Phenolic compounds from Chaenomeles speciosa alleviate inf lammation in lipopolysaccharide-treated RAW264.7 macrophages via the NF-κB and MAPK pathways
Fuxi Hu, Cho Liu, Fengqin Wng, Chngxin Zhou, Motong Zhu,Dongxio Sun-Wterhouse*, Zhosheng Wng,*
a Key Laboratory of Food Processing Technology and Quality Control in Shandong Province, College of Food Science and Engineering,Shandong Agricultural University, Taian 271018, China
b Key Laboratory of Novel Food Resources Processing, Ministry of Agriculture and Rural Affairs, Key Laboratory of Agro-Products Processing Technology of Shandong Province, Institute of Agro-Food Science and Technology, Jinan 250100, China
c School of Chemical Sciences, The University of Auckland, Private Bag 92019, New Zealand
Keywords:Chaenomeles speciosa Phenolic extract Purif ication Anti-inf lammatory
A B S T R A C T Chaenomeles speciosa (Sweet) Nakai cultivated widely in temperate regions possesses anti-inflammatory properties, however, the underlying molecular mechanisms remain not fully understood. In this study, a purif ied phenolic extract of C. speciosa rich in chlorogenic acid, procyanidin B1 and catechin (determined by HPLC-Q-TOF-MS/MS) exhibited dose-dependent anti-inflammatory effects on lipopolysaccharide(LPS)-treated RAW264.7 macrophages. The extract at 30 μg/mL was most potent and enabled most cells in normal morphology under LPS stimulation without causing cytotoxicity. The extract suppressed the levels of nitric oxide (NO), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β), and the mRNA and protein expressions of nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). The mechanisms underlying such anti-inf lammatory actions included the regulation of phosphorylation of related proteins to monitor the expressions of inflammatory mediators and genes in the nuclear factor kappa-B(NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. Therefore, the phenolic extract from C. speciosa is a desirable anti-inf lammatory agent for inf lammatory conditions to meet the rising demand for natural and cost-effective therapeutics.
1. Introduction
Inf lammation is a common biological response of body tissues as a defense/healing process to harmful stimuli and microbial invaders(e.g. irritants, damaged cells or infectious pathogens), or even occurs in the absence of infection or overt tissue damage due to tissue stress and malfunction [1]. Inflammation, especially prolonged/chronic inf lammation, can mediate a range of diseases, including hay fever, allergies, periodontal disease, inflammatory bowel disease,osteoarthritis, cancer, type II diabetes, lipid metabolism disorders,chronic obstructive pulmonary disease and nervous system-related disorders (such as Parkinson’s disease, Alzheimer’s disease, stroke and other cognitive impairment symptoms) [2,3]. When inf lammation occurs, a variety of inf lammatory mediators, chemokines, cytokines and reactive oxygen species (ROS) are activated, such as nitric oxide (NO), tumor necrosis factor (TNF)-α, interleukin (IL)-1β,IL-6, IL-8, inducible NO synthase (iNOS), cyclooxygenase (COX)-2 and some other immune cells [4,5]. The nuclear factor-kappa B(NF-кB) signaling pathway is the main pathway involved in inflammation and immunity (especially innate immune response) [6].The NF-кB signaling pathway is closely related to intracellular signaling pathways like mitogen-activated protein kinase (MAPK)pathways. In response to inflammatory conditions, the activation of both types of inflammatory signaling pathways leads to the release of proinflammatory cytokines and eventually inflammatory response [7,8].Besides intracellular stimuli, extracellular stimuli can also activate MAPKs e.g. extracellular stresses activates the p38 MAPK signaling pathway that plays an essential role in regulating cellular processes and acts as a therapeutic target in inflammatory diseases [9].Therefore, attenuating inflammatory response is crucial for sustaining local and systemic homeostasis and maintaining human health.
Macrophages are key players of host defense against foreign pathogens. Stimuli (e.g. lipopolysaccharide (LPS)) activate macrophages,resulting in the production of ROS, pro-inflammatory cytokines and inflammatory mediators [10,11]. The produced NO can combine with superoxide radical-induced nitrosative stress to form reactive nitrogen species (RNS), causing imbalanced redox and rising inflammatory burden [10]. Since inflammation and oxidative stress are interconnected processes, effective therapeutic approaches against inflammatory conditions/disorders include the monitoring of free radicals and inflammatory mediators. Phenolic compounds possess anti-inflammatory activities [12,13], which may be associated with their effects on enzymatic and signaling systems, and their actions as antioxidants [14,15].
Chaenomeles speciosa(Sweet) Nakai (Rosaceae family),also known as Begonia, Xuan Mugua, Tang Mugua, etc., is rich in nutrients, and bioactive compounds e.g. phenolic compounds(including flavonoids), terpenoids and their derivatives (including saponins), polysaccharides and essential oils [16]. These bioactive components ofC. speciosaare closely associated with its antiinflammatory, antioxidant, immunoregulatory, analgesic and antimicrobial antitumor effects [16]. AlthoughC. speciosahas been used to treat inflammation, the molecular mechanisms underlying the anti-inflammatory actions of its phenolic compounds have not yet been fully elucidated. Therefore, this study aimed to make a contribution to understanding such molecular mechanisms. Phenolic compounds were extracted fromC. speciosausing an aqueous ethanolic solution and analyzed by HPLC-Q-TOF-MS/MS. Then,the anti-inflammatory capacity of the phenolic compounds fromC. speciosaand the underlying molecular mechanism were examined.
2. Materials and methods
2.1 Reagents and materials
FreshC. speciosafruits were harvested from Linyi, Shandong Province, China. Methanol was provided by Oceanpak Alexative Chemical Co., Ltd. (Sweden), and ethanol was provided by Komeo Chemical Reagent Co., Ltd. (Tianjin, China). LPS, catechin,chlorogenic acid and procyanidin B1 were procured from Yuanye Biological Technology Co., Ltd. (Shanghai, China). Cell counting kit-8 (CCK-8) and NO assay kit were obtained from Beyotime Biotechnology Co., Ltd. (Shanghai, China). Fetal bovine serum(FBS) was obtained from Gemini Ltd. (California, USA). Dulbecco’s Modified Eagle Medium (DMEM), phosphate buffered saline (PBS),and trypsin-EDTA (0.25%) were purchased from Thermo Fisher Biochemical Products Co., Ltd. (Beijing, China). Enzyme-linked immunosorbent assay (ELISA) kits were obtained from Servicebio Biological Technology Co., Ltd. (Wuhan, China).
2.2 Preparation of purified phenolic extract from the C. speciosa
C. speciosawas dried in the open air at 45 °C at a constant temperature, pulverized and sieved (60-80 mesh). TheC. speciosaparticles that passed through the sieve were extracted under the following conditions: sodium dodecyl sulfate (SDS) concentration,0.40 g/L; ethanol concentration, 58% (V/V, in water); material-toliquid ratio, 1/33 g/mL; ultrasonic treatment, 51 °C, 200 W, for 30 min. The obtained extract was centrifuged at 4 000 r/min and room temperature for 10 min using a centrifuge (LXJ-IIB, Anting Scientific Instrument Factory, Shanghai, China). The supernatant was collected as the crude phenolic extract, and further purified by a column packed with LSA-900C resin under the following conditions:sample concentration, 2.0 mg/mL; pH 3; sample loading flow rate,1.0 mL/min; concentration of aqueous ethanol solution (as the eluent),50%; elution flow rate, 1.5 mL/min; volume of eluent for elution, 120 mL.Finally, the purified phenolic extract fromC. speciosawas concentrated by rotary evaporation and freeze-dried for further experiments.
2.3 HPLC-Q-TOF-MS/MS and HPLC analyses
The dry purified phenolic extract ofC. speciosawas reconstituted in a 50% aqueous methanol solution (chromatographic grade; in water), filtered through an organic film (0.45 μm), and collected into a sample bottle for HPLC-Q-TOF-MS/MS analysis. UPLC analysis was performed with an Acquity UPLC system (Waters Co., MA,USA), equipped with an Agilent SB-C18 column (4.6 mm × 250 mm,5 μm; Agilent Technologies, Palo Alto, CA, USA). The mobile phases consist of 0.1% aqueous formic acid (A) and methanol (B), with the elution scheme as follow: 0-10 min, 15% B; 10-20 min, 15%-18% B;20-28 min, 18% B; 28-45 min, 18%-20% B; 45-50 min, 20% B;50-60 min, 20%-25% B; 60-70 min, 25% B. The flow rate was 0.8 mL/min with the column temperature at 25 °C and injection volume as 2 μL. The MS analysis conditions were as follows:capillary voltage, 3.5 kV; drying gas (N2) temperature, 200 °C;nebulizer gas (N2) pressure, 2 × 105Pa; range ofm/z20-1 500.
The 3 main phenolic components were verified by HPLC using an Inertsil ODS-SP liquid chromatography column (4.6 mm × 250 mm,5 μm, Japan). The injection volume was 10 μL, with the flow rate as 0.8 mL/min and the column temperature at 35 °C. The composition of mobile phase and its gradient elution scheme were the same as those for the HPLC-Q-TOF-MS/MS analysis. The wavelength for detection was 280 nm.
2.4 Evaluation of anti-inflammatory capacity
2.4.1 Cell culture
The RAW264.7 mouse cells were grown at 37 °C in atmosphere of 5% CO2in DMEM/high glucose medium with 10% FBS and 1%penicillin/streptomycin mixture (100 U/mL penicillin; 100 μg/mL streptomycin) [17].
2.4.2 Cell viability assay
The cells were cultured until they reach 80%-90% confluence,then the cell density was adjusted to 5 × 104cells/mL with 100 μL/well in a 96-well plate. After 24 h of culture, RAW264.7 cells were treated with fresh medium containing the purified phenolic extract fromC. speciosaat different concentrations (0-100 μg/mL).After further culture for 24 h, an aliquot (10 μL) of the CCK-8 solution was added followed by an incubation at 37 °C for 2 h. Then,the optical density (OD) at 450 nm was measured with an enzyme labeling instrument (Bio Tek, Vermont, USA).
2.4.3 NO production assay
LPS as a prototype of inflammatory stimuli was used to activate mouse macrophages RAW264.7 cell line to construct an inflammatory response cell model. RAW264.7 cells were handled according to section 2.4.2. After 24 h of culture, RAW264.7 cells were treated with LPS (0, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 2.0 or 5.0 μg/mL), and after the 24-h culture, the NO produced was measured using Griess kit(Beyotime, China). Then, the amount of LPS was determined through comparing the amount of NO released.
The amount of released NO was determined as follows. The cells were cultured for 24 h, followed by the treatments with LPS in the absence/presence of the treatment with the purified phenolic extract fromC. speciosaat different concentrations. A blank group (normal cell culture), a model group (treated with LPS, 0.8 μg/mL), and a treatment group (purified phenolic extract (5, 10, 20 or 30 μg/mL)along with LPS (0.8 μg/mL)). After 24 h of culture, the amount of released NO was measured using the Griess kit. The absorbance at 540 nm was measured by the enzyme labeling instrument to evaluate the influence of the purified phenolic extract fromC. speciosaon NO release in the inflammatory response cell model.
2.4.4 Crystal violet staining assay
RAW264.7 cells were seeded in 6-well plates at a density of 1 × 106cells/well and allowed culture for 24 h. The cells were cotreated for 24 h with the purified phenolic extract (5, 10, 20 or 30 μg/mL) and LPS (0.8 μg/mL). Then, the cells were fixed with 4%paraformaldehyde for 10-20 min, washed with PBS, stained with 0.5% crystal violet solution for 10 min, and washed with PBS several times (until the dye stopped coming off). Finally, the stained cells were examined under an inverted microscope.
2.4.5 ELISA
RAW264.7 cells were handled according to section 2.4.4. The culture medium was harvested, and the levels of TNF-α, IL-6 and IL-1β were measured according to the manufacturer’s instructions for the ELISA kits.
2.4.6 Real-time PCR analysis
RAW264.7 cells were handled according to section 2.4.4. After the treatments, the TRIzol reagent (Servicebio, Wuhan, China) was used to extract the total RNA, and the cDNA was synthesized from the extracted total RNA using a reverse transcription kit (Servicebio,Wuhan, China). The RT-qPCR analysis was carried out using 2 × SYBR green qPCR Master Mix (None ROX). The relative expression levels were calculated by the comparative Ct method(2-ΔΔCt). The mouse GAPDH expression was used as an internal control. The primers were synthesized by Servicebio Biological Technology Co., Ltd. (Wuhan, China) and are shown in Table 1.
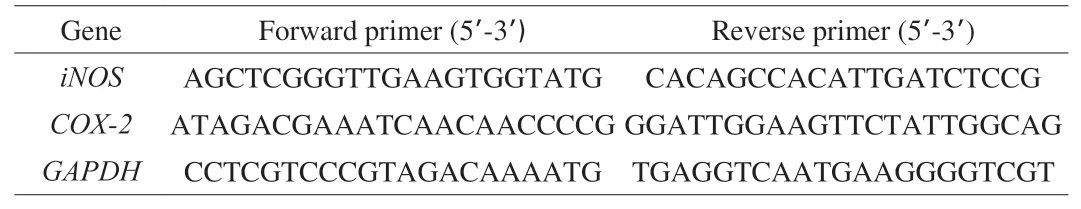
Table 1Primer sequences used in this study for RT-qPCR amplification.
2.4.7 Immunofluorescence assay
RAW264.7 cells were handled according to section 2.4.4. After the treatments, the cells were treated with 4% paraformaldehyde solution at 4 °C for 30 min and blocked with 5% BSA at 37 °C for 30 min. Finally, RAW264.7 cells were incubated overnight with NF-кB p65 antibody (1:100) at 4 °C. The treated cells were examined by an ortho fluorescence microscope (Nikon Eclipse C1, Nikon, Japan).
2.4.8 Western blot analysis
RAW264.7 cells were handled according to section 2.4.4. After the treatments, total cell proteins were extracted and quantified with a bicinchoninic acid (BCA) kit (Beyotime, Haimen, China). The extracted protein sample was then mixed with the loading buffer and boiled in boiling water for 10 min to allow denaturation. Proteins were separated using a 10% SDS-polyacrylamide gel electrophoresis (SDSPAGE) system and transferred to polyvinylidene fluoride (PVDF)membranes (Millipore, Bedford, MA, USA). Then, the membranes were incubated first with 5% nonfat milk for 2 h at room temperature and further incubated at 4 °C overnight with primary antibodies including iNOS (ab178945, Abcam), COX-2 (12282, CST), p65(GB11997, Servicebio), phospho-p65 (GB11142-1, Servicebio), IκBα(4812, CST), phospho-IκBα (5209, CST), p38 (ab170099 Abcam),phospho-p38 (ab195049, Abcam), Jun N-terminal kinase (JNK)1/2/3(ab179461, Abcam), phospho-JNK1/2/3 (ab76572, Abcam),β-actin (GB12001, Servicebio), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 5174T, CST). Then, the incubated membranes were washed three times with Tris-buffered saline and Tween-20 (TBST, pH 7.4), and incubated with a proper secondary antibody. Finally, the enhanced chemiluminescence (ECL) agent(Beyotime Biotechnology, China) was used to allow visualization of the target proteins.β-Actin or GAPDH were used as references.
2.5 Statistical analysis
All data were reported as “mean ± standard deviation” of at least triplicate measurements per sample. One-way analysis of variance(ANOVA) and Duncan’s test were conducted using the SPSS 17.0,and theP< 0.05 was considered statistically significant.
3. Results and discussion
3.1 Analysis of the key phenolic compounds in the purified extract of C. speciosa
As shown in the total ion chromatograms, HPLC chromatograms(λ280nm) of the purified phenolic extract fromC. speciosaand the MS2spectra (Fig. 1 and Table 2), 9 peaks (peaks 1-9) were found in the spectra of the purified extract fromC. speciosa. Among the 9 peaks, 5 were identified as phenolic compounds i.e. procyanidin trimers (peaks 1 and 6), procyanidin B1 (peak 5), catechin (peak 7),and chlorogenic acid (peak 8).C. speciosawas reported to contain these compounds and other phenolic species such as cinnamic acid,caffeic acid, (-)-epicatichin, quercetin, rutin, pelargonidin 3-O-β-D-glucopyranoside, pelargonidin-3-galactoside, cyanidin 3-β-Oglucoside, cyanidin 3-O-β-galactopyranoside, 4-hydroxybenzoic acid,3,4-dihydroxybenzoic acid, gallic acid, vanillic acid, ferulic acid,p-coumaric acids, and protocatechuic acid ethyl ester [18-21]. The actual phenolic composition ofC. speciosadepends on the sample nature and analysis methods [22].
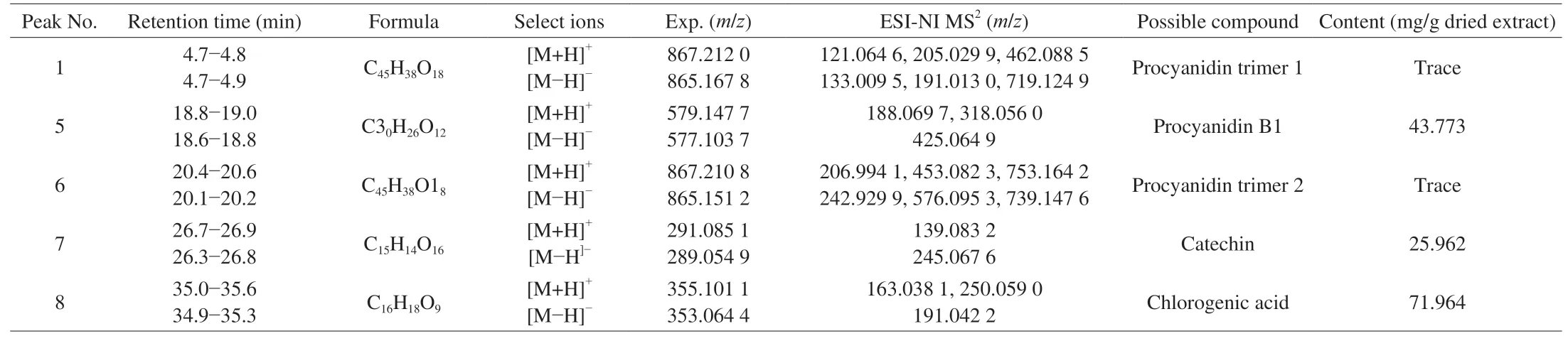
Table 2Phenolic compounds in the extract of C. speciosa analyzed by HPLC-Q-TOF-MS/MS.
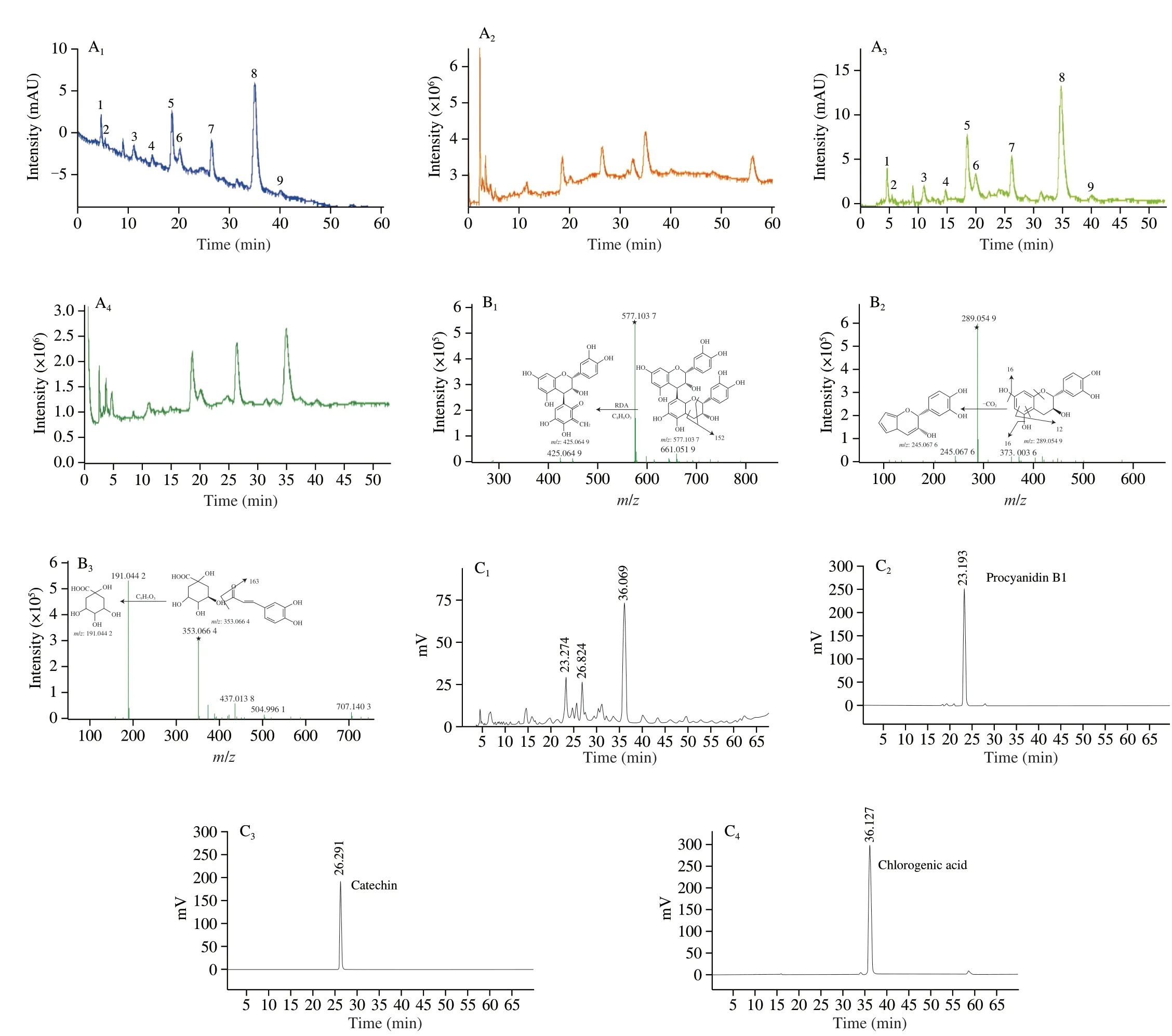
Fig. 1 Analysis of the phenolic compounds in the purified extract from C. speciosa. (A) The HPLC phenolic profiles (280 nm) (A1) and the total ion chromatograms (TICs) (A2) in positive, and the HPLC phenolic profiles (280 nm) (A3) and the TICs (A4) in negative mode; (B) MS2 spectra and the proposed fragmentation pathways of procyanidin B1 (B1), catechin (B2), chlorogenic acid (B3) in negative ion mode. The symbol ★ indicates the precursor ion, [M-H]-;(C) HPLC chromatograms (280 nm) of the phenolic extract of C. speciosa (C1) as well as procyanidins B1 (C2), catechin (C3), and chlorogenic acid (C4) standards.
The fragment ion atm/z577.103 7 was from [M-H]-of peak 5,due to the loss of a hydrogen ion (with a mass number of 1 in the negative ion mode). Peak 5 was tentatively identified as procyanidin B1, which was consistent with the finding of a previous study [23].As shown in the MS2spectrum of peak 5 (Figs. 1B1-B3), a fragment ion atm/z577.103 7 (precursor ion mass) was detected, along with a fragment at MS2m/z425.064 9. The MS2m/z425.064 9 likely resulted from a retro Diels-Alder (RAD) reaction of the dimer, causing the breakdown of the C-ring of a flavanol and the loss of a neutral structure C8H8O3withm/z152. After the comparison with a previous report, peak 5 was tentatively identified as procyanidin B1 [24].Them/z289.054 9 associated with [M-H]-(along with the MS2m/z245.067 6) likely came from catechin (peak 7). Them/z245.067 6 might result from the loss of CO2from the A ring. The identification of catechin (peak 7) agreed with that of a previous report [25].Peak 8 was likely chlorogenic acid based on them/z353.064 4 (as[M-H]-) and the MS2m/z191.044 2. The MS2m/z191.044 2 might arise owing to the loss of a C9H7O3structure withm/z163. Such identification of chlorogenic acid was based on a previous report [26].
In the HPLC profile (λ280nm) of the purified phenolic extract fromC. speciosa, the main peaks 5, 7 and 8 had retention times as 23.274,26.892 and 36.069 min, respectively, which matched the retention times of the standards of procyanidin B1, catechin and chlorogenic acid. These results further confirmed that compounds 5, 7 and 8 were procyanidin B1, catechin, and chlorogenic acid, respectively (which were consistent with the results obtained based on MS2spectra). In this study, the calibration curves of these compounds all showed good linearity (R2values higher than 0.999 2). The contents of the three key phenolic compounds were in the order of chlorogenic acid >procyanidin B1 > catechin (Table 2), with the sum of their contents being about 141.70 mg/g dry purified extract. Chlorogenic acid had the highest amount in the purified phenolic extract fromC. speciosa.
3.2 Anti-inflammatory activities of the purified phenolic extract from C. speciosa
3.2.1 Effects of the purified phenolic extract on the viability of RAW264.7 cells
Macrophages play a key role in the anti-inflammatory process [27].To examine the potential cytotoxicity of the substances used to treat RAW264.7 cells, CCK-8 assay was used to determine cell viability.Compared to the control group, the treatment with the purified phenolic extract fromC. speciosaat a concentration up to 30 μg/mL led to cell survival rates higher than 90% without any cytotoxic effects(Fig. 2A). However, the treatment with the purified extract at 40, 50 or 100 μg/mL decreased significantly cell viability (greater than 20%),indicating that the concentration of the purified phenolic extract above 40 μg/mL could lead to a certain toxic effect on RAW264.7 cells. A previous study also revealed that a green tea extract at an elevated concentration exhibited a significant toxic effect on RAW264.7 cells [28]. Therefore, the concentration not higher than 30 μg/mL(5, 10, 20 or 30 μg/mL) was selected for subsequent experiments.
3.2.2 Effects of the purified phenolic extract on NO production and morphology of RAW264.7 cells
Macrophages can release a large amount of NO upon activation,therefore, NO production is an indicator of macrophage activation. As shown in Fig. 2B, the NO content increased significantly with the LPS concentration till 0.8 μg/mL, and exhibited a slight initial decrease then remained unchanged when LPS was higher than 0.8 μg/mL.Such LPS-induced changes indicate that the treatment with LPS at a very high concentration could disrupt or even destroy normal macrophages [29]. Therefore, a LPS concentration of 0.8 μg/mL was selected as the concentration for induction.
Compared with the control, the stimulation with LPS at 0.8 μg/mL increased significantly the release of NO (by 5 times) (Figs. 2C and D),indicating that the LPS-induced RAW264.7 cell inflammation model was successfully established. Upon the pre-treatment with the purified phenolic extract, no significant difference was found in NO concentration between the model group and the treatment group with the purified extract at 5 μg/mL, whilst the purified extract at 10-30 μg/mL led to a significant suppression on NO production(P< 0.01) (Fig. 2D). Upon the co-treatment with the purified phenolic extract and LPS, similar phenolic extract concentration-dependent suppressive effects on NO concentration were detected. However, at the same concentration of purified phenolic extract, the suppressive effect was greater for the 24 h co-treatment with the phenolic extract and LPS than the 2 h pre-treatment with the phenolic extract(Figs. 2C and D). A high level of NO can cause inflammation and inflammatory diseases [30]. Therefore, the purified phenolic extract fromC. speciosahad a significant NO-inhibitory effect, and help prevent and treat inflammation and inflammatory diseases (indicated by the pretreatment experiments and co-treatment experiments,respectively). In the subsequent experiments, the co-treatment with the purified phenolic extract (10, 20 or 30 μg/mL) and LPS for 24 h was applied to place a focus on the ability of the phenolic extract to treat inflammation.
The crystal violet staining allowed the visualization of cells(Fig. 2E). Normal RAW264.7 cells were oval or round and full with smooth cell surface. Compared with the normal group, LPS induced distinct dendritic morphology and increased cell size of RAW264.7 cells, likely due to cell differentiation and the increases in the numbers of pseudopodia, bubbles and granular substances.Such changes were suppressed significantly by the purified phenolic extract, with different concentrations of the phenolic extract exerting different extents of suppression. For example, there were still a significant number of differentiated cells at the phenolic extract of 10 μg/mL, whilst most cells had normal morphology at the phenolic extract at 30 μg/mL.
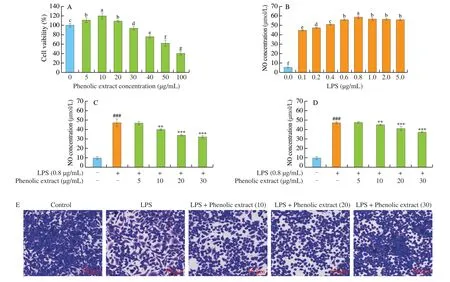
Fig. 2 Effects of the purified phenolic extract from C. speciosa on LPS-induced injury in RAW264.7 cells. (A) The influence of purified extract concentration on cell viability; (B) influence of LPS concentration on cell viability; (C) influence of the co-treatment with the purified phenolic extract and LPS for 24 h;(D) influence of the pretreatment with the purified phenolic extract for 2 h; (E) influence of the co-treatment with purified phenolic extract and LPS on the morphology of RAW264.7 cells. The data are shown means (column heights) ± standard deviations (error bars) (n = 3 independent observations). #P < 0.05,##P < 0.01, ###P < 0.001 compared with the control group, *P < 0.05, **P < 0.01, ***P < 0.001 compared with the LPS-treated group. Different lowercase letters represent significant differences at P < 0.05.
3.2.3 Effects of the purified phenolic extract on the inflammatory cytokines and inflammation-related enzymes
While inflammatory responses vary with the nature of initial stimulus/stimuli, place of occurrence and host’s health status, the activation of inflammatory pathways, release of inflammatory markers, and increases in the levels of inflammatory mediators and inflammation-related enzymes (e.g. iNOS and COX-2) are commonly found [31]. Activation of iNOS can significantly increase the concentration of NO and accelerate the development of inflammation [32,33]. Therefore, down-regulating iNOS and COX-2 expression levels can effectively inhibit inflammation. The levels of inflammatory factors reflect the degree of inflammatory damage and anti-inflammatory effects of therapeutic agents [34]. As shown in Figs. 3A-C, the treatment with LPS alone raised remarkably the levels of TNF-α, IL-6 and IL-1β as well as the protein and mRNA expressions of iNOS and COX-2, whilst the co-presence of the purified phenolic extract at 10, 20 or 30 μg/mL significantly suppressed such LPS-induced increases in a dose-dependent manner.Among the doses of the purified phenolic extract fromC. speciosa,the dose of 30 μg/mL led to the greatest anti-inflammatory effect:greatest decreases in the TNF-α, IL-1β and IL-6 levels (by 66.31%,71.09% and 66.67%, respectively) (Figs. 3A1-A3); greatest decreases in the mRNA expressions ofiNOSandCOX-2(by 82.87% and 79.26%, respectively) (Figs. 3B1and B2); greatest decreases in the expressions of iNOS protein and COX-2 protein (by 81.06% and 63.09%, respectively) (Fig. 3C). Therefore, the anti-inflammatory actions of the purified phenolic extract fromC. speciosaincluded the inhibition of secretion of TNF-α, IL-6 and IL-1β, suppression of NO production, and down-regulation of the expression of iNOS and COX-2 proteins and genes. Phenolic compounds such as 3,4-dihydroxybenzoic acid and quercetin were reported able to inhibit the production of TNF-α by about 23% and 33%,respectively, in RAW264.7 macrophage cells [35].
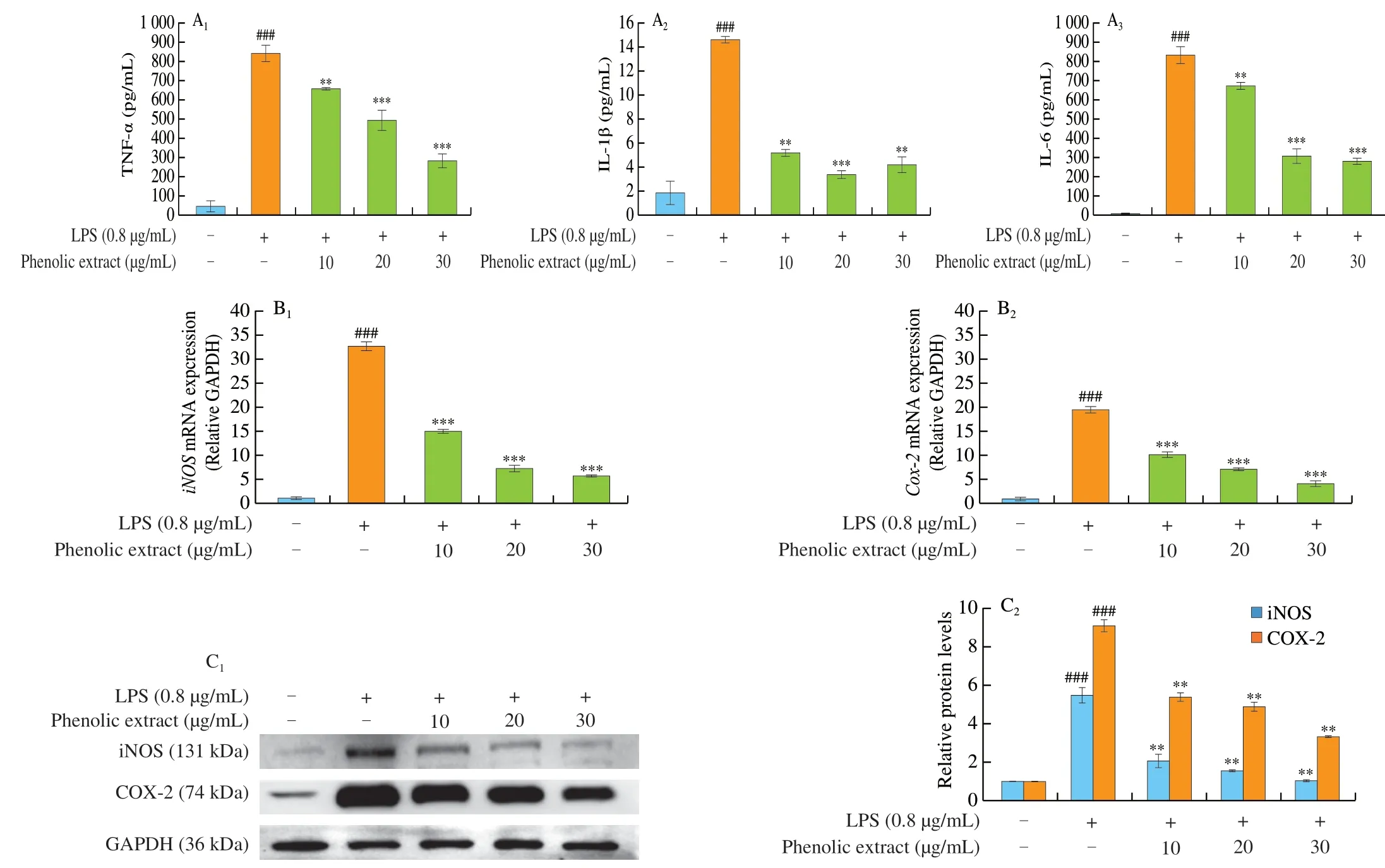
Fig. 3 Effects of the purified phenolic extract from C. speciosa on LPS-induced injury in RAW264.7 cells. (A) The expression levels of TNF-α (A1), IL-1β (A2)and IL-6 (A3); (B) the mRNA expressions of iNOS (B1) and COX-2 (B2); (C) the protein expressions of iNOS and COX-2. The data are shown means (column heights) ± standard deviations (error bars) (n = 3 independent observations). #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the control group, *P < 0.05,**P < 0.01, ***P < 0.001 compared with the LPS-treated group.
3.2.4 Effects of the purified phenolic extract from C. speciosa on the NF-κB and MAPK pathways in LPS-induced RAW264.7 cells
NF-κB, as a heterodimeric transcription factor involving in inflammation and immune responses, typically contains p65 and p50 subunits. The NF-κB pathway induces/regulates the expression of inflammatory mediators and inflammation-related enzymes and genes, thereby playing a critical role in inflammation development and regulation [7,34]. NF-κB regulates the gene expressions ofCOX,LOX,TNF,iNOS,IL-1,IL-6,IL-8, and cell cycle regulatory molecules [36]. The activation of NF-κB is closely associated with the regulatory proteins, the IκB family (including IκBα, IκBβ and IκBε). In resting/normal state, NF-κB exists in the cytoplasm as an inactive complex with IκBs. When cells/cell-surface receptors are stimulated (e.g. by pro-inflammatory stimuli), NF-κB can be activated [37,38]. Activation of NF-κB may take place via ubiquitindependent degradation of IκB in the 26S proteasome or through phosphorylating and activating IκB kinases [39]. The activated NF-κB as released dimers (p65 and p50) is phosphorylated,before translocating from the cytoplasm to the nucleus (involving intermediate mechanisms e.g. dephosphorylation, demethylation,or deacetylation) and initiating the transcription of target genes (by which the levels and/or activities of pro-inflammatory factors are monitored) [7,40,41]. Accordingly, the influence of the purified phenolic extract fromC. speciosaon NF-κB activation during the inflammatory response was examined in this study.
As shown in Fig. 4A, the phosphorylation levels of IкBα and p65 in the cells increased by 4.12 and 3.91 times, respectively, upon LPS stimulation. Compared with the model group, the treatment with the purified phenolic extract fromC. speciosasignificantly suppressed LPS-induced increases in the levels of p-IкBα and p-p65. The most effective dose of the phenolic extract for such suppressions was 30 μg/mL, causing decreases by 87.86% and 72.63%, respectively.Similar results were reported previously. For example, the extract of the rhizome ofAtractylodes lanceaimparted the anti-inflammatory effect via the NF-κB signaling pathway to reduce NO production as well as the mRNA and the protein expressions of iNOS and COX-2 [42]. As shown in Fig. 4B, NF-κB p65 was mainly distributed in the cytoplasm under normal conditions (the control group). After LPS alone treatment, most of the NF-κB p65 translocated into the cell nucleus with an increase of the content. The presence of the phenolic extract fromC. speciosaduring LPS stimulation could significantly reduce the NF-κB p65 content in the nucleus, compared with the cells treated solely with LPS. Therefore, the phenolic extract fromC. speciosapolyphenols can inhibit the phosphorylation of IκBα and p65 proteins, prevent NF-κB from entering the nucleus,thereby inhibiting the expression of genes in relation to inflammation(anti-inflammatory action).

Fig. 4 Effects of the purified phenolic extract from C. speciosa on LPS-induced injury in RAW264.7 cells. (A) The expressions of p-p65, p65, p-IκBα, and IκBα; (B) the nuclear translocation of the NF-κB p65 subunit; (C) the expressions of p-p38, p38, p-JNK, and JNK. The data are shown means (column heights) ±standard deviations (error bars) (n = 3 independent observations). #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the control group, *P < 0.05, **P < 0.01,***P < 0.001 compared with the LPS-treated group.
MAPKs, as critical regulators for the communications between the receptors on the cell surface and the DNA in the nucleus, can direct cellular responses to various stimuli, transmit extracellular signals via consecutive phosphorylation events, and monitor the production of pro-inflammatory cytokines and downstream signaling associated with inflammation [43,44]. There are 7 distinct MAPK pathways in mammalian cells, including extracellular signal-regulated kinases 1 and 2 (ERK1/2), JNK1/2/3, p38MAPK, ERK3/4, ERK5, ERK7/8, and Nemo-like kinase [39]. MAPKs can regulate NF-κB activation via various mechanisms, including the regulation of NF-κB activation by IκB via MAPKs to induce specific phosphorylation. Previous studies reported that the MAPK pathway activated by LPS can indirectly activate the NF-κB pathway and other associated proteins to exert anti-inflammatory effects [45]. Accordingly, the involvement of the MAPK pathway in the regulation of inflammatory response was also investigated in this study. As shown in Fig. 4C, LPS stimulation increased greatly the levels of p-p38 and p-c-Jun N-terminal kinase(p-JNK) proteins (by 2.20 and 2.59 times, respectively). The presence of the purified phenolic extract fromC. speciosaeffectively suppressed LPS-induced increases in the expressions of p-p38 and p-JNK proteins, with the dose of 30 μg/mL being the most effective(suppressed by 30.45% and 51.35%, respectively, for p-p38 and p-JNK expressions). Similar anti-inflammatory actions of phenolic compounds were reported previously, for example, the polyphenols from Shanxi-aged vinegar alleviated inflammation through regulating the MAPK/NF-κB pathway [46].
In summary, the phenolic extract fromC. speciosacan significantly suppress the nuclear translocation of NF-κB, the phosphorylation of the p38 and JNK proteins, as well as the expressions of pro-inflammatory cytokines and inflammatory mediators via the MAPK pathway in RWA264.7 cells. A previous study onC. speciosareported that chlorogenic acid was responsible for the anti-inflammatory effect ofC. speciosa[47].The procyanidin B1 fromC. speciosawas found to exhibit significant anti-inflammatory activity, as it might compete with LPS for the binding sites of toll-like receptor 4-MD-2 heterodimer and inhibiting the downstream activation of NF-κB and MAPK (p38) signaling pathways [48]. As an antagonist of the immune response, procyanidin B1 can participate in the development and progression of many inflammatory diseases at translational levels (via suppressing phosphorylated NF-κB, p38 and MAPK) and also at transcriptional levels (via suppressing NF-κB, myeloid differentiation-2 (MD-2) and TNF receptor associated factor 6 (TRAF-6)) [49]. A previous report,although not onC. speciosa, has linked the anti-inflammatory action of catechin with its ability to monitor the activation/deactivation of inflammation and/or oxidative stress related cell signaling pathways(e.g. NF-κB, MAPKs, transcription factor nuclear factor (erythroidderived 2)-like 2 (Nrf2), signal transducer and the activator of transcription 1/3 (STAT1/3) pathways), and suppress the production and reaction of inflammatory mediators and inflammation-related enzymes (e.g. cytokines, chemokines, iNOS and COX-2) [50].
4. Conclusions
The purified phenolic extract ofC. speciosarich in chlorogenic acid,procyanidin B1 and catechin (determined by HPLC-Q-TOF-MS/MS)clearly demonstratedin vitroanti-inflammatory properties. In LPStreated RAW264.7 macrophages, the purified phenolic extract suppressed NO production and TNF-α, IL-6 and IL-1β levels, and reduced the mRNA and protein expressions of iNOS and COX-2.The mechanisms underlying the anti-inflammatory actions of the phenolic extract ofC. speciosaat least included its regulation of the phosphorylation of related proteins to monitor the expressions of inflammatory mediators and inflammation genes associated with the NF-κB and MAPK signaling pathways. Therefore,C. speciosais a natural source of anti-inflammatory agents and can be developed into therapeutic products for inflammatory conditions and diseases.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgements
This study was supported by the Key R&D Program of Forestry Shandong Province (2021TZXD014), and the Science and Technology Innovation Breakthrough Project of Heze Ctiy (2021KJTP10).
 食品科學(xué)與人類(lèi)健康(英文)2023年4期
食品科學(xué)與人類(lèi)健康(英文)2023年4期
- 食品科學(xué)與人類(lèi)健康(英文)的其它文章
- Call for Papers from Journal of Future Foods
- GUIDE FOR AUTHORS
- Cross-protective effect of acid adaptation on ethanol tolerance in Salmonella Enteritidis
- Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans
- Ameliorative effect of Lacticaseibacillus rhamnosus Fmb14 from Chinese yogurt on hyperuricemia
- Study on the interaction between β-carotene and gut microf lora using an in vitro fermentation model
