Taxonomic Composition and Spatial Distribution of Meiofauna Community from a Sandy Intertidal Zone in Sishili Bay, Yellow Sea Coast (Northern China)
PAVLYUK Olga N., TREBUKHOVA Yulia A., SHCHERBAKOV Ilya A., TARASOVA Tatiana S., LUTAENKO Konstantin A., CHEN Linlin, SONG Bo, LI Xiaojing, and LI Baoquan, *
Taxonomic Composition and Spatial Distribution of Meiofauna Community from a Sandy Intertidal Zone in Sishili Bay, Yellow Sea Coast (Northern China)
PAVLYUK Olga N.1), TREBUKHOVA Yulia A.1),SHCHERBAKOV Ilya A.,TARASOVA Tatiana S.1), LUTAENKO Konstantin A.1), CHEN Linlin2), SONG Bo2), LI Xiaojing2), and LI Baoquan1), *
1) A. V. Zhirmunsky National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Sciences, Vladivostok 690041, Russia 2) Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China
The meiofauna composition and trophic groups of the nematode communities have been studied at a sandy intertidal zone in Sishili Bay along the Yantai coast (Yellow Sea, China). Nematoda was dominant among the 11 groups of meiofauna. The meiofaunal densities were low, ranging between 111±47 and 542±131ind./10cm2. Results obtained from the correlation analysis made it possible to determine several factors that influence species composition and distribution. Generally, sediment granulometry significantly affected the communities. The highest abundance was found in fine-grained sediments (318.77±126 ind./10cm2) compared to coarse-grained sediments (175±82 ind./10cm2). Nematodes were predominant in all studied sites. A total of 70 species belonging to 52 genera and 18 families were identified in the study area. There was a significant difference in nematode abundance (ANOVA, F=2.38,<0.05) between the three sites. Nematode communities were characterized by a high level of species diversity in sediments composed mainly of very-fine sands (>63μm), with dominant species belonging to the genus,,,. The trophic groups were dominated by non-selective deposit feeders (40.7%) and epistrate feeders (41.29%). In contrast, the species composition in medium grain sands was characterized by a low species diversity index and a high Simpson dominance index. Nematode communities showed similar dominant species compositions belonging to a single genus,,and, and in terms of trophic structure-representatives of deposit feeders (46.15%), predators, and omnivores (64.71%) prevailed. The divergence of meiofaunal community structures can be explained by abiotic factors such as sediment grain size, salinity, and organic carbon content. Specifically, sediment particle size can explain the diversity of nematodes at the level of in abundance, diversity indices, and trophic structure. The highest nematode density and diversity coincided with the highest percentage of very fine sands.
meiofauna; nematodes; sandy intertidal zone; sediment grain size; Yellow Sea
1 Introduction
Sishili Bay is located in the northern Yellow Sea, along the coastline of Yantai City, China. Yantai is a harbor city enclosures fast development with over 3 million citizens, which has posed threats to the marine ecosystem. Pollutant input, shipment of YantaiPort,and intensive coastal raft aquaculture for scallop over the last two decades have had a major impact on the bay’s marine environment. For this reason, there is an increasing interest in studying the environmental quality of the intertidal zone of the Yantai coast.
Studies on the macrobenthic communities in coastal waters of Sishili Bay have been conducted since the 1990s (Wu and Zhang, 1994; Wang., 1995; Li., 2013; Wang and Li, 2013). Moreover, a few studies focused on the changes of the macrobenthic community influenced by the intensive scallop culture and other environmental variables (Zhou., 2006; Han., 2013; Li., 2013; Zhou., 2018). Recently, there have been numerous researches on the distribution of contaminants (.., heavy metals, industrial and domestic wastewater disposal,.), nutrients in the sediments around Yantai (Hao., 2011; Li., 2013; Wang., 2018; Zhou., 2018; Zhao., 2019), and microplastic pollution (Zhang., 2019). The temporal and spatial differences in the distribution of macroalgae communities in the littoral of Yantai were also studied (Han and Liu, 2014). Long-term studies of nutrient and chlorophyll anomalies during red tide have been carried out (Hao., 2011). The distribution and pollution of arsenic and mercury in the surface sediments of the littoral zone of Sishili Bay were assessed (Hao., 2011). Although many aspects of marine ecology and environmental conditions have been studied in Sishili Bay, the meiofauna community in this area is still unknown due to their essential functionings.
Many studies on the meiofauna and their organisms have been carried out along the China coastline, from the intertidal zone (Hua., 2016а, b; Yin., 2017) to subtidal (Liu., 2007, 2015; Gao and Liu, 2018). Recently, meiofauna is beingextensively studiedin the Bohai Sea (Zhang., 2001; Guo., 2002a, 2002b; Mu., 2002; Zhang., 2010; Khima., 2018; Yang., 2019). In the East China Sea (Lin., 2004; Zhang., 2004), studies show that the meiobenthic ecosystem has been degenerating since the 1990s due to anthropogenic activities such as pollution. However, few studies have been conducted in the Yantai waters, particularly for meiofauna and free-living nematodes. The analysis of the meiofauna community structure could offer some vital information that cannot be achieved by using macrofauna analysis (Giere, 2009; Semprucci., 2016). Moreover, because meiofaunal organisms are bound to the sediment throughout their all life history and are often sensitive to many pollutants (Coull and Chandler, 1992; Guo., 2001). Furthermore, it has been shown that meiofauna features are good indicators of environmental conditions and changes caused by anthropogenic activity.
The present work provides a preliminary pioneer study on meiofauna communities in Sishili Bay, the northern Yellow Sea coast. The main goal is to clarify the density, species diversity, structure of meiofauna communities, particularly free-living nematodes in the intertidal zone.
2 Materials and Methods
2.1 Study Area
Meiofauna samples were collected at the intertidal zone of Sishili Bay in June 2018. Sishili Bay is a 130km2embayment on the Yellow Sea coast with an average water depth of 8–9m (Fig.1). Sandy beaches largely represent the intertidal zone except for the rocky substratum on the northern side of Yangma Island. The tidal regime in the bay is semidiurnal, with a maximum tide range from1 to 3m. The maximum tidal current along the periphery is almost 40cms?1, decreasing to about 10cms?1inside the bay (Zhang and Dong, 1990). Thus, the hydrodynamic of Sishili Bay features significant mixing currents and weak stratification, which conversely affects pollutant diffusion and sediment deposition.
To compare different marine environments, 3 differentsites (sites A, B, and C) were selected to collect meiofauna samples in the intertidal zone of Sishili Bay. Site A is characterized by a high urbanization development intensity near Yangma Island. The sediments of the intertidal zone were represented by slightly silted, very fine black sand.

Fig.1 Location of the studied sandy beaches and sampling stations (indicated by numbers).
At site A with very fine sandy grain size (63–125μm), three transects (.., A, A', and A") were located perpendicular to the waterline, with 100m from each other. Three sampling stations were chosen on each transect (A1, A2, A3; A'1, A'2, A'3; A"1, A"2, A"3), which respectively represented the high, middle and low tidal zones. Site B is located in Tianyue beach, with medium sandy grain size (250–500μm) and lower human activities than site A. One transect (.., B1, B2, B3) was established at site B. Site C with fine sandy grain size (125–250μm) is located in the area of Yantai University. Only 2 stations (.., C2 and C3) were set up at site C, representing middle and low tidal zones due to its narrow geographical features.
2.2 Sediments Sampling and Laboratory Processing
Meiofauna samples were taken during a low tide in triplicates using hand cores (3.6cm in diameter and 30cm in height) from the upper 10cm layer. Three replicate samples were taken for meiofauna at each station, and three replicates were taken for organic content, salinity, and grain size analysis. Extracted samples were fixed and stored in a 4% neutralized formalin solution. Samples for particle size analysis and total organic content were stored at ?20℃ until analysis.
Animals were extracted from the sediments by Ludox flotation (Heip, 1985), the meiofauna were separated from the sediment using the LudoxTM centrifugation technique. The samples were washed into a centrifuge tube with a small amount of fresh water. Approximately 20mL of Ludox was added to the centrifuge tube. A variable-speed vortex mixer set at the maximum was used to mix the sample for 30s, then the sample was mixed for 4min at the lowest setting. Each sample was centrifuged at 900×for 5min. The solution containing the meiofauna was then poured over a 32μm sieve. The contents of the sieve were washed with water to remove any Ludox from the sample. The meiofauna sample was then washed over sieves of varying sizes (500, 250, 125, 63, 32μm), starting with the largest sized sieve to separate the meiofauna according to size. In the laboratory, all samples were stained in 70% ethanol with Rose Bengal for more than 24h. The meiofaunal samples were washed into a petri dish, and the meiofauna was sorted and counted under a stereoscopic microscope.
The water depth, bottom water temperature, and salinity were measured, and some bottom water samples were taken to measure DO (dissolved oxygen). The sieving method and sedimentation method were jointly used for grain size analysis. The sieving method was used to analyze particles over 63μm, and the sedimentation method was used to analyze particles below 63μm. Grain size composition was classified into fractions: coarse sand (500–2000μm), medium sand (250–500μm), fine sand (125–250μm), very fine sand (63–125μm), and silt (<63μm). Total Carbon and TON were measured: all the carbon in the sample, consisting of both inorganic and organic carbon; TOC=Total Organic Carbon: material derived from decaying vegetation, bacterial growth, and living organisms; TN=Total Nitrogen.
All samples were stored in plastic bags and frozen immediately for further analysis. For organic carbon content analysis, samples were firstly treated using 1molL–1HCl solution for 12h to remove inorganic carbon. Then soil organic carbon (TOC) and total nitrogen (TN) contents of acidified samples were determined using an elemental analyzer (Vario MACRO cube, Elementar, Germany). The sediment grain size was analyzed using a laser particle analyzer (Malvern Mastersizer 2000F, Malvern 130 Panalytical, England).
The meiofauna was sorted into major taxa using an inverted microscope. From each sample, about 200 nematodes (or all individuals if fewer) were picked out, transferred to pure glycerol, mounted onto permanent slides, and identified to genus/putative morphospecies using the pictorial keys (Platt and wick, 1988; Warwick,1998), taxonomic articles, and the NeMys Database (Be- zerra., 2019). The morphology of buccal cavities classified the trophic types of nematodes: 1A, selective deposit feeders; 1B, non-selective deposit feeders; 2A, epigrowth feeders; 2B, omnivorous/predators) (Wieser, 1953).
2.3 Data Analysis
Data were analyzed to 1) compare the distribution of environmental factors between intertidal areas, 2) characterize the community distribution considering composition, density, and feeding groups, and 3) find possible differences between areas with different environmental factors. All meiofaunal densities were expressed as individuals per 10 cm2for each sample.
Statistical differences among stations were tested by two-way analysis of variance (ANOVA) using the quantitative information about the meiofaunal communities (number of taxonomic groups, density). Spatial variation of meiofaunal community structure was displayed using a non-metric multidimensional scaling plot (-MDS), based on the Bray-Curtis similarity matrix calculated from the square-root-transformed abundance data. To test the hypothesis that the density of meiofauna is different at three tidal zones (high, middle, and lower) and to decipher if it is significantly different (or not) between the various levels, we calculated a matrix of dissimilarity scores for eve- ry pair of sites.The Pearson correlation coefficient was used to investigate the relationships between meiofaunal abundance and environmental variables.
Nematode species abundance data (ind./10cm2) were used to calculate the number of species per sample (), Pielou’s evenness (′), Shannon-Wiener diversity ('), and the Simpson’s domination indices (). The significance of differences in univariate measures between sites was tested using a one-way ANOVA. Nematode species richness was calculated as the total number of species collected for each site. All indices were calculated in the PRIMER 7 software (Clarke and Gorley, 2015). Similarity matrices for multivariate data (.., nematode community structure) were built using the Bray-Curtis similarity measure of square root-transformed abundance data. Analysis of similarity (one-way ANOSIM) was used to assess the significance of differencesbetween intertidal zones (high, middle, and lower) from all areas. The similarity percentages analysis (SIMPER) was applied to determine the contribution of higher meiofaunal taxa towards the discrimination of groups (Clarke, 1993). Differences in environmental variables between sites were then tested using a one-way ANOVA.The same software also performed the Principal Component Analysis (PCA).
3 Results
3.1 Environmental Conditions
During the study period, salinity in Sishili Bay sediments fluctuated between 21‰ and 28‰.The surface- water temperature and oxygen concentrations showed a similar pattern, varying from 18.1 to 19.2℃ and 4.88 to 8.67molL–1, respectively. Organic matter content in all sediments was generally low, ranging from 0.0028% to 0.152%. These parameters at site A differed slightly from sites B and C, with the lowest sediment TOC and salinity (Table 1). The granulometric composition of the bottom sediments differed between stations (Table 1). At site A grain size was characterized by the dominance of the very fine sand fraction at most stations surveyed. The sediments at site B were denser than at transect A and was characterized by coarse and medium sandy sediments. Site C was characterized by fine sand sediments. A principal component analysis (PCA) was applied based on the environmental characteristics to visualize the most prominent environmental gradients (Fig.2). Statistically significant differences between grain size characteristics were found (Table 2).
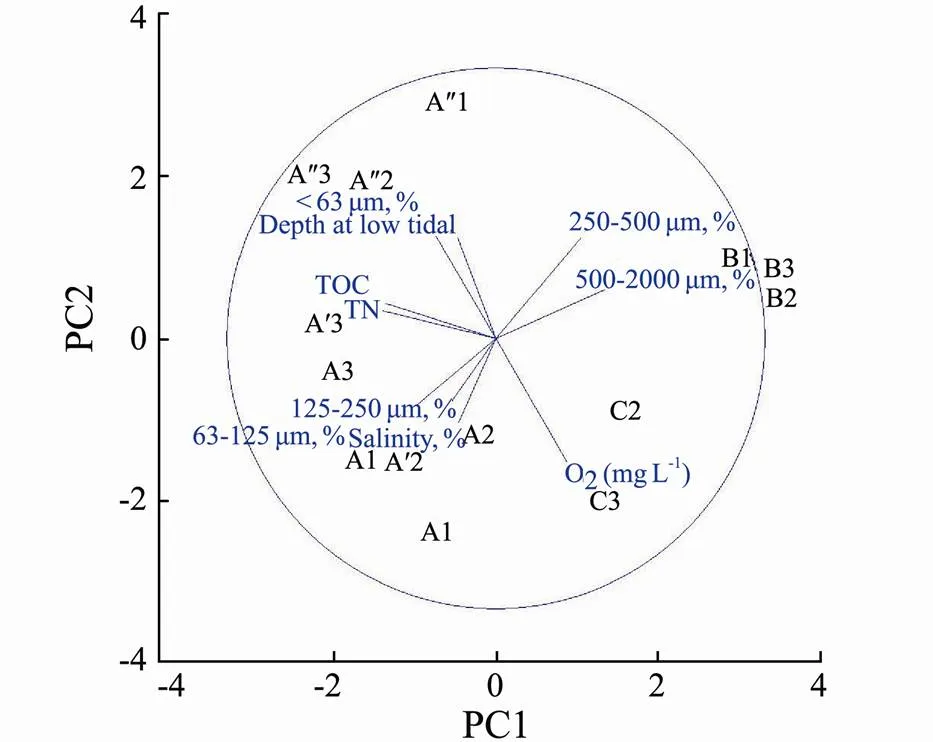
Fig.2 PCA plot of sediment grain size, TOC, and salinity across sites.

Table 1 Environmental characteristics of the beaches

Table 2 Coefficients on the linear combination of variables making up the PC’s for three sandy beaches of Sishili Bay
There is a significant difference among the three sites in the median diameter of sediments (ANOVA, F=4.44,<0.05). At site A, the sediments are mainly composed of very fine sand, with silt percentage (fraction <63 μm) up to 9.33%. Moreover, the median grain size of site A was lower than the other two sites. Sediment in the sampling stations at site B consisted mainly of medium sand (250–500μm), and the median grain size was the highest compared to the other two sites (A and C), which characterized by a lower median and consisted of very fine to fine grains (Table 1).The sorting coefficient 1.05–1.09, an index for well-sorted sediments) was not significantly different among the three sites (ANOVA, F=6.45,< 0.05).
3.2 Meiofaunal Density and Taxonomic Composi-tion in Three Intertidal Zones of Yantai Coast
The density of meiofauna communities was relatively low, with uneven spatial distributions in the Sishili Bay tidal zone (Fig.3). The highest density was found in the middle tidal zone at all transects. The highest density and diversity levels were reported for the transect А′ (430±150 ind./10cm2). The total meiofauna density was the lowest at transect B (175±44ind./10cm2).
A total of 11meiofaunal higher taxa were identified, of which the dominant group was marine nematodes (Fig.4). Harpacticoids, Ostracods, and Polychaetes were the three most common taxa, contributing to 97% of the total meiofauna. The meiofaunal community was dominated mainly by the nematodes (.., 80% in all investigated sites), followed by the harpacticoid copepods (.., 9%). Polychaetes are the third dominant group with higher numbers than ostracods (Fig.4). Ostracods comprised 3% of the total meiofauna. The rest groups such as Turbellaria, Gastropoda, Amphipoda, Bivalvia, Isopoda, Amphipoda, Oligochaeta, and Cumacea were uncommon in the study areas (see Fig.4, ≤0.10ind./10cm2in average). Cumacea and Amphipoda were found only at stations А, А′ and А″. Nematodes abundance was significantly lower at medium sandy (site B) and fine sandy (site C) than at site A. The remaining groups did not significantly vary among the three studied sites (Fig.4).
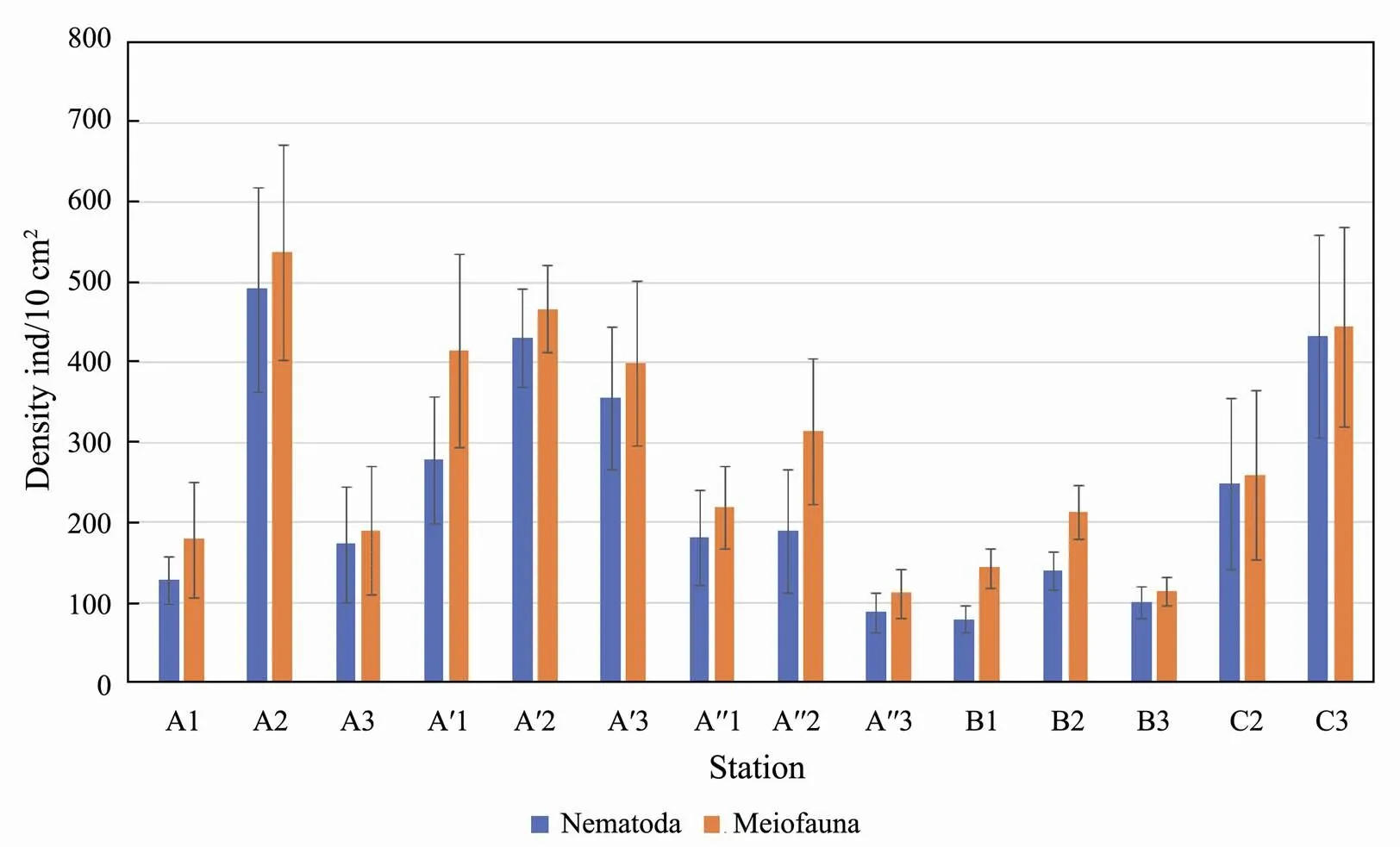
Fig.3 Mean abundance (mean ± SD, ind./10cm2) of meiofauna and nematodes.

Fig.4 Relative abundance (proportion) of the meiofaunal taxa at each sampling station.
In our study, meiofauna showed considerable spatial variability between stations. This pattern was more likely controlled by the distribution of nematodes due to their high densities in meiofauna communities. Nematodes dominate meiofauna communities comprising more than half of the total meiofauna, and harpacticoids are usually subdominant. This was the case for all sites during our study except for station B2 in the middle tide zone. The number of higher meiofaunal taxa at sampling stations ranged from 3 to 7 (Fig.4).

Fig.5 Non-metric multidimensional scaling on square-root transformed meiofauna abundance data across three sandy beaches.
The numerical relationship between meiofaunal taxa varies. These differences were explained mainly by Poly- chaetes and Ostracods, which were significantly higher in terms of abundance and percentage contribution to total meiofaunal abundance in the study areas (Fig.5).
The two-way ANOSIM showed that two groups of sampling stations differed in their taxa composition. Specifically, taxa composition at site A differed significantly from those at site B (global=0.556,<0.05) and site C (global=0.444,<0.05). However, the taxa compositions between sites B and C are not significantly different.
When examining differences between sampling units across all tide zone groups, similarity analysis showed that differences between site groups were statistically sig- nificant (ANOSIM,=0.568,<0.05). The ANOSIMencompasses a low-value (0.218), which indicates an even taxa distribution within and between studied groups. The densities of total meiofauna recorded in the middle tide zones at all sites were significantly higher than the high and low zones, a most obvious difference is seen (=0.481,<0.05).
3.3 Correlation Analysis with the Environmental Factors
Meiofaunal abundances differed significantly among the sampling sites (<0.01), indicating high spatial abun- dance variability in the study area. The spatial differences of meiofaunal assemblages were correlated with the environmental factors, using Pearson correlation coefficients between faunal and environmental factors (Table 3). According to the correlation results, some environmental factors were positively related to the meiofaunal abundance except TON, which was negatively related to the community. The main environmental factors that influenced the distribution of the meiofaunal abundance were the grain size composition, salinity, and organic matter concentration (see Table 3, TOC and TON concentrations were not high in the sediment during our survey). Therefore, only grain size composition was taken as the main factor during further community structure analysis. There was an overall increase in meiofaunal densities with decreasing sediment grain size, although Ostracods were more abundant in larger grain-sized sediments at site B (Fig.6). Increasing salinity positively affected the abundance of harpacticoid Copepods (=0.44,<0.05). More- over, organic content was an essential factor in the abundance of Copepods (=0.62,<0.05).
The density and taxonomic diversity of meiofauna communities were uneven in silted and sandy areas. The analyses revealed apparent differences in the community structures between the sites, particularly between site A and the other two sites (Fig.6). The content of TOC in the sediment was low throughout the study period, so no significant influence over the meiofaunal assemblage structure was observed. The density of nematodes showed substantial differences between very fine sediments (site A) and fine to medium sandy zones (sites B and C).

Table 3 Pearson correlation between meiofauna and environmental factors
Note: Marked correlations are significant at<0.05.

Fig.6 Non-metric multidimensional scaling (n-MDS) plots, based on a Bray-Curtis distance matrix calculated from the square root-transformed, bubble size equals relative nematodes, turbellarians, harpacticoids, and polychaetes abundance (ind./10cm2) at each beach.
3.4 Abundance and Structure of Nematode Communities
The average nematode density was low in the tidal zone of the Sishili bay, which was 243.6±116.51ind./10cm2. The highest average density (492.0±80.00ind./10cm2) was found in the middle tidal zone at all transects. The lowest density, except for station A", is noted in the upper tidal zone (station В1, 80.0±16.52ind./10cm2).
A clear separation distinction of the three sites tested was confirmed by ANOSIM across all different zones (=0.887,<0.001). Based on the results of nMDS analysis, two groups of sites were distinguished, i) nine stations from site A, and ii) consisted of all stations B and C (Fig.7). The characteristics of nematode assemblages differed between all stations (Fig.3), and the station groups show- ed significant differences on one-way ANOSIM (=0.580,<0.05).

Fig.7 MDS-analysis of sites on the fourth root transformed data using the Bray-Curtis index of similarity.
The abundance of nematodes varied significantly between the three study sites (ANOVA, F=2.38,<0.05), with the highest value found at site A and the lowest at site B. The average density of nematodes at site A was 466.8±275ind./10cm2. At site C (.., medium sandy sediments), the average nematode density was 342±72ind./10cm2.
3.5 Nematode Abundance and Diversity
A total of 70 species were identified in the area, belonging to 52 genera and 18 families. The present study has shown that five families of nematode species dominated the Sishili Bay in the study period: Enoplidae, Oncholaimidae, Tripyloididae, Chromadoridae, and Comesomatidae. The percentage of dominant nematode genera and species signi?cantly varied between stations regarding the nematode assemblages.
The dominant species in community A differed from those in community B and C. In contrast, sites B and C had common dominant species (Table 4). The four famili- es dominated At site A, which were Chromadoridae (accounted for 16%), Comesomatidae (15.8%), Tripyloididae (9%), and Xyalidae (6.7%). 45 genera and 68 species were found, dominated bysp. 1 (16.8%),(12.5%),sp. (10.3%),sp. (7.7%), and(5.7%). At site B, three families were dominant: Enoplidae (41.1%), Tripyloididae (29.9%), and Oncholaimidae (18.6%). Fourteen genera and 19 species were found,dominated (29.9%),sp. 1 (13.3%),sp. 2 (21.5%), and(12.6%). The same families dominated site C as at site B. 21 genera, and 26 species were found, dominated by(41%),(22%),(22.5%), andsp. 2 (8.6%). As a whole, a high number of putative nematode species were recognized in the study. We have described a new speciesof the family Enchelidiidae (Enoplida), when working with samples from Sishili Bay (Zograf., 2020).
As for the species diversity of nematodes, site A had the highest diversity indices, despite the Simpson’s dominance index () reaching the lowest value at this site (Table 4). Specifically, site A had the highest species diversity index () and the lowest dominance index (), thus indicating a more diverse nematode community. Moreover, species richness (Margalef index) was found to be significantly higher (ANOVA, F=4.82,<0.05) in comparison with that at sites B and C. The species richness indices, Simpson’s dominance () and Pielou’s evenness ('), were lower at sites B and C.
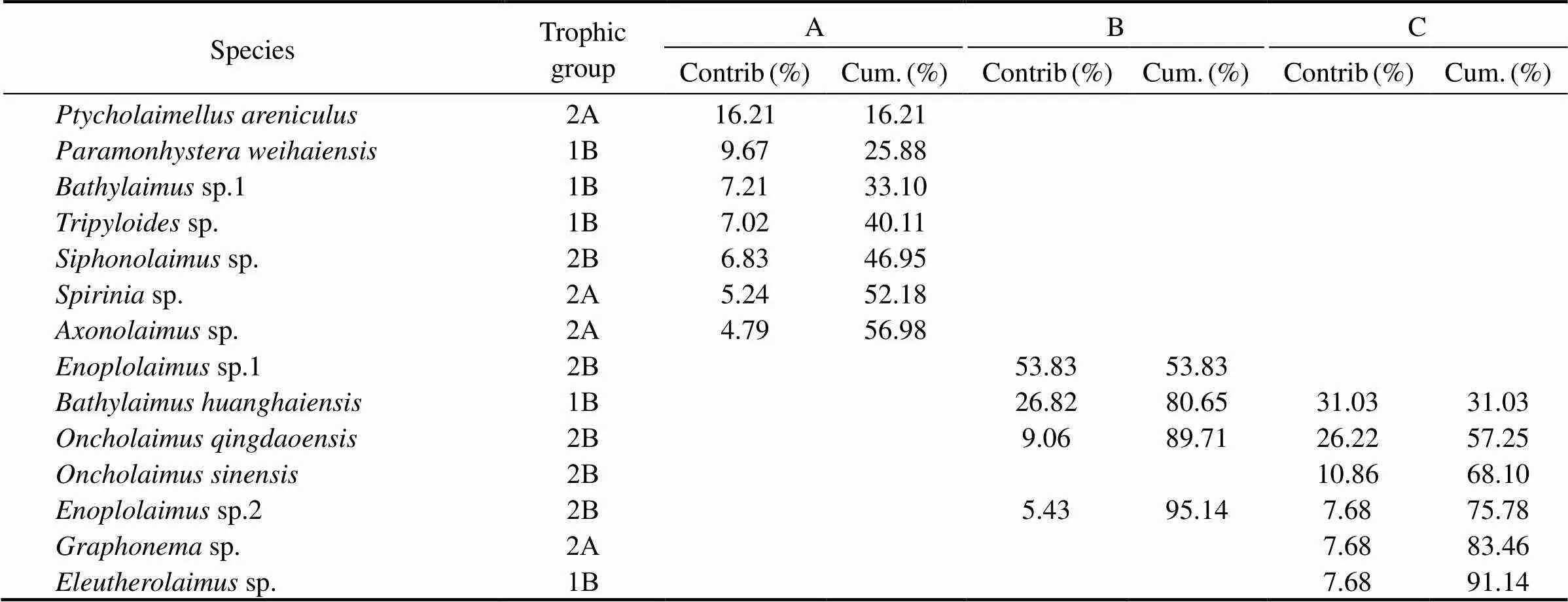
Table 4 Top discriminating species (>5% of the community and shared species) for three sandy beaches of Sishili
The highest densities and diversity of nematodes coincided with the highest percent of very fine sands (site А). The Shannon-Wiener diversity index was significantly different (ANOVA, F=13.91,<0.05) among the three study sites (Table 4). In contrast, Simpson’s dominance was lowest at A and significantly different from the other two sites (ANOVA, F=12.63,<0.05). The′ index was also significantly different among sites (ANOVA, F=3.74,<0.05), being lowest at sites B and C due to the dominance of certain specific, such asand. At site A, species richness (Margalef’s index) was significantly higher (ANOVA, F=4.82,<0.05) compared to both other sites (B and C).
Site A was dominated by non-selective deposit feeders 1B (40.7%) and epistrate-feeders 2A (41.29%); the pro- portion of predators and omnivores 2B made up 17.76%. Site B was dominated by members from group 2B, 64.71%, and the proportion of detritus-feeders 1B was 32% with 3.1% epistrate-feeders. Site C was dominated by deposit feeders 1B (46.15%) and predators/omnivores (41.55%), with 7% epistrate-feeders. Selective deposit feeders were recorded only from site A, with their proportion being less than 0.1 % (Fig.8).

Fig.8 Proportion of the trophic groups of nematodes at different sites.
4 Discussion
Many studies have reported the spatial distribution of meiofauna along with coastal areas in China. Generally, meiofauna densities ranged between 4.67±2.08ind./10 cm2to 294.00±180.40ind./10cm2, with a middle range value of 162.31±84.36ind./10cm2recorded in northwest Taiwan (Cai., 2020). Moreover, in other parts of around the world, low values for temperate sandy beaches (361±128ind./10cm2) were also previously recorded from the North Sea in France (Kotwicki., 2005), the Baltic coast (161±23ind./10cm2), and the Mediterranean region (638±208ind./10cm2) (Gheskiere., 2005). Compared to previous studies, the meiofauna density reported in the present study was very low at 297.21±129.9 ind./10cm2. In addition, significant variability was observed in the meiofauna abundance, composition, and structure of communities simultaneously with the variability of environmental conditions.
In the Yellow Sea, some studies were performed in Jiaozhou Bay and Qingdao Bay (Zhang., 1993; Huang., 2005; Hua., 2016). However, there are no available studies concerning meiofauna data from Sishili Bay. There were significant differences between meiofaunal abundance in the nine sandy beaches at six latitudinal gradients along Chinese coasts (Hua., 2016). Overall, the meiofauna abundance reported in the present data was lower than in other studies, such as sandy beaches in Qingdao Bay (from 1006 to 2170 ind./10cm2, Hua., 2016). The average density and taxonomic composition in the current study sites were also lower than other sandy littoral zones, for example, in Zhoushan (495±250ind/10cm2, Xu., 2013) and Xiamen City (Hua., 2016). Moreover, the taxa number of meiofauna is much lower than that found in 9 beaches studied by Hua. (2016).
It is known that the structure of meiofauna on sandy beaches is affected by various physical parameters, such as temperature, salinity, oxygenation, and others. Generally, sediment granulometry significantly affected the meiofauna community composition. As responses to the sediment characteristics (grain size, silt content, median size.) inside our studied area, meiofauna community showed significant differences throughout the study period being the sedimentary type of very fine sands the most important, jointly with other variables, such as salinity and oxygen content.Abiotic factors can explain the low meiofauna density in Sishili Bay, since the Yantai coast and adjacent marine ecosystems suffered from dense population and fast development of economy and agriculture. Our study recorded the highest densities at the middle tidal zone (Fig.3). Many authors also noted the same fact (Gheskiere., 2004; Rodriguez, 2004; Kotwicki., 2005a; Pereira., 2018). The mid-tide level represents an intermediate zone where hydrodynamics and low-tide exposure are generally more at equilibrium, allowing more species to co-exist (Wu., 2019). As far as food availability is concerned, one of the main factors explaining the meiofaunal variations could be food patchiness in the intertidal zones. For example, the local distributions of bacteria and diatoms are the most likely factors affecting small-scale distribution patterns of meiofaunal assemblages (Blome., 1999). The bacteria composition and microphytobenthos distribution may also be responsible for the patchy pattern of meio- benthos (Galtsova, 1991; Vanaverbeke., 2000; Tchesunov, 2006; Mokievsky, 2009).
The overall meiofauna community showed the typical features described in other beaches in China, as nematodes were the dominant group. In the study areas, the average density of nematodes accounted for 243.6± 116.51ind./10cm2, which was relatively low compared to the other sandy beaches in China (Dang., 1996; Fan., 2006; Du., 2011; Hua., 2016). However, some other China localities, such as Xiamen and Zhoushan, also showed low values of nematode density ranging from 87 to 161ind./cm2(Hua., 2016).
In the Sishili Bay, the sampling sites were dominated by fine to very fine sands throughout the entire study period. The abundance of nematodes strongly correlated with median sediment grain size (see Table 3, 250–500 μm). The sediment at site A, with the highest density of nematodes recorded, was mainly composed of very fine sand (particle size between 63 and 125μm). The sediment at site B, characterized by the lowest abundance of nematodes, was composed mainly of medium-grained sand (see Table 1, grain size between 250 and 500μm). At site C, the density of nematodes was slightly higher than site B, and the sediment was dominated by fine-grained sand (Table 1). These results showed the sediment composition and texture as essential factors determining the nematode abundance at the studied sites. The dominant species can also confirm the correlation between sediment types and community composition. Specifically, the genera at site A were most commonly found on sandy beaches, for example,,,,, and(Heip., 198; Nicholas and Hodda, 1999; Gheskiere, 2005; Gingold., 2010; Fonseca and Fehrlauer-Ale, 2012; Lee and Riveros, 2012; Moens., 2014). At sites B and C, genera,, andare typical sandy beach representatives (Heip., 1985).
Nematode taxonomic diversity indices differed between the studied sites. Site A showed the highest index of species diversity () and the lowest index of dominance (), indicating that nematodes belonged to more diverse taxonomic positions. In contrast, only a few species dominated 90% of the nematode communities in sites B and C. In the present study, almost all the taxonomic diversity indices correlated with the environmental variables, except for the evenness index (').Furthermore, the differences in taxonomic diversity were statistically significant between all three study sites (Table 5).
The concentration of food material in bottom sediments also plays a major role in the nematode density distribution. Site A was dominated by epistrate-feeders (2A, 41%) and non-selective deposit feeders (40%). Both feed on the same benthic microalgae but have different feeding strategies: deposit-feeders ingest algal cells, while epistrate-feeders cleave or pierce cells and suck out the contents (Moens., 2014; Moens and Beninger, 2018). Thus, it can be assumed that the diatom content was quite high in the bottom sediments of the study area.
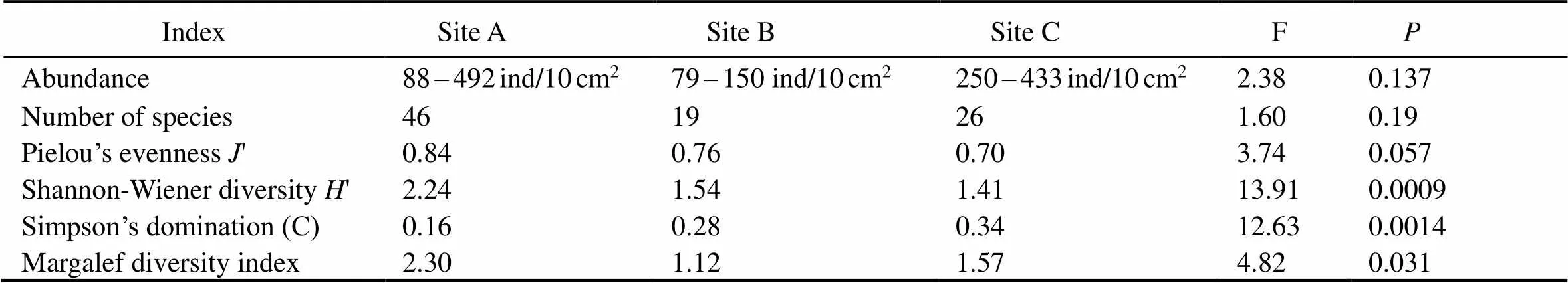
Table 5 Nematode abundance and diversity indices across three beaches
Note:<0.05.
Sites B and C were dominated by predatory/omnivo- rous species of the genera,, and a large-size speciessp., a typical non- selective detritus-feeders at sandy beaches (Heip., 1985). Predatory/omnivorous nematodes are very flexible in terms of nutrition mode. Depending on the competition with other consumers, they can switch to predation on heterotrophic protozoa or even on herbivores and bacte- rivores (Moens., 1999c; Hamels., 2001; Moens., 2004; Franco., 2008; Moens., 2014;Moens and Beninger, 2018)., although it is a detritus feeder, can also transform into predators feeding mainly on ciliates and bacteria (Moens and Vincx, 1997). Thepopulation was more than 30% in the study area (Table 5). Therefore, it can be assumed that there was a high abundance of ciliates at sites B and C.
5 Conclusions
The meiofauna composition and environmental conditions were studied in the intertidal zone of Sishili Bay. Sediment composition explained most of the differences observed in meiofauna spatial distribution. The density and taxonomic diversity of meiofauna communities were uneven in very fine sands and in coarse sandy areas. The meiofaunal community was dominated mainly by the marine nematodes (.., from 61% to 84% in all investigated sites). The nematode communities differed significantly between the three study sites. At site A, the very fine sediments resulted in a high level of species diversity. At site B, the sediments were mainly composed of mediumgrain sands, and the species composition was characterized by a low diversity index and high domination index. Non-selective deposit feeders and epistrate-feeders dominated site A, while predators and omnivores dominated site B. Deposit feeders, predators, and omnivores formed the most essential trophic group at site C. The most abundant nematodes weresp.1 andat site A;sp. andat site B;andat site C. There was a significant difference in nematode abundance among the three sites (ANOVA, F=2.38,<0.05). The highest nematode density and diversity coincided with the highest percentage of very fine sands.
Acknowledgements
We thank Drs. Zhengquan Zhou, Lang Yan, Chunyun Liu, and Shaoyu Jiang for their help in sampling and pre-treatment samples. The authors declare no conflict of interest, and this article does not contain any studies with human participants or animals performed by any of the authors.
Bezerra, T. N., Decraemer, W., Eisendle-Fl?ckner, U., Hodda, M., Holovachov, O., Leduc, D.,., 2019.Availabe from: http://nemys.ugent (accessed 17 November 2019).
Blome, D., Schleier, U., and van Bernem, K. H., 1999. Analysis of the small-scale spatial patterns of free-living marine nematodes from tidal flats in the East Frisian Wadden Sea., 133 (4): 717-726.
Cai, L., Fu, S., Zhou, X., Tseng, L. C., and Hwang, J. S., 2020. Benthic meiofauna with emphasis on nematode assemblage response to environmental variation in the intertidal zone of the Danshuei River Estuary, Northwest Taiwan., 35 (5): 857-870.
Cai, L. Z., Li, H. M., and Zou, C. Z., 2000. Species composition and diversity of marine nematode community on intertidal mudflat in Zhongzhai, Xiamen., 39 (5): 669-675.
Clarke, K. R., 1993. Non-parametric multivariate analyses of changes in community structure., 18 (1): 117-143.
Clarke, K. R., and Gorley, R. N., 2015. PRIMER Version 7.0. 12: User Manual/Tutorial. PRIMER-E, Plymouth.
Coull, B. C., and Chandler, G. T., 1992. Pollution and meiofauna: Field, laboratory, and mesocosm studies., 30: 191-271.
Dang, H, Huang, B., andZhang, Z., 1996. Study on marine benthos in an organically polluted intertidal beach of Qingdao bay: II. The pollution ecology of meiobenthos., 37: 91-101 (in Chinese with English abstract).
Du, Y. F., Xu, K. D., Lei, Y. L., and Dai, R. H., 2011. Annual quantitative distribution of meiofauna in relation to sediment environment in Qingdao Bay., 31 (2): 431-440.
Du, Y., Xu, K., Warren, A., Lei, Y., and Dai, R., 2012. Benthic ciliate and meiofaunal communities in two contrasting habitats of an intertidal estuarine wetland., 70: 50-63.
Fan, S. L., Liu, H. B., Zhang, Z. N., Deng, K., and Yuan, W., 2006. Study on the abundance and biomass of meiofauna in the sandy beach of Taiping Bay, Qingdao., 36 (3): 98-104.
Fonseca, G., and Fehlauer-Ale, K. H., 2012. Three in one: Fixing marine nematodes for ecological, molecular, and morphological studies., 10 (7): 516-523.
Franco, M. A., Soetaert, K., Costa, M. J., Vincx, M., and Vanaverbeke, J., 2008. Uptake of phytodetritus by meiobenthos using13C labelled diatoms andin two contrasting sediments from the North Sea., 362 (1): 1-8.
Franco, M. A., Soetaert, K., Van Oevelen, D., Van Gansbeke, D., Costa, M. J., Vincx, M.,., 2008. Density, vertical distribution and trophic responses of metazoan meio-benthos to phytoplankton deposition in contrasting sediment types., 358:51-62.
Galtsova, V. V., 1991. Meiobenthos in marine ecosystems, with special reference to freeliving nematodes. Proc Zool Inst USSR AS Leningrad, 240pp.
Gao, C., and Liu, X., 2018. Spatio-temporal distribution of meiofaunal assemblages and its relationship with environmental factors in a semi-enclosed bay., 131:45-52.
Gheskiere, T., Hoste, E., Vanaverbeke, J., Vincx, M., and Degraer, S., 2004. Horizontal zonation patterns and feeding structure of marine nematode assemblages on a macrotidal, ultra-dissipative sandy beach (De Panne, Belgium)., 52 (3): 211-226.
Giere, O., 2008. Meiobenthology: The microscopic motile fauna of aquatic sediments. Springer Science and Business Media.
Gingold, R., Mundo-Ocampo, M., Holovachov, O., and Rocha- Olivares, A., 2010. The role of habitat heterogeneity in structuring the community of intertidal free-living marine nematodes., 157 (8): 1741-1753.
Guo, Y. Q., Somerfield, P. J., Warwick, R. M., and Zhang, Z. N., 2001. Large-scale patterns in the community structure and biodiversity of freeliving nematodes in the Bohai Sea, China., 81 (5): 755-763.
Hamels, I., Moens, T., Muylaert, K., and Vyverman, W., 2001. Trophic interactions between ciliates and nematodes from an intertidal flat., 26 (1): 61-72.
Han, Q., and Liu, D., 2014. Temporal and spatial variations in the distribution of macroalgal communities along the Yantai coast, China., 32 (3): 595-607.
Han, Q., Wang, Y., Zhang, Y., Keesing, J., and Liu, D., 2013. Effects of intensive scallop mariculture on macrobenthic assemblages in Sishili Bay, the northern Yellow Sea of China., 718 (1): 1-15.
Hao, Y., Tang, D., Yu, L., and Xing, Q., 2011. Nutrient and chlorophyllanomaly in red-tide periods of 2003–2008 in Sishili Bay, China., 29 (3): 664-673.
Heip, C., Vincx, M., and Vranken, G., 1985. The ecology of marine nematodes., 23: 379- 389.
Hongyue, D., Bo, H., and Zhinan, Z., 1996. Study on marine benthos in an organically polluted intertidal beach of Qingdao Bay II. The pollution ecology of meiobenthos..
Hua, E., Mu, F., Zhang, Z. N., Yang, S., Zhang, T., and Li, J., 2016. Nematode community structure and diversity pattern in sandy beaches of Qingdao, China., 15 (1): 33-40.
Huang, Q., Olenin, S., Jiang, T., Sun, S., and De Troch, M., 2019. Assessing environmental effects of the bay scallopculture in China: Using abiotic and biotic indicators., 499: 316-328.
Huang, Y., Zhang, Z., and Liu, X., 2005. Studies on the community structures of meiofauna and marine nematode at six stations in the southern Yellow Sea, China., 4 (1): 34-42.
Khim, J. S., Park, J., Song, S. J., Yoon, S. J., Noh, J., Hong, S.,., 2018. Chemical-, site-, and taxa-dependent benthic community health in coastal areas of the Bohai Sea and northern Yellow Sea: A sediment quality triad approach., 645: 743-752.
Kotwicki, L., De Troch, M., Urban-Malinga, B., Gheskiere, T., and W?slawski, J. M., 2005. Horizontal and vertical distribution of meiofauna on sandy beaches of the North Sea (The Netherlands, Belgium, France)., 59 (4): 255-264.
Kotwicki, L., Deidun, A., Grzelak, K., and Gianni, F., 2014. A preliminary comparative assessment of the meiofaunal communities of Maltese pocket sandy beaches.,, 150:111-119.
Lee, M. R., and Riveros, M., 2012. Latitudinal trends in the species richness of free-living marine nematode assemblages from exposed sandy beaches along the coast of Chile (18– 42°S)., 33 (3): 317-325.
Li, B., Keesing, J. K., Liu, D., Han, Q., Wang, Y., Dong, Z.,., 2013. Anthropogenic impacts on hyperbenthos in the coastal waters of Sishili Bay, Yellow Sea., 31 (6): 1257-1267.
Li, B., Wang, Q., and Li, B., 2013. Assessing the benthic ecological status in the stressed coastal waters of Yantai, Yellow Sea, using AMBI and M-AMBI., 75 (1-2): 53-61.
Lin, A., Liang, J., Gu, D., and Wang, D., 2004. On the relationship between convection intensity of South China Sea summer monsoon and air-sea temperature difference in the tropical oceans., 23 (2): 267-278.
Liu, X. S., Zhang, Z. N., and Huang, Y., 2007. Sublittoral meiofauna with particular reference to nematodes in the southern Yellow Sea, China., 71 (3-4): 616-628.
Liu, X., Huang, D., Zhu, Y., Chang, T., Liu, Q., Huang, L.,., 2015. Bioassessment of marine sediment quality using meiofaunal assemblages in a semi-enclosed bay., 100 (1): 92-101.
Moens, T., and Beninger, P. G., 2018. Meiofauna: An inconspicuous but important player in mudflat ecology. In:, Springer, 91-147.
Moens, T., and Vincx, M., 1997. Observations on the feeding ecology of estuarine nematodes., 77 (1): 211-227.
Moens, T., Braeckman, U., Derycke, S., Fonseca, G., Gallucci, F., Gingold, R.,., 2013. 3. Ecology of free-living marine nematodes. In Volume 2 Nematoda (109-152). De Gruyter.
Moens, T., Vafeiadou, A. M., De Geyter, E., Vanormelingen, P., Sabbe, K., and De Troch, M., 2014. Diatom feeding across trophic guilds in tidal flat nematodes, and the importance of diatom cell size., 92:125-133.
Moens, T., Verbeeck, L., and Vincx, M., 1999. Feeding biology of a predatory and a facultatively predatory nematode (and)., 134 (3): 585-593.
Mokievsky, V. O., 2009.. KMK Scientific Press, Moscow, 286pp.
Mu, F. H., Zhang, Z. N., and Gao, Y. Q., 2001. Abundance and biomass of the benthic meiofauna in the Bohai Sea.,, 31 (6): 897-905.
Nicholas, W. L., and Hodda, M., 1999. The free-living nematodes of a temperate, high energy, sandy beach: Faunal composition and variation over space and time., 394: 113-127.
Nordstrom, K. F., 1992. Estuarine Beaches: An introduction to the physical and human factors affecting use and management of beaches in estuaries, lagoons, bays and fjords. Springer Science and Business Media, 217pp.
Pereira, T. J., Gingold, R., Villegas, A. D. M., and Rocha-Oli- vares, A., 2018. Patterns of spatial variation of meiofauna in sandy beaches of northwestern Mexico with contrasting levels of disturbance., 34 (1): 53-63.
Platt, H. M., and Warwick, R. M., 1988. Freeliving marine ne- matodes: Part II. British Chromadorida.. No.38. Brill,E. J.,., eds., Backhuys for the Linnean Society of London and the Estuarine and Brackish-Water Sciences Associatio, 123.
Rodr??guez, J. G., Lastra, M., and López, J., 2003. Meiofauna distribution along a gradient of sandy beaches in northern Spain., 58: 63-69.
Semprucci, F., Balsamo, M., and Sandulli, R., 2016. Assessment of the ecological quality (EcoQ) of the Venice lagoon using the structure and biodiversity of the meiofaunal assemblages., 67: 451-457.
Steyaert, M. P. M. J., Herman, P. M. J., Moens, T., Widdows, J., and Vincx, M., 2001. Tidal migration of nematodes on an estuarine tidal flat (the Molenplaat, Schelde Estuary, SW Netherlands)., 224:299-304.
Tchesunov A. V., 2006.. KMK Scientific Press Ltd., Moscow, 367.
Vanaverbeke, J., Gheskiere, T., and Vincx, M., 2000. The meiobenthos of subtidal sandbanks on the Belgian Continental Shelf (Southern Bight of the North Sea)., 51 (5): 637-649.
Wang, C. C., Pan, D. W., Han, H. T., and Hu, X. P., 2018. Distribution and contamination assessment of arsenic and mercury in surface sediments from the intertidal zone of Yantai Sishili Bay, China.:, 24 (8): 2024-2035.
Wang, Q. C., and Li, B. Q., 2013. Community structure of macrobenthos in coastal water off Yantai, East China., 44 (6): 1667-1680.
Wang, Y., Liu, D., Richard, P., and Li, X., 2013. A geochemical record of environmental changes in sediments from Sishili Bay, northern Yellow Sea, China: Anthropogenic influence on organic matter sources and composition over the last 100 years., 77 (1-2): 227-236.
Warwick, R. M., Platt, H. M., Somerfield, P. J., 1998. Free- living marine nematodes. Part III: British monhysterids. In: Synopses of the British Fauna, New Series, No. 53. Field Studies Council, Shrewsbury, 296.
Wieser, W., 1953. Die Beziehung zwischen Mundhhlengestalt. Ernhrungsweise und Vorkommen bei freilebenden marinen Nematoden,, 4:439-484.
Wu, X., Vanreusel, A., Hauquier, F., and Moens, T., 2019. Environmental drivers of nematode abundance and genus composition at two spatial scales on an estuarine intertidal flat., 846 (1): 193-214.
Wu, Y., and Zhang, B., 1994. Ecological characters of macrobenthos in Zhifu Bay, Yantai., 13 (3): 1-6.
Xu, S. H., Zhou, H., Hua, E., Lei, Y., Zhang, K., and Sun, X. Y., 2013. Distribution pattern of intertidal meiofauna in a sandy beach of zhoushan in summer and winter and its influencing factors., 43 (8): 60- 68.
Wang, X., Xu, Z. F., and Zhou, X. J., 1995. Benthic animal survey in Yantai inshores., 141 (1): 6-10.
Yang, X., Lin, C., Song, X., Xu, M., and Yang, H., 2019. Effects of artificial reefs on the meiofaunal community and benthic environment–A case study in Bohai Sea, China., 140: 179-187.
Yin, S., Tan, P., Yuan, C., Hu, J., and Liu, X., 2017. Seasonal dynamics of meiofaunal distribution in the Dagu River Estuary, Jiaozhou Bay, China., 36 (12): 79-86.
Guo, Y. Q., Zhang, Z. N., and Mu, F. H., 2002. Biomass of meiofauna in the Bohai Sea, China., 24 (6): 76-83.
Zhang, B., Wu, D., Yang, X., Teng, J., Liu, Y., Zhang, C.,., 2019. Microplastic pollution in the surface sediments collected from Sishili Bay, North Yellow Sea, China., 141:9-15.
Zhang, Q., Wang, X., and Hu, G., 2010. Evaluation of estimation methods for meiofaunal biomass from a meiofaunal survey in Bohai Bay., 28 (1): 82-87.
Zhang, R., and Dong, Y., 1990. Analysis of conditions of natural environment in sea areas for pollutant discharge and the study on pathways of pollutant transport in Yantai., 9 (2): 35-44.
Zhang, Z., Gu, F., and Yu, Z., 1990. A study on spatial pattern of marine nematodes in the subaqueous delta of the Huanghe River., 21 (1): 11-19.
Zhang, Z. N., Li, Y. G., Tu, L. H., and Yu, Z. S., 1989. A preliminary study of meiobenthos in the submarine delta of Huanghe River estuary and its adjacent waters., 20: 97-207.
Zhang, Z., Dang, H., and Yu, Z., 1993. A study on the meiobenthic community in an organically polluted beach of Qingdao Bay., 23 (1): 83-91.
Zhang, Z., Zhou, H., Yu, Z. S., and Zhou, H., 2001. Abundance and biomass of the benthic meiofauna in the northern soft-botiom of the Jiaozhou Bay., 32 (2): 139-147.
Zhao, M., Wang, E., Xia, P., Feng, A., Chi, Y., and Sun, Y., 2019. Distribution and pollution assessment of heavy metals in the intertidal zone environments of typical sea areas in China., 138:397-406.
Zhang, Z. N., Lin, K. X., Zhou, H., Han, J., Wang, R. Z., and Tian, S. Y., 2004. Abundance and biomass of meioben- thos in autumn and spring in the East China Sea and the Yellow Sea., 24 (5): 997-1005.
Zhou, Y., Yang, H., Zhang, T., Liu, S., Zhang, S., Liu, Q.,., 2006. Influence of filtering and biodeposition by the cultured scallopon benthic-pelagic coupling in a eutrophic bay in China., 317:127-141.
Zhou, Z., Li, X., Chen, L., Li, B., Liu, T., Ai, B.,., 2018. Macrobenthic assemblage characteristics under stressed waters and ecological health assessment using AMBI and M- AMBI: A case study at the Xin’an River Estuary, Yantai, China., 37 (5): 77-86.
Zograf, J. K., Pavlyuk, O. N., Trebukhova, Y. A., and Li B. Q., 2020. New species of(Enoplida, Enchelidiidae) from the Yellow Sea and redescription of.Belogurova., 1986., 4845(2): 239-252.
May 6, 2021;
April 12, 2022;
May 3, 2022
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
. E-mail:bqli@yic.ac.cn
(Edited by Ji Dechun)
 Journal of Ocean University of China2022年5期
Journal of Ocean University of China2022年5期
- Journal of Ocean University of China的其它文章
- Stress Analysis of Wire Strands by Mesoscale Mechanics
- Effect of Temperature on the Carbon, Nitrogen, and Phosphorus Nutrient Budgets of Steelhead Trout (Oncorhynchus mykiss) with Different Sizes
- Impact of Evaporation Duct on Electromagnetic Wave Propagation During a Typhoon
- A New α-Cyclopiazonic Acid Alkaloid Identified from the Weizhou Island Coral-Derived Fungus Aspergillus flavus GXIMD 02503
- Design of Copper Oxide Nanosheets-Loaded Zeolite with Efficient Inhibition of Marine Bacteria
- 3-D Marine CSEM Modeling in General Anisotropic Media by Using an Adaptive Finite Element Approach Based on the Vector-Scalar Potential
