Naringenin inhibits lipid accumulation by activating the AMPK pathway in vivo and in vitro
Xiaoyu Cai, Songxu Wang, Huali Wang, Suwn Liu,Guishan Liu, Huiin Chn, Ji Kang*, Hao Wang*
a State Key Laboratory of Food Nutrition and Safety, College of Food Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China b China National Center for Food Safety Risk Assessment, Beijing 100022, China
c College of Food Science and Technology, Hebei Normal University of Science and Technology, Qinhuangdao 066004, China
d School of Food and Wine, Ningxia University, Yinchuan 750021, China
e College of Life Sciences, Fujian Normal University, Fuzhou 350117, China
Keywords:Naringenin Lipid accumulation Molecular docking
A B S T R A C T The aim of this study was to explore the lipid-lowering effect of naringenin and the underlying mechanism in high-fat-diet-fed SD rats and 3T3-L1 cells. In this study, SD rats were divided into the normal chow diet group (NCD), high fat diet group (HFD), three treatment groups feeding high-fat diet with naringenin (100, 200,400 mg/kg) for 12 weeks. Results indicated that naringenin treatment decreased total cholesterol (TC),triglyceride (TG) and the non-high-density lipoprotein cholesterol (non-HDL-C) levels in serum. Naringenin also alleviated hepatic steatosis and reduced the adipocyte size in the epididymis in high-fat-diet-induced SD rats. In addition, naringenin (25-75 μg/mL) decrease TG and TC levels in 3T3 mature adipocytes.The molecular mechanism of naringenin in the treatment of obesity were predicted by using network pharmacology. Real-time PCR analysis results showed that naringenin regulated the expression of lipid metabolism genes. Meanwhile, naringenin increased the AMPK (AMP-activated protein kinase) activity and the expression of AMPK phosphorylated protein in 3T3 mature adipocytes. And the inhibitory effect of naringenin on lipid accumulation in 3T3 adipocytes was abolished by Compound C. Molecular docking results indicated that naringenin could bind to AMPK protein. These results indicated naringenin reduced lipid accumulation through AMPK pathway.
1. Introduction
With the rapid changes of lifestyles and eating habits, obesity has become a serious health problem [1]. Obesity is associated with hypertension, asthma, arthritis, diabetes and a chronic inf lammatory disease, which already affects human health [2,3]. With the increasing number of overweight and obesity, people pay more attention to the harm of obesity and the related diseases. In addition,drugs are an important adjunct to the treatment of obesity, especially in overweight patients with obesity risk. Indeed, orlistat, which is an anti-obesity drug already on the market, has been shown to prevent fat absorption by inhibiting lipase activity [4,5]. However,the resulting side effects, such as diarrhea and abdominal pain,limit long-term use in obese patients. Natural products have the characteristics of diverse structure, good activity and less toxic and side effects, among which flavonoids, alkaloids and other natural products are considered to have a clear anti-obesity effect. In recent years, natural products have become a popular target in the search for safe and healthy obesity treatments [6,7].
Grapefruit peel is rich in flavonoids which range 1%-6% and are mainly dihydroflavones, such as naringin, naringenin and hesperidin.Previous studies have shown that naringenin could reduce hepatic lipid accumulation, increase fatty acid oxidation, improve dyslipidemia in zebrafish larvae [8]. In addition, naringenin has positive effects on regulating glucose and lipid metabolism in type 2 diabetes (T2D) rats through down-regulating related inflammation response [9]. Study has found that naringenin suppressed macrophage infiltration into adipose tissue in an early phase of high-fat diet-induced obesity [10].
Although the lipid-lowering effect of naringenin has been reported, the specific molecular mechanism needs to be further studied. In some other researches, network pharmacology is the network analysis of biological systems based on the theory of systems biology [11], was used to predict the targets and pathways of the interactions between naringenin and disease [12]. Both of them identified the targets and pathwaysin vivoorin vitroexperiments.And then, molecular docking was used to verify naringenin’s ability to bind to these pathway proteins. In these researches, molecular docking showed strong ability to study the interaction between receptors and ligands and predict their affinity and binding patterns [12]. Therefore,in this study, we used network pharmacology to analyze the molecular mechanism of naringenin in the treatment of obesity, providing a new direction for the treatment of obesity. Moreover, we used high fat diet to feed SD rats, 3T3 mature adipocyte model and molecular docking to further explore the molecular mechanism of naringenin’s lipidlowering effect, seeking for a new natural remedy for obesity.
2. Materials and methods
2.1 Chemicals
Naringenin (≥ 97%), 3-isobutyl-1-methylxanthine (IBMX),dexamethasone (DEX), and insulin were obtained from Aladdin (China). Oil Red O, trypsin-EDTA and primers were bought from Beijing Dingguo Changsheng Biotechnology Co., Ltd. (Beijing,China). Fetal bovine serum (FBS) and high-glucose Dulbecco’s 12 Modified Eagle’s Medium (DMEM) were obtained from Gibco (USA). triglyceride (TG), total cholesterol (TC), highdensity lipoprotein cholesterol (HDL-C), aspartate aminotransferase(ALT) and alanine transaminase (AST) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).Compound C (6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine) were obtained from Sigma-Aldrich(St Louis, MO, USA). The specific antibodies were used from Cell Signaling Technology, Inc. (Danvers, MA, USA). Other reagents used in this study were purchased from local reagent vendors.
2.2 Network pharmacological
The target proteins corresponding to naringenin was obtained from TCMSP and SYMMAP database. And the target proteins were imported into the UNIPROT database for “protein-gene”name conversion to obtain Naringenin action gene name. Obesityrelated genes were searched from Gamecards database, with the key word “obesity”. Then the genes that naringenin targets for obesity were obtained from the intersection of naringenin action genes and obesity-related genes. The STRING database was used to construct the protein-protein interaction network map (PPI network map)for the intersection genes, and the score value was set as 0.700.David database was used for Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analysis of intersection genes, and Image GP was used forP< 0.05. Cystoscope version 3.8.0 was used to draw the network diagram of naringeningene-obesity.
2.3 Animals and cell models
Male Sprague Dawley (SD) rats ((250 ± 20) g) were purchased from Beijing SpAFu Biotechnology Co., Ltd. (China). The rats were reared under suitable conditions (temperature, (23 ± 2) °C;relative humidity, 50%-70%; 12 h/12 h light-dark cycle). The animal experiment in this study was approved by the Animal Experiment Ethics Committee of drug Safety Evaluation Center of Tianjin University of Science and Technology.
Prior to the study, all the mice were fed a normal chow diet for a week to adjust to the diet form. After 1 week of adaptation, SD rats were randomly divided into 5 groups, and were continuously given normal diet (NCD), high fat diet (HFD) and a high-fat diet with naringenin (100, 200, 400 mg/kg) for 12 weeks, with 12 rats in each group. The diet of NCD (13.5% of total energy as fat) and HFD (43.2%of total energy as fat) were designed in accordance with the American Institute of Nutrition’s 1993 guidelines. The diets in this experiment were provided in Table S1. The rat chows were added with different doses of naringenin. To ensure the correct dosage, each group’s intake was measured daily, and the proportion of naringenin in the rat diet was adjusted once based on previous intake results.
3T3-L1 preadipocytes were seeded into 6-well plates until confluent, then the growth medium was replaced with a differentiation medium (10% FBS, 0.5 mmol/L IBMX, 1 μmol/L DEX, and 10 μg/mL insulin (MDI)) for incubation for 48 h [13]. Then differentiation medium was replaced with an adipocyte maintenance medium containing 10% FBS and insulin (10 μg/mL). The adipocyte maintenance medium was changed every 48 h. After induction for 8 days, the cells were fully differentiated when the lipid droplets were formed. The cells were fully differentiated between 8 days after induction as evidenced by lipid droplet formation. From day 0,3T3-L1 preadipocytes were treated with or without naringenin (25,50, 75 μg/mL) until the end of cell differentiation.
2.4 Measurement of serum parameters
The method referred to our previous study [14]. TG, TC, HDL-C levels in serum were determined according to the instructions of the commercial Kits (Nanjing Jiancheng, China). The formula for calculating non-HDL-C is as follows:

2.5 Pathological histology analyses
The method referred to our previous Hematoxylin-eosin staining (H&E) experiment [14]. Epididymal white adipose tissues(EWATs) and liver tissue were removed from 10% formalin solution,paraffin-embedded, and sectioned. Then, sections were stained with H&E. The pathological status of EWATs and liver in each group was observed by optical microscope.
2.6 Cell toxicity and proliferation assay
3T3 cells were seeded in 96-well plates at a concentration of 2 × 104/well. After culturing for 24 h, 3T3 cells were treated with high-glucose DMEM (10% FBS) and naringenin (25, 50, 75 μg/mL)for 24 h. After the old culture was abandoned, high sugar medium(containing 10% MTT) was added to incubate for 4 h. Finally,150 μL/well methyl sulfoxide (DMSO) was added into each well in order to dissolve formazan precipitate for 10 min. Colorimetry was performed at 492 nm.
2.7 Lipid content in cells
Oil Red O staining method referred to the previous staining method of our laboratory [15]. After 3T3 mature adipocytes were stained with Oil Red O, 3T3 mature adipocytes were observed under the laser confocal microscope (Olympus CKX41, Japan) and photographed. Then, 1 mL isopropanol was added to each well,and the absorbance was measured at 510 nm with a microplate reader (Thermo, MA, USA).
2.8 TG, TC content
TC and TG were measured according to the previous laboratory method [15]. The TC, TG and protein content of 3T3 mature adipocytes were determined according to the instructions of the commercial kits (Nanjing Jiancheng, China).
2.9 RT-PCR
mRNAs were extracted and detected according to our previous method [16]. The forward and reverse primers of peroxisome proliferator-activated receptor alpha (PPARα), acetyl-coa carboxylase(ACC), carnitineO-palmitoyl transferase 1 (CPT-1), 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), sterol regulatory element-binding protein 1c (SREBP-1C) and fatty acid synthase (FAS)were shown in Table 1.β-Actinexpression was regarded as standard.

Table 1Sequence of primers used in real-time PCR.
2.10 Western blotting analysis
Protein was extracted and detected according to our previous method [17]. The protein was isolated by 7% SDS-PAGE, then translated to PVDF membrane (Millipore, Burlington, MA, USA).After that, the membrane was hatched at 4 °C in specific antibody overnight. Then, the membrane was washed 3 times for 10 min,and hatched with a secondary antibody at room temperature for 2 h. Optical density was observed by the gel imaging analyzer, and analyzed by Image J. Optical density was observed by the gel imaging analyzer, and analyzed by ImageJ.
2.11 Molecular docking
PT1 (2-chloro-5-[[5-[[5-(4,5-dimethyl-2-nitrophenyl)-2-furanyl]methylene]-4,5-dihydro-4-oxo-2-thiazolyl] amino] benzoic acid), a novel small molecule activator, can bind to AMPK protein and has the ability to regulate lipid metabolism [18].
In this research, the docking of interaction was studied between two compounds (PT1, naringenin) and AMPK protein. The enzyme crystal structure (PDB:4CFH) and small molecules of molecular docking were obtained from the PDB database (http://www.rcsb.org)and Chemical Book (https://www.chemicalbook.com), respectively.The amino acid sequences of CPT1A (UniProKB-P50416),FASN (UniProKB-P49327), HMGCR (UniProKB-P04035),PPARα (UniProKB-Q07869), SREBP1 (UniProKB-P36956) were downloaded from the UNIPROT Protein Database (http://uniprot.org). Discover Studio 3.5 (Accelrys, CA, USA) software was used to dock small molecules (PT1, naringenin) with proteins (AMPK) and analyze their binding energy and interacting amino acids.
2.12 Data analysis
The results were expressed as mean ± SD and processed statistically by GraphPad Prism software V7.0 (Graph Pad Software,San Diego, CA, USA). One-way ANOVA and Tukey’s post-hoc test was used for data analysis It was statistically significant that the probability value was less than 0.05 (P< 0.05).
3. Results
3.1 Network Pharmacology
Forty targets of naringenin in the treatment of obesity were screened (Fig. 1A). The details of the 40 target proteins are shown in Table S1. To elucidate the strength of protein-protein interactions, we obtained a PPI network of 40 targets. The line thickness of two targets represents the strength of supporting data. The thicker the line, the stronger the interaction. Those with a score of 0.7 or above are given priority. As shown in Fig. 1B, the key targets of naringenin-obesity included FASN, CPT1A, SREBF1, PPARα, ACACA, and HMGCR,and these protein-protein interactions are strong. And the network diagram of naringenin-genes-obesity was shown in the Fig. 1C. In order to reveal the potential mechanism of naringenin in the treatment of obesity, the top 10 GO enrichment results of cellular component (CC),biological process (BP), and molecular function (MF) were chosen according toP-value. As for BP, mitochondrion, endoplasmic reticulum, endoplasmic reticulum membrane, and cytosol were significantly enriched (P< 0.01, Fig. 1C). In addition, GO results showed that overlapping targets were enriched in CC, including response to drug and oxidation-reduction process (P< 0.01, Fig. 1D).For MF, protein homodimerization activity, identical protein binding,and NADP binding were significantly enriched (P< 0.01, Fig. 1E).In order to further understand the relationship between naringenin and obesity, we enriched the naringenin-obesity-related signaling pathways. We found that common targets were mainly concentrated in pathways in cancer, AMPK signaling pathway, and Toxoplasmosis(P< 0.01, Fig. 1F).
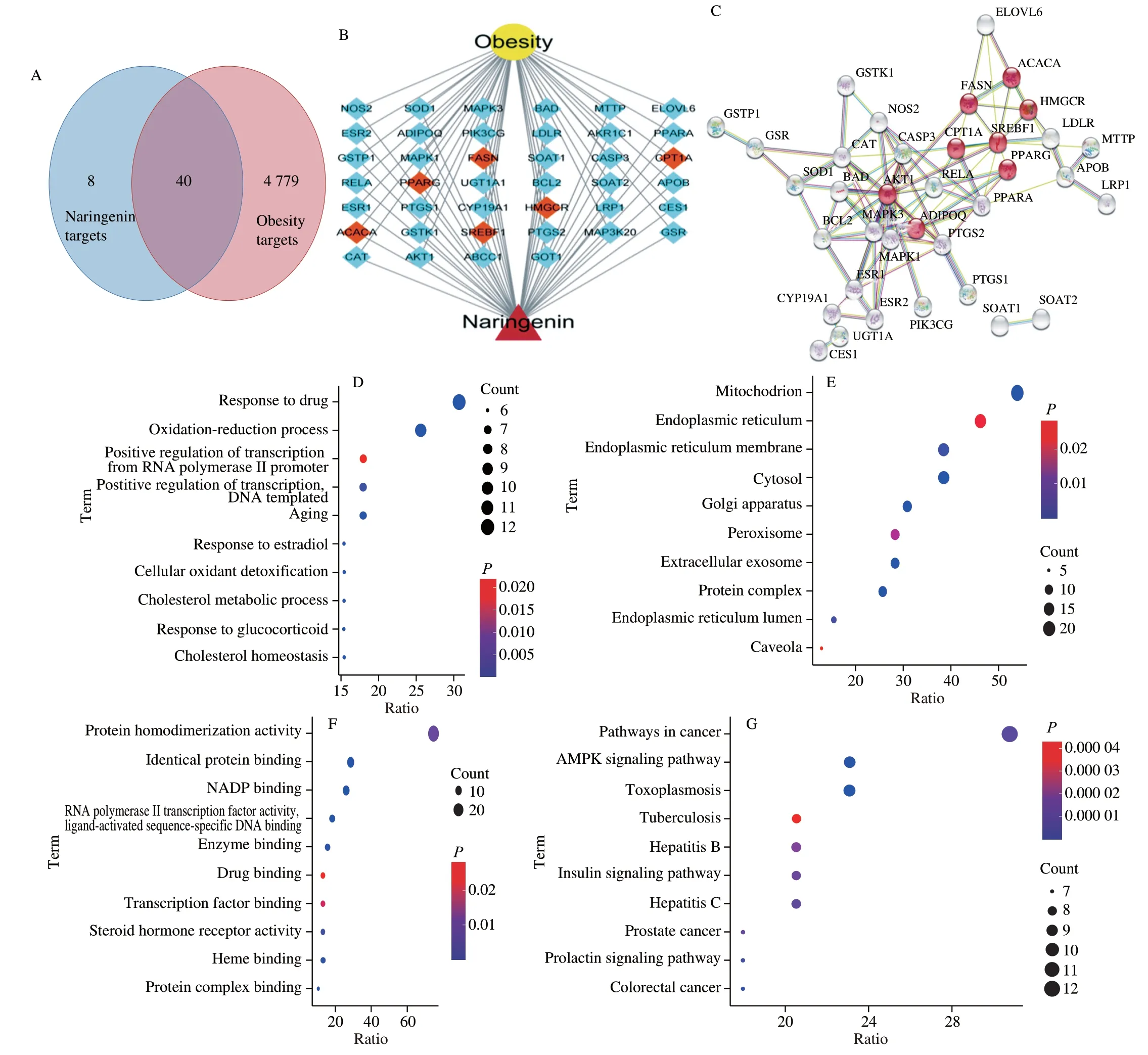
Fig. 1 Venn diagram of naringenin-obesity common targets. (A) The intersection of the two circles shows 40 common targets. (B) The naringenin-obesity targetsnetwork. (C) The PPI of naringenin-obesity targets, the red circles represent important targets. Function enrichment analysis for GO terms: (D) BP, (E) CC,(F) MF; (G) KEGG pathway.
3.2 Body weight, food intake and serum index
Compared with NCD group, the body weight of HFD group was increased markedly (P< 0.05, Fig. 2A). However, naringenin treatment (100, 200, 400 mg/kg) resulted in significant weight loss (Fig. 2A). There was no obvious difference in food intake between groups on the high-fat diet (Fig. 2B). Body weight parameters and organ weight parameters were shown in Table S2. After 12 weeks of naringenin (400 mg/kg) treatment, the body weight and liver weight decreased by 13.9% and 19.6%, respectively, compared with HFD group. Serum TC and TG levels were significantly increased and serum HDL-C levels were decreased in HFD group compared with the NCD group (P < 0.01, Fig. 2). Naringenin (400 mg/kg) treatment significantly decreased serum TC, TG and non-HDL-C levels, and increased serum HDL-C levels contrast with HFD group (P< 0.01,Fig. 2). In addition, the activity of serum ALT and AST in the HFD group were significantly higher than the NCD group. Naringenin treatment alleviated a high-fat diet-induced liver damage by inhibiting the activity ALT and AST (Fig. S1,P< 0.05).
3.3 Pathological analysis of liver and EWAT
To further verify the effect of naringenin on obesity,histopathological analysis of EWATs and liver were performed (Fig. 3).The average size of EWATs in the HFD group were obviously increased, but they were markedly decreased after the treatment of naringenin (Fig. 3A). The liver cells in NCD group had intact structure, orderly arrangement and clear nuclei, while the liver cells in HFD group showed a large number of fat vacuoles,and the number and volume of lipid droplets in the cytoplasm increased, which was consistent with the observation of weight gain of SD rats fed with high fat diet for 12 weeks. In addition,naringenin treatment reduced liver lipid content caused by a high-fat diet (Fig. 3B).
3.4 Cell viability
The effect of naringenin on the viability of 3T3-L1 cells was detected by MTT assay. Naringenin had no significant effect on the viability of 3T3 preadipocytes in the concentrations of 25, 50 and 75 μg/mL, while naringenin reduced cell viability in the concentration of 100 μg/mL compared with the control group (Fig. 4C). Thus, the final concentrations of naringenin in subsequent experiments were 25,50 and 75 μg/mL.
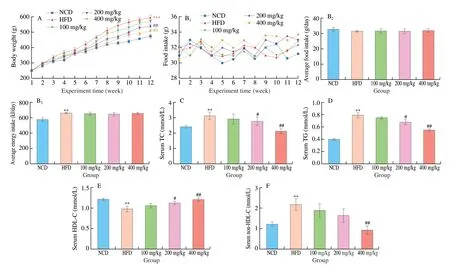
Fig. 2 Effect of naringenin on the (A) body weight, (B1) food and energy intake, (B2) averge food intake and (B3) averge energy intake. Concent of (C) TC,(D)TG, (E) HDL-C and (F) non-HDL-C in serum. *P < 0.05 and **P < 0.01 vs NCD group, respectively. #P < 0.05 and ##P < 0.01 vs HFD group, respectively.
3.5 Inhibiting effect on lipid accumulation in cells
To investigate the effect of naringenin on lipogenesis, the differentiating 3T3-L1 cells were pretreated with different concentrations of naringenin (25, 50, 75 μg/mL), and lipid accumulation was measured by Oil Red O staining, TC and TG assay kits. We found that the number of lipid droplets, the OD510nmvalue of Oil Red O staining, and TC and TG contents were significantly increased after 3T3-L1 were fully differentiated when treated with a differentiation medium as compared to the control cells (no treatment with a differentiation medium). However, 25-75 μg/mL naringenin notably decreased the number of lipid droplets, the OD510nmvalue of Oil Red O staining, TG and TC contents (Fig. 4). These results indicated that naringenin effectively inhibited lipid accumulation in 3T3 cells.
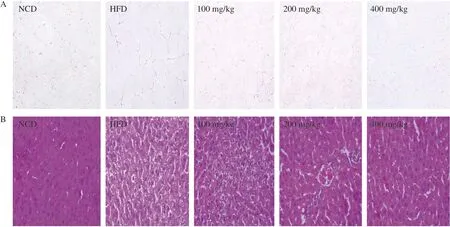
Fig. 3 Effect of naringenin on (A) epididymal white adipose tissues and (B) liver tissues (H&E staining, magnification 200×).

Fig. 4 Naringenin inhibited lipid accumulation in 3T3 cells. (A) The structural formula of naringenin. (B) Preadipocytes were induced to differentiate in the presence of naringenin for 8 days. (C) Cytotoxicity of 3T3 cells caused by naringenin. (D) Oil Red O staining (400× original magnification). The content of intracellular (E) lipids, (F) TC and (G) TG content was measured. **P < 0.01 and ****P < 0.000 1 vs control group, #P < 0.05, ##P < 0.01, ###P < 0.001 and####P < 0.000 1 vs induction group (MDI).
3.6 Regulating mRNA expression levels of lipid mentalism
AMPK is crucial to cell energy homeostasis, which is the main control factor of lipid metabolism. In this study, KEGG function analysis showed that naringenin treatment against to obesity was related to AMPK pathway. In order to further clarify whether the anti-obesity effects of naringenin was related to AMPK pathway, the expression of related genes (SREBP-1C,FAS,ACC,CPT-1,PPARα,andHMGCR) in liver were detected by using RT-PCR (Fig. 5A).In vivo, the expression levels of genes includingFAS,ACCandHMGCRwere significantly increased compared with the control group,while naringenin (200 mg/kg) treatment notably decreased their levels.Meanwhile, naringenin (200 mg/kg) treatment for 10 weeks notably decreased the mRNA expression levels ofCPT-1,PPARα. These results indicated naringenin reduced obesity induced by high fat diet in rats.
In 3T3-L1 adipocytes, the mRNA expression levels of genes related to AMPK pathway were detected. As shown in Fig. 5B,differentiated 3T3 cells up-regulated the expression of genes related to fatty acid synthesis and cholesterol synthesis includingFAS,ACC,SREBP-1CandHMGCR, and down-regulated the expression ofCPT-1related to fatty acid oxidation. However, with the treatment of naringenin (25-75 μg/mL), the mRNA expression levels ofFAS,ACC,SREBP-1CandHMGCRwere decreased, and the mRNA expression levels ofCPT-1were obviously increased. These results indicated 25-75 μg/mL naringenin inhibited 3T3-L1 cell differentiation and lipid accumulation. And these results showed naringenin treatment against to obesity through AMPK signaling.
3.7 AMPK signaling pathway is critical for lipid mentalism
Furthermore, we further determined whether naringenin treatment activated AMPK. As shown in Fig. 6, naringenin (25-75 μg/mL)treatment significantly increased the expression of p-AMPK,without changing the total protein. TheAMPKmRNA expression was significantly decreased in MDI group, while naringenin (50,75 μg/mL) significantly increased the mRNA expression ofAMPKin 3T3-L1 adipocytes (Fig. 6C).
Compound C is an AMPK inhibitor, which can directly inhibit the expression of AMPK [19]. After treatment with Compound C, the effect of naringenin on reducing intracellular TC and TG content were reversed (Fig. 6). These results indicated that the decrease of TC and TG content in naringenin treatment was related to AMPK.
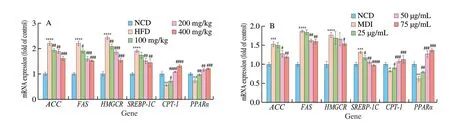
Fig. 5 The effect of naringenin on mRNA expression of ACC, FAS, HMGCR, ACC, SREBP-1C, CPT-1 and PPARα relating AMPK signaling pathway. (A) Liver,(B) 3T3-L1. **P < 0.01, ***P < 0.001 and ****P < 0.000 1 vs (A) NCD group, (B) control group, #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.000 1 vs(A) HFD group, (B) induction group (MDI).

Fig. 6 The effect of naringenin on AMPK protein in 3T3 adipocytes cells. (A) Protein expression was analyzed by Western blot, and (B) quantitative data of p-AMPK/AMPK. (C) mRNA expression of AMPK in 3T3-L1 adipocytes. (D, E) Intracellular TC and TG content. **P < 0.01, ***P < 0.001 and ****P < 0.000 1 vs control group, #P < 0.05, ##P < 0.01, ###P < 0.001 and ####P < 0.000 1 vs induction group (MDI).
3.8 Molecular docking
In molecular docking, the binding energy is commonly used to evaluate stability of the binding between small molecules and protein molecules. In molecular docking, the lower the numerical values of binding energy between small molecules and protein molecules, the stronger the stability of their binding [20].
Moreover, recent studies have shown that PT1 bound to AMPK protein, which had the effect to reduce lipid accumulation. Thus,we docked small molecules (PT1, naringenin) with AMPK protein,respectively. The interaction residues and binding energies are described in Table S3. Computer simulation results showed that the binding energy of PTI to AMPK protein was -195.91 kcal/mol, while the binding energy of naringenin to AMPK protein was -280.99 kcal/mol.These results indicated that the binding stability of naringenin was stronger than that of PT1. As shown in Fig. 7, PT1 and naringenin shared common pockets formed by amino acid residues including LYS 260, GLU232, ARG263, PRO278, ASN335, ARG132,LYS261. In summary, PT1 and naringenin were docked into the same active center and might have the same effect to reduce lipid accumulation. In addition, naringenin has binding effect with CPT1A(-42.960 1 kcal/mol), FASN (-99.3446 3 kcal/mol), HMGCR(-38.393 6 kcal/mol), PPARα (-37.185 kcal/mol), SREBP1(-258.687 kcal/mol) and ACACA (-88.539 2 kcal/mol). These results showed that naringenin can bind to obesity-related protein.
4. Discussion
Many studies of naringenin or other flavonoids in citrus fruits had worked on regulation of energy intake and expenditure, regulation of lipid metabolism [7,21,22]. Although naringenin is verified that treats lipid metabolic diseases, the specific molecular mechanism remained to be unknown. Network pharmacology and molecular docking had been used to study the molecular mechanism of naringenin in many areas [12,23]. In this work, we studied the effect of naringenin on obesity by network pharmacology, besides lipid accumulation and molecular mechanism in high-fat-diet-fed SD rats and 3T3-L1 preadipocytes. The present study showed naringenin effectively inhibited lipid accumulation, alleviated the development of obesity.
In vivo, the experimental results showed that naringenin (200,400 mg/kg) treatment for 12 weeks alleviated the weight gain and the serum levels of TC, TG and non-HDL-C in male SD rats induced by high fat diet (Fig. 2). In previous studies, Namkhah et al. [21]found that naringenin (200 mg/kg) treatment improved lipids and anthropometric parameters in NAFLD patients, including TC, TG,LDL, HDL, and body weight. And this is related to the study that adding 3% (m/m) naringenin to high fat diet alleviated weight gain in C57BL/6 mice induced by high fat diet and plasma TG content in Lepob/obmice [24]. At the same time, naringenin treatment alleviated the hepatic steatosis and adipocyte size associated with a high-fat diet (Figs. 3, S1). Similarly, Hansi et al. [25] found that 25 mg/kg naringenin alleviated adipocyte enlargement in diabetic rats induced by a high-fat diet. These results suggested that naringenin alleviates obesity induced by a high-fat diet.
In vitro, 25, 50, 75 μg/mL naringenin had no significant effect on the viability of 3T3 preadipocytes (Fig. 4C). 25, 50, 75 μg/mL naringenin showed positive effects on inhibiting lipid accumulation.25-75 μg/mL naringenin significantly reduced lipid content, TC and TG levels (Fig. 4), and this finding was similar with a previous report that 25 μg/mL naringenin had partial inhibition in adipocytes of 3T3 cells and reduced insulin sensitivity [26]. And 25 μg/mL naringenin had a significant effect on Oil Red O staining of mature 3T3 cells [26].

Fig. 7 Computational Analysis of naringenin and PT1. The global diagrams of the docking between small molecules (PT1 (A), naringenin (D) and AMPKprotein; the details of PT1 (B) and naringenin (E) docking with AMPK protein; the 2D diagrams of the maximum interaction forces of PT1 (C) and naringenin (F))combining with AMPK.
In addition to, the abovein vivoandin vitroexperiments have proved that naringenin has a good inhibitory effect on obesity, we also conducted a specific study on the underlying mechanism of naringin’s effect on obesity. KEGG pathway enrichment results showed that obesity was significantly correlated with AMPK signaling pathway,which regulated lipid metabolism. Recent research had verified that AMPK was the potential target for obesity treatments [27,28]. In addition, some enzymes, such as ACC and FAS, had also shown the ability of adjusting lipids balance [29,30]. PPARα played an important role in regulating adipose differentiation and function [31].In this study, naringenin down-regulated the expression of genes related to fatty acid synthesis includingFAS,ACC,HMGCR, andSREBP-1C,up-regulated the expression of fatty acid oxidation gene (CPT-1)in high-fat-diet-fed SD rats and 3T3-L1 preadipocytes (Fig. 5).In addition, 50-75 μg/mL naringenin treatment significantly promotedAMPKmRNA expression in 3T3 adipocytes cells(Fig. 6C,P< 0.05). Moreover, naringenin treatment significantly promoted AMPK phosphorylation level (Fig. 6). These results were lined with a previous study that naringenin treatment in HFD-fed mice significantly reduced the mRNA expression ofACCandFAS,increased the mRNA expression ofCPT-1[32,33]. In addition, the effects of naringenin on the contents of TC and TG in 3T3 cells were reversed by Compound C (Fig. 6). These results indicated that naringenin reduced lipid accumulation in 3T3-L1 cells may be related to AMPK.
Furthermore, the computer simulation results showed that naringenin and PT1 bound to the same pockets of AMPK, which caused AMPK to be activated. It verified that naringenin and PT1 might have similar mechanisms of action to activity AMPK.
5. Conclusion
Naringenin showed strong ability to inhibit the lipid accumulation in high-fat-diet-fed SD rats and 3T3 differentiated cells. Naringenin treatment not only down-regulated the mRNA expression ofPPARα,FAS,ACC,HMGCR, andSREBP-1C, but also up-regulated the mRNA expression ofCPT-1. Besides, naringenin treatment significantly promoted p-AMPK, which meant that the effect of naringenin on lipid accumulation might be related to AMPK signaling pathway.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.10.043.
- 食品科學與人類健康(英文)的其它文章
- Call for Papers from Journal of Future Foods
- GUIDE FOR AUTHORS
- Cross-protective effect of acid adaptation on ethanol tolerance in Salmonella Enteritidis
- Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans
- Ameliorative effect of Lacticaseibacillus rhamnosus Fmb14 from Chinese yogurt on hyperuricemia
- Study on the interaction between β-carotene and gut microf lora using an in vitro fermentation model

