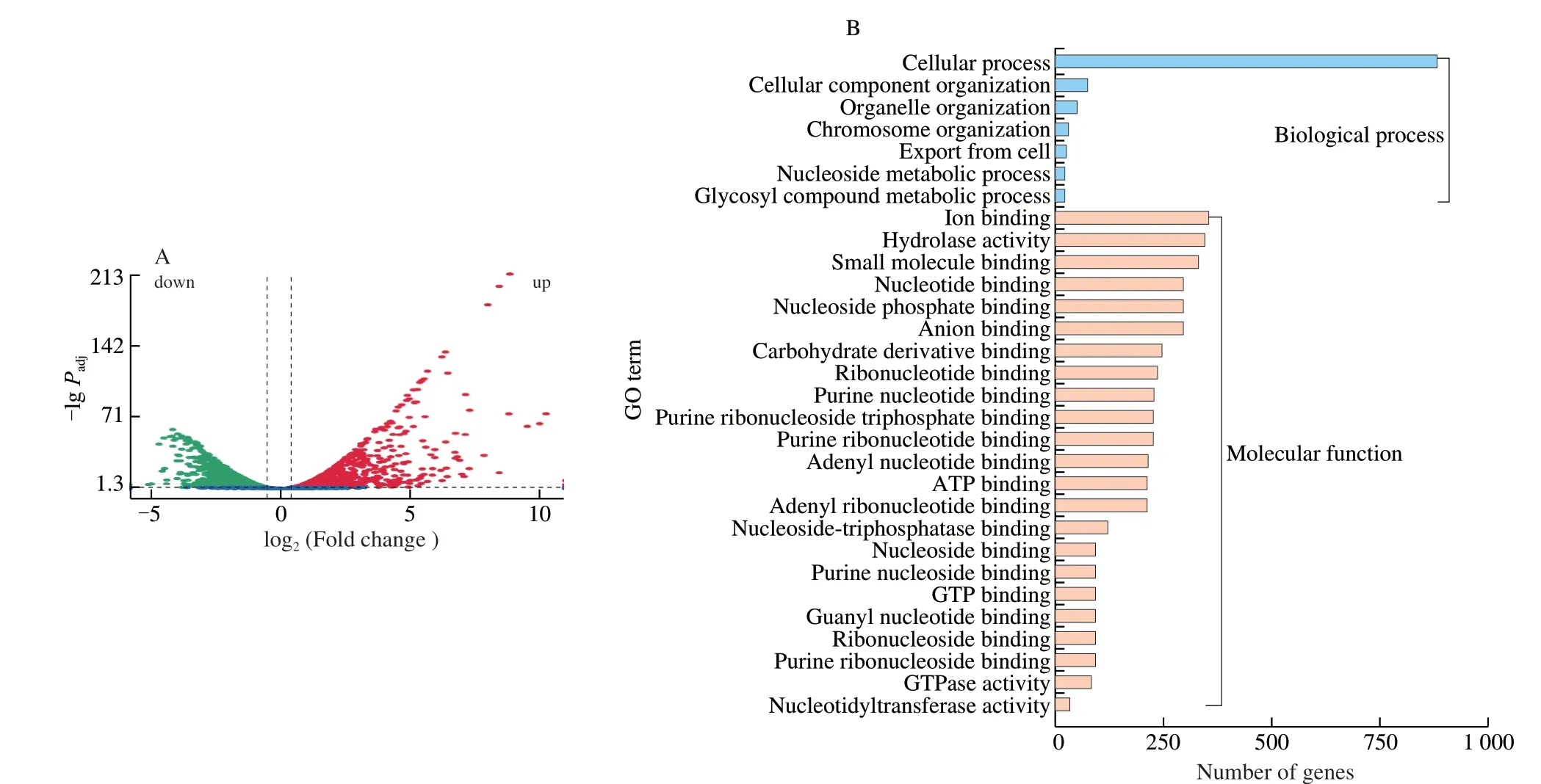
Fig. 3 Volcano plot (A) and Gene Ontology analysis (B) of differential expressed genes. (A) Green dots denote down-regulated genes. Red dots denoteup-regulated genes. (B) Blue columns denote biological processes. Orange columns denote molecular functions.
Brevilaterin B influenced multiple KEGG pathways inL. monocytogenes, including peptidoglycan synthesis, membrane transport (ATP-binding cassette (ABC) transporters, two-component systems), cellular metabolism (amino acid metabolism, lipid metabolism), oxidative phosphorylation (ATP synthesis), quorum sensing, and cellular motility (bacterial chemotaxis, flagellum assembly) etc. (Table 1). Among them, some vital pathways will take place on the cytomembrane, such as the assembly and transport of Lipid II, ATP synthesis, and membrane transport. And several metabolisms may have an indirect relationship with membranes, such as amino acid metabolism and lipid metabolism. Therefore, the results showed that brevilaterin B also have an interaction with the membraneat sub-MIC, which causedL. monocytogenesto exhibit a series of membrane-associated response at transcription level as mapped in Fig. 4. These significant DEGs and associated KEGG pathways were analysed as follows.

Fig. 4 KEGG pathways of L. monocytogenes influenced by brevilaterin B. Up-regulated genes are written in red, and down-regulated genes are written in green.The dashed line represents multiple-step reactions.

Table 1Differentially expressed genes? in the main KEGG pathways.
3.4.1 Effect on cell wall synthesis
Peptidoglycan, the backbone of the cell wall, is regarded as the first barrier against environmental stress in gram-positive bacteria [27]. Peptidoglycan biosynthesis mainly involves the intracellular biosynthesis of repeating units ofN-acetylglucosamine,N-acetylmuramic acid and peptide chains; assembly and transport of Lipid II in the membrane; and cross-linking of peptidoglycan [28].After treatment with brevilaterin B, DEGs includingmurC,murE,murYandmurGwere all down-regulated. These DEGs are involved in the biosynthesis of the precursor Lipid II of peptidoglycan,which mainly took place on the membrane. However, the genes encoded glycosyltransferase, DD-transpeptidase (pbpA-2,LMOATCC19117_2292abbreviated aslmoa2292) and DD-carboxypeptidase (dacA), which are involved in the cross-linking of peptidoglycan, were up-regulated. The RT-qPCR assay further verified thatmurEexpression was decreased by 5.93-folds compared to the control level, whereaslmoa2292expression was increased by 2.42-fold (Fig. 5A).
The results suggested that brevilaterin B may interfere with peptidoglycan biosynthesis by the accumulation of upstream precursors (Lipid I and Lipid II) on the membrane or down-regulation of enzymes involved in the cross-linking process. Inhibition of peptidoglycan biosynthesis is general regard as an important antibacterial mechanism of antibiotics [29]. But recently AMP was also reported similar action. Typically, membrane-active nisin can bind to Lipid II to inhibit the biosynthesis of the cell wall and form stable pores on the membrane [30].
The cell wall of gram-positive bacteria usually contains teichoic acids, which consist of anionic polymers composed of phosphorus polyol [31]. The down-regulation oftagDand teichoic acid ABC transporters (tagGandtagH) indicated that brevilaterin B might inhibit the biosynthesis and transport of teichoic acids. Meanwhile,we found that the cation antimicrobial peptide resistant genes(dltA,dltB,dltCanddltD) ofL. monocytogeneswere all up-regulated.These resistance genes can encode proteins to exportD-alanine,which can modify teichoic acids to reduce the electrostatic adsorption of cation AMPs [32]. Brevilaterin B is a cation peptide containing three positively charged amino acid residues (ornithine and 2 lysine). Therefore, it can be predicted that cationic brevilaterin B may first electrostatically adsorb onto teichoic acids. In response to this electrostatic action,L. monocytogenesattempted to neutralize the negative charges of teichoic acids viaD-alanine esterification [33]. Similar phenomena have been reported for other AMPs, such as polymyxin B, vancomycin, and colistin.L. monocytogenesexhibited resistance to nisin upon theD-alanine esterification of teichoic acids [34].
3.4.2 Effect on membrane transport
The cell membrane plays a vitally important role in bacterial substance transport and physiological metabolism, thereby providing a universal antimicrobial target for AMPs [35,36]. After treatment with brevilaterin B, 43 down-regulated DEGs associated with ABC transporters, mainly glycine betaine/proline transporters(gbuA,gbuBandgbuC), galactose oligomer/malto-oligosaccharide transporters (lmoa2148,lmoa0263,lmoa2147andlmoa0295),cystine/D-methionine transporters (lmoa2346,lmoa2347,lmoa2348andhisP), oligopeptide transporters (oppA,oppB,oppC,oppDandoppF), and teichoic acid transporters (tagGandtagH), were observed.Of these,oppCwas verified by RT-qPCR to be down-regulated by 6.46-fold versus the control (Fig. 5A), which was consistent with the results of transcriptomic analysis. ABC transporters comprise a large transmembrane protein family. They can couple ATP, transport ions, amino acids, and oligosaccharides to the other side of the cell membrane, which is of great significance for bacteria to maintain cellular metabolism [37]. Hence, the down-regulated DEGs of ABC transporters indicated brevilaterin B might inhibit the metabolism of a small molecule on the membrane, including amino acid, oligopeptides,and oligosaccharides. The co-culture of nisin-producingLactococcus lactisDY-13 withL. monocytogenesin milk was found to decrease the expression of the stress-related osmotic genegbu[38].
Meanwhile, 20 up-regulated DEGs encoded ABC transporters,including phosphate (pstB,pstCandpstF), hemolysin (lmoa1008andlmoa1009) and biotin transporters (lmoa2644,cbiO-1andcbiO-2).Of these, phosphate transporters participate in osmotic regulation and phosphate utilization. Under phosphate starvation, the bacterial pstSCAB operon could be activated to improve the transport of inorganic phosphate [39]. The up-regulated ofpstgenes indicated that brevilaterin B influenced the transport and utilization of phosphate inL. monocytogenes.
In addition, thekdpD,kdpE,kdpA,kdpBandkdpCgenes were up-regulated by brevilaterin B in this study.kdpDandkdpEencode KdpD and KdpE, respectively, which form two-component systems to regulate the protein expression of KdpABC in response to K+limitation [40]. Therefore, the up-regulated of these DEGs indicated thatL. monocytogenestriggered the stress response to K+limitation induced by brevilaterin B. Many AMPs were discovered to induce ion leakage by damaging the cell membrane [41]. Gramicidin S and valinomycin induced the formation of new ion channels that selectively carry ions, thereby inducing ionic stress [42,43].
3.4.3 Effect on ATP synthesis
ATPase plays an important role in ATP synthesis and energy metabolism. DEGs encoding F-type ATPase and oxidoreductase were all down-regulated, includingatpA,atpB,atpC,atpD,atpE,atpF,atpG,atpHandppaC. Consistent with the transcription results,RT-qPCR verified the significant down-regulation (P< 0.05) ofatpBby 4.24-fold versus the untreated control (Fig. 5A). As illustrated in Fig. 5B, compared to the untreated group (CK), ATP levels inL. monocytogeneswere significantly decreased by brevilaterin B exposure in a concentration-dependent manner (P< 0.05). The positive control gramicidin decreased ATP levels to a similar extent(33.91%). The down-regulated DEGs and decreased ATP concentration further proved the brevilaterin B inhibited ATPase activity.

Fig. 5 Relative gene expression of L. monocytogenes in response to brevilaterin B as determined by quantitative real-time PCR (A). Green columns denote untreated groups (NaCN-glu). Blue columns denote groups treated with 1 × MIC of brevilaterin B (NaCN). ATP level of L. monocytogenes following brevilaterin B exposure (B). CK: untreated group. GS: the group treated with 1 × MIC of gramicidin solution. Values are the mean ± SD from 3 trials.**, significant difference (P < 0.01). *, significant difference (P < 0.05).
In addition, ATP synthesis is dependent on the proton motive force in the cell membrane of prokaryotes. Our previous findings proved that brevilaterin B induced membrane depolarization [14].Therefore, brevilaterin B might exert a significant inhibitory effect on energy metabolism inL. monocytogenes. Similar findings were also reported in the literature. Gramicidin S and polymyxins disturb membrane integrity, thereby inhibiting cytochrome bd quinol oxidase and malate: quinone oxidoreductase, respectively [44]. The small-molecule peptide MP196 was found to delocalize cytochrome oxidase c in the peripheral membrane, thereby interfering with cell wall biosynthesis and energy production [45]. Membrane-active gold nanoparticle induced similar changes with the decreases of ATPase activity and ATP levels [46].
3.4.4 Effect of brevilaterin B on cellular metabolism
Brevilaterin B influenced multiple amino acid metabolic pathways inL. monocytogenes, including valine/leucine/isoleucine biosynthesis, serine biosynthesis, glycine/threonine biosynthesis,glycerophospholipid metabolism, and fatty acid biosynthesis. In the glycerophospholipid metabolism pathway, glycerone-P can be used to synthesize phosphatidylglycerol.tagD, encoding glycerol-3-phosphate cytidylytransferase, was down-regulated.pgsA, which encodes CDP-diacylglycerol-glycerol-3-phosphate 3-phosphatidyltransferase and further stimulates phosphatidylglycerol production, was significantly down-regulated [47]. Phosphatidylglycerol is a negatively charged component of the positive bacterial membrane.The degradation and conversion of phosphatidylglycerol may have been induced by the up-regulation DEGs (plcB,cls-1andcls-2).Generally, cation AMPs prefer to bind to phosphatidylglycerol,and then damage the cytomembrane based on electrostatic and hydrophobic effect. The up-regulation DEGs suggest thatL. monocytogenesmay intend tochange the lipid composition of membrane, such as reduce the content of phosphatidylglycerol to resist the binding of brevilaterin B to the membrane [48]. This change provided evidence of membrane interaction of brevilaterin B.
3.4.5 Effect of brevilaterin B on bacterial chemotaxis,quorum sensing, and cellular stress response
Bacterial chemotaxis is a sensing and response process to environmental chemical concentrations [49]. In this study, 6 DEGs related to bacterial chemotaxis (cheA,cheY,lmoa0759,fliM,fliN-1,andfliN-2) were significantly up-regulated, whereas 5 DEGs were down-regulated (cheR,cheV,fliG,motAandmotB). However, fingered citron essential oil exposure inL.monocytogenesdown-regulated genes related to bacterial chemotaxis [50]. Chemotaxis genes were up-regulated inEscherichia colitreated with gold nanoparticles [51].These different findings illustrated that gene expression is differentially altered by different treatments. Although different tendencies were observed in this pathway, the results indicated thatL.monocytogenesinfluences the motility ofL.monocytogenesto respond tobrevilaterin B.
DEGs (agrA,agrB,agrC,oppA,oppB,oppC,oppDandoppF) related to quorum sensing were significant down-regulated.L. monocytogenescan release autoinducers to sense cell intensity and facilitate communication between cells via the quorum sensing system. The increased cytoplasm intensity will result in the production of toxin and biofilm, which can protect bacteria and cause pathogenicity [46]. The down-regulation of genes involved in quorum sensing suggested that brevilaterin B might block signal transduction related bacterial communication. Fengycins eliminatedS. aureusby inhibiting quorum sensing, which influences the response of bacteria to changes in population density through altered gene regulation [52].
To resist damage induced by brevilaterin B,L. monocytogenesmay activate a serial of protection pathways as revealed via transcriptomic analysis. It was discovered that the cation antimicrobial peptide resistance genesdltA/B/C/DandliaF/S/Rwere all up-regulated by brevilaterin B. The resistance genesdltA/B/C/Dencode D-alanine, which can modify teichoic acids to decrease the electrostatic adsorption of the cation AMPs polymyxin B,vancomycin, and colistin [53]. TheliaF/S/Rrelated to the LiaFSR system was also significantly up-regulated. The LiaFSR system is a two-component system that controls stress responses to cell membrane-active antibiotics and cationic AMPs, such as bacitracin and vancomycin [54]. It was reported that the lipopeptide daptomycin can attack the bacterial membrane. The LiaFSR pathway of vancomycin-resistantEnterococcican be activated and induce cell membrane rearrangement to resist daptomycin [55]. Therefore, the up-regulation of genes (liaF/S/R) provided evidence that brevilaterin B attacks the cell envelope.
4. Conclusion
In conclusion, brevilaterin B induced growth inhibition rather than direct membrane lysis inL. monocytogenesat the MIC.Transcriptomic analysis identified 1 779 DEGs, including 895 up-regulated and 884 down-regulated genes. GO and KEGG analyses indicated that brevilaterin B influenced multiple membrane-related pathways, including inhibition of peptidoglycan biosynthesis,membrane transport, cellular metabolism, ATP synthesis, and stress response activation at the gene level. These pathways were mainly occurred on bacterial membrane, indicating that brevilaterin B has inhibitedL. monocytogenesby influencing gene expression associated with membrane-associated pathways. This work provided the first evidence of the effects of brevilaterin B againstL. monocytogenesat the gene level and further insights into its membrane-active mechanism at the transcriptomic level. These results will lay a theoretical foundation to promote the development and application of lipopeptide brevilaterin B in the food industry.
Declaration of competing interest
All authors have read and agreed to the published version of the manuscript. The authors declare that they have no conflict of interest.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (31771951, 32072199, 31801510); the Beijing Natural Science Foundation (KZ201810011016).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.037.
- 食品科學(xué)與人類健康(英文)的其它文章
- Call for Papers from Journal of Future Foods
- GUIDE FOR AUTHORS
- Cross-protective effect of acid adaptation on ethanol tolerance in Salmonella Enteritidis
- Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans
- Ameliorative effect of Lacticaseibacillus rhamnosus Fmb14 from Chinese yogurt on hyperuricemia
- Study on the interaction between β-carotene and gut microf lora using an in vitro fermentation model

