Metabolic enzyme inhibitory abilities, in vivo hypoglycemic ability of palmleaf raspberry fruits extracts and identif ication of hypoglycemic compounds
Jun Tan, Danshu Wang, Yu Lu, Yehan Wang, Zongcai Tu, Tao Yuan*, Lu Zhang*
National R&D Center for Freshwater Fish Processing, Engineering Research Center of Freshwater Fish High-Value Utilization of Jiangxi Province,College of Life Science, Jiangxi Normal University, Nanchang, Jiangxi 330022, China
Keywords:Pal mleaf raspberry Extraction and identif ication Enzyme inhibition HPLC-QTOF-MS/MS Hypoglycemic activity
A B S T R A C T The unripe palmleaf raspberry, namely Fupenzi (FPZ), is an important medicinal and edible food. This study aims to evaluate the potential of FPZ extracts prepared with different approaches in attenuating hyperglycemia,gout, Alzheimer’s disease, and pigmentation, to obtain the enriching fraction and to identify the major active compounds. Results indicated that FPZ extracts showed weak activity against acetylcholinesterase,considerable ability against tyrosinase and xanthine oxidase, but excellent inhibition on α-glucosidase.Ultrasound-assisted 40% ethanol extract (40EUS) gave the highest phenolics content, and the best α-glucosidase inhibition (IC50 = 0.08 μg/mL), which is 877-fold higher than that of positive control acarbose. The 40%ethanol eluting fraction of 40EUS showed the strongest α-glucosidase inhibition with the IC50 value of 37.79 ng/mL, it could also effectively attenuate the fasting blood glucose level and oral glucose tolerance of C57BL/6 mice. Twenty-six compounds were identif ied from 40% ethanol fraction by using HPLC-QTOF-MS/MS,hydrolysable tannins (including 11 ellagitannins and 4 gallotannins) were the major compounds, phenolic acids came to the second. Above results could provide important technical supporting for the further application and research of FPZ in health foods and drugs against diabetes.
1. Introduction
Palmleaf raspberry (Rubus ChingiiHu), belonging to family Rosaceae, is a perennial deciduous shrub widely cultivated in East and Southeast of China, especially in Jiangxi, Zhejiang, Jiangsu,Anhui, Fujian provinces. The ripe red-colored fruits can be consumed as fresh fruit or used to produce jams, juices, and preserved food,and so on. The green unripe fruit, namely Fupenzi (FPZ) has been used as traditional Chinese medicine to treat several aliments since ancient times, such as nephropathy, enuresis, impotence, and prospermia, and so on [1]. Accumulating pharmacological researches indicated that extracts or effective ingredients from FPZ exhibited a variety of biological activities, e.g. antioxidant, anti-proliferative,anti-complementary, anti-cancer, hypoglycemic, anti-inflammatory,and anti-hypotensive activities, and so on [2,3]. Flavonoids, phenolic acids, ellagitannins, essential oils, and terpenoids were the major chemical constituents responsible for above health-promoting effects,especially for f lavonoids and ellagitannins. Up to data, more than 230 compounds have been isolated and identif ied from FPZ [2,4].
Diabetes mellitus, gout , pigmentation, and Alzheimer’s disease are well-known chronic diseases widely occur in developed and developing country, which have become global health problems,and caused serious bad effect to human life. There are some of the key enzymes involved in the development and progression of these diseases, such asα-glucosidase, xanthine oxidase, tyrosinase,butyrylcholinesterase, and acetylcholinesterase. Suppressing the activity of these enzymes can impact the mechanisms involving these enzymes that lead to the progression of these diseases [5-7]. However,many clinical enzyme inhibitors like acarbose, allopurinol, kojic acid,and galantamine exhibit undesirable gastrointestinal problems and toxic effects [8,9]. Thus, searching for the inhibitors from natural sources is a good choice as their low toxicity. Polyphenols derived from medical plants and foods are promising inhibitors of various metabolic enzymes due to their less or negligible side effects. Of late, increasing attentions are paid to screen and identify polyphenols containing promising biological activities from plant resources [10,11].
The recovery efficiency of active constituents from plant materials depends on the extraction methods to a larger extent, and the optimal method for different materials varied due to the distinction in materials’ properties and chemical composition [12,13]. It is important to develop an efficient method for extracting active constituents from a given material. FPZ is a popular traditional Chinese medicine with various pharmacological activities, but no study analyzes the influence of solvents in various polarities on the hypoglycemic,anti-gout, anti-Alzheimer’s disease, and skin whitening capacity of FPZ extracts. The appropriate extraction and enriching methods for active constituents have not been investigated neither.
The purpose of current research was to evaluate the potential of FPZ in attenuating hyperglycemia, gout, Alzheimer’s disease,and pigmentation, and to obtain the appropriate extraction and enriching approach. Bioactive constituents were extracted via ultrasound-assisted extraction and maceration, respectively, with various concentrations of ethanol and methanol (20%, 40%, 60%,80%, and 100%) as solvents. The yield and extraction efficiency on phenolics and flavonoids were determined, the hypoglycemic,anti-gout, anti-Alzheimer’s disease, and skin whitening activities potential of extracts were evaluated throughα-glucosidase, xanthine oxidase, acetylcholinesterase, and tyrosinase inhibitory ability assay,respectively. The sample giving the highest bio-activity was then fractionated with macroporous resin to yield enriching fraction, which was further subjected to chemical profiling characterization by using high-performance liquid chromatography-quadrupole time-of-flightmass spectrometry (HPLC-QTOF-MS/MS), thein vivopostprandial hypoglycemic activity was also evaluated through C57BL/6 mice.
2. Materials and methods
2.1 Chemicals
Dried unripe FPZ were purchased from Dexing Town (grown locally), Jiangxi Province, China, on Sep. 2017, and identified by Prof.Lan Cao from Jiangxi University of Traditional Chinese Medicine,who engaged in the research of traditional Chinese medicine. They were pulverized with YF8-1 multifunction pulverizer (Ruian Yongli Pharmaceutical Machinery Co. Ltd., Ruian, China), placed in self-sealing bags, and stored at 4 °C. Methanol and formic acid were HPLC grade and from Merck (Waters, USA). Tyrosinase from mushroom (EC 1.14.18.1), acetylcholinesterase (AChE) from electric eel (EC 3.1.1.7),α-glucosidase fromSaccharomyces cerevisiae(EC 3.2.1.20), xanthine oxidase from bovine milk (EC 1.17.3.2),acetylthiocholine iodide, 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB),3,4-dihydroxy-D-phenylalanine (L-DOPA),p-nitrophenyl-α-Dglucopyranoside (pNPG), xanthine, allopurinol, galantamine, kojic acid, 1,1-dipheny-l,2-picrylhydrazyl (DPPH), and 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) were bought from Sigma-Aldrich (St. Louis, MO, USA). C57BL/6 male mice used in this research (8 weeks old) was purchased from Charles River(Beijing, China). Ultra-pure water was prepared with Millipore water purification system. All other reagents were analytical grade and bought from Solarbio Chemical Co. (Shanghai, China).
2.2 Extraction with different solvents by ultrasound and maceration
Dried FPZ powders were dispersed into 20%-100% methanol and 20%-100% ethanol aqueous solution, respectively, at a solid/liquid ratio of 1:10 (m/V). For ultrasound -assisted extraction, the mixtures were treated at 400 W, 50 °C for 1 h with a KH-500DE ultrasonic cleaners(Hechuang Ultrasonic Equipment Co., KunShan, China). In case of maceration, the mixtures were shaken in a temperature-controlled shaker at 50 r/min, 25 °C for 24 h. After centrifugation, the supernatants were collected, the residues were re-extracted under the same condition for another time. Subsequently, the supernatants were combined, filtered and evaporated to obtain dry extracts. The percentage yield of each solvent was calculated as follows:

where,m1is the weight of dry round-bottom flask and extract,m2is the weight of dry round-bottom flask,Mis the weight of dry FPZ powder.
To simplify the presentation of all samples, 20-100MUS and 20-100EUS refer to the extracts prepared by ultrasound-assisted extraction with methanol and ethanol solution as solvent, respectively.20-100MMT and 20-100EMT refer to the extracts prepared by maceration with methanol and ethanol solution as solvent,respectively. The data before the abbreviation indicates the concentration of solvent. All extracts were dissolved in corresponding extraction solvent for further analysis.
2.3 Determination of total phenolic and total flavonoid contents
The total phenolic content was determined with Folin-Ciocalteu’s method as previously described [14]. The results were expressed in milligram gallic acid equivalent per gram of extract (mg GAE/g)through the calibration curve (y= 0.006 7x+ 0.157 8,R2= 0.998 9)plotted with gallic acid as reference standard.
The total flavonoid content was measured according to the AlCl3-NaOH-NaNO3method reported by Muniyandi et al. [15] with minor modifications. Five hundred microliter of samples at proper concentration were mixed with 0.1 mL of 5% NaNO2, 6 min later,0.1 mL of 10% AlCl3was added and allowed to react for another 6 min. Finally, 1.0 mL of 4% NaOH and 1.0 mL distilled water were mixed and shaken thoroughly. After 15 min of incubation at 25 °C, 200 μL of reaction solution was pipetted into 96-well micro-plate, and absorbance at 510 nm was recorded with a Synergy H1 Multi-mode microplate reader (BioTek, USA). The system prepared with all reagents excepted for 10% AlCl3was used as blank group. Quercetin was used as standard solution to plot the calibration curve (y= 0.938 6x- 0.015 9,R2= 0.998 4), results were expressed in milligram quercetin equivalent per gram of extract (mg Que/g).
2.4 Evaluation of enzymes inhibitory abilities
The hypoglycemic activity was measured byα-glucosidase inhibitory ability assay with acarbose as positive control [16]. The anti-hyperuricemia capacity was determined through xanthine oxidase inhibitory ability assay according to previous published procedures [17], allopurinol was used as positive control. The acetylcholinesterase inhibitory assay was employed to determine the potential against Alzheimer’s disease [18] with galanthamine as positive control. The skin whitening ability was assayed through tyrosinase inhibition based on the procedures descried by Zengin et al. [19], kojic acid was taken as reference standard. All assays were carried out in triplicate, the results were expressed as IC50value, which is the concentration required to inhibit 50% of enzymatic activity.
2.5 Enriching of bio-active constituents
To enrich the bio-active constituents present in FPZ, FPZ powders (3.0 kg) were extracted by ultrasound-assisted extraction following the procedures described above, 40% ethanol was used as extraction solvent. Finally, all supernatants were evaporated to yield 456.7 g dry extract with a RV10 rotary evaporator (IKA, Germany).Subsequently, the extract was dissolved in deionized water, and loaded onto a AB-8 chromatography column overnight, following by eluting with 0%, 10%, 40%, 60%, 80% and 95% ethanol in sequence to obtain 6 fractions. All fractions were further evaporated and stored at -20 °C for further analyze.
2.6 HPLC-QTOF-MS/MS analysis
The fraction gave the strongestα-glucosidase inhibition was subjected to chemical profiling identification with an Exion LC AD HPLC coupled to a Triple TOF?6600 high resolution tandem mass spectrometry (HPLC-QTOF-MS/MS). The compounds were separated on a GL inertsil ODS-3 column (4.6 mm × 250 mm, 5 μm,GL Sciences, Japan) at a flow rate of 0.75 mL/min. Mobile phase A and B consisted of 0.1% formic acid and methanol respectively.The gradient elution program was: 0 min, 10% B; 30 min, 50% B;31 min, 100% B, 40 min, 100% B. The injection volume was 5 μL,the column temperature was 29 °C.
The MS and MS/MS data were acquired under negative ion mode in the scan range of 50-1 200 Da. The major parameters were: Electrospray ionization (ESI) source, spray voltage,-4.5 kV; atomization temperature, 550 °C; curtain gas pressure,170.64 kPa; sheath gas and auxiliary gas pressure, 350.25 kPa; sheath and auxillary gas, nitrogen; collision energy, 35 eV. The MS and MS/MS data were processed using software PeakView 1.2.
2.7 Postprandial hypoglycemic assays
The mice assay was carried out on Nanchang University,C57BL/6 mice were housed in animal room at 21-25 °C with a 12 h light/dark cycle. After 7 days of acclimation free access to water and food, they were randomly divided into 4 group (n= 8 for each group): control group (sucrose group), positive group (sucrose +5 mg acarbose/(kg·day)), high dose FPZ group (sucrose + 60 mg fraction/(kg·day), FPZ-H), and low dose FPZ group (sucrose + 30 mg fraction/(kg·day), FPZ-L). The 40% ethanol fraction (10 mg/mL) and sucrose solution (15 mg/mL) was prepared with 40% 12-propanediol and double-distilled water, respectively. The control group was gavaged with equal volume of 40% 1,2-propanediol (vehicle). After 7 days of gavage, the mouse was fasted overnight individually(12-14 h), and the glucose level was measured with portable glucometer (Bioland Technology, Shenzhen, China). Then mice were gavaged with vehicle, acarbose (5 mg/kg body weight), high,and low dose of FPZ fraction. Thirty minutes later, gavaged the mice with sucrose solution at a dose of 3 g/kg body weight (gavage volume: 20 times body weight, with the unit of μL), the blood glucose level was determined at times 30, 60, 90, and 120 min after sucrose loading. The protocol strictly complied with the national guidelines for Experimental Animal Welfare (MOST of China, 2006), and were approved by the Institutional Animal Care and Use Committee of
Nanchang University (SYXK (Gan) 2018-0021).
2.8 Statistical analysis
The assays for extraction, content measurement, and bio-active evaluation were performed in triplicate. The results were represented as means ± standard deviation, one-way analysis of variance followed by Tukey’s test was carried out by SPSS version 13.0 to assess the significant differences (P< 0.05) among data.
3. Results and discussion
3.1 Variation in the yield of extracts
In the present study, different concentrations of ethanol and methanol aqueous solutions were chosen as extraction solvent due to the fact that aqueous-organic mixture solvents are usually more effective in recovering polyphenols from plant materials than absolute organic solvents [13,20], and they are more environmental friendly as compared with acetone, ethyl acetate, chloroform andn-hexane.As shown in Table 1, the yield of dry extract varied from 23.00%to 8.10%, the value decreased with increased ethanol and methanol concentration when the concentration was over 60%. 20EUS and 40EUS displayed the highest yield, with the individual value of 23.00% and 22.60% (P> 0.05), which is about 2.5-2.8 fold of the lowest yield found in 100% ethanol extract. The better yield obtained through 20%-40% solvent than 80%-100% solvent could be that solvent at strong polarity promoted the swelling and permeability of cell tissue, leading to better molecular diffusion, and more substances at high polarity such as phenolic acid, sugar and protein were recovered [12,13]. In addition, excepted for 100% ethanol, 20%and 40% methanol extracts, ultrasound-assisted extraction presented superior recovery efficacy than maceration (P< 0.05). This could be resulted from the cavitation phenomena and implosion of cavitation bubbles generated by ultrasound, subsequent disruption of plant cell walls and penetration of more solvents accelerated the solubility and mass transfer of inner substances, leading to improved extraction yield [21]. Higher extraction efficiency of ultrasound than maceration on polyphenols from other plant materials has also been observed by many researchers [22,23].
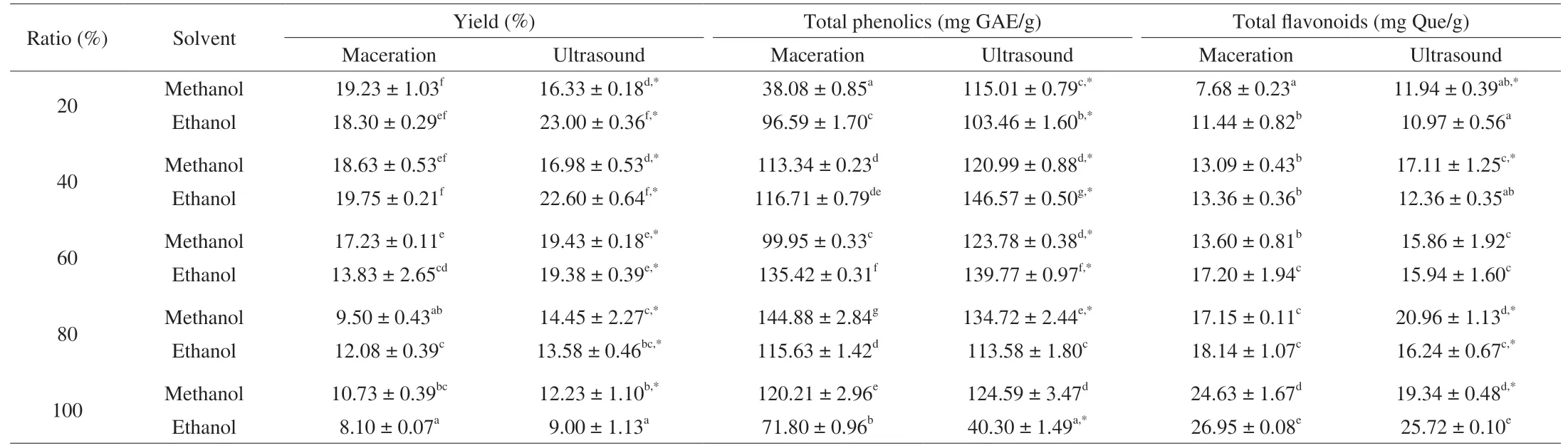
Table 1Influence of solvent polarity on extraction yield and the extraction efficacy of phenolics and flavonoids from palmleaf raspberry fruits.
3.2 Content of total phenolics and total flavonoids
The content of phenolics and flavonoids in extracts prepared with different solvents and methods were displayed in Table 1, it is clear that both extraction solvent and method affect the recovery of phenolics and flavonoids greatly. The total phenolic and total flavonoid content varied from 146.57 mg GAE/g to 38.08 mg GAE/g and from 26.95 mg Que/g to 7.68 mg Que/g, respectively. The highest total phenolic content was found in 40EUS and 80MMT with the corresponding content of 146.57 and 144.88 mg GAE/g (P> 0.05), following by 60EUS(139.77 mg GAE/g). However, the highest total flavonoid content was detected in 100EMT and 100EUS (25.72-26.95 mg Que/g,P> 0.05), 20 MMT possessed the lowest value (7.68 mg Que/g). In case of maceration, the content of flavonoids increased with improved ethanol and methanol concentration. This demonstrates that the flavonoids present in FPZ are more soluble in high concentration of ethanol/methanol, while the phenolics are prone to middle polar solvents (40%, 60%, 80%). Taken the yield into account, the yield of 40EUS is 2.5-2.8 fold of that of 100EMT and 100EUS, but the total flavonoid content in 100EMT and 100EUS is only about 2 fold of that of 40EUS. The 40EUS and 80MMT possessed similar total phenolic content, but the yield of 40EUS is 2.4 fold of that of 80MMT.Therefore, to sum up, ultrasound-assisted extraction with 40% ethanol as solvent is the appropriate protocol for recovering the phenolics and flavonoids present in FPZ.
3.3 Inhibitory activity on α-glucosidase, acetylcholinesterase,tyrosinase, and xanthine oxidase
To get an insight into the application potential of FPZ extracts against multiple commonly chronic diseases, and the influence of solvents polarity, theα-glucosidase, tyrosinase, acetylcholinesterase,and xanthine oxidase inhibitory abilities of samples were evaluated.α-Glucosidase is responsible for the final step of carbohydrates hydrolysis by catalyzing oligosaccharide into absorbable monosaccharide, leading to increased postprandial glucose levels [14].Acetylcholinesterase catalyzes the hydrolysis of acetylcholine,high level of activity will reduce acetylcholine levels in the synaptic area and even terminate nerve impulses [5]. Tyrosinase plays an vital role in catalyzing the hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine and finally to dopaquinone, its inhibitors can hinder melanogenic pathways and prevent overproduction of melanin in the skin, slowing down the occurrence of many skin disorders (hyperpigmentation, melanoma, etc.) [24]. Xanthine oxidase plays an vital role in the conversion of hypoxanthine to xanthine and xanthine to uric acid, its inhibitors can prevent the formation of uric acid, subsequently alleviate the prevalence of hypeluricemia and gout [25]. Thereby, inhibitors ofα-glucosidase, acetylcholinesterase,tyrosinase, and xanthine oxidase is considered as effective strategy for the management of hyperglycaemia, Alzheimer’s disease,hyperpigmentation and hypeluricemia, respectively. These 4 enzymes are also the most common targets widely used to screen drugs against related diseases.
Theα-glucosidase, acetylcholinesterase, tyrosinase, and xanthine oxidase inhibition of FPZ extracts were depicted in Fig. 1.All extracts had inhibitory ability on tested enzymes, the most noteworthy inhibitory ability was found onα-glucosidase with the IC50values ranged from 0.08 μg/mL to 1.49 μg/mL, which is much lower than that of positive control acarbose (70.20 μg/mL) (Fig. 1A),demonstrating excellentα-glucosidase inhibition of FPZ extracts. In addition, 40EUS gave the strongest inhibition, followed by 40MUS and 80MUS, 20EMT exhibited the weakest ability. This could be due to their relative higher total phenolic content, especially for ellagitannins. Currently, many ellagitannins were reported to show strong suppression on the activity ofα-glucosidase [26,27], and ellagitannins were also found to be the major components responsible for theα-glucosidase inhibition of FPZ extract [4]. In case of samples prepared with 20%-80% ethanol and methanol, ultrasound extracts always showed superiorα-glucosidase inhibition than corresponding maceration extracts. Combing the extraction yield (Table 1), 40%ethanol combined with ultrasound extraction was considered to be suitable for extractingα-glucosidase inhibitors from FPZ.
As shown in Fig. 1B, significant differences in acetylcholinesterase inhibition were observed among samples prepared with different solvents and methods. 80MUS and 100MUS gave the strongest ability against acetylcholinesterase with the lowest IC50values at 44.22-47.86 μg/mL, but it was much higher than that of its positive control galantamine (IC50= 0.19 μg/mL). The weakest inhibition was found on 40MMT, and the IC50value (282.04 μg/mL) was roughly 6 folds of that of 80MUS. This could be resulted from the relatively low acetylcholinesterase inhibition of chemical components present in FPZ extract. Notable memory-enhancing effects of water extract and ethyl acetate extract of FPZ have been reported by Huang et al. [28],but no data about the acetylcholinesterase inhibition of FPZ extract is available before this.

Fig. 1 Effect of extraction solvents and methods on the inhibition of FPZ extracts on (A) α-glucosidase, (B) acetylcholinesterase, (C) tyrosinase and (D) xanthine oxidase.
Fig. 1C reveals that the IC50values declined with increasing ethanol or methanol concentration, 100EMT showed the lowest IC50(17.23 μg/mL), which suggested considerable tyrosinase inhibition of FPZ extract even through it is a little higher than positive control kojic acid (IC50value of 2.26 μg/mL). Relative weak tyrosinase inhibitory ability was also found by Hsieh et al. [29],who reported that the percentage inhibition was 34.6% for 70%ethanol extract when the ratio of extract/enzyme was at 2:1. For xanthine oxidase, considerable inhibition potential was only detected on 20%-100% ethanol extracts, the IC50values ranged from 71.85 μg/mL to 176.85 μg/mL. In addition, ethanol extracts showed significantly higher inhibition than the methanol extracts recovered under the same conditions, the best inhibition was found on 80EMT.To the best of our knowledge, this is the first report on the xanthine oxidase inhibition of FPZ. Unexpectedly, superior enzyme inhibition of ultrasound extracts than macerated extracts were only observed onα-glucosidase and acetylcholinesterase when 40%-80% ethanol or methanol was used, demonstrating that ultrasound treatment could not promote the recovery of xanthine oxidase and tyrosinase inhibitors from FPZ. In addition, for acetylcholinesterase and tyrosinase,the inhibition was enhanced with increasing ethanol/methanol concentration, but no obvious trend was found onα-glucosidase and xanthine oxidase. Above data demonstrated that the optimal extraction methods for different bioactive ingredients varied greatly.
3.4 Fractionation of 40EUS and α-glucosidase inhibition
The 40EUS was selected for further separation andα-glucosidase inhibition evaluation due to its highest extraction yield and strongestα-glucosidase inhibition among tested samples. After being fractionated with AB-8 macroporous resin, 6 eluting fractions were yielded, including water (156.19 g), 10% (17.87 g), 40% (67.97 g),60% (39 g), 80% (4.05 g) and 95% ethanol (1.3 g) fractions, theirα-glucosidase inhibitory ability are shown in Fig. 2. 40% ethanol eluting fraction presented the strongestα-glucosidase inhibition (IC50value of 37.79 ng/mL), which is about 2.12 and 1 859-fold of that of 40EUS and acarbose, respectively, 60% ethanol fraction come to the second (76.94 ng/mL), revealing significantly enriching effect of AB-8 macroporous resin. But no obvious inhibition was found on water fraction (IC50> 0.2 mg/mL). Therefore, the phytochemical profiling of 40% ethanol eluting fraction was further identified with HPLC-QTOF-MS/MS to analyze the major bioactive components.

Fig. 2 α-Glucosidase inhibitory ability of 40EUS and its fractions. Different letters above the column indicate significant difference among data (P < 0.05).
3.5 Chemical profiling identification by HPLC-QTOF-MS/MS
The 40% ethanol fraction of 40EUS was selected for further phytochemical profiling identification due to the strongestα-glucosidase inhibition, the base peak chromatogram recorded under negative ion mode was shown in Fig. 3. The MS and MS/MS data were processed with PeakView, structure characterization was carried out by matching the obtained MS and MS/MS data with that reported in references and that documented in database Scifinder and Metlin. Twenty-six compounds were identified or tentatively identified, including 11 ellagitannins, 4 gallotannins, 7 phenolic acids and 4 flavonoids, Table 2 summarized the mass information used for structural identification, including retention time, accurate precursor ion, molecular formula, fragment ions, proposed name and references.

Table 2The retention time (RT), precursor ion, formula, fragment ions, proposed name and references of major compounds in the 40% fraction of 40EUS obtained via HPLC-QTOF-MS/MS under negative mode.

Fig. 3 Base peak chromatogram of 40% ethanol fraction of 40EUS under negative ion mode.
3.5.1 Hydrolysable tannins
Hydrolysable tannins were one class of the major active compounds present in 40% ethanol eluting fraction. The tentative structure can be proposed by taking into account the accurate molecular weight of precursor ion and fragment ions, along with their characteristic fragmentation pattern. Commonly, the diagnostic fragment ions of hydrolysable tannins under negative mode were:m/z151 and 169 for gallic acid or galloyl derivatives,m/z301 for ellagic acid or hexahydroxydiphenoyl (HHDP) group,m/z463 indicated the HHDP-glucose moiety,m/zat 331 for galloyl-glucose moiety. In addition, characteristic neutral loss of glucosyl group(162 Da), pentosyl (132 Da), glucuronyl (176 Da), galloyl group (152 Da),gallic acid (170 Da), HHDP (302 Da) and galloyl-glucose (332 Da),etc. can also been detected [30].
Totally, 15 hydrolyzable tannins were deduced, including 11 ellagitannins (4 pedunculagin/casuariin isomer, 4 ellagic acid derivatives, ellagic acid, casuarinin, and casuarictin) and 4 gallotannins (galloyl-HHDP-glucose). Peaks 1, 3, 8 and 12 were tentatively identified as pedunculagin/casuariin isomer [31] based on the same precursor ion [M-H]-atm/z783.04 (C34H24O22) and characteristic fragment ions atm/z631.05 [M-H-galloyl]-andm/z301.00 [HHDP-H]-.Pedunculagin and casuariin have previously been described in FPZ [3].Peaks 2, 4, 5, and 7 presented the same parent ion [M-H]-atm/z633.05,characteristic MS/MS ions ofm/z463.04 [M-H-170]-andm/z301.00[M-H-332]-indicated the existence of a galloyl, a HHDP and a glucose moiety. They were thus assigned as galloyl-HHDP-glucose [30].
Compared with peaks 1 and 5, the precursor ion of peaks 10 and 11 ([M-H]-atm/z935.04, C41H28O26) showed am/zshift difference of 302 (C14H6O8), which corresponds to the HHDP group. Base peak fragments atm/z633.054 3 [M-H-HHDP]-andm/z300.993 [HHDP-H]-allowed the identification of casuarinin and casuarictin [31], respectively, which have been identified in FPZ [3].Peaks 23 (m/z477.013 4, C20H14O14), 25 (m/z433.040 8, C19H14O12)and 26 (m/z300.988 8, C14H6O8) were proposed as ellagic acid glucuronide, ellagic acid pentoside and ellagic acid, respectively,according to their accurate parent ions and fragmentation pattern [27,32].Peaks 13 and 14 with molecular ion ofm/z467.046 1 andm/z551.020 3were proposed as ellagic acid derivatives due to the characterized MS/MS ions atm/z300.995 1 [ellagic acid-H]-andm/z169.012 8[gallic acid-H]-.
3.5.2 PhenolicsPeak 15 with [M-H]-ion atm/z491.121 9 (C26H20O10) was identified as salvianolic acid C due to diagnostic fragments atm/z445.124 4 [M-H-HCOOH]-,m/z293.085 9 [C17H10O5-H]-,m/z151.041 2 [C8H8O3-H]-andm/z149.043 6 [C8H6O3-H]-[33].It is reported that salvianolic acid C can bind withα-glucosidase through hydrophobic interaction and hydrogen bonds, and inhibits its activity in a mixed-competitive manner with IC50of 3.03 μmol/L [34].Peaks 17 and 22 yielded parent ion atm/z297.051 9 (C13H14O8) andm/z281.056 9 (C13H14O7) was proposed as caffeoylthreonic acid and coumaroylthreonic acid, respectively. Fragment ions atm/z179.036 7andm/z163.039 2resulting from the cleavage of threonic acid (m/z135.029 7) corresponded to the deprotonated caffeic acid and coumalic acid accordingly [35]. These two compounds have been detected inRubusidaeusL. andR.fruticosusL. [32].
Peak 18 produced precursor ion atm/z291.005 4 (C13H8O8),diagnosed MS/MS ions atm/z247.023 2 [M-CO2-H]-,m/z219.029 7[M-CO2-CO-H]-andm/z191.034 1 [M-CO2-2CO-H]-allowed to the assignment of brevifolin carboxylic acid [36], which has been reported from FPZ by Chai et al. [37]. Peak 20 gave parent ion atm/z197.039 5([M-H]-, C9H10O5), characterized MS/MS ions ofm/z169.014 3 andm/z125.024 5 were resulted from the consecutive loss of CH2CH2and H2O, it was assigned as ethyl gallate by matching the fragmentation pattern with the known compounds in FPZ [2]. In terms of peak 21 ([M-H]-,m/z247.017 2), fragment ions ofm/z219.029 3,m/z191.039 5,m/z173.024 1,m/z145.029 7 were produced from the successive neutral loss of CO, CH2CH2, H2O and CO, it was then identified as Rubusin A. By the same way, peak 24 ([M-H]-,m/z475.165 0) were proposed as Darendoside B [33], MS/MS ions atm/z429.172 1,m/z339.048 4,m/z325.121 7,m/z265.095 1, andm/z235.075 4 corresponds to the successive loss of C2H4O, 3CH2O, CH2, 2CH2O,and CH2O, respectively, indicating the presence of one acetyl and two monosaccharide unit.
3.5.3 Flavonoids
Only 4 flavonoids were identified from the 40% ethanol fraction of FPZ. Peaks 6 (m/z577.112 3, C30H26O12) and 9 (m/z289.061 9,C15H14O6) was tentatively identified as procyanidin dimmer and epicatechin/catechin, respectively, by matching the MS and MS/MS data with that reported in references [33], they are well-known active constituents of herbs, vegetables and fruits. Peak 16 gave a parent ionm/zat 449.093 1 (C21H22O11), characterized aglycone ion atm/z287.052 6 [M-H-C6H10O5]-suggested the linkage of a hexose.MS/MS ions atm/z269.043 9 andm/z259.059 8 resulted from the dehydration and decarboxylation of aglycone ion, corresponding to the aglycone of eriodictyol or aromadendrin [38,39], but it was finally proposed as aromadendrin-O-hexoside since only aromadendrin has been found in FPZ [2].
Peak 19 was proposed as apigenin-rhamnoside due to the same parent ion atm/z415.158 5 and fragmentation pattern with that reported in reference [33]. Aglycone ion atm/z269.101 9was yielded from the elimination of rhamnosyl (146 Da). Combing with the ion ofm/z161.044 2it was allowed to the assignment of apigenin or emodin [40]. However, apigenin has been found in the leaves ofR. idaeusL, andR. fruticosusL. [32], and apigenin glycosides has also been reported inR. ulmifoliusSchott fruit [41], but no emodin was described inRubusspecies. Thus,emodin-rhamnoside was excluded.
3.6 In vivo postprandial hypoglycemia activity
To confirm thein vivopostprandial hypoglycemia activity of the 40% ethanol eluting fraction of 40EUS, the animal experiments were performed through C57BL/6 mice. As shown in Fig. 4. after 7 days of gavage, there was a significant (P< 0.05) reduction in the fasting blood glucose (FBG) level in group treated with FPZ fraction, the value for FPZ-L and FPZ-H group is 4.47 and 4.43 mmol/L, respectively,which is much lower than that of control group (7.32 mmol/L) and acarbose group (6.47 mmol/L). The alleviation on FBG level detected in this research was much better than that reported by Xie et al. [42],who gavaged mice at a low, middle, and high dose of 0.5, 1.0, and 2.0 g extract/(kg·day), after 4 weeks of treatment, the FBG level was only decreased by 10.7%, 20.2%, and 19.8%, respectively. While, in this research, the gavaged doses were 30 and 60 mg fraction/(kg·day),indicating the positive efficacy of extraction optimization and enrich on hypoglycemic components. After 30 min of sucrose loading, the postprandial blood glucose level was 9.95, 6.7, 6.3, and 8.1 mmol/L, respectively, for control group, FPZ-L, FPZ-H, and acarbose group, in addition, the area under curve of FPZ-H and FPZ-L group were also much lower than control and acarbose group (Fig. 4B), indicating superior efficacy of 40% ethanol eluting fraction on improving oral glucose tolerance than acarbose (5 mg/kg).Therefore, it can be concluded that the 40% ethanol eluting fraction of 40EUS exhibited promising amelioration on FBG by inhibitingα-glucosidase, and could increase oral glucose tolerance.Hydrolyzable tannins, especially for ellagitannins might be the major contributor since they were detected to be the dominant compounds (Table 2). In addition, hydrolyzable tannins have been reported to be the major anti-diabetic components of many plants,such as pomegranate flower [26] andAcer palmatumleaves [27].

Fig. 4 Effects of 40% ethanol eluting fraction of FPZ on blood glucose level of C57BL/6 mice subjected to an oral glucose tolerance test. (A) glucose content,(B) area under the curve (AUC) for postprandial glucose content. The significant difference was analyzed by comparing the value of FPZ-H, FPZ-L and acarbose group with that of control group. * indicated significant difference between control group and FPZ/FPZ-H/acarbose group.
4. Conclusions
This research firstly compared the metabolic enzyme inhibitory activity of FPZ extracts recovered through various methods and solvents. The yield of bio-active constituents and bioactivity of extracts varied greatly depend on the extraction solvents and methods used. Ultrasound-assisted extraction with 40% ethanol is the proper approach for recovering the phenolics in FPZ, while, ultrasound with 80% methanol is more suitable for flavonoids. All samples extracted with 40%-100% methanol and ethanol gave excellentα-glucosidase inhibition, considerable tyrosinase and xanthine oxidase inhibition, and weak acetylcholinesterase inhibition. The strongest activity was found on 40EUS, 100EMT, 80EMT and 80MUS,respectively, with individual IC50value of 0.08, 17.23, 71.85 and 44.22 μg/mL. Solvent at low polarity showed better extraction efficacy on acetylcholinesterase and tyrosinase inhibitors, US showed better recovery efficacy onα-glucosidase inhibitors than maceration. The 40% ethanol eluting fraction of 40EUS displayed excellentin vitroandin vivohypoglycemic activity, and the activity was much stronger than clinical hypoglycemic agent acarbose.Twenty-six compounds were identified or tentatively identified from the eluting fraction, including 15 ellagitannins, 7 phenolic acids and 4 flavonoids, hydrolysable tannins were the dominant hypoglycemic constituents. Overall, the 40% ethanol eluting fraction of 40EUS is promising in preventing and treating of diabetes through attenuating the postprandial blood glucose level. But the precise structure andα-glucosidase inhibitory activity of tentatively identified compounds,as well as the inhibition behavior and mechanism need further study.
Conflict of interest
The authors declare there is no conflict of interest.
Acknowledgement
The authors gratefully acknowledge the financial support of National Natural Science Foundation of China (31860475), Key Youth Foundation of Jiangxi Province (20192ACB21011) and Jiangxi“Shuangqian” Program (JXSQ2018101008).
- 食品科學(xué)與人類健康(英文)的其它文章
- Call for Papers from Journal of Future Foods
- GUIDE FOR AUTHORS
- Cross-protective effect of acid adaptation on ethanol tolerance in Salmonella Enteritidis
- Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans
- Ameliorative effect of Lacticaseibacillus rhamnosus Fmb14 from Chinese yogurt on hyperuricemia
- Study on the interaction between β-carotene and gut microf lora using an in vitro fermentation model

