Protective Effects of Sepia Ink Melanin on Hepatic Tissue in Streptozotocin-Induced Diabetic Mice
WANG Xiaopeng, HAN Meng, DONG Hui, ZHAO Yun, SONG Weiwei,WANG Chunlin, MUChangkaoand LI Ronghua
Protective Effects of Sepia Ink Melanin on Hepatic Tissue in Streptozotocin-Induced Diabetic Mice
WANG Xiaopeng1), HAN Meng1), DONG Hui1), ZHAO Yun1), SONG Weiwei1), 2),*,WANG Chunlin1), 2),*, MUChangkao1), 2), and LI Ronghua1), 2)
1),,,315832,2),315832,
Diabetes mellitus (DM) has emerged as a serious public health concern, due to the high morbidity and mortality resulted from its complications, such as diabetic nephropathy, diabetic cardiovascular complication, and diabetic neuropathy,. In this study, we investigated the beneficial effects of sepia ink melanin (SIM) on hyperglycaemia and the restoration of diabetic symptoms in streptozotocin (STZ)-induced diabetic model mice. At first, the normal experimental mice were performed with intraperitoneal injec- tion of STZ (40mg(kgBW)?1) (BW, body weight) to attain diabetes and then were treated with different concentrations of SIM (120, 240 and 480mg(kgBW)?1) for four weeks. After treatment, significant decrease in gluconeogenesis were determined, accompanied by a notable increase in both glycolysis and oxidative enzyme activities in SIM-treated groups, such as liver marker enzymes in the serum and key antioxidant enzymes in liver. qPCR results revealed the transcriptional alterations in SIM-treated groups. SIM exposure in- creased the expression levels of several genes related to insulin transduction and PI3K/Akt pathway, including,,, andMeanwhile, expression levels of Dicarbonyl/l-xylulose reductase (Dcxr) and UDP-glucose dehydrogenase (Ugdh), which are in- volved in pentose-glucuronate interconversion pathway, were also elevated in SIM-treated groups. Furthermore, histological observation results indicated that nuclear deformation and organelle dissolution were improved, thus could enhance the liver function. These results demonstrated that SIM can be effective in ameliorating diabetic symptoms and improving disease management for diabetic patients.
diabetic hepatopathy; sepia ink melanin; PI3K/Akt pathway; pentose-glucuronate interconversion pathway
1 Introduction
Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycaemia with disturbances in carbohy- drate metabolism, involving complications in glycol-lipid metabolism and insulin deficiency or insulin resistance lead- ing to highly elevated glucose levels (Fiorino, 2012; Meng, 2019). Presently, the pharmacological agents used to treat diabetes mellitus include sulfonylurea, bigua- nide, and α-glycosidase inhibitors, which have been restrict- ed use due to side effects and failing to significantly alter the course of diabetes (Balamurugan, 2014). The high prevalence of diabetes and the severity of diabetes-asso- ciated complications has led to an ongoing search for hypoglycaemic agents derived from natural sources.
Cephalopod ink is a great source for decreasing various health problems and could be used widely in both pharma-ceuticals and food industries (Hossain, 2018). The inkcontains melanin, proteins, peptidoglycans, amino acids, li- pid, metals, tetrodotoxin (Li, 2018). Sepia ink mela-nin (SIM), a eumelanin extracted from Sepia ink, composedof an indole monomer 5,6-dihydroxyindole (DHI), and 5,6- dihydroxyindole-2 carboxylic acid (DHICA), might play an overall protective role against exogenous and endoge- nous oxidative stressors(Novellino, 1998;Borges, 2001; Xie, 2021). Melanin has also manifested anti- inflammatory and anti-tumour properties (Sha, 2014), through scavenging free radicals (Chen, 2007) and enhancement of immune function (Lei, 2012), as de- monstrated by the effect of squid ink melanin on regula- tion of lipid metabolism in hyperlipidaemia mice. Our pre- vious study demonstrated that exposure to sepia melanin enhanced anti-oxidant action and regulated glycosylation end products accumulation in a model of D-galactose in- duced aging mice (Zhou, 2015), suggesting that me- lanin could affect carbohydrate metabolism.
The liver is an insulin sensitive tissue, which plays a vi- tal role in maintaining energy homeostasis by providing balance to the pathways of gluconeogenesis and glycoly- sis (Prabakaran and Ashokkumar, 2012). Under the persis- tent state of hyperglycaemia, the activities of enzymes re- lated to glycolysis and pentose phosphate pathway are im- paired, while the activities of enzymes relevant to gluco- neogenesis and glycogenolysis are increased, leading to a deterioration of the diabetic condition (Ferre, 2003).Moreover, persistent hyperglycaemia increases susceptibi- lity to oxidative stress and can result in increased produc- tion of reactive oxygen species (ROS) through glucose auto- oxidation and non-enzymatic protein glycation,which dis-turbs enzymes involved in antioxidant defence (Hamed, 2018). Furthermore, hyperglycaemia-induced oxida- tive stress results in the activation of several damaging pathways involved in the pathogenesis of diabetes and re- lated complications (Johansen, 2005).
A primary function of the hormone insulin is to decrease blood glucose concentration through the rapid promotion of liver glycogen synthesis and the inhibition of glycogen metabolism. However, deregulation of the insulin signal- ling pathway members PI3K/Akt can lead to the impaired insulin secretion, reduce glycogen synthesis and enhance glycogen breakdown (Zhang, 2020). Therefore, an approach to improve the control of blood glucose level in diabetes can involve in normalizing the activities of PI3K/ Akt in the insulin signalling pathway. This can stimulate or repair remaining pancreatic beta-cells for insulin secre- tion, which will enhance glycogen synthesis and inhibit glu- coneogenesis. Furthermore, this may result in a reduction in the glycosylation end products accumulation suffering from the long-term elevated glucose level and improving diabetes complications resulting from oxidative damage (Chen, 2020).
The model of STZ-induced experimental hyperglycae- mia has often been used to study the activity of hypogly- caemic agents (Abolfathi, 2012). The mechanism by which STZ brings about diabetic state including selective destruction of insulin-producing beta-cells in the pancre- atic islets, leading to hyperinsulinemia, decreased glucose levels, and hyperglycaemia (Burns and Gold, 2007).In thepresent study, the STZ-induced diabetic mice were app-lied, we examined the effect and mechanisms of SIM onhepatic metabolism enzymes in diabetic mice, including en- zymes involved in gluconeogenesis, glycolysis, transduction of the insulin signalling pathway, and anti-oxidized re- sponses.
2 Methods
2.1 Chemicals and Reagents
Streptozotocin was purchased from Sigma-Aldrich (St. Louis, Mo, USA). RT-PCR and qRT-PCR reagents werepurchased from Trans Co. (Beijing, China). All other chemi- cals and solvents were of analytical grade and purchased from Jiancheng Research Institute (Nanjing, China). Blood glucose meters and urine sugar tests were purchased from Qiangsheng Co. (USA).
2.2 Extraction of Sepia Ink Melanin
Wild sepias () (258.4g±18.6g per sepia, total 5.3kg) were purchased from Lulin aquatic products wholesale market (Ningbo, China). The ink melanin ex-traction method was based on our previous study (Zhou, 2015). Briefly, the sepia ink sac was squeezing into a three layer-gauze filter, repeated twice, and centrifuged at 8000for 10min at 4℃, followed by freeze-drying of the melanin crude product. Alkaline protease extraction in- volved combining of 2% crude melanin and 1.5% alkaline protease at pH 10.3 and followed by holding at 50℃ for 4–5h. The products of enzymolysis were centrifuged 6 times at 6000for 10min at 4℃ to obtain highly purified me- lanin, the purity of extracted melanin was derived from the sum of DHI and DHICA contents measuring by CE ana- lysis, which is similar to the method reported previously (Ito and Jimbow, 1983). The absorbance at 222.5nm is usedas a relative indicator for determining the SIM content. De-tailed methods for the characterization of melanin were provided in the supplementary materials.
2.3 Experimental Animals
All experiments in this study were conducted in com- pliance with the Chinese legislation regarding the use and care of laboratory animals and were approved by the Ani- mal Care and Use Committee of Ningbo University.
Eighty healthy male ICR strain mice were provided by the Zhejiang Province Animal Center, with the Certificate NO. SCXK 2014-0001 (Zhejiang, China). Animals were raised at the animal centre of Ningbo University for four weeks under standard conditions (12h light and 12h dark cycle, 25℃±3℃) and were fed a diet of standard mouse chow with wateruntil body weight reached 20.8±3.6g.
2.4 Experimental Induction of Diabetes in Mice
The diabetic model mice were induced using STZ as pre- viously described (Wei, 2003). Briefly, diabetes was induced in mice that had fasted for 8h intraperitoneal in- jection of STZ (40mg(kgBW)?1), dissolved in freshly pre- pared citrate buffer (0.1molL?1, pH 4.5), for three con- secutive days. STZ injected mice were allowed to drink 10% glucose solution to overcome drug-induced hypo- glycaemic mortality. Control mice were injected with samevolume of citrate buffer alone. After 72h, plasma glu- cose levels were measured. Mice with fasting blood glu- cose more than 11.1mmolL?1were considered as diabetesand incorporated into the experimental groups used in this study.
2.5 Experimental Design
Mice were randomly divided into 6 groups with 10 micein each group: NC(Normal group), MG (STZ-induced mo- del group), PG (Positive control group using metformin hy- drochloride), LD(MG+Low dose of melanin group), MD(MG+Medium dose of melanin group), HD (MG+High dose of melanin group). Treatment vehicle was 0.9% saline. Single doses of melanin (120, 240, and 480mg(kgBW)?1) (BW, body weight) and single doses of metformin hydro- chloride (240mg(kgBW)?1) were suspended in 0.9% sa- line and were administered orally every day for four weeks (Table 1).
Body weight, food and water intake, and glucose levels were measured weekly during the experiment. Four weeksafter treatment, experimental mice were euthanized by de- capitation, blood was collected, and serum was separated immediately.

Table 1 Experimental design
2.6 Oral Glucose Tolerance Test (OGTT)
After fasting 8h in the final treatment cycle, a baseline 0min blood sample was taken from all mice. Without de- lay, a glucose solution (2g(kgBW)?1) was administered orally. Blood samples were taken at 30, 60, 90 and 120min after glucose administration, respectively. Blood sam- ples were measured with blood glucose meter.
2.7 Biochemical Evaluations
2.7.1 Serum biochemical parameters
Serum biochemical parameters, including plasma livermarker enzymes, such as alanine aminotransaminase (ALT), aspartate aminotransferase (AST) activities, alkaline pho- sphatase (ALP), and lactate dehydrogenase (LD) were de- tected. Plasma insulin concentration was measured by the automated clinical analyzer (Cobas Integra 400) at the af- filiated hospital of Ningbo University.
2.7.2 Estimation of glucokinase, glucose-6- phosphatase, and glycogen in liver
Glucokinase and glucose-6-phosphatase were assayed by a human glucokinase ELISA kit (96T) and G6PT ELISA kit, respectively, according to the manufacturer’s instruc- tions (Yaji Biochemical Tech. Co., Shanghai, China). Gly- cogen was measured by the mouse glycogen ELISA (96T) kit according to the manufacturer’s instructions (Yaji Bio- chemical Tech. Co., Shanghai, China).
2.7.3 Determination of key antioxidant enzymes in liver
The mice’s liver was removed immediately after the death, and washed in normal saline, and homogenate was pre- pared in 1.15% (w/v) of potassium chloride. The homo- genate was centrifuged at 7000for 10min at 4℃ and su- pernatant was used for measurement of antioxidant enzy- mes’ activities. Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and the concentra- tion of malondialdehyde (MDA), were measured by the re- spective analysis kits according to the manufacturer’s in- structions (Nanjing Jiancheng Bioengineering Institute, Nan- jing, China).
2.7.4 Hematoxylin and Eosin (H-E) staining
Isolated liver tissues were preserved in 10% formalin so- lution and embedded in paraffin. The tissues were sliced into 4μm sections, dewaxed and rehydrated. Finally, sam- ples were stained with H-E and examined under a stan- dard light microscopy.
2.7.5 Transmission electron microscopy(TEM) analysis
Liver tissues were isolated immediately and cut into se- veral blocks of 1mm3. The tissue blocks were fixed in the fixative solution overnight at 4℃. Following several wash- es in phosphate-buffered saline (PBS), the tissue blocks were postfixed in 1% (w/v) osmium tetroxide. The tissue blocks were dehydrated with a series of washing with dif- ferent concentrations of ethanol as follows: 50% (v/v) etha- nol for 15min, 75% (v/v) ethanol for 15min, 95% (v/v) ethanol for 15min (twice) and absolute ethanol for 30min (twice). The tissue blocks were finally dehydrated with ab- solute acetone for 10min (twice). The tissue blocks were then embedded with Spur’s resin and cured at 60℃ in an oven for 48h. Ultrathin sections (90nm thickness) of the tissue blocks were cut using Leica EMUC7 ultrathin sli- cer and the sections were then collected on a 150-mesh cop-per grid. The sections on the copper grid were double-stain- ed with uranyl acetate and lead citrate. The ultrathin sec- tions were viewed under H-7650 Transmission Electron Microscope (Hitachi-Science & Technology, Japan).
2.7.6 qRT-PCR analysis
The qRT-PCR experiment was performed as previously reported (Wang, 2020). Briefly, total RNA was ex- tracted from frozen liver tissues using TRIZOL reagent (Invitrogen Corp., Carlsbad, CA) according to the manu- facturer’s instructions. Reverse transcription was perform- ed using Trans RT-PCR reagents (Trans Co., Shanghai, Chi-na) according to the manufacturer’s instructions. SYBR Green PCR Mater Mix reagent kits (Trans Co., Shanghai, China) were used according to the manufacturer’s instruc- tions. Primers specific for murine genes were designed for the genes of interest using Primer Express software-Pri- mer5. β-actin was used as a housekeeping gene for nor- malization. Primer sequences, PCR product length, anneal- ing temperature, and number of cycles are shown in Table 2. The active expression ratio () of a target gene was ex- pressed for the sample versus the control in comparison to the β-actin gene.was calculated based on the following equation:=2???Ct.

Table 2 The primer sequences, product length, annealing temperature and cycles of genes
2.8 Statistical Analysis
The Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA), version 19.0, was used for statistical analysis. All data were presented as mean±SD. The data obtained were tested by ANOVA, followed by Duncan’s post hoc multiple comparison test.0.05 was consider- ed statistically significant and0.01 was considered high- ly significant.
3 Results
3.1 Effect of SIM on the Physiological Indexes
All groups were investigated for the physiological in- dexes, including body weight, food and fluid intake. Re- sults were shown in Table 3. Compared with the control group, the fluid and food intake of diabetic model mice were significantly higher, yet with considerably lower body weight, which were in line with the typical diabetes symp-toms. As we can see, the symptoms were improved after four-weeks melanin and metformin hydrochloride treatment (0.05), respectively.
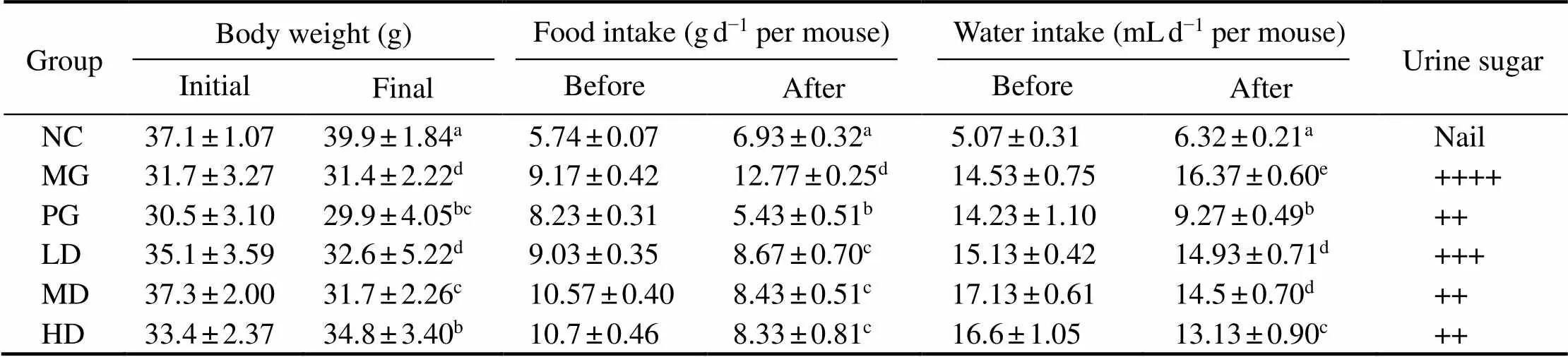
Table 3 Body weight, food, water intake and urine sugar in STZ-induced diabetic mice before and after treatment with melanin
Notes: Values are given as mean±SD for 10 mice in each group. For each data column, groups not sharing a common superscript letter(a–e)differ significantly at0.05 (DMRT).
3.2 Fasting Blood Glucose (FBG), Plasma Insulin Levels and Oral Glucose Tolerance Test (OGTT)
The FBG levels of diabetic mice were remarkably high- er than the NC group, after the repeated treatment of low- dose STZ (Fig.1A). However, significant decrease of bloodglucose levels were measured in the HD group and PGgroup after one-week SIM administration. Notably, it seem- ed that metformin took into effect more quickly than high dosage of SIM, as PG group demonstrated a significant de- crease at the first week, while the HD group gradually re- stored to a comparable level with metformin after fourweeks. Moreover, the blood glucose of LD and MD groupswere also significantly lower than the MG group, which retained a considerably higher level. Similarly, the plasma insulin level of three SIM treated groups and PG group were markedly lower than the MG group by the end of theexperiment (Fig.1B). The OGTT results were shown in Fig.1C, the peaks of all treated groups and NG group ap- peared at 60min. Compared with the MG group, the AUC value of PG and SIM treated groups were significantly low- er, with a considerably lower peak value. These results de- monstrated that SIM exhibited the potency for diabetic treat- ment.
3.3 Effects of SIM on Serum Levels of Markers
The liver has been confirmed as a sensitive organ to in- sulin, which can maintain the dynamic stability of glucose metabolism by regulating glycolysis and gluconeogenesis(Prabakaran and Ashokkumar, 2012). As an essen- tial catalyst in metabolism, transaminase is one of the in- dices to reflect liver function. As shown in Fig.2A, com- pared with the NC group, the serum level of AST and ALT were significantly increased (0.05). After four weeks of SIM administration, the AST and ALT levels in all the SIM treated groups were remarkably decreased when com- pared with the MG group, suggesting that the feeding ofSIM could ameliorate the liver damage caused by diabetes.

Fig.1 The blood glucose and plasma insulin levels of all experimental mice. (A) The FBG levels of experimental mice un- der different repeated treatment in each week. * P<0.05 vs. Model Group; ** P<0.01 vs. Model Group; (B) The blood glu- cose level and plasma insulin level after four-week treatment. For each group, groups not sharing a common superscript letter (a–e) differ significantly at P<0.05; (C) The OGTT results. Each value is mean±S.D. for ten mice in each group.
Moreover, the widely distributed ALP in the liver were used as the biomarker to evaluate the obstruction of bile duct. Due to the constantly high sugar environment, car- diovascular disease becomes the most common diabetic complication, then the LDH level was introduced to detectmyocardial function. Fig.2B indicated that the ALP and LDH values of the MG group were significantly increased (0.05) compared with the NC group, indicating the bileduct obstruction and cardiovascular damage. However, af- ter four weeks SIM feeding, these indexes in all the SIM treated groups were significantly lower than that in the MG group and restored to the similar level with the NC group.

Fig.2 Effects of SIM on plasma liver enzyme activities in experimental mice. (A) ALT and AST activities; (B) ALP and LDH activities. Each value is mean±S.D. for ten mice in each group. ALT-alanine aminotransaminase, AST-aspartate ami- notransferase, ALP-alkaline phosphatase, LDH-Lactate dehydrogenase. For each measured group, groups not sharing a common superscript letter (a–e) differ significantly at P<0.05.
3.4 Effects of SIM on Carbohydrate Metabolism Enzymes
Glucose-6-phosphatase and glucokinase are two hepa- tic glucometabolic related enzymes, which are responsible for the gluconeogenesis and glycolysis, respectively. The activities of glucose-6-phosphatase, glucokinase, and hepa-tic glycogen content in all groups were determined (Fig.3A).Compared to MG group, the activity of glucose-6-phos-phatase was significantly decreased (0.05), while the activity of glucokinase and hepatic glycogen levels were significantly increased (0.05) in SIM treated groups.
3.5 Effects of SIM on Key Liver Antioxidant Enzymes
As shown in the Fig.3B, the activities of antioxidant en- zymes, namely SOD, CAT and GSH-Px, were significant- ly lower in the MG group than the NC group, while the level of MDA was remarkably higher than that of the NC group. After SIM treatment, activities of all three determin- ed antioxidant enzymes were significantly increased, and all SIM treated groups exhibited similar trends. Meanwhile, the MDA content in the liver was considerably (0.05) decreased in all dosage of SIM feeding groups, compared with the MG group.

Fig.3 Effects of SIM on carbohydrate metabolism enzymes and antioxidant enzymes in experimental mice. (A) Glucose- 6-phosphatase and glucokinase activities; (B) SOD and CAT activities; (C) GSH-Px activity and MDA content. For each measured group, groups not sharing a common superscript letter (a–d) differ significantly at P<0.05.
3.6 Effects of SIM on mRNA Expression of Genes Regulating Glucose Metabolism Signal Pathways
To investigate the effect of SIM on the pentose-glucu- ronate interconversion pathway, we examined the relative expression levels ofand, which were respon- sible for two vital rate-limiting enzymes in this pathway. As shown in Fig.4A, the expression levels of two genes were significantly up-regulated in three SIM treated groups, compared with the MG group. Meanwhile, SIM seemed to exhibit dose-independent effect, while the increased le- vel sequentially varied from the SIM feeding dosage.
The expression levels of related genes involved in the insulin signal transduction pathway were also determined, in which PI3K and Akt represented two major enzymes. Asshown in Fig.4B, expression level ofincreased 0.34-,1.16-, and 1.61-fold (0.05) in the livers of SIM treated group with the dosage of 120, 240, or 480mg(kgBW)?1, respectively. With the increased expression of, the ex- pression ofandwere improved correspondingly (Fig.4B). Compared with the NC group, the expression level ofwas significantly decreased in the MG group. After four weeks SIM feeding, theexpression level was increased by 0.83 times (0.01) in the HD group, which was restored to the normal level. Meanwhile, the expression level ofin MD and LD groups were sig- nificantly upregulated, compared with that of MG group. Moreover, under the same treatment, the expression level of, a downstream gene of insulin signaling path- way, were 27%, 43% and 52% lower in the LD, MD and HD groups, respectively, compared with that of the MG group.

Fig.4 Effects of melanin on the expression of pentose-glucuronate interconversion pathway genes and insulin signal trans- duction pathway related genes. (A) Expression levels of Dcxr, Ugdh, Akt and Gsk-3β genes; (B) Expression levels of Insr, Irs-2 and PI3K genes. Values are given as mean±S.D. for ten mice in each group. For each gene, groups with different superscript letters (a–c) differ significantly at P<0.05 in mRNA expression.
3.7 Histological Observation on Mice Liver Tissue
To further explore the potential beneficial effects of SIM on hepatic tissue, as well as verifying the improvement cor- responding to the biochemical parameters, we performed the histological observation. As shown in Fig.5A, the li- ver histology results indicated that the NC group exhibitednormal hepatic cell architecture and morphology. How-ever, severe liver damages were observed in the MG group, including fibrosis and cell deformation. After four weeks of SIM treatment, the intercellular space became slightly filled, fibrosis and hepatocytes deformation were allevi- ated, thus the liver function might be improved.
As shown in the electron microscope results in Fig.5B, in the MG group,apoptosis of mitochondria and other or- ganelles were observed, the apoptosis products were around the nucleus, and the chromatin accumulated in the nucleus, without observable fat particles. After four weeks of SIM feeding, the nucleus was filled with many clear tubular mi- tochondria, with the observable endoplasmic reticulum, the nuclear content was clear, and there was no significant dif-ference in nuclear shape between the SIM treated group and the NC group. Therefore, it is suggested that SIMcan enhance liver function by preventing nuclear deformation and organelle dissolution.
4 Discussion
Diabetes mellitus is a disease characterized by abnor- mal carbohydrate metabolism and mainly linked with chro- nic hyperglycaemia and insensitivity of target organs to insulin(Hamed, 2018). Currently available drug re- gimens for treating and improving type I diabetes mellitus have certain drawbacks, thus it is urgent to find safer and more effective antidiabetic treatments(Grover, 2000).In this study, we characterized the role of cuttlefish ink me- lanin in protecting against the death of pancreatic beta-cells caused by STZ-induced diabetes. Moreover, treat- ment with melanin significantly ameliorated STZ-induced alterations, indicating the utility of SIM as an antioxidant. In addition, our results demonstrated that melanin mightbe able to modulate key hepatic enzymes function involved in carbohydrate metabolism, and improve the insulin sig- nalling pathways in STZ-induced diabetic mice.

Fig.5 Effects of SIM on hepatic microstructure and ultrastructure changes of experimental mice observed by optical mi- croscope (A) and electron microscope (B), respectively. NC, normal control group; MG, model samples; HD, model sam- ples with high dosage of SIM treatment.
STZ-induced diabetic model mice is also characterized by severe dehydration, increased water and food intake, and weight loss (Wei, 2003). Body weight loss could be attributed to the protein wasting causing by the unavail- ability of carbohydrates for energy metabolism(Balamu- rugan, 2014). Oral administration of SIM improved body weight and decreased food and water intake in diabetic model mice. The result might be ascribed to the improved cellular glucose utilization, which can alleviate the loss of glucose in the urine and mitigate the stimulus for food and water intake. Moreover, SIM feeding effectively prevent- ed the increasing glucose level in the fasting blood and avoided the hyperglycaemic state, possibly resulting from the restoration of damaged pancreatic beta-cells or stimu- lus for the secretion of insulin from the remaining beta- cells. Therefore, SIM treatment demonstrates an improve- ment in glycemic control.
The liver plays an important role in glucose homeostasisby regulating glucose uptake and glucose storage, however, STZ-induced diabetic mice tend to suffer from liver necro- sis(Ohaeri, 2001). Activities of the AST, ALT, and ALP en- zymes are indicators for liver damage, and the increased activities of AST and ALT indicate hepatic dysfunctioncaused by diabetes. In this study, increased activities of se- rum enzymes including AST, ALT, ALP, and LD in STZ- induced diabetic mice may induce the breakdown of pro- tein, which lead to the enhanced amino acid catabolism andprovided substrates for gluconeogenesis.Our results de- monstrated that oral administration of SIM significantly re-duced the activities of these enzymes in diabetic model mice, which could be attributed to the alleviation of liver damage and simultaneous restoration of liver function.
Long-lasting hyperglycaemia is associated with suscep- tibility to elevated free radical levels, followed by produc- tion of reactive oxygen species (ROS), which could lead to increased lipid peroxidation, alter antioxidant defences, and further impair glucose metabolism(Choudhary, 2012). Chronic treatment with melanin significantly im-proved the levels of endogenous antioxidant enzymes (SOD,CAT and GSH-Px) and prevented membrane damage by decreasing lipid peroxidation, which indicated that mela- nin might demonstrate the antioxidant properties by reduc- ing free radicals(Dong, 2016). Recently, melanin has been used as an antioxidant against the inflammatory liver damage, oxidative stress, and lipid peroxidation inducedintraperitoneally by gold nanoparticles (GNPs), and the results verified the beneficial use of melanin together with GNPs for alleviating its toxicity (Abdelhalim, 2018). Moreover, considering their o-diphenolic structures, DHI and DHICA are highly oxidizable, and can provide an efficient barrier against a broad range of cytotoxic oxi- dants, which are produced during chronic hyperglycaemia(Jiang, 2010). Therefore, melanin might serve as an anti-oxidant toprevent the destruction of beta cells fromthe peroxidation chain reaction and might be helpful in the management and restoration of diabetes.
The liver is the primary site of endogenous glucose pro-duction through gluconeogenesis or glycogenolysis, with a minor contribution from the kidneys (Meyer, 2004). Glycolysis and gluconeogenesis are the two primary com- plementary events balancing glucose levels. A partial or to- tal deficiency and over-reliance on insulin cause derange- ment in carbohydrate metabolism and decrease the acti- vity and amount of several key enzymes, including gluco- kinase and glucose-6-phosphatase (Choudhary, 2012), which play pivotal roles in the deregulation of glucose me- tabolism leading to imbalance of systemic glucose. In the present study,glucokinase activity decreased in the liver of diabetic mice possibly due to insulin dependence, and treatment with melanin increased insulin secretion, which elevated the activity of glucokinase. Increased glucokinase activity can decrease the glucose levelin blood by increas- ing glucose utilization. On the other hand, decreased acti- vity of glucose-6-phosphatase, a key rate-limiting enzyme in glycolysis, leads to a severe metabolic disorder mainly characterized by hypoglycaemia; its activity is stimulated by cAMP (cyclic adenosine monophosphate) and repress- ed by insulin (Kurosaki, 2003; Soty, 2016). In- sulin deficiency or insulin dependence in experimental dia-betic mice increases glucose-6-phosphatase activity, whichin turn increases blood glucose. Here we demonstrated that administration of melanin diminished the activities of glu- cose-6-phosphatase in STZ-induced diabetic mice. There- fore, we concluded that the reduced glucose-6-phospha- tase activity might lead to decreased gluconeogenesis, hence reducing the endogenous glucose production.
Hepatic glycogen deposition is a physiological response to an increase in blood glucose concentration after a meal (Gomis, 2003). Glycogen content plays an impor- tant role in balancing glucose metabolism and blood glu- cose stability, and serves as the reflection of insulin acti- vity, as insulin promotes intracellular glycogen deposition by stimulating glycogen synthesis and inhibiting glycogenphosphorylase. Diabetes mellitus impairs the normal capa- city of the liver to synthesize glycogen, and glycogen le- vels are significantly decreased in STZ-induced diabetic mice. Oral administration of melanin for four weeks sig- nificantly improved liver glycogen content, indicating an improved insulin activity in the diabetic mice treated with melanin.
Dcxr is a highly conserved and phylogenetically wide- spread enzyme that converts l-xylulose into xylitol and re-duces highly reactive α-dicarbonyl compounds (DCs), thus performing a dual role in carbohydrate metabolism and de- toxi?cation in the pentose-glucuronate interconversion pa- thway. Xylitol is an intermediate carbohydrate, which can be absorbed through cells in the absence of insulin to af- fect glucose metabolism (Lee, 2013). DCs are rou- tinely generated during various normal metabolic reactions, widely reactive and tend to be converted into advancedglycation end-products (AGEs) (Bohlender, 2005), which are frequently accumulated in the plasma proteins and tissues of diabetics, and are associated with kidney failure and advanced aging. Dcxr effectively reduces DCs and detoxifies endogenous and xenobiotic carbonyl com- pounds. Thus, deficiency of thegene causes human clinicalcondition, and low Dcxr activity is implicated in age-related diseases including cancers and diabetes. Here, we demonstrated that SIM treatment could up-regulate the expression level ofin diabetic mice. After four weeks of melanin administration, the expression levels ofincreased 1.40-fold, 1.62-fold, and 2.45-fold in LD, MD, and HD groups, respectively. Our results are consistent with prior reports that up-regulation of thegene can regulate glucose metabolism in diabetic mice in a positive way (Edhager, 2018).
Ugdh is another key enzyme in carbohydrate metabo-lism in the pentose-glucuronate interconversion pathway(Roman, 2003). Up-regulation of thegene canpromote glycolysis and induce galactose metabolism, there- by resulting in a decrease in blood glucose level in diabe- tic mice. Melanin treatment mediated the pentose-glucu- ronate interconversion pathway and resulted in a 1.06-fold,1.16-fold, and 2.16-fold increase inexpression inLD, MD, and HD groups, respectively, compared to theMG group. Therefore, we speculated that the reduction in blood glucose by melanin treatment could be attributed to the increased expression of thegene.
Moreover, we further studied the insulin signalling path- way genes, which mediate liver glyco- gen synthesis (Wang, 2013). Insulin signalling is ini- tiated through activation of insulin receptor (), and ex- cessexpression can enhance the sensitivity of insu- lin target cells and lead to the increased insulin levels. The insulin receptor substrate (IRS), downstream of, is a tyrosine kinase receptor, which is constituted of two iso- forms, namely, IRS-1and IRS-2.IRS-1/2 expression is in- volved in insulin-mediated glucose metabolism in skeletal muscle and liver. Additionally, IRS is susceptible to tyro- sine phosphorylation when insulin is bound to InsR, lead- ing to downstream activation of the PI3K/Akt signal path- way, which can further promote glycogen synthesis. PI3K and Akt are two key protein kinases and play a vital role in activatingin signal transduction pathways (Yu, 2011). The activation of Aktmight be affected by the upstreamsignalling cascade and can mediate downstream inhibitory phosphorylation of, which is involved in glycogen synthesis. Conversely, when the activity of Akt is inhibited, gene expression level ofis elevated, leading to the blockage of glycogen syn- thesis (Xia, 2011). Our data indicated that the ex- pression levels of,,, andin liver were significantly increased in diabetic mice in response to me- lanin treatment, while gene expression ofwas de- creased, suggesting that SIM inhibited the GSK-3β by ac- tivating and up-regulating the PI3K/Akt insulin signal trans- duction pathway. This promoted the conversion and storage of glycogen, further ameliorating plasma glucose levels in STZ-induced diabetic mice.
5 Conclusions
In this study, the protective effects of Sepia melanin ink on STZ-induced diabetic mice and alleviation of liver tis- sue damage were studied for the first time. Results demon- strated that typical diabetes symptoms, such as excess food and water intake, and severe body weight loss were im- proved. Biomarkers in the serum and liver were ameliorated, indicating the restored body functioning. Meanwhile, en- hanced antioxidant enzyme activities alleviated the oxi- dant stress damage. Up-regulated transcription factors in PI3K/Akt, as well as pentose-glucuronate interconversion pathways in hepatocytes indicated the positive regulation of SIM in treating diabetes. Moreover, the histological ex- amination demonstrated the repairment of damaged liver cells, which is consistent with the biochemical investiga- tion results. Cumulatively, these data indicated that SIM ex- hibited beneficial aspects to alleviate diabetes and demon- strated reservoir of heuristic therapeutic value in the future.
Abbreviations
SIM: sepia ink melanin;
Dcxr: dicarbonyl/l-xylulose reductase;
Ugdh: UDP-glucose dehydrogenase;
DHI: 5,6-di-hydroxyindole;
DHICA: 5,6-dihydroxyindole-2 carboxylic acid;
ROS: reactive oxygen species;
STZ: streptozotocin;
ALT: alanine aminotransaminase;
AST: aspartate aminotransferase;
ALP: alkaline phosphatase;
LD: lactate dehydrogenase;
SOD: superoxide dismutase;
GSH-Px: glutathione peroxidase;
CAT: catalase;
MDA: malondialdehyde;
TEM: transmission electron microscopy;
FBG: fasting blood glucose;
OGTT: oral glucose tolerance test;
cAMP: cyclic adenosine monophosphate;
DCs: dicarbonyl compounds;
AGEs: advanced glycation end-products.
Acknowledgements
This research was supported by the Natural Science Foundation of Zhejiang Province (No. LY18C190006) and sponsored by K. C. Wong Magna Fund in Ningbo Univer- sity.
Abdelhalim, M. A. K., Moussa, S. A. A., Qaid, H. A., and Al-Ayed, M. S., 2018. Effect of melanin on gold nanoparticle-in- duced hepatotoxicity and lipid peroxidation in rats., 13: 5207-5213.
Abolfathi, A. A., Mohajeri, D., Rezaie, A., and Nazeri, M., 2012. Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats., 2012: 740671.
Balamurugan, R., Duraipandiyan, V., and Ignacimuthu, S., 2014. Antidiabetic activity of γ-sitosterol isolated fromL. in streptozotocin induced diabetic rats., 30: 410-418.
Bohlender, J. M., Franke, S., Stein, G.,and Wolf, G., 2005. Ad- vanced glycation end products and the kidney., 40: 742-755.
Borges, C. R., Roberts, J. C., Wilkins, D. G., and Rollins, D. E., 2001. Relationship of melanin degradation products to actual melanin content: Application to human hair., 290: 116-125.
Burns, N., and Gold, B., 2007. The effect of 3-methyladenine DNA glycosylase-mediated DNA repair on the induction oftoxicity and diabetes by the beta-cell toxicant streptozotocin., 95: 391-400.
Chen, S. G., Xue, C. H., Xue, Y., Li, Z. J., and Ma, Q., 2007. Studies on the free radical scavenging activities of melanin from squid ink., 26:24-27.
Chen, T. S., Lai, C. H., Shen, C. Y., Pai, P. Y., Chen, R. J., Pad- maViswanadha, V.,., 2020. Orally administered resvera- trol enhances the therapeutic effect of autologous transplanted adipose-derived stem cells on rats with diabetic hepatopathy., 95: 37-45.
Choudhary, S. K., Chhabra, G., Sharma, D., Vashishta, A., Ohri, S., and Dixit, A., 2012. Comprehensive evaluation of anti-hy- perglycemic activity of fractionatedseedextract in alloxan-induced diabetic rats., 2012: 293650.
Dong, H., Wang, L. D., Wang, C. L., Song, W. W., Mu, C. K., and Li, R. H., 2016. Immunomodulatory effects of melanin fromink on hypoimmune mice., 33:27-30.
Edhager, A. V., Povlsen, J. A., L?fgren, B., B?tker, H. E., and Palmfeldt, J., 2018. Proteomics of the rat myocardium during development of type 2 diabetes mellitus reveals progressive alterations in major metabolic pathways., 17: 2521-2532.
Ferre, T., Riu, E., Franckhauser, S., Agudo, J., and Bosch, F., 2003.Long-term overexpression of glucokinase in the liver of trans- genic mice leads to insulin resistance., 46: 1662-1668.
Fiorino, P., Evangelista, F. S., Santos, F., Motter Magri, F. M., Delorenzi, J. C., Ginoza, M.,., 2012. The effects of green tea consumption on cardiometabolic alterations induced by ex- perimental diabetes., 2012: 309231.
Gomis, R. R., Favre, C., Garc??a-Rocha, M., Fernández-Novell, J. M., Ferrer, J. C., and Guinovart, J. J., 2003. Glucose 6-phos- phate produced by gluconeogenesis and by glucokinase is equal- ly effective in activating hepatic glycogen synthase., 278: 9740-9746.
Grover, J. K., Vats, V., and Rathi, S. S., 2000. Anti-hyperglyce- mic effect ofandin experimental diabetes and their effects on key metabolic en- zymes involved in carbohydrate metabolism., 73: 461-470.
Hamed, A. E., Elsahar, M., Elwan, N. M., El-Nakeep, S., Na- guib, M., Soliman, H. H.,., 2018. Managing diabetes and liver disease association., 19: 166-179.
Hossain, M. P., Rabeta, M. S., and Husnal, A. T., 2018. Medici- nal and therapeutic properties of cephalopod ink: A short re- view., 3: 188-198.
Ito, S., and Jimbow, K., 1983. Quantitative analysis of eumelanin and pheomelanin in hair and melanomas., 80: 268-272.
Jiang, S., Liu, X. M., Dai, X., Zhou, Q., Lei, T. C., Beermann, F.,., 2010. Regulation of DHICA-mediated antioxidation by dopachrome tautomerase: Implication for skin photoprotection against UVA radiation., 48: 1144-1151.
Johansen, J. S., Harris, A. K., Rychly, D. J., and Ergul, A., 2005. Oxidative stress and the use of antioxidants in diabetes: Link- ing basic science to clinical practice., 4: 5.
Kurosaki, E., Nakano, R., Momose, K., Shimaya, A., Suzuki, T., Shibasaki, M.,., 2003. Hypoglycemic agent YM440 sup- presses hepatic glucose output via gluconeogenesis by reduc- ing glucose-6-phosphatase activity in obese Zucker rats., 468: 151-158.
Lee, S. K., Le, T. S., Choi, H. J., and Ahnn, J.,2013. Dicarbonyl/L-xylulose dehydrogenase(DCXR): The multifunctional pen- tosuria enzyme., 45: 2563-2567.
Lei, M., Zhao, M., and Liu, Q., 2012. Immunomodulatory ef- fects of sepia melanin on hypoimmune mice., 33: 397-400.
Li, F., Luo, P., and Liu, H., 2018. A potential adjuvant agent of chemotherapy: Sepia ink polysaccharides., 16: 106.
Meng, J. M., Cao, S. Y., Wei, X. L., Gan, R. Y., Wang, Y. F., Cai, S. X.,., 2019. Effects and mechanisms of tea for the pre- vention and management of diabetes mellitus and diabetic com- plications: An updated review., 8: 170.
Meyer, C., Woerle, H. J., Dostou, J. M., Welle, S. L., and Gerich, J. E., 2004. Abnormal renal, hepatic, and muscle glucose me- tabolism following glucose ingestion in type 2 diabetes., 287: E1049-1056.
Novellino, L., d’Ischia, M., and Prota, G., 1998. Nitric oxide-in- duced oxidation of 5,6-dihydroxyindole and 5,6-dihydroxyin- dole-2-carboxylic acid under aerobic conditions: Non-enzy- matic route to melanin pigments of potential relevance to skin (photo)protection.–, 1425: 27-35.
Ohaeri, O. C., 2001. Effect of garlic oil on the levels of various enzymes in the serum and tissue of streptozotocin diabetic rats., 21: 19-24.
Prabakaran, D., and Ashokkumar, N., 2012. Antihyperglycemic effect of esculetin modulated carbohydrate metabolic enzymes activities in streptozotocin induced diabetic rats., 4: 776-783.
Roman, E., Roberts, I., Lidholt, K., and Kusche-Gullberg, M., 2003. Overexpression of UDP-glucose dehydrogenase inresults in decreased biosynthesis of K5 poly- saccharide., 374: 767-772.
Sha, L., Wu, W. H., Wu, H. B., Hou, Y. Y., and Xu, J. F., 2014. Anti-inflammatory effect of the extract fromvisceral organs., 23: 629-633.
Soty, M., Chilloux, J., Delalande, F., Zitoun, C., Bertile, F., Mi- thieux, G.,., 2016. Post-translational regulation of the glu-cose-6-phosphatase complex by cyclic adenosine monophos- phate is a crucial determinant of endogenous glucose produc- tion and is controlled by the glucose-6-phosphate transporter., 15: 1342-1349.
Wang, C. C., Shi, H. H., Xu, J., Yanagita, T., Xue, C. H., Zhang, T. T.,., 2020. Docosahexaenoic acid-acylated astaxanthin ester exhibits superior performance over non-esterified asta- xanthin in preventing behavioral deficits coupled with apop- tosis in MPTP-induced mice with Parkinson’s disease., 11: 8038-8050.
Wang, X., Wang, Z., and Chen, Y., 2013. The functions of PI3K/AKT signaling pathway in glucose homeostasis., 25: 133-139.
Wei, M., Ong, L., Smith, M. T., Ross, F. B., Schmid, K., Hoey, A. J.,., 2003. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes., 12: 44-50.
Xia, X., Yan, J., Shen, Y., Tang, K., Yin, J., Zhang, Y.,., 2011. Berberine improves glucose metabolism in diabetic rats by in- hibition of hepatic gluconeogenesis., 6: e16556.
Xie, J. W., Li, H. Y., Che, H. X., Dong, X. F., Yang, X. H., and Xie, W. C., 2021. Extraction, physicochemical characterisa-tion, and bioactive properties of ink melanin from cuttlefish ()., 56: 3627-3640.
Yu, X., Shen, N., Zhang, M. L., and Pan, F. Y., 2011. Egr-1 de-creases adipocyte insulin sensitivity by tilting PI3K/Akt and MAPK signal balance in mice.,30:3754-3765.
Zhang, H. J., Chen, C., Ding, L., Shi, H. H., Wang, C. C., Xue, C. H.,., 2020. Sea cucumbers-derived sterol sulfate allevi- ates insulin resistance and inflammation in high-fat-high-fructose diet-induced obese mice., 160: 105191.
Zhou, Y. Y., Wang, C. L., Mu, C. K., Li, R. H., and Song, W. W., 2015. Research on the extraction method of the melanin fromink., 2: 28-32.
Zhou, Y. Y., Wang, L. D., Du, M. F., Wang, C. L., Mu, C. K., Li, R. H.,., 2015. Antioxidant effects of melanin fromon subacute aged model mice., 27: 1664-1667.
April 16, 2021;
June 28, 2021;
October 5, 2021
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
.E-mail: songweiwei@nbu.edu.cn
E-mail: wangchunlin@nbu.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年5期
Journal of Ocean University of China2022年5期
- Journal of Ocean University of China的其它文章
- Stress Analysis of Wire Strands by Mesoscale Mechanics
- Effect of Temperature on the Carbon, Nitrogen, and Phosphorus Nutrient Budgets of Steelhead Trout (Oncorhynchus mykiss) with Different Sizes
- Impact of Evaporation Duct on Electromagnetic Wave Propagation During a Typhoon
- A New α-Cyclopiazonic Acid Alkaloid Identified from the Weizhou Island Coral-Derived Fungus Aspergillus flavus GXIMD 02503
- Design of Copper Oxide Nanosheets-Loaded Zeolite with Efficient Inhibition of Marine Bacteria
- 3-D Marine CSEM Modeling in General Anisotropic Media by Using an Adaptive Finite Element Approach Based on the Vector-Scalar Potential
