A narrative review on inhibitory effects of edible mushrooms against malaria and tuberculosis-the world’s deadliest diseases
Ashim Y. Mouss, Bojun Xu*
a Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Cairo 11566, Egypt
b Food Science and Technology Program, BNU-HKBU United International College, Zhuhai 519087, China
Keywords:Antituberculosis Antiplasmodial Macrofungi Mushrooms Structure activity relationship
A B S T R A C T The isolated secondary metabolites from 39 edible mushrooms are reported, among which 107 compounds were active, 61 demonstrated antitubercular activities with IC50 range of 0.2-50 μg/mL and 46 manifested antimalarial effects with IC50 range of 0.061-36 μg/mL. While more than 2 000 strains of edible mushrooms are identified, this review shows the paucity of research in these rich organisms featuring a vital culinary ingredient worldwide. A thorough search was conducted on basidiomycetes to discuss the chemistry and biology of the isolated compounds, structure activity relationships (SAR) as well as the cytotoxicity prof iles of, primarily, the active anti-plasmodial and antitubercular molecules. With a safe cellular prof ile, lanostane triterpenoids were found to be the only molecules with combined activities against both diseases. SAR correlations reviewed here indicated the significance of 3β- and 7α-hydroxylation in the anti-tuberculosis activity and the terminal unsaturated moiety between C-4 and C-28 in the antimalarial activity in the same terpene skeleton. This review will attract the attention of medicinal chemists, and food scientists to optimize and rationalize the use of mushrooms both as unexploited sources of novel molecules and as nutraceuticals to treat two of the deadliest infectious diseases, malaria, and tuberculosis.
1. Introduction
Tuberculosis (TB) and malaria, being linked to poverty and low hygienic standards, are specifically spread among third world countries [1]. Perhaps a common feature between malaria and TB is the need for strict therapeutic regimens to reach favorable outcomes,namely fast cure without relapses or development of multi-resistant strains [2]. While treatment regimens prescribed for TB encompass at least two effective drugs to be taken for a long period of time, malaria requires the use of artemisinin drug combinations for at least 3 weeks(18-40 days) unless a case of relapse was detected. The issuance of the malaria vaccine marked a hopeful startup, but the price remains a barrier and many doubts if it will ever reach poor countries in sufficient quantities [3,4]. In both cases, the patient’s adherence to therapeutic regimen coincides with successful elimination of these deadly microbes. In low-income populations, where TB and malaria are the deadliest, sufficient patient awareness and education are lacking; meanwhile, the capacity of the healthcare system is not sufficient to report misuse or adequate patient follow-up. Taken together, these factors contribute to a great extent to hindering the eff iciency of antimicrobial agents [5,6].
TB reemergence with serious multi-resistant strains is still endangering people in wide parts of the world, even in countries that have previously succeeded in its eradication. This often occurs with immunocompromised patients and those with co-infections as in human immuno-def iciency virus (HIV). From this perspective, natural resources of fungi, plants, insects, and marine organisms are sought after to provide potential drug candidates to treat the new emerging forms of TB [7,8].
Natural products from plant kingdom, marine sources and microorganisms have been extensively studied for antitubercular and anti-plasmodial diseases with minimum inhibitory concentration(MIC) values from 5 μg/mL to 50 μg/mL; as ursane, seco-taraxane,oleanane and lanostanes and other triterpene carbon skeletons with recorded anti-TB activity [9], yet no reports documented the yield of drugs against these two diseases from edible mushrooms even though macrofungi do possess activities, even of their crude extracts, against several pathogens multi-resistant forms [10,11]. Fungi is a prolific source of bioactive natural products; from nematocidal [12], antiviral,cytotoxic, antimicrobial agents to antidiabetic, anti-cholestermic and immune boosting activities. The genetic structure of fungi does hold the flexibility required to satisfy the superior chemical diversity needed for the aspirated sought for carbon skeletons capable of halting the ever increasing resistant microbial and parasitic strains.
Mushrooms belonging to either ascomycetes or basidiomycetes have been used for therapeutic and culinary purposes for many centuries; particularly, the edible species are popular of their nutraceutical values and use as functional foods [13]. For instance, the entomopathogenic fungus cordyceps grown in the Himalayas, Tibet,Yunnan, Qinghai, and China at high altitude possesses great value in Chinese medicine as therapeutic agent for the following aliments:diabetes, obesity, hepatic diseases, cardiovascular diseases (CVD),cancer and hyperlipidemia [14].
According to the Food and Agriculture Organization figures,mushrooms use is on the rise over the previous decades with escalating efforts to list and identify the edible vs poisonous species to maximize their utilization as alternative food; specifically, in poor countries [15]. Recently, tiger milk mushroom (Lignosus rhinoceros)was studied for its clinical implications on respiratory system and revealed efficacy in respiratory diseases, oxidative stress and immune symptoms based on its traditional use in indigenous communities [16].Many mushrooms are well known from the ethnomedicinal point of view to treat various ailments, yet scientific evidence and clinical approaches have been always lacking to support their use as adjuvant therapies. Alternatively, more funds are needed to be raised in this field to encourage drug discovery of novel bioactive molecules, which if separated, identified, and subjected to clinical studies could present better leads with lower microbial resistance [17-19].
Few literatures are available on mushrooms and their effect on malaria and TB [20,21]; thus, we found it necessary to embark on writing this review to cover this untapped topic. Based on two pillars,the first is drug discovery of new molecules of novel carbon skeletons,and the second is the rationalization of the ethnomycological uses of these mushrooms and perhaps affecting consumption habits with respect to amounts (doses) and methods of preparation of these culinary components as nutraceuticals by local poor people. Using a combination of search words of anti-plasmodial, antimalarial,antitubercular, TB, and antimycobacterial, together with the word mushrooms, basidiomycetes in a collection of search engines; mainly,PubMed, Scopus, Web of Science, SciFinder, Google Scholar and ScienceDirect, a total of 107 compounds were found to be active either against TB or malaria from edible mushrooms.
2. Bioactive compounds from edible mushrooms against malaria and TB
2.1 Antitubercular compounds from edible mushrooms
2.1.1 Polyacetylenes
Polyacetylenes were isolated from a wide variety of organisms,plants, fungi, algae, marine corals, animals and insects. Studies revealed that those detected in fungi are far less than their counterparts in plants even though the chemical diversity is higher. This was shown mostly in the fruiting bodies [22].
FromPolyporus biformisliquid culture,the antibioticbiformin(1)(Fig. 1) was isolated after inoculating agar discs grown on Czapek-Dox medium and revealed activity against virulentMycobacterium tuberculosisas well asStaphylococcus aureusin as low as 0.56-1 μg/mL IC50value together with no demonstrated chemotherapeutic effect on mice [23,24]. FromPoria corticolaandPoria tenuis,two antibiotics named nemotin A(2)and nemotinic acid(3)were isolated and identified revealing activities againstM. smegmasandM. tuberculosisH37RVwith MIC values as potent as 1-4 μg/mL for the former and 7 μg/mL for the latter [25].
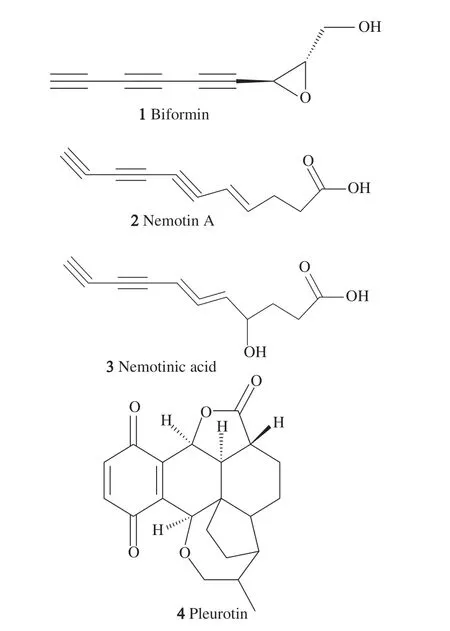
Fig. 1 Chemical structures of polyacetylene compounds isolated from edible mushrooms.
2.1.2 Polyketides
Although polyketides are almost produced by all living organisms,they are prolifically biosynthesized in basidiomycetes and are distinguished for their therapeutic potential as antibiotics, antifungals and anti-hyperchloremic agents. As the genomic era of mushrooms was set later than ascomycetes, and being reflected in the number of polyketide synthases and peptide synthases, the number of discovered polyketides from edible mushrooms is still few compared to that of gene clusters per basidiomycete species [26].
Colonies ofPleurotus griseusgrown on modified Czapek-Dox medium were able to inhibit the growth ofM. tuberculosisresistant strain H234 grown on yeast peptone agar and virulentM. tuberculosisH37 grown on Herrold egg medium although a better activity was noted againstStaphylococcus aureus. In the liquid culture, the antimycobacterial effect was not attributed to the antibiotic pleurotin(4)isolated from the same fungus since pure pleurotin was proved to be ineffective against the sameM. tuberculosisstrain [27], but it was noteworthy that the mycelium extract showed more potent effect than pleurotin(4), which warranted further investigation of other antibiotic molecules. The effectiveness of pleurotin in the inhibition of thioredoxin-thioreductase system was proved by studying different species of basidiomycetes and modified fermentation procedures were adopted to maximize its culture production up to > 300 mg/L [28,29].
The alcoholic extract of the mushroomPleurotus ostreatuswas tested againstMycobacteriaspp. revealing an IC50values from 2.5 mg/mL to 15 mg/mL. Most of the compounds detected by LC/MS were phenolics, flavonoids such as chalcone glucoside (m/z435.0),naringenin-7-O-glucoside, pantothenic acid hexose (m/z381.0) and flavones apigenin (m/z268.1),p-coumaric acid, sinapic acid, galliccaffeic acid ester, myricetin, apigenin whose antimicrobial effect against many pathogens is well studied; nevertheless,M. tuberculosiswas resistant to all detected metabolites and was only susceptible to the crude extract effect. Therefore, the extract activity was attributed to a synergistic effect between the bioactive components [30].
2.1.3 Sterols and terpenes
Terpenes are among the most widely spread metabolites in mushrooms as well as in plants with interesting biological activities that include but is not limited to anti-inflammatory and ant infectious effects. Whereas this class is comprised of diterpenes,sesquiterpenes, sesterterpenes, triterpenes are more frequent in the fungal metabolites. Besides the highly oxidized lanostane triterpenes, more than 80 terpenoids were isolated from the ganoderic acid derivatives inGanoderma lucidium. With antihypertensive,antineoplastic, antihistaminic effects [31]. Steroids is a class of compounds biosynthetically derived from terpenes but possess a four cycloalkane rings with characteristic arrangement; they form the basis of cholesterol, sex hormones as testosterone and estradiol. Herein we compiled the mushroom derived sterols and triterpenes with reported activities against TB and malaria.
Regarding sterols, while theTrametessp. extract inhibited
M. tuberculosiswith IC50of 6.18 μg/mL;Cerrena unicolorwas a source of ergosterol peroxide(5)whose SAR correlations were studied by partial synthesis of its derivatives and revealed the importance of the 3-OH and C-17 long chain, and the endoperoxide moiety was not vital for the antitubercular activity [11]. Cerevisterol(6)is another potent sterol metabolite of 6 μg/mL potency, as compared to a positive control (rifampin MIC 0.25 μg/mL) (Fig. 2).

Fig. 2 (Continued)
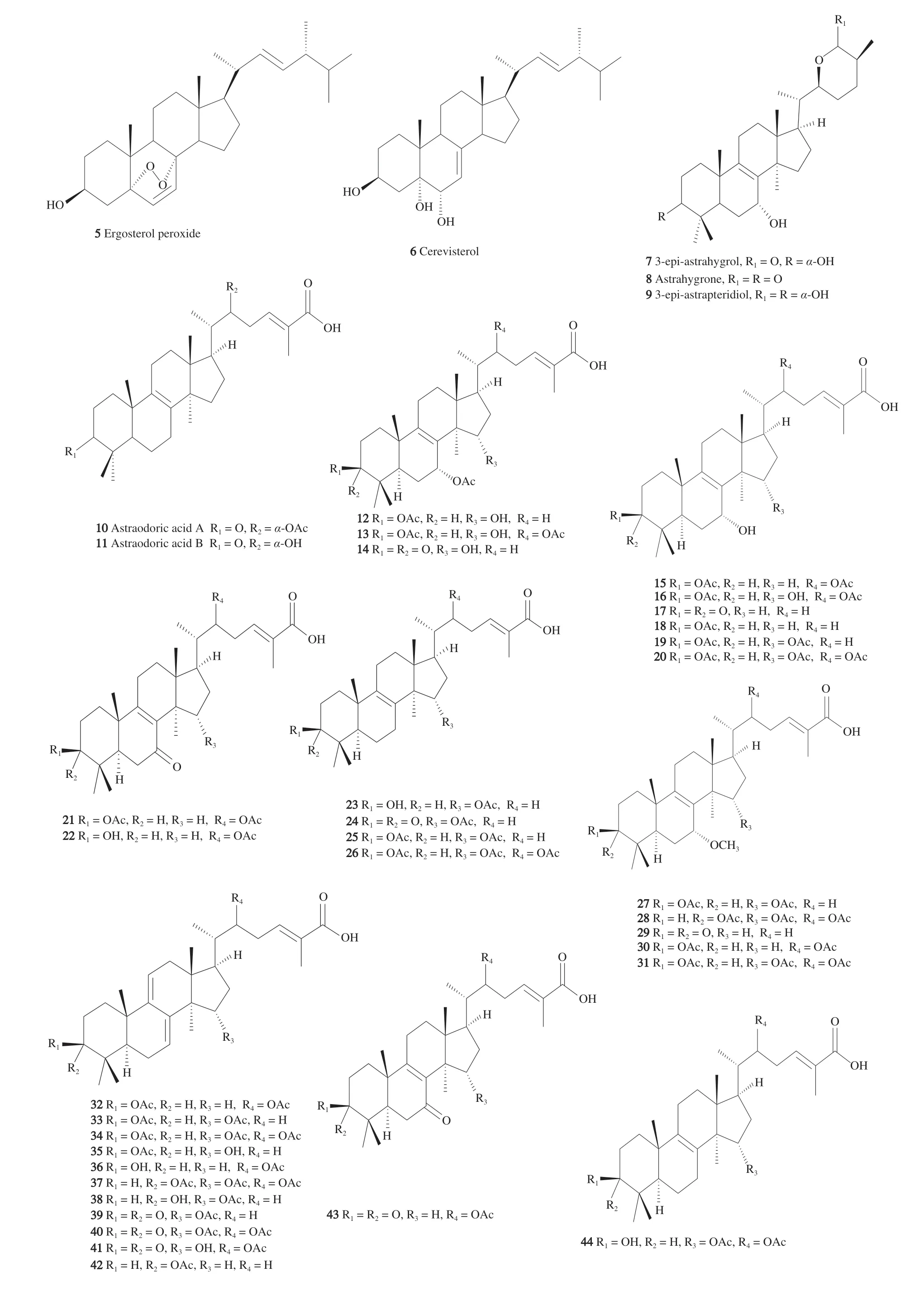
Fig. 2 Chemical structures of terpene compounds with anti-tubercular activity isolated from edible mushrooms.
Astraeus pteridis(Shear) Zeller, belongs to family Astraceae,order Boletales and phylum Basidiomycota is characterized by its unpalatable taste and truffle like nature. From the fruiting bodies,three new lanostane triterpenes were isolated and their structure elucidated by 1D and 2D NMR, high resolution mass spectrometry and X-ray crystallography; their antitubercular activity was recorded.The components of the 95% ethanolic extract were detected, isolated,and characterized as six lanostane triterpenes. These included 3-epi-astrahygrol(7), astrahygrone(8)and 3-epi-astrapteridiol(9),whichrevealed moderate activity againstM. tuberculosisH37Rv(ATCC27294) using the Alamar Blue microplate assay with MIC values of 34, 58, 64 μg/mL, respectively, denoting a 90% decrease in fluorescence with the least concentration. Astrapteridone,astrapteridiol andL-phenylalanine betaine were of negligible activity of > 36 μg/mL. All the compounds were reported to be safe against the Vero cell lines with > 100 μg/mL [32,33].
BothAtraeus asiaticusandA. odoratusare two expensive edible mushrooms (Hed Phor Fai and Hed Phor Nang) widely spread in Thailand and Thai food. Nine compounds were isolated from the false earthstar mushroom (A. odoratus) and tested againstM. tuberculosisH37Ra. While astraodoric acid A(10)and B(11)showed moderate activity with MIC of 50 and 25 μg/mL, astraodoric acid C and D were inactive. The lack of astraodorol activity could be explained by its C-25-R relative configuration compared to 3-epi-astrahygrol with a C-25-S-configuration. However, the confirmed influence of the C-25 stereochemistry required further studies that involve testing the compounds on the same strains ofMycobacteriumH37Rv(ATCC27294). It is worth mentioning that C-3 and C-22 functional groups were vital features in the structure activity relationship of the antitubercular mode of action since the carbonyl group presence was favored in astraodoric acids A and B rather than its absence in the C and D derivatives. Regarding C-22, acetyl or hydroxy groups, were adequately required to demonstrate the anti-TB activity although the hydroxyl group leads to a more active astraodoric acid B than the acetyl group in the astraodoric acid A. In short,the C-3 carbonyl group in conjunction with the C-22 acetyl or OH groups were active in anti-TB activity, yet the C-3 OH group was inactive with either the C-22 OH or acetyl groups; additionally, the carboxylic acid side chain is essential in this diversity. Cytotoxicity of astraodoric acid B recorded an IC50values of 34.69 and 18.57 μg/mL against KB (human epidermoid carcinoma) and 19.99 and 48.35 μg/mL against NCI-H187 (human small cell lung cancer).The new hypaphorine and its 5-OH derivative were devoid of any antitubercular effect [9].
Upon close screening of steroids and their antitubercular effect,it was evident that the polar group and flexible nonpolar phytyl tail with its substituents are critical for the biological activity andMycobacteriumH37Rv strain disruption [34].
Ganodermatriterpenes were widely recognized for their triterpene content and their promising biological activities [35,36].Lately, scientists have been targeting the increased production yield ofGanodermatriterpenes through multiple ways. One way is better understanding of the gene functions and their role in gibberellins (GA)biosynthesis as part of the mevalonate pathway. Another alternative is the submerged culture approach where various media compositions were tried with different parameters and elicitors; moreover,genetic engineering role have been highly regarded to regulate the biosynthesis of key enzymes; particularly, the rate limiting enzymes involved in biosynthesis [36].
Ganodermaor Lingzhe is one of the safe remedies mentioned in the Shen Nong Ben Caosa foundation of traditional Chinses medicine(TCMs) dating back to 2 200 years ago and that can be used over
long periods of time without adverse effects. Basidiocarps are core producers of bioactive lanostane triterpenes, for example, ganoderic acid, with more than 250 derivatives isolated fromGanodermaand similar mushrooms. Extending over two hundred years, not onlyG. lucidiumwas an ingredient of many Chinese health products in East Asia but alsoG. applanatum,G. amboinense,G. carnosumandG. colossumwere related species recognized as components in similar products. As the antitubercular activity of more than 50 myceliumassociated lanostane terpenes(12-47)(Fig. 2), isolated from threeGanodermastrains, were assayed againstM. tuberculosisH37Ra by Isaka et al. [37], plausible SAR correlations were proposed based on the critical 3β- and 15α-acetoxy groups. The first groups that assumed distinctive influence over the biological activity were C-3, C-7, C-15,and C-22; furthermore, (24E)-3β,15α-diacetoxylanosta-7,9(11),24-trien-26-oic acid(37)was regarded as the most potent compound as revealed from its MIC value of 0.391 μg/mL. Continuing with the next highest activity compounds,29and38that reported an MIC value of 0.781 μg/mL. Comparisons between the various derivatives sharing the 7,9-diene, 22Sacetoxy and 15αacetoxy indicated that 3β-acetoxy group is indispensable for the anti-TB activity [37,38].Whereas the 22-acetoxy group reduces the anti-TB activity, 15αacetoxy was suggested to enhance it. The MIC values indicated that C-7 bulky methoxy group was not favored and the 7,9(11)-diene was more active than its 8(ene) congeners. The SAR characteristics based on the close comparison between (24E)-3β,15α-diacetoxylanosta-7,9(11),24-trien-26-oic acid(37)or ganoderic acid and its congeners,which is obeyed by all the compounds except for the 7-oxo derivatives. Despite the unknown antitubercular mechanism of action of lanostane terpenes, they were regarded as safe molecules based on their weak cytotoxic activities [39-42].
In continuation of the previous report, more than forty ganoderic acid derivatives were isolated and characterized from the fruiting bodies and cultures of Ganoderma orbiforme, strain BC16642. Among them is the C-3 epimer of ganoderic acid T(39)that demonstrated a significant antitubercular activity with MIC value of 0.7 μg/mL while the whole mycelial extract showed MIC of 6.25 μg/mL;furthermore, the scaling up fermentation was conducted to produce larger quantities of these oxygenated lanostane bioactive compounds.Weaker cytotoxicity was reported for most of the lanostane terpenes against the non-malignant Vero cell lines within the range of IC50>50 μmol/L except for compounds12,16,19-21,25,26,28,30,31,34,35,37(Fig. 2). Most of the ganoderic acid derivatives were safe regarding their cytotoxic effect except for compounds26,34,35showing an IC50value of 13-15 μmol/L [43].
Unique in their biological activity, lanostane triterpenes are among the very few compounds showing combined anti-TB and anti-malarial activities. It is noteworthy that Isaka et al. [43] reported many of the isolated lanostanoids shared withG. lucidium, yet 16 compounds were new, and the SAR analysis of all isolated compounds revealed that the 3β- and 7-αacetoxy groups were vital to anti-TB activity [43].
FromG. orbiformeBCC 22324 cultivated in Thailand forest biodiversity, ganorbiformin A, D, E, F(46), G, ganoderic acids P, S(48), T, and the C-3 epimer of ganoderic acid T(39)were isolated and characterized by 1D and 2D NMR, HRMS and subjected to anti-TB, anti-plasmodial and cytotoxicity assay protocols on both cancer (NCI-H187, MCF-7 and KB) and normal cell lines (Vero cells). Whereas ganorbiformin derivatives as well as ganoderic acids P and S were either inactive or displayed very weak activities,ganoderic acid T(37)and its C-3 epimer(39)showed promising antimycobacterial activity with MIC 10 and 1.3 μmol/L, respectively.Despite having powerful antimycobacterial potential, the previously mentioned bioactive compounds were slightly cytotoxic to cells,which demonstrated the need for medicinal chemists to introduce modifications where these molecules were considered as future drug leads [44-47]. While compounds49and50manifested an antimalarial of 7.6, 6.3 μg/mL; anti-TB of 25, 12.5 μg/mL; and cytotoxic effects of 17 and 18 μg/mL of the mentioned values, respectively,compounds51and52showed antimalarial activity of 7.4, 3.8 μg/mL;anti-TB effects of 25, 50 μg/mL; and cytotoxic effects of 34 and 28 μg/mL, respectively.
Lepiotaprocerin I(53)(Fig. 2) was isolated from the edible mushroomMacrolepiota proceragrown in Poland and its absolute configuration assigned based on electronic circular dichroism(ECD) calculations and crystal X-ray diffraction analysis. UsingM. tuberculosisH37Ra, the antimycobacterial activity was reported indicating a weak activity with MIC value of 50 μg/mL compared to the standard isoniazid 0.046 9 μg/mL [48]. Cytotoxicity was measured for the same compound against 5 cell lines, SMMC-7721 human hepatocellular carcinoma, HL-60 (ATCC CCL-240) human myeloid leukemia, A-549 (ATCC CCL-185), SW-480 (ATCC CCL-228)human colon cancer lung cancer, and MCF-7 (ATCC HTB-22)breast cancer and recorded IC50values of 10.7, 14.5, 18.8, 16.7, and 25.8 μmol/L, respectively.
Probably the first antitubercular agent isolated from mushrooms was grifolin(54), the trimethyl docadecanyl hydroxy phenyl terpenoid antibiotic fromGrifola confluensandAlbatrellus ovinus.This compound revealed an MIC value of 1.6 g/kg body weight using two species ofMycobacterium,aviumandphlei, and was stable even after heating to 200 °C [49,50].
Hypsizigus marmoreusis an edible popular mushroom cultivated in Asia by different culture methods and recognized for its various biological activities. From the nonpolar fraction of its extract, fifteen compounds were isolated comprised of sterols and polyisoprenepolyols and tested againstM. tuberculosisH37Rv using the Alamar Blue microplate assay. The sterols, ergosterol peroxide(5),cerevisterol(6), 6-epicerevisterol(55), 22,23-dihydrocerevisterol(56),6-O-methylcerevisterol(57)and (22E,24R)-5α,6α-epoxyergosta-8,22-diene-3β,7β-diol(58)and polyisoprenepolyols; hypsiziprenol A9(59)and hypsiziprenol BA10(60)(Fig. 2), revealed a minimum inhibitory concentration in the range of 1-51 μg/mL whereas the rest of the compounds were inactive. The study concludes that the sterols from the lipids ofH. marmoreuswere more significantly contributing to the antitubercular action rather than the hypsiziprenols with ergosterol peroxide and cerevisterol, the only among the 8 tested hypsiziprenols to exhibit potent MIC values of 1 and 6 μg/mL, respectively [51].
SAR correlations proposed that epoxidation at C-6α,8αor both C-5αand C-6αhydroxylation imparts better activity to the 3β-hydroxy sterols as well as the C-7βhydroxylation. It is noteworthy to mention that sterols demonstrated superior antimycobacterial effect whereas hypsiziprenols were of less importance [51].
2.1.4 Phenolics
Phenolic compounds are major components of edible mushrooms and contribute to their beneficial effect as nutraceuticals. Known to be high in protein content, low in fat and cholesterol and rich in vitamins and minerals, mushrooms are postulated as the perfect human diet with proven effects such as antiviral, antimicrobial, anti-inflammatory,antiallergenic actions and excellent antioxidant actions, the latter is imparted by the diverse phenolic constituents in these macrofungi.Furthermore, phenolics exert pronounced antibacterial and antifungal properties; herein this review will introduce the latest pure phenolic compounds with significant activities against malaria or TB or both.
FromRamaria cystidiophora, thecoral fungi mushroom, two linear unbranched new groups of butenolides named ramariolides,
a spirooxiran and a spirooxetane butenolides were isolated from
the fruiting bodies and their structures were elucidated. Among the unusual polyketides observed in nature, spiro oxetanebutenolide &oxeran butenolides were isolated for the second time in nature. Upon assaying the anti-TB effect, the MIC of ramariolide A(61)(Fig. 3)was 8 μg/mL againstM. smegmatuswith a unity ratio between the MIC and minimum bactericidal concentration (MBC), like isoniazid,denoting the bactericidal effect against TB bacteria. Another control drug that was used is spectinomycin whose action was bacteriostatic with a ratio of 2-4 between the MBC and MIC value of 32 μg/mL.Ramariolide A(61) with its novel carbon skeleton largely unmatched in nature except for hygrophorones F and G, revealed a 50%inhibitory concentration of 53 μg/mL and MIC of 64-128 μg/mL [52].
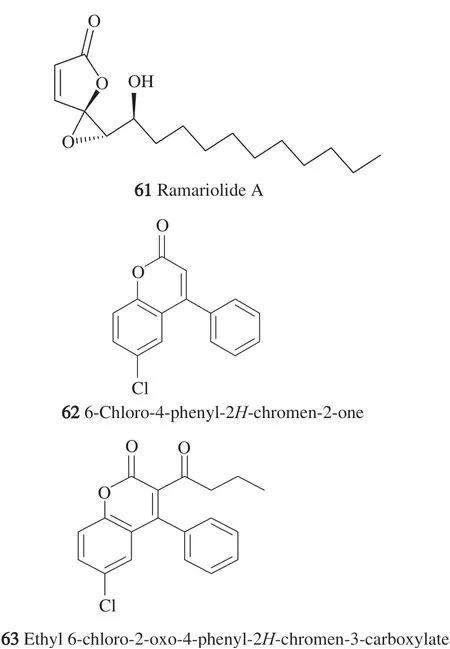
Fig. 3 Chemical structures of phenolic compounds isolated from edible mushrooms.
Fomitopsis officinalisis one of the very polypore fungi,which infects wood rotting commonly found in old forests of the Northern United States. Except for a few chemical reports, the unique triterpenoids in this fungus were largely untouched despite its traditional use to treat TB, asthma, and pneumonia. In a study of the mycelial ethanolic culture ofF. officinalis, two new natural chlorinated coumarins previously synthesized and characterized as 6-chloro-4-phenyl-2H-chromen-2-one(62)and ethyl 6-chloro-2-oxo-4-phenyl-2H-chromen-3-carboxylate(63)were isolated, and their structure confirmed by chemical synthesis. Furthermore, two analogs were synthesized to further profile the spectrum of activity
againstM. tuberculosis. Cytotoxicity assays were conducted against
Vero cell lines for the 4 compounds and showed the IC50values of >100, 36, 30 and 39 μg/mL, respectively. Moreover, the concentrations used were the same causing the bactericidal effect against TB. The target required for the progression of TB drug leads is a SI (IC50/MIC) more than unity; however, it was not evident if this new class of compounds inF. officinalisis responsible for the antitubercular activity encountered by the whole crude extract in ethnomedical uses [53].
To shed light over the mechanism of action of the four coumarins, two assays were carried out, microplate Alamar blue assay (MABA) and low oxygen recovery assay (LORA) designed to measure replicating and non-replicatingM. tuberculosisrespectively.Regarding SAR correlations, the 7-chloro congener with the ethyl ester side chain revealed the highest activity rather than the olefinic 3-substitution, which was slightly less potent. Similarly, the 6-chloro congeners, compounds62and63, with the ethyl ester substitution were of superior antitubercular effect. Yet, the 6-choloro structural variation was dominant in its superior activity if containing ethyl ester. Compounds with promising activity in the LORA carried more significance since they were expected to persist non-replicating bacteria, the primary cause of bacterial resistance; therefore,shortening the therapy duration. Of all the four coumarins, the synthetic 7-chloro-4-phenyl-2H-chromen-2-one was the most potent with MIC values of 23 and 21 μg/mL against both replicating and
non-replicatingM. tuberculosis[53].
2.1.5 Polysaccharides and nucleotide analogues.
Polysaccharides are another type of biologically active constituents in higher basidiomycetes with diverse chemical structures; particularly, theβ-D-glucans were reported to manifest autoinflammatory, anticancer, anti-asthmatic, and immunomodulating effects due to their high molecular weight and uniqueβ-linkage both(β-1-3) and (β-1-6).
FromLentinus edodes(shitake mushroom), the most widely used edible mushroom in the global market, the immunomodulator branched (1,3)-β-D-glucan polysaccharide, lentinan(64), was isolated from the mycelia and fruiting bodies, and its protective effect againstM. tuberculosiswas studied bothin vitroandin vivo.Thein vivostudy was conducted through intraperitoneal injection of lentinan(64)(Fig. 4) in mice prior to TB infection. A dose of 1 mg/kg was administered 3 times in 2 days and monitored through histological examinations, colony forming units (CFU) counts ofM. tuberculosisin spleen, lung, and liver as well as body temperature and spleen weight. Lentinan(64)revealed useful clinical outcomes of experiments through reducing the growth ofMycobacteriainside spleen and liver with a killing index (KI) of 2.03 and 7.9 after 2 and 24 hours, respectively; furthermore, histological results showed marked spleen activation, mobilization of host defense potential and

Fig. 4 Chemical structures of polysaccharide and nucleotide analogue compounds isolated from edible mushrooms.
M. eradication[54,55].
It was pleasant and cheerful to live in the old place now: themother superintended the household, and the father looked after things out-of-doors, and they were indeed very prosperous.
Mushrooms effect on TB has attracted attention since 1 946 when scientists discovered that the juice ofClitocybe nebularispossessed inhibitory effects againstM. tuberculosisandM. phlei, a short time before the isolation of nebularine(65), the purine-9-β-Dribofuranoside, from the same fungus [7,56].
2.2 Antiplasmodial compounds from edible mushrooms
2.2.1 Terpenoids.
Although sesquiterpenes are abundant in Basidiomes, three novel carbon skeleton sesquiterpenes sterostrein A, B, C and two furancontaining illudalanes, sterostrein D and E were isolated from cultures of the BasidiomyceteStereum ostreaBCC 22955, long known for its hirsutanes, cadinanes, stereumanes and sterpuranes terpenes [57,58].The structures stereochemistry was confirmed by modified Mosher’s method and a possible biogenetic pathway was proposed for the dimeric compounds where the dehydroproducts of illudalane and nor-illudalane form a cycloadduct by Diels-Alder reaction followed by aromatization and oxidation of the alcoholic primary group to aldehyde to yield sterostrein C; subsequent oxidation to the acid and decarboxylation allows the formation of sterostreinD. SterostreinA(67)demonstrated activity against the parasitePlasmodium falciparumK1, an IC50of 2.3 μg/mL [59], see Table 1.

Table 1 (Continued)
Table 1The biologically active natural products and their mushroom sources.
The methanolic extract of the air-driedHypoxylon fuscummushroom grown in Nigeria was assayed for its antimalarial effect against two plasmodial strains, the chloroquine resistant W2 and the chloroquine sensitive D6 strain revealing its effectiveness with IC50value of 8.33 and 6.98 μg/mL, respectively, compared to chloroquine and artemisinin whose IC50was less than 0.025 6 μg/mL.As far as reported standards about anti-plasmodial activity are concerned, extracts with less than 10 μg/mL may be called highly active, those within the range of 10-50 μg/mL may fall under the moderate activity and those larger than 50 μg/mL may be regarded as inactive; accordingly,H. fuscumis considered as a potent source of antimalarial molecules, yet the cytotoxicity profile was significant with a cytotoxic concentration of the extracts to cause death to 50% of viable cells (CC50) of 8.60 and 3.33 μg/mL against rhabdomyosarcoma andArtemia salina(brine shrimp), respectively. This was evident when theHypoxylonfruiting bodies extract was tested using the MTT viability assay and the brine shrimp lethality assay (BSLA), and results suggested significant effect in the former, CC50= 8.60 μg/mL,as well as on the latter, LD50= 3.33 μg/mL [60].
In thePlasmodium bergheistrain ANKA, thein vivoeffect ofAgaricus sylvaticussupplementation on oxidative stress was studied.Significant reduction in plasmodial infection marked with higher levels of Trolox equivalent antioxidant capacity (TEAC) was noted in groups of animals receiving either thiobarbituric acid reactive substances (TBARS) or theA. sylvaticuscrude extract with the latter group showing the highest value. What is known as the cytoadherent phenomena is especially characteristic forP. bergheiwhere the disease is marked with ischemia, free radical formation and respiratory distress; therefore, parasitemia is inevitable in this type of severe malaria, which necessitates the use of antioxidants as a major component of the treatment strategy; moreover, nitrites and nitrates(NN) levels were maintained in high levels in groups of animals taking N-acetyl cysteine (NAC) compared to theA. sylvaticus, which simulated in its effect the negative control. The study concluded that adding the mushroom extract or the NAC to rats diet contributed to the reduction of plasmodial infection and parasitemia, which puts forwardA. sylvaticusas a possible adjuvant remedy to malaria [61].A. sylvaticuspossesses highly valued group of antioxidant molecules with superior activity among all natural products [62]. The aqueous extract of the mushroomA. sylvaticuswas incapable of reducing cell viability in both tumor cells (OSCC-3) and non-tumor cells (NIH3T3)at all tested concentrations; thus, the authors deduced its human safe consumption [63].
From the large-scale culture ofPhellinus linteusgrown in Thailand, the methylene chloride and methanolic crude extracts of the mushroom were shown to possess antimalarial activities with IC50of 3.08 and 3.15 μg/mL againstP. falciparum, the multi resistant strain.Their cytotoxicity assays were also significant reporting IC50of 25.7 and 17.3 μg/mL in MCF7 cells; 17.8 and 19.1 μg/mL in NCI-H187 cells, 42.5 and 48.4 μg/mL in Vero cell lines [64].
UsingG. lucidumfruiting bodies aqueous extract, anin vivostudy was conducted on Swiss albino mice whereP. bergheiwas injected intraperitoneal at a concentration of 1 × 107compared to the standard chloroquine for 4 consecutive days, through which the parasite growth was monitored as well as the liver enzymes; alkaline phosphatase (ALP), serum aminotransferases (AST and ALT), and gamma glutamine transpeptidase (γ-GT) to refer to the effect on the mice liver growth compared to the untreated mice. Results pointed out thatG. lucidumethanolic extract effectively inhibited the malaria parasite growth and reversed the damage caused to liver due to the infection. The anti-plasmodial effect was related to the synergistic or single action of sterols, terpenes, and flavonoids ofG. lucidumpreviously isolated [65-67].
From the medicinal mushroomGanoderma boninensefruiting bodies ethyl acetate extract, a group of nortriterpenes were isolated for the first time in nature and named ganoboninketals A-C(68-70). The new skeleton was a rearranged 3,4-seco-27-norlanostane with highly intricate polycyclic system. While ganoboninketals A-C(68-70)manifested an IC50values of 4.0, 7.9 and 1.7 μmol/L,respectively againstP. falciparum, they displayed weak cytotoxicity using the A549 and HeLa cell lines. The new skeleton features of ganoboninketals A(68)and B(69)were a 2,9-dioxabicyclo (4.2.1)nonane moiety with a 6-6-5 ring system whereas the ganoboninketal C(70)(Fig. 5) possessed a decahydro-2’,7’-dioxaspiro-(9α,3α-(epoxymethano) cyclopenta(a)naphthalene-3,3’-bicyclo (2.2.1)heptane [68].
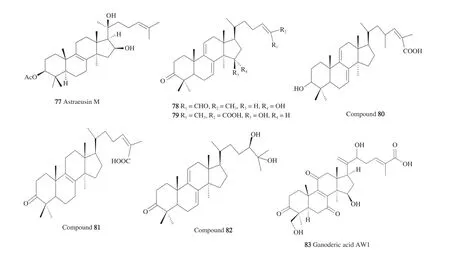
Fig. 5 (Continued)

Fig. 5 Chemical structures of terpene compounds with anti-plasmodial activity isolated from edible mushrooms.
From the fruiting bodies of theG. boninense(Polyporaceae), 6 new nor-lanostane terpenenoids with a unique tetradecahydrobenzo[4,5]indeno[1,7a-c] furan skeleton was isolated. The antimalarial effect was measured using thein vitroassay onP. falciparumrevealing IC50values of 27, 15 and 2 μmol/L for ganoboninone A, B, F(71-73),respectively. Ganoboninketals A-C(68-70)were recorded to possess antimalarial activity with IC50values of 4.04, 7.88, and 1.72 μmol/L [69]. From the fruiting bodies of the artificially cultivated basidiomyceteGanodermasp. BCC 21329, 6 new lanostane triterpenes were isolated and identified based on their 1D and 2D NMR data and mass spectrometry; the absolute configuration was detected according to the modified Mosher method. Moderate anti plasmodial effect was recorded for the 4 compounds74-77by using the microculture radio-isotope technique againstP. falciparumK1 with IC50of 3.8-7.6 μg/mL; furthermore, the antitubercular effect was evaluated by the green fluorescent protein (GFP) microplate assay but turned out to be insignificant [70].
From the edible mushroomRussula nigricans, commonly known for being sold as food in Thai markets, 3 marasmane sesquiterpenes given the trivial names russulanigrins A-C and one normarasmane russulangirin D were isolated and identified from the fruiting bodies with another 6 known sesquiterpenes named isolactarorufin,lactarorufin A, lactarorufin methyl ether and three other russulanigrin D derivatives. All the structures were elucidated based on the 1D and 2D NMR and mass spectrometric information. Russulanigrin D,normarasman and marasman lactone were assayed for both antimycobacterial and anti-plasmodial activities usingM. tuberculosisH37Ra andP. falciparumK1, respectively, demonstrating insignificant MIC values at concentrations of 50 μg/mL for the anti-TB, and even 10 μg/mL was inactive for the antimalarial assay.Moreover, cytotoxicity tests were conducted against several cell lines(KB, MCF-7, and NCI-H187) and revealed inactivity [72,73].
From the food mushroomAstraeus asiaticus, 7 new lanostane triterpenes; astraeusin M-Q, 26-epi-astrasiaone and 26-epi-artabotryol C1 were isolated with 17 known compounds comprised of astraodoric acid and astraeusin derivatives. All the isolated compounds were assayed againstPlasmodium falcipariumK1, yet only astraeusin M(77)revealed significant effect with IC50value of 3 μg/mL [74].
The fruiting bodies ethyl acetate extract ofG. lucidumor Lingzhe revealed a marked antimalarial effect of 79% inhibition at 4.9 μg/mL.Seven lanostanes and a benzofuran derivative of ganomycin B were isolated and assayed for their biological activity againstP. falciparum.Their results proved that the effectiveness of the polar fractions containing ganoderic acids was far less than the nonpolar lanostanes whose inhibition ranged from 40% to 79% while the former was less than 10% inhibition. From the eight compounds, ganoderic aldehyde TR(78)manifested the highest activity with IC50of 6 μmol/L followed by 23-hydroxyganoderic acid S(80)and ganoderic acid S(48)reporting an IC50of 11 μmol/L. Ganoderic acid DM(81)showed lower activity probably due to the lack of the conjugated 7,9(11)-diene.Ganodermanondiol(82)(13 μmol/L) was more active than ganoderic acid TR(47)and ganoderic acid TR 1(79)with IC50of 20 and 18 μmol/L,respectively. Both ganoderic acid DM and ganofuran B, the furan derivative of ganomycin B were inactive at 20 μmol/L. Therefore, the lanostanes reported from 6 to more than 20 μmol/L activities against malaria parasite [75].
Another ganoderic acid derivative, ganoderic acid AW1(83)was isolated from an EgyptianGanodermasp. and showed anti-plasmodial effect againstP. falciparumchloroquine sensitive strain with IC50250 nmol/L and negligible cytotoxic effect on Vero cell line [76].
From the basidiomycetesFomitopsis feei, three new methyl lanostane triterpene were isolated together with four known compounds. The structure of fomitopsin D was proved after the acidic conversion reaction of fomitopsin D into a mixture of fomitopsin E and F [77-79]. Ganoboninone A, B, F and ganobonineketal B, C showed promising anti-plasmodial effects against theP. falciparum3D7 strain. While compounds such as ganoboninone A and B manifested anti-plasmodial effect of 27 and 15 μmol/L, others such as ganobonineketals C, D, and E were shown to be inactive within IC50values of > 100 μmol/L. Core values that direct the SAR of antiplasmodial activity might be attributed to the terminal unsaturated moiety between C-4 and C-28 d.
2.2.2 Polyketides
From the edible genusAnthracophyllum,belonging to family Marasmiaceae, the broth and cell culture extracts ofAnthracophyllumsp. BCC18695 revealed a promising antimalarial effect for the strainP. falciparumK1 with minimal cytotoxicity using the MCF-7 and Vero cell lines, which put these extracts forward as good candidates for isolating effective anti-plasmodial molecules. Subsequently,the isolation procedures led to the separation of 2 new spirosesquiterpenes, anthracophyllic acid, anthracophyllone [80] and 7 known compounds, among them three compounds, aurisins A(84), G(85),and K(86),(Fig. 6) demonstrated the antiparasitic effect under investigation with IC50values of 1.43, 0.27 0.69 μg/mL, respectively.In this study, aurisin A stereo chemistry was determined for the first time by X-ray crystallography, and aurisin G proton and carbon NMR data were reported. The active components ofAnthracophyllumsp.BCC18695 manifested close resemblance to the chemical profile of the luminescent mushroomNeonothopanus nimbibelonging to the same family, possibly pointing out to the Marasmiaceae chemotaxonomic markers [81]. It is to be borne in mind that aurisin A and k revealed in another study significant antitubercular activities together with cytotoxic effect in NCI-H187 and cholangiocarcinoma
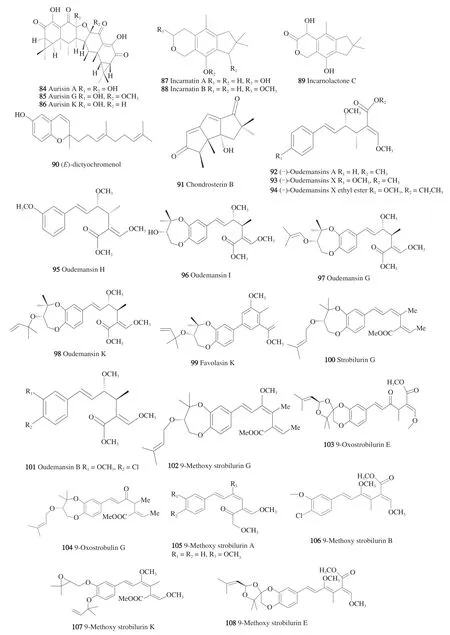
Fig. 6 Chemical structures of polyketide compounds isolated from edible mushrooms.
cell lines ranging from 1.5 μmol/L to 50 μmol/L [82].
Chemical profiling of the crude extracts ofGloeostereum incarnatumBCC41461 mushroom yielded 11 unprecedented compounds: the illudane type structures; incarnatins A-B(87,88),incarnolactone C(93); incarnatin E, incarnetic acid, incarnatenin;and the alliacane type structure, incarnate methyl ester; additionally,(E)-dictyochromenol(90), and chondrosterin B(91)were two known compounds obtained from both the broth and cell cultures of the fungus in malt extract agar. After the chemical spectroscopic identification involving 1D and 2D NMR of the compounds, both antimalarial and anti-TB assays were conducted against all the compounds, which were isolated from the culture broth but for
incarnatin B(88), incarnolactone A and (E)-dictyochromenol(90),
which were purified from the cell culture. Incarnatin A exhibited potent anti-plasmodial (K-1 strain) effect with IC50value of 9.8μg/mL, similarly, incarnatin B(88)and chondrostean B revealed significant antimalarial activity with IC50values of 3.9 and 3.1 μg/mL,respectively. The known compounds chondrosterin B(91)and (E)-dictyochromenol(90)manifested an activity againstM. tuberculosisH37Ra with MIC value of 12.5 μg/mL. Cytotoxicity of the isolated compounds was assayed using KB, MCF-7, NCI-H187, and Vero cells cell lines and showed low cytotoxic potential of incarnatin A(87)and incarnolactone C and most of the compounds except for chondrosterin B(91)and (E)-dictyochromenol(90),which were of strong cytotoxic effect. Biological activities suggested that the lack of hydroxyl group in incarnatin B and C at C-11 was probably responsible for the anti-TB and antimalarial bioactivities as well as for the cytotoxicity. The hydroxyl group at C-6 in incarnatin A(87)was probably involved in the antimalarial role it imparts at a concentration of 50 μg/mL [83].
From the mushroomXerulasp. BCC56836, family Physalacriaceae, order Agaricales, isolated from the Thai forest soil in Chiang Rai province, Thailand, twelve new polyketide compounds were isolated from the cell and broth cultures. Identification unveiled the nature of the compounds as (+)-oudemansin A lactone, (+)-11-epidihydrooudemansinol, oudemansin A acid,(-)-oudemansin X ethyl ester, (+)-xeruhydrofuranol, xerucitrinic acids A, (-)-dihydrooudemansinol, (+)-oudemansin X lactone and 9 known compounds: (-)-oudemansins A, and X, (3Z,5E)-3-methyl-6-phenylhexa-3,5-dien-1-ol (13), 2-(E-hept-5-en-1-yl)-3-methyl-6,7,8,8a-tetrahydro-4H-pyrrolo[2,1-b][1,3]oxazin-4-one) [84].Besides, the cytotoxic effect was assessed and proved to be low in both normal (Vero) and cancerous cell lines (MCF-7), KB, and NCI-H187 for most of the compounds by using resazuin microplate assay. The used assay for the multidrug resistant anti-plasmodial effect was microculture radioisotope technique and revealed a potent activity for (-)-oudemansins A and X(92, 93)and (-)-oudemansin X ethyl ester(94)againstP. falciparum, K1 strain with IC50range from 1.19 μmol/L to 13.70 μmol/L [85].
From theMarasmiellussp. BCC 22389, new sesquiterpenes were isolated and named marasmiellins A and B belonging to the hirsutane type compounds commonly found in basidiomycetes, yet they showed negligible activities both in the antimalarial and antitubercular assays despite being safe and inactive in cytotoxicity. The structures and absolute configuration were assigned based on the 1D and 2D NMR,HRMS and modified Mosher method, respectively [86,87].
From the mycelial culture ofFavolaschiasp. BCC 18686 was collected from a Thai bamboo tree and the closely relatedFavolaschia caloceraBCC 36684 was collected from unidentified twigs in mixed Thai forest [88], the methoxy acrylic acid derivatives; strobilurins and oudemansins were isolated and structure activity relationships of their antimalarial activities were proposed. Based on the unique HPLC and NMR chemical profiles shown by the mycelial fungal cultures, which differed from its previously discussed close speciesF. tonkinensisBCC 18689 [89], the authors embarked on studying their active components and antimalarial effects against the multidrug-resistant strainP. falciparumK1. The new compounds were oudemansins H, I, G, and K(95-98), oudemansinols A and B, O(phenylacetyl) oudemansinol B, favolasins A, G, and K(99), and favolasinin as well as three polyketides, and (R,E)-2,4-dimethyl-5-phenyl-4-pentene-2,3-diol fromFavolaschiasp.BCC 18686.Furthermore, favolasin E and 9-oxostrobilurin E were isolated fromF. caloceraBCC 36684 for the first time. The known compounds were strobilurin G(100), oudemansins A(92)and B(101),9-methoxystrobilurins A, G(102)and K, 9-oxostrobirulins E(103)and G(104), 11-epihydrooudemansinol, (S,E)-3-(hydroxymethyl)-5-methoxy-4-methyl-5-styrylfuran-2(5H)-one fromF. caloceraBCC 36684 and nineβ-methoxy acrylate-type derivatives. Using the microculture radioisotope technique for assaying the anti-plasmodial activity, some compounds revealed strong antimalarial effects such as 9-methoxystrobilurins A, B(105, 106)and G whose IC50values recorded 0.061, 0.089, and 0.140 μmol/L, respectively, while their cytotoxicity was the minimum against Vero cells (African green monkey kidney fibroblasts [90].
Because of the occurrence of oudemansinol derivatives along with oudemansin ones inFavolaschiasp., they were proposed to share a common biosynthetic pathway, which indicated a similarity in the configuration of C-9 in both classes. Moreover, hydroxystrobilurins conversion to the biphenyl structures was seen as co-metabolites with favolasins [91], which suggested that the former might be the biogenetic precursors of the latter [77]. In short,Favolaschiasp.demonstrated a unique diversity in its chemical molecules, andF. calocerarevealed the most potent anti-plasmodial action through the action of 9-methoxy strobilurin derivative; additionally, other(E)-β-methoxyacrylate-type polyketides as oudemansin A(92)and B(101)were significant malaria suppressors with the least cytotoxicity to Vero cells. As far as SAR is concerned, bulky nonpolar groups attached to the benzene ring with the methyl enol ether at C-9 of the polyene chain were important. 9-Methoxy strobilurins K(107), G(102),and E(108)were the most potent in their anti-plasmodial effect with IC50values of 0.089, 0.061, and 0.140 μmol/L, respectively [88].Based on their own spectroscopic NMR data, the authors concluded that 9-oxotrobilurins are derivatives of their methyl enol ether congeners 9-methoxy strobilurins when exposed to acidic medium.
Due to theβ-methoxy acrylate moiety, oudemansins and stobilurins revealed wide biological activity profiles, among which are insecticidal, antiviral, antitumor activities. As far as antimalarial activity is concerned, the structures correlated with the activity since the methoxy strobilurins manifested a clear categorization in potency againstP. falciparum. While 9-methoxystrobilurins K(107)and G(102)showed significant effect with IC50of 0.089 and 0.061 μmol/L,9-methoxystrobilurin E was less effective with IC50of 0.14 μmol/L,and 9-methoxystrobilurins A and B with IC50of 1.35, 0.85 μmol/L were the weakest in their action.
Regarding oudemansins and following the aforementioned trend,substituents of the aromatic ring; particularly, the ether-linked isoprene group played a vital role in SAR correlations where oudemansins G and K exhibited a more active nature than oudemansins A, B, I, and H.The 9-methoxy group manifested a remarkable importance to boost the antimalarial effect as demonstrated in the IC50value difference between 9-methoxystrobilurin G and strobilurin G; likewise, the 9, 10 double bonds of the 9-methoxy strobilurins compared to oudemansins and the oxostrobilurins. Inactive or weak compounds were mostly polyketide derivatives or lacking the (E)-β-methoxy acrylate moiety.It is worth mentioning here that the most active compounds againstP. falciparumwere the ones with the least cytotoxicity as proven by their calculated selectivity indices.
The nonpolar fraction ofPleurotus ostreatusrevealed its nutraceutical potential with a yield of 0.93% (m/m) when assayed for its antimalarial effect through the plasmodium lactate dehydrogenasein vitroassay demonstrating an IC50of 25.18 μg/mL compared to the standard chloroquine whose IC50reported 0.016 μg/mL. The selectivity index was measured to be greater than 4 due to the extract mild cytotoxic effect, and ergostan-5,7,22-trien-3-ol was isolated and identified with various spectroscopic techniques from the extract [13].
3. Discussion
More than 2 160 known edible mushrooms were identified to date, yet only a few were adequately studied in literature [15]. Edible mushrooms are abundant sources of bioactive compounds that remains to a great extent untapped. The quest for anti-TB and antiplasmodial compounds urges the use of novel sources with creative genetic systems that could be propitious to the production of drug leads invulnerable to antibiotic resistance. Chemistry of TB active compounds revealed dominance of the terpenes, sterols, polyketides,polyacetylenes, and phenolic compounds, the kind of carbon skeletons tailored with supremacy in mushrooms genetic system, which sets these organisms forward as promising source of future drug leads for these diseases.
Based on the analysis carried out in this review, 273 compounds targeting malaria and TB were isolated from 39 medicinal mushrooms,out of which, 108 were active carbon skeletons.Ganodermaspecies recorded both activities, and this was a hallmark for the lanostane triterpene skeletons. In lanostane triterpenes, SAR analysis manifested that 3βand 7-αhydroxylation was vital to the anti-TB activity, which was enhanced by further hydroxylation at C-5α,6αor epoxidation at C-6α,8α. In coumarins, the ethyl ester side chain was favored in the 7-chloro or the 6-chloro congeners whose structural variation was preferred for better activity. In sterol molecules, the 3-OH and the C-17 long chain were emphasized; furthermore, 15αacetoxy and not the 22-acetoxy was suggested to enhance the anti-TB effect. 3β- and 7α-acetoxy groups were vital to the antitubercular activity as well as the 7,9(11)-diene.
Concerning the antimalarial effect, the terminal unsaturated moiety of lanostanes between C-4 and C-28 is important. Within the oudemansin nucleus, the potency results suggested the significance of the bulky nonpolar groups attached to the benzene ring as etherlinked isoprene groups; additionally, the methyl enol ether at C-9 of the polyene chain is a key player in anti-plasmodial mechanism. Extra boosting effect can be made by the 9-methoxy group in oudemansins and the 9,10 double bond of the 9-methoxy strobilurins. Inactive oudemansin derivatives could be due to the polyketide nature or the lack of the (E)-β-methoxy acrylate moiety.
The IC50values of the anti-TB molecules showed the most potent compound as (24E)-3β,15α-diacetoxylanosta-7,9(11),24-trien-26-oic acid(37)as revealed from its MIC value of 0.391 μg/mL followed by with the next highest activity compounds,29and38fromGanodermasp.The antimalarial most significant molecule was 9-methoxy strobilurin G and K with IC50values of 0.061, 0.089 μg/mL recorded to be produced from genusFavolaschi calocera; interestingly, the mechanism of action and deep molecular studies are lacking in most of the studies reported herein. Probably more focus on the molecular targets would impart an idea about the changes medicinal chemists would need to do to optimize these active carbon skeletons
Medicinal mushrooms can be used as a nutraceutical in poor countries to treat and limit malaria and TB. Medicinal mushrooms are not exploited, and a lot of research funds are required in this field. Mushrooms are rich in their metabolites, and the fact that it represents a part of human diet in many parts of the world encourage our propensity to discover new bioactive molecules from them;especially, the molecular requirements for TB active drugs and antimalarial drugs are well satisfied by the mushroom biosynthetic nuclei. The taxonomical efforts need to be intensified to properly characterize the edibility of mushrooms as many species still lie under the title (unrecognized) while they produce effective molecules against malaria and TB. The number of active crude extracts indicates that even the current list of edible mushrooms warrants further investigation to isolate possible drug leads.
Although mushrooms use is pronounced as nutraceuticals in various diseases; for instance, autoimmune diseases, heart,inflammation, hypertension, hyperlipidemia, and to promote the nature of gut microbiota, their use as antimalarial and anti-TB nutraceuticals is almost lacking despite the notable variation in their antimicrobial effects among the various basidiomycetes [92].
4. Conclusions
In this review study, the promising use of edible mushrooms as nutraceuticals against TB and malaria is highlighted; particularly,Ganodermafor their rich triterpenes in the former andFavolaschiasp.polyketides in the latter. Beyond the notable numbers of isolated bioactive compounds, many remain to be identified from these rich depositories. The structure activity relationship was evaluated and key features such as hydroxylation, esterification or acetoxy group additions were manifested in lanostane triterpenes and coumarins to boost their anti-TB effect. The methoxylation, the presence of a bulky nonpolar group and unsaturation of a terminal position in oudemansin and strobilurin polyketides, respectively, contributed to a better antimalarial effect. Generally, none of the mentioned compounds showed significant cytotoxic affect, which support our hypothesis that edible mushrooms are safe to use for these two fatal diseases.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
This work is jointly supported by two grants (UIC202007 and UIC202107) from BNU-HKBU United International College.
- 食品科學(xué)與人類健康(英文)的其它文章
- Call for Papers from Journal of Future Foods
- GUIDE FOR AUTHORS
- Cross-protective effect of acid adaptation on ethanol tolerance in Salmonella Enteritidis
- Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans
- Ameliorative effect of Lacticaseibacillus rhamnosus Fmb14 from Chinese yogurt on hyperuricemia
- Study on the interaction between β-carotene and gut microf lora using an in vitro fermentation model

