Transcriptome Profile Based on Protein-Protein Interaction Networks Provides a Set of Core Genes for Understanding the Metabolic Mechanisms of the Egg-Protecting Behavior in Amphioctopus fangsiao
BAO Xiaokai, LI Zan, ZHANG Jianbai, LI Yan, CHEN Xipan, WANG Weijun,SUN Guohua, XU Xiaohui, LIU Xiumei, FENG Yanwei, and YANG Jianmin
Transcriptome Profile Based on Protein-Protein Interaction Networks Provides a Set of Core Genes for Understanding the Metabolic Mechanisms of the Egg-Protecting Behavior in
BAO Xiaokai1), LI Zan1), ZHANG Jianbai2), LI Yan1), CHEN Xipan1), WANG Weijun1),SUN Guohua1), XU Xiaohui1), LIU Xiumei3), FENG Yanwei1), *, and YANG Jianmin1), *
1),,264025,2),264004,3),,264005,
Marine organisms cannot grow and reproduce without proper metabolic regulation. Within a metabolic network, problems with a given link will affect the normal life activities of the organism. Many metabolic mechanisms associated with behaviors ofare still unclear. Moreover, as a factor affecting the normal growth of, egg protection has rarely been considered in previous behavioral studies. In this research, we analyzed the transcriptome profile of gene expression inegg-unprotected larvae and egg-protected larvae, and identified 818 differentially expressed genes (DEGs). We used GO and KEGG enrichment analyses to search for metabolism-related DEGs. Protein-protein interaction networks were constructed to examine the interactions between metabolism-related genes. Twenty hub genes with multiple protein-protein interaction relationships or that were involved in multiple KEGG signaling pathways were obtained and verified by quantitative RT-PCR. We first studied the effects of egg protection on the metabolism oflarvae by means of protein-protein interaction networks, and the results provide va- luable gene resources for understanding the metabolism of invertebrate larvae. The data serve as a foundation for further research on the egg-protecting behavior of invertebrates.
; egg-protecting behavior; transcriptome; protein-protein interaction networks; metabolism
1 Introduction
Cephalopods are marine mollusks that are widely distri- buted throughout the world’s oceans. Due to the difficulty associated with wild catches, the level of artificial breed- ing of cephalopods has gradually risen in recent years (Ar- khipkin, 1985; Budelmann, 1995; Jiang., 2020a, 2020b; Schnell., 2021).has become a sought-after breed for its high nutritional value, shortgrowth cycle, rapid growth, strong adaptability to the breed-ing environment, and ease of reproduction (Boletzky, 1975; Navarro., 2003; Rosas., 2013; Wei., 2015). In China, the research onlarvae has mainly fo- cused on survival, feeding, and palatability factors, with relatively less research on the breeding methods ofduring the hatching period (Boletzky, 1989; Li., 2019).
There are many factors that affect the growth and deve- lopment oflarvae, including water quality, dis- solved oxygen, light, temperature, and egg protection. The egg-protecting behavior of femalecan reduce the occurrence of hatching diseases and improve the hat- ching rate (Li., 2019). Similarly, in marine vertebrates such as catfish, perch, and other fishes, parents may display egg-protecting behavior, and this has an impact on the birth rate and bodily functions of fish larvae. The protec- tion of eggs influences the growth oflarvae, and determines the immune and metabolic functions of the larvae to a certain extent (Webb, 2000; Baldridge., 2013;Foster., 2016).
Metabolism is the synthesis and biochemical processes of cells and tissues (Harper., 2020). Metabolic pro- cesses are complex and diverse, and metabolic processes can be linked together in multiple networks. Changes in one metabolic process often lead to changes in other process- es. A good understanding of metabolic functions is con- ducive to the study of the physiological and biochemical functions of organisms (Hosseini., 2016; Matsumoto., 2018; Xu., 2020). In studies of invertebrate me- tabolism, energy accounts for a large proportion; for ex- ample, glucose metabolism in marine invertebrates or the comparison of energy metabolism between Bivalvia and Brachiopoda (Yepiz-Plascencia., 2012; Bruning., 2013; Payne., 2014). Studies of cephalopod meta- bolic impact factors mainly focus on external environmen- tal factors such as water depth and habitat temperature,while other studies consider metabolic mechanisms through starvation, individual size, and other aspects. To date, in our knowledge, there are no studies on the metabolism of egg-protecting behavior (Seibel., 1997; Pimentel., 2012; Ikeda, 2016; Speers-Roesch., 2016).
In this research, the eggs spawned by anfe- male were divided into a protected group (Pro) and un- protected group (Unp). We used the primary incubationlarvae for transcriptome sequencing and bioinfor- matics analyses, including gene function annotation, diffe- rentially expressed genes (DEGs) analysis, simple sequencerepeats (SSR), Gene Ontology (GO) functional enrichment, Kyoto Encyclopedia of Genes and Genomes (KEGG) en- richment analysis, and metabolism-related protein interac- tion networks. Finally, 20 hub genes were identified and va-lidated by quantitative RT-PCR. The results provide new ideas for further understanding the effect of egg-protect- ing behavior on the metabolism oflarvae. The data provide a meaningful reference for subsequent artifi- cial breeding of.
2 Materials and Methods
2.1 Sample Collection and RNA Preparation
The wild parent used in the experiment was taken from the Rizhao sea area. After spawning, the eggs were equal- ly divided into two groups. One group (the egg-protected group) was cared for and incubated by the female parent, and the other group (the egg-unprotected group)hatched in flowing natural seawater. During the incubation process, the seawater temperature was maintained at 19– 20.8℃, and the incubation lasted approximately 29 days. Primary incubation larvae were collected and immediate- ly stored in liquid nitrogen until RNA extraction.
A TRIzol kit (Invitrogen, USA) was used to extract to- tal RNA according to the manufacturer’s instructions, and an RNase-free DNase I (TaKaRa, China) kit was used to remove residual DNA contamination. The quality of the ob- tained RNA was evaluated by 1.5% agarose gel electropho- resis and spectrophotometry with a NanoPhotometer Pearl spectrophotometer.
Nine primary incubation larvae were randomly selected from each group for RNA extraction. For each group, equalmolar ratios of RNA from any three of nine larvae were pooled as one replicate to provide templates for RNA-Seq library construction; any three of the remaining six larvae were pooled as the second replicate; and the final three larvae were pooled as the third replicate. The remaining RNA was reserved for quantitative RT-PCR validation.
2.2 Library Construction and Illumina Sequencing
Following the manufacturer’s suggestions, the library was constructed using the NEBNext?Ultra?RNA Library Prep Kit for Illumina?(#E7530L, NEB, USA), and index codes were added to attribute sequences to each sample. Poly-T oligo-attached magnetic beads were used to purify the mRNA, and then divalent cations were added to NEB- Next First-Strand Synthesis Reaction Buffer (5×) to degrade the RNA. RNase H and random hexamer primer were used to synthesize first-strand cDNA. Then, dNTPs, buffer, DNA polymerase I, and RNase H were used to synthesize second-strand cDNA. The cDNAs were assessed using Agilent Bio-analyzer 2100 system (Agilent Technologies, CA, USA) and ABI StepOne-Plus?Real-Time PCR System (ABI, USA). Finally, Illumina Hiseq 4000 platform was used to sequence the library.
2.3 Gene Expression Level Analysis and Function Annotation
In this study, the reference sequence was obtained by splicing Trinity with RSEM, and the clean reads were map- ped to the reference sequence. Then, we obtained the num- ber of read counts of each sample mapped to each gene. The read count was transformed into fragments per kilo- base of transcript per million mapped reads (FPKM) to ana-lyze the gene expression and abundance, and expression levels of samples were positively correlated with FPKM. Finally, the structure and function of unigenes were anno- tated and compared to several databases including NR, NT, KOG, Pfam, GO, KO, and SwissProt.
2.4 SSR and SNP Analyses
MISA software was used to perform SSR detection on the genes, and the detection parameters were 1-10, 2-6, 3- 5, 4-5, 5-5, and 6-5. SAMtools, Picard-Tools, and other pro- fessional tools were used to sequence the chromosomal co- ordinates of the results, and to remove repeated reads; dif- ferent bases covering the same location were used to de- termine and predict the occurrence of SNP sites.
2.5 DEGs Screening and Analysis
We used DESeq2 software to screen for DEGs, using a-value≤0.05 and |log2(Fold change)|≥1 as the screening criteria. The significantly differentially expressed genes in the two groups were screened in GO functional enrich- ment analysis to obtain the GO terms and the distribution of DEGs, and the statistical analyses of DEGs were con- ducted. In order to further understand the functions of DEGs,KEGG signaling pathways analyses were performed for up- and down-regulated genes, and we annotated the metabo- lic pathways involving DEGs. Finally, we screened signal- ing pathways in which DEGs were significantly enriched, and the species and numbers of DEGs in the pathways were counted.
2.6 Functional Protein Association Network Construction
Protein-protein interaction networks were constructed using STRING v11.0 (http://string-db.org/) with default pa- rameters to further investigate the relationships of genesin the metabolic regulatory pathways (Szklarczyk., 2019).
2.7 Quantitative RT-PCR Validation
In order to verify the accuracy of the RNA-Seq results, we used quantitative real-time RT-PCR (qRT-PCR) to va- lidate 20 selected genes. Three biological replicates of each group were used in the experiment, and we used Primer Premier 5.0 to design gene-specific primers. Table 1 lists the 20 selected genes and their corresponding primer se- quences. The expression level oftend-ed to be stable, and it was used as an endogenous control in this experiment. The qRT-PCR was performed with a 20μL solution containing 10ng of template cDNA and SYBR Premix Ex Taq II (TaKaRa) in a LightCycler 480 at 95℃ for 5min pre-incubation followed by 45 cycles of 95℃ for 15s and 60℃ for 45s. Finally, the melting curve was analyzed to detect single amplification. Fluorescent signal accumulation was recorded at the 60℃, 45s phase during each cycle by using a LightCycler 480. The rela- tive quantities of the target genes expressed as fold varia- tion overwere calculated using the 2?DDCtcom- parative Ct method.

Table 1 List of the primers used for quantitative RT-PCR validation
3 Results
3.1 Sequencing Results and Quality Assessment
In order to explore the difference in the metabolic me- chanisms between egg-protected and egg-unprotected lar- vae, we sequenced each sample by RNA-Seq. The results are shown in Table 2.

Table 2 Summary of sequencing results
3.2 Gene Function Annotation
The function annotation of the assembled unigenes is shown in Table 3. A total of 178898 unigenes were anno- tated, and 50.13% of the unigenes were annotated in at least one database. The Pfam, GO, and NR databases had the highest annotation rates.

Table 3 Unigene annotation and annotation rate
3.3 SSR and SNP Analyses
As a new research species, SSR and SNP markers ofwere the focus of our research. In this study we detected 348676 SSR loci in 178898 unigenes, and there was at least one SSR marker among 74122 gene sequences.In total, 153196 SNPs were identified in our transcriptome of, with 104918 transition sites and 152865 transversion sites.
3.4 Differential Expression Analysis
Differential expression analysis indicated that there were 818 DEGs between the Pro and Unp groups. Among them, 446 DEGs were up-regulated, and 372 DEGs were down- regulated (Fig.1). The heatmap (Fig.2) intuitively shows the clustering distribution of DEGs.
3.5 GO and KEGG Enrichments of DEGs
In this study, we used David v6.8 to enrich the 818 DEGs. The results of GO functional enrichment analysis showed that the DEGs were divided into three categories: biologi- cal process (21 level-3 subclasses), cellular component (13 level-3 subclasses), and molecular function (15 level-3 sub-classes). Then, we identified the top 10 level-3 terms of the three categories (Fig.3). Similarly, KEGG enrichment ana- lysis helped us to further understand the function of genes by enriching DEGs in different pathways. Of the 818 se- lected DEGs, 536 DEGs were enriched into 226 le-vel-2 KEGG classes pathways (Fig.4). Table 4 shows the 11 me- tabolism-related pathways that were significantly enriched.

Fig.1 Volcano plot of DEGs distribution trends between Pro and Unp groups. log2 (Fold change) indicates the meanexpression level of each gene. Each dot represents onegene. Red dots represent up-regulated DEGs; green dots are down-regulated DEGs; blue dots indicate the absence of DEGs.

Fig.2 Heatmap analysis of hierarchical clustering of DEGs in the two groups. Clusters were obtained using the hierarchi- cal-means method based on 818 DEGs. Each row represents a gene, and each column represents a group. The colors range from blue to red, indicating the level of expression from low to high.

Fig.3 Gene ontology (GO) enrichment analysis of DEGs. Distribution of level-3 GO annotation in three categories. The x-axis indicates the gene functional classification based on GO; the y-axis lists the corresponding number of DEGs.

Fig.4 Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of DEGs. The x-axis indicates the corre- sponding number of DEGs; the y-axis lists level-2 KEGG classes.
3.6 Construction of Metabolism-Related Protein Interaction Networks
Proteins are important components of all cells and tis- sues in living organisms.All important parts of the orga- nism need protein participation, which is the main under- taker of life activities.The construction of protein-protein interaction networks can help discover the key genes in- volved in a metabolic process. In this study, protein-pro- tein interaction networks were constructed using the pro- tein sequences of 26 genes in 11 significantly enriched me- tabolism-related signaling pathways.Fig.5 shows the pro- tein-protein interaction networks, and Table 5 lists the re- levant parameter information. These proteins interacted sig- nificantly better than randomly selected groups of proteinsof similar size This enrichment indicates that these pro- teins cooperate in a group function.
3.7 Analysis of Key DEGs Related to Metabolic Processes
This study focuses on the interaction relationship of key metabolism-related genes. Twenty key DEGs (Table 6) with multiple interaction relationships or involved in multiple signaling pathways were obtained from the KEGG path- way and protein-protein interaction networks. They were then used to explore the interaction mechanism among them. Although these DEGs do not belong to the same family, and cannot be classified accurately, they are closely related to the egg-protecting metabolism of.
3.8 Validation of DEGs Using qRT-PCR
We used qRT-PCR to detect the expression of 20 me- tabolism-related genes in the two groups, and verified the DEGs identified by RNA-Seq through the consistency of their expression. The qRT-PCR results showed that all DEGs measured were single products. A comparison of the expression profiles from qRT-PCR and RNA-Seq indicatedthat the qRT-PCR results were significantly correlated with the RNA-Seq results, and the two methods showed the same trend pattern (Fig.6).
4 Discussion
4.1 Purpose and Significance of This Research
has become increasingly popular because of its rich nutritional value and delicious meat (Boletzky, 1975; Wei., 2018). As an economically important spe- cies in mariculture, the artificial breeding ofis an issue of concern. In previous studies, we observed that octopus displayed egg-protecting behavior, and this beha- vior plays a significant role in the development of octopus larvae. The eggs ofcannot incubate with- out egg protection, while the unprotected eggs ofcan hatch, but the hatchability and survival rate of theeggs are significantly lower than those of protected eggs (Budelmann, 1995; Navarro., 2003). Therefore, it is important to understand the effects of egg protection on thegrowth and artificial breeding oflarvae. In this study, 818 DEGs were screened, and we believe that these genes have important relationships with the metabolic me- chanisms oflarvae. The heatmap showed that there were significant differences between the Pro and Unpgroups. The analysis further indicated that the metabolic mechanisms of hatchlings without egg protection were quite different from those of normal hatchlings. We obtained 11 metabolism-related KEGG pathways through DEGs enrich- ment. These pathways interact with each other and share metabolic functions. Finally, 26 genes in these pathways were used to construct protein-protein interaction networksto understand the metabolic mechanisms oflar- vae.
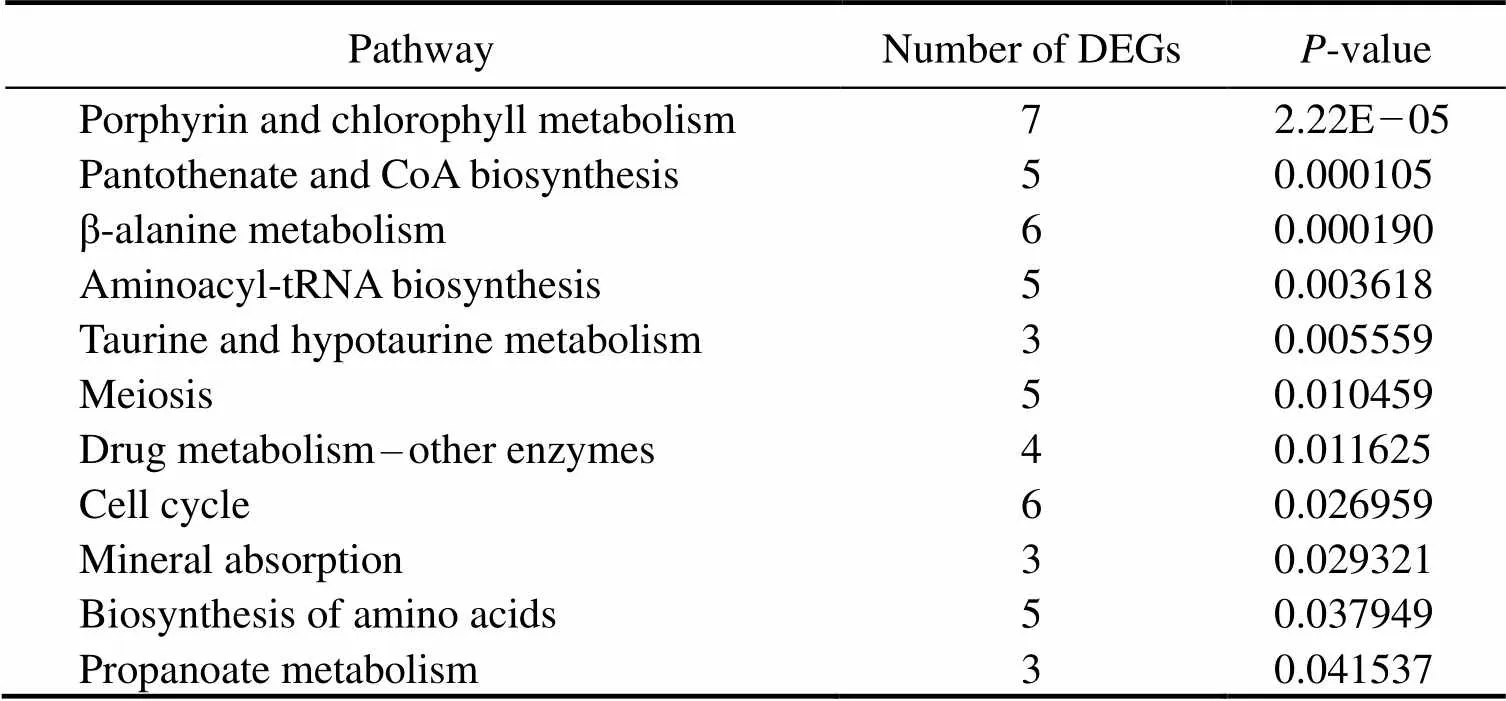
Table 4 Summary of 11 significant metabolism-related signaling pathways

Fig.5 Metabolism-related protein-protein interaction networks. Network nodes represent proteins. The legend represents the relationships between nodes.

Table 5 Network statistics of metabolism-related proteins

Table 6 Summary of 20 key DEGs

Fig.6 Comparison of the expression levels from RNA-Seq and qRT-PCR. The transcriptional expression levels of selected DEGs were normalized to the transcriptional expression levels of the β-actin gene.
4.2 Enrichment of Metabolism-Related GO Terms and KEGG Pathways
Fig.3 shows the top 10 level-3 GO terms of biological process, cellular component, and molecular function. A large number of metabolism-related functions, includingtransmembrane transport, oxidation reduction process, celladhesion, and proteolysis, were significantly enriched in biological process. Moreover, significantly enriched KEGG pathways such as β-alanine metabolism, cell cycle, ami- noacyl-tRNA biosynthesis, pantothenate, and CoA biosyn- thesis are closely related to metabolism. Functional enrich- ment analysis of GO and KEGG based on the 818 DEGs showed that there were abundant metabolism-related genes in the transcriptome oflarvae, indicating com- plex metabolic reactions occurred in the larvae. A compre- hensive analysis of these GO terms and KEGG signaling pathways will help us to explore the molecular mechanismsand metabolic systems ofegg-protecting be- havior, thereby aiding our artificial breeding work.
4.3 Speculation of Hub Genes
Protein is the basis of biological activities. The research on the interaction between proteins helps us to understand the metabolic mechanism of organisms. In this study, 26 key DEGs of metabolism-related signaling pathways were used to construct protein-protein interaction networks. Theresults showed that there were clear interactions among pro- teins, and some proteins were associated with up to eight proteins. Therefore, we suggested that such nodes with more edges were hub proteins in metabolism. The genes corre- sponding to these hub proteins were identified as hub genes for further study and verification.
4.4 Functional Analysis of Hub Genes and KEGG Signaling Pathways
We used transcriptome profiling to analyze and com- pare the metabolic mechanisms of egg-protected and egg- unprotected larvae. It helps us to further understand theeffect of egg-protecting behavior on metabolic mechanismof. Finally, we speculated and screened 20 hub genes involved in multiple protein-protein interaction re- lationships or KEGG pathways, and discussed these hub genes, KEGG signaling pathways, and the metabolic dif- ferences between the two groups.
4.4.1Cell cycle signaling pathway and meiosis signaling pathway
It is well known that the cell is the basic unit of orga- nism structure and function. Except for viruses, all orga- nisms are composed of cells. The cell cycle is closely re- lated to cell metabolism, as the cell cycle regulates celldivision and differentiation (Zheng., 2007; Takubo., 2013; Kaplon., 2015). In this study, two cellcycle related KEGG signaling pathways (cell cycle signal-ing and meiosis signaling pathways) were significantly en- riched, and both are related to cellular metabolism. The meiosis signaling pathway plays an important role in con- trolling the metabolic processes related to nutritional star- vation. The meiosis signaling pathway affects the metabo- lic processes of organisms by regulating the content of glu- cose (Honigberg., 2003; Kaplon., 2015). As the main component of signaling pathways, gene expressionaffects the function of the pathways. In our results, fourhub genes enriched in the above two signaling pathwayswere screened. As one of the indispensable enzymes inorganisms, the enzyme separase plays a key role in the cell cycle. Overexpression of separase leads to a variety of pro- blems such as aneuploidy formation.encodes sepa- rase and regulates the separation of sister chromatids (Wirth., 2006; Huang., 2009; Mukherjee., 2011). In this study, the expression ofwas down-regulated in the above two signaling pathways in Pro group larvae compared with Unp larvae, indicating that the protected lar-vae had a lower mutation rate and a higher survival rate. STAG1 and STAG2 can promote sister chromatid binding and ensure cell survival. Only down-regulation of STAG1 or STAG2 can eliminate the sticking tendency of sister chromatids, and the apoptosis rate and mitotic mutation rate also decrease (Lelij., 2017). Down-regulation of STAG1and STAG2 at the same time can reduce the rate of cell proliferation and prevent the occurrence of tumors (Bene- detti., 2017; Lelij., 2017). In our results, both genes were simultaneously down-regulated in Pro, and this may explain the high survival rate of Pro compared with Unp. CDC14 is a regulator of the cell cycle that controls cell proliferation and differentiation. Moreover, CDC14 alsoregulates cell proliferation (Zheng., 2007; Urbano., 2009; Sopko., 2014; Neitzel., 2018). The high expression of. The specific mecha- nisms of these signaling pathways and hub genes in the pro- cess of egg protection inneed further study.
4.4.2 Pantothenate and CoA biosynthesis signaling pathway and β-alanine metabolism signaling pathway
As an important regulator, pantothenic acid is widely dis-tributed in most organisms, which can regulate the syn- thesis rate of CoA to regulate the metabolic process of or- ganism (Vallari., 1987). CoA is a coenzyme of ace- tylation reactions that plays an important role in the me- tabolism of sugars, fats, and proteins. It is involved in the metabolism and oxidative stress of fatty acids, and it can be used as a biological module to participate in the me- tabolism of cofactors. Furthermore, CoA can promote the metabolism of fats, fatty acids, and vitamins, factors that help organisms maintain metabolic balance (Guo., 2018;Cui., 2020; Satoh., 2020). Meanwhile, the β-ala- nine metabolism signaling pathway was significantly en- riched in this study, suggesting that it is one of the key components in the metabolism of. β-alanine is a nonessential amino acid synthesized in the liver. It is close- ly related to the biosynthesis of pantothenic acid and CoA, and thus can affect the metabolic processes of fatty acids and other cofactors by regulating the anabolism of CoA and pantothenic acid (Dunnett., 1999; Harris., 2006; Tomita., 2014). Moreover, β-alanine is involvedin regulating the synthesis of amino acids. Some amino acids can be used as intermediates in the synthesis of ac- tive compounds that can affect the metabolic rate of orga- nisms. Other amino acids that are synthesized, such as crea- tine and phenylalanine, also affect the metabolic process- es of organisms (Trakatellis.,1970; Shuttleworth., 1984; Velí?ek., 2006). In our study, the above two sig- naling pathways were significantly enriched. They are not only closely related but also play an important role in the metabolic processes of organisms. Most of the DEGs en- riched in the above two signaling pathways were up-re- gulated in Pro group, indicating that thelarvae in Pro had a stronger metabolic capacity and higher sur- vival rate compared with those in Unp group.
4.4.3Taurine and hypotaurine metabolism signaling pathways
Taurine has a wide range of sources. It can be obtained not only by the decomposition of sulfur-containing amino acids but also by the metabolism of β-alanine. Taurine is involved in protein metabolic activities, in hypoglycemia, and in antioxidant reactions (Guerin., 1995; Kuzmi- na., 2010). Although taurine is found at low levels in aquatic animals compared with terrestrial mammals, it has an important impact on the development and reproduction of aquatic species. An appropriate taurine content is bene- ficial for gamete maturation and embryo development. Fur- thermore, taurine can stimulate aquatic larvae to produce insulin, thereby reducing blood sugar and promoting thesynthesis of glycogen, fat, and protein (Guerin., 1995;Cherif., 1996; Matsuda., 2012). Taurine metabo- lism can inhibit disorders of the expression of amino acid and fatty acid metabolism-related genes (Huxtable, 1992; Matsuda., 2012). This signaling pathway was signi- ficantly enriched in the present study, and most of the genes that were enriched in this pathway were significantly up- regulated in Pro. This phenomenon indicates that Pro had better ability to regulate metabolic balance, and this was beneficial to the growth of larvae compared with Unp.
4.4.4 Analysis of two important hub genes
Among all hub genes,andwere enrich- ed in more than two metabolism-related signaling pathways, and they possessed higher numbers of protein-protein in- teractions. GADL1 plays a key role in the metabolism of neurotransmitters and neuroprotective substances. The up- regulation of GADL1 can catalyze the decarboxylation of cysteine, aspartic acid, and other amino acids to produce β-alanine, taurine, and hypotaurine. At the same time, it can promote the metabolism and activity of larvae (Liu.,2012; Raasakka., 2018).In this study,washighly expressed in Pro compared with Unp. This resultshows that egg-protected larvae had a higher metabolic ac-tivity and stronger ability to regulate their metabolism.DYPS can control metabolism by regulating the binding ofmetabolic substrates and the stability of enzymes. It can also lead to disorders of the uracil metabolic pathways by changing the activities of several enzymes (Hiratsuka., 2015; Nakajima., 2017; Yokoi., 2020). In our research, the up-regulation of thegene in Pro sug- gests that egg-protected larvae had more stable metabolic processes. However, the specific functions of GADL1 and DYPS in larval metabolism need to be further explored.
4.4.5 Other signaling pathways and hub genes
In addition to the above signaling pathways and hub genes, our results also identified other pathways and key genes involved in the metabolism of cephalopod larvae, including aminoacyl-tRNA biosynthesis signaling pathway,mineral absorption signaling pathway, thegene, and thegene. The two signaling pathways are close- ly related to metabolism and are essential metabolic pro- cesses for life (Ibba., 1997; Yang., 2012; Sa- rayloo., 2019; Guo., 2020).Both EARS2 and HOMX1 were involved in the two metabolic pathways. EARS2 is a form of t-RNA synthetase that can catalyze glutamic acid to charge mitochondria. It is also a key gene in mitochondrial protein synthesis (Barbosa-Gouveia.,2020). HOMX1 is an anti-inflammatory and cell-protective stress protein that regulates cell proliferation and cell cy- cle metabolism (Durante., 2019; Yamamoto., 2019).The signaling pathways and genes of the metabolic mechanisms of cephalopod egg-protecting behavior need to be further elucidated in the future.
5 Conclusions
We performed transcriptome profiling of gene expres- sion in larvae from protected and unprotected eggs and constructed a protein-protein interaction network. Twenty hub genes with multiple protein-protein interaction rela- tionships or that were involved in multiple KEGG signal- ing pathways were identified. In our research, protein- protein interaction networks were first used to study the effects of egg protection onlarval metabolism, and the results provide valuable gene resources for under- standing the metabolism of invertebrate larvae. Meanwhile, the data serve as a foundation for further research into the egg-protecting behavior of invertebrates.
Acknowledgements
This research was supported by the earmarked fund for the Modern Agro-industry Technology Research System (No. CARS-49), the Natural Science Foundation of Shan- dong Province (No. ZR2019BC052), and the National Na- tural Science Foundation of China (No. 42006077).
Arkhipkin, A. I., 1985. Reproductive system structure, develop- ment and function in cephalopods with a new general scale formaturity stages., 12: 63-74.
Baldridge, A. K., and Lodge, D. M., 2013. Intraguild predation between spawning smallmouth bass () and nest-raiding crayfish (): Implications for bass nesting success., 58(11): 2355-2365.
Barbosa-Gouveia, S., Gonzalez-Vioque, E., Hermida, A., Suarez, M. U., Martinez-Gonzalez, M. J., Borges, F.,., 2020. Iden-tification of a novel variant inassociated with a severe clinical phenotype expands the clinical spectrum of LTBL., 11 (9): 1028.
Benedetti, L., Cereda, M., Monteverde, L., Desai, N., and Cic- carelli, F. D., 2017. Synthetic lethal interaction between the tumour suppressorand its paralog., 8 (23): 37619-37632.
Boletzky, S. V., 1975. A contribution to the study of yolk absorp-tion in the cephalopoda., 80 (3): 229-246.
Boletzky, S. V., 1989. Recent studies on spawning, embryonic de-velopment, and hatching in the cephalopoda., 25 (6): 85-115.
Bruning, A., González, A., Gaitán-Espitia, J. D., Bartheld, J. L., Toader-Williams, A., Mondaca, F.,., 2013. Energy meta- bolism, heart rate and physiological differentiation in the pul- monate gastropod cornu aspersum., 79 (3): 257-262.
Budelmann, B. U., 1995. Cephalopod sense organs, nerves and the brain: Adaptations for high performance and life style., 25 (1-3): 13-33.
Cherif, H., Reusens, B., Dahri, S., Remacle, C., and Hoet, J. J., 1996. Stimulatory effects of taurine on insulin secretion by fe- tal rat islets cultured., 151 (3): 501-506.
Cui, Y. F., Cao, K. R., Lin, H. Y., Cui, S. N., Shen, C. K., Wen, W. H.,., 2020. Early-life stress induces depression-like behavior and synaptic-plasticity changes in a maternal sepa- ration rat model: Gender difference and metabolomics study., 11: 102.
Dunnett, M., and Harris, R. C., 1999. Influence of oral β-alanine and L-histidine supplementation on the carnosine content of the gluteus medius., 31 (30): 499-504.
Durante, W., El-Achkar, G. A., Mrad, M. F., Mouawad, C. A., Badran, B., Jaffa, A. A.,., 2019. Heme oxygenase-1- de- pendent anti-inflammatory effects of atorvastatin in zymosan-injected subcutaneous air pouch in mice., 14 (5): e0216405.
Foster, J. G., Algera, D. A., Brownscombe, J. W., Zolderdo, A. J., and Cooke, S. J., 2016. Consequences of different types of lit- toral zone light pollution on the parental care behaviour of a freshwater teleost fish., 227 (11): 404.
Guerin, P., and Menezo, Y., 1995. Hypotaurine and taurine in ga- mete and embryo environments:synthesisthe cy- steine sulfinic acid pathway in oviduct cells., 3 (4): 333-343.
Guo, D. D., Ding, M. H., Song, X. L., Sun, Y. Y., Li, G. P., Li, Z. H.,., 2020. Regulatory roles of differentially expressed MicroRNAs in metabolic processes in negative lens-induced myopia cuinea pigs., 21 (1): 13.
Guo, Y. S., and Tao, J. Z., 2018. Metabolomics and pathway ana-lyses to characterize metabolic alterations in pregnant dairy cows on D 17 and D 45 after AI., 8 (1): 5973.
Harper, M. E., and Patti, M. E., 2020. Metabolic terminology: What’s in a name?, 2 (6): 476-477.
Harris, R. C., Tallon, M. J., Dunnett, M., Boobis, L., Coakley, J., Kim, H. J.,., 2006. The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis., 30 (3): 279-289.
Hiratsuka, M., Yamashita, H., Akai, F., Hosono, H., Hishinuma, E., Hirasawa, N.,., 2015. Genetic polymorphisms of di- hydropyrimidinase in a Japanese patient with capecitabine-in- duced toxicity.,10 (4): e0124818.
Honigberg, S. M., and Purnapatre, K., 2003. Signal pathway in- tegration in the switch from the mitotic cell cycle to meiosis in yeast., 116 (11): 2137-2147.
Hosseini, S. R., Martin, O. C., and Wagner, A., 2016. Phenotypic innovation through recombination in genome-scale metabolic networks., 283 (1839): 20161536.
Huang, X. X., Andreu-Vieyra, C. V., Wang, M. Z., Cooney, A. J., Matzuk, M. M., and Zhang, P. M., 2009. Preimplantation mouse embryos depend on inhibitory phosphorylation of separase to prevent chromosome missegregation., 29 (6): 1498-1505.
Huxtable, R. J., 1992. Physiological actions of taurine., 72 (1): 101-163.
Ibba, M., Curnow, A. W., and Soll, D., 1997. Aminoacyl-tRNA synthesis: Divergent routes to a common goal., 22 (2): 39-42.
Ikeda, T., 2016. Routine metabolic rates of pelagic marine fishes and cephalopods as a function of body mass, habitat tempe- rature and habitat depth., 480: 74-86.
Jiang, D. H., Zheng, X. D., Qian, Y. S., and Zhang, Q. Q.,2020a. Development of(Mollusca: Cepha- lopoda) from eggs to hatchlings: Indications for the embryonic developmental management., 2 (1):24-30.
Jiang, D. H., Zheng, X. D., Qian, Y. S., and Zhang, Q. Q., 2020b. Embryonic development ofunder ele- vated temperatures: Implications for resource management and conservation., 225:105479.
Kaplon, J., Dam, L. V., and Peeper, D., 2015. Two-way commu- nication between the metabolic and cell cycle machineries: The molecular basis., 14 (13): 2022-2032.
Kuzmina, V. V., Gavrovskaya, L. K., and Ryzhova, O. V., 2010. Effect on exotrophia and metabolism in mammals and fish., 46 (1): 19-27.
Lelij, P. V. D., Lieb, S., Jude, J., Wutz, G., Santos, C. P., Falken- berg, K.,., 2017. Synthetic lethality between the cohesin subunitsand, 6: e26980.
Li, Z., Fan, T. T., Liu, X. T., Liu, X. M., Wang, W. J., Wang, Q. Q.,., 2019. Characterization and functional study oninterleukin-17., 18 (6): 1443-1450.
Liu, P. Y., Ge, X. M., Ding, H. Z., Jiang, H. L., Christensen, B. M., and Li, J. Y., 2012. Role of glutamate decarboxylase-like protein 1 (GADL1) in taurine biosynthesis., 287 (49): 40898-40906.
Matsuda, M., and Asano, Y., 2012. A simple assay of taurine con-centrations in food and biological samples using taurine di- oxygenase., 427 (2): 121-123.
Matsumoto, T., Tanaka, T., and Kondo, A., 2018. Sortase A-as- sisted metabolic enzyme ligation infor en- hancing metabolic flux., 1772: 125-136.
Mukherjee, M., Ge, G. Q., Zhang, N. G., Huang, E. Y., Nakamu- ra, L. V., Minor, M.,., 2011. Separase loss of function cooperates with the loss of p53 in the initiation and progres- sion of T- and B-cell lymphoma, leukemia and aneuploidy in mice., 6 (7): e22167.
Nakajima, Y., Meijer, J., Dobritzsch, D., Ito, T., Zhang, C. H., Wang, X.,., 2017. Dihydropyrimidinase deficiency in four East Asian patients due to novel and raremutations af- fecting protein structural integrity and catalytic activity., 122 (4): 216-222.
Navarro, J. C., and Villanueva, R., 2003. The fatty acid compo- sition ofparalarvae reared with live and inert food: Deviation from their natural fatty acid profile., 219 (1-4): 613-631.
Neitzel, L. R., Broadus, M. R., Zhang, N. L., Sawyer, L., Wal- lace, H. A., Merkle, J. A.,., 2018. Characterization of anull allele in., 7 (7):bio035394.
Payne, J. L., Heim, N. A., Knope, M. L., and McClain, C. R., 2014. Metabolic dominance of bivalves predates brachiopod diversity decline by more than 150 million years., 281 (1783): 20133122.
Pimentel, M. S., Trübenbach, K., Faleiro, F., Boavida-Portugal, J., Repolho, T., and Rosa, R., 2012. Impact of ocean warming on the early ontogeny of cephalopods: A metabolic approach., 159 (9): 2051-2059.
Raasakka, A., Mahootchi, E., Winge, I., Luan, W. S., Kursula, P., and Haavik, J., 2018. Structure of the mouse acidic aminoacid decarboxylase GADL1.–, 74(1): 65-73.
Rosas, C., Valero, A., Caamal-Monsreal, C., Uriarte, I., Farias, A., Gallardo, P.,., 2013. Effects of dietary protein sources on growth, survival and digestive capacity ofjuveniles (Mollusca: Cephalopoda)., 44 (7): 1029-1044.
Sarayloo, F., Dionne-Laporte, A., Catoire, H., Rochefort, D., Houle, G., Ross, J. P.,., 2019. Mineral absorption is an enriched pathway in a brain region of restless legs syndrome patients with reducedexpression., 14 (11): e0225186.
Satoh, S., Ozaki, M., Matsumoto, S., Nabatame, T., Kaku, M., Shudo, T.,., 2020. Enhancement of fatty acid biosynthe- sis by exogenous acetyl-CoA carboxylase and pantothenate ki-nase in., 42 (12): 2595-2605.
Schnell, A. K., Amodio, P., Boeckle, M., and Clayton, N. S., 2021. How intelligent is a cephalopod? Lessons from comparative cognition., 96 (1): 162-178.
Seibel, B. A., Thuesen, E. V., and Gorodezky, L. A., 1997. De- cline in pelagic cephalopod metabolism with habitat depth re- flects differences in locomotory efficiency., 192 (2): 262-278.
Shuttleworth, T. J., and Goldstein, L., 1984.-alanine transport in the isolated hepatocytes of the elasmobranch., 231 (1): 39-44.
Sopko, R., Foos, M., Vinayagam, A., Zhai, B., Binari, R., Hu, Y. H.,., 2014. Combining genetic perturbations and proteo- mics to examine kinase-phosphatase networks inembryos., 31 (1): 114-127.
Speers-Roesch, B., Callaghan, N. I., MacCormack, T. J., Lamar- re, S. G., Sykes, A. V., and Driedzic, W. R., 2016. Enzymatic capacities of metabolic fuel use in cuttlefish () and responses to food deprivation: Insight into the metabolic organization and starvation survival strategy of cephalopods.–, 186 (6): 711-725.
Szklarczyk, D., Gable, A. L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J.,., 2019. STRING v11: Protein-protein as-sociation networks with increased coverage, supporting func- tional discovery in genome-wide experimental datasets., 47 (D1): D607-D613.
Takubo, K., Nagamatsu, G., Kobayashi, C. I., Nakamura-Ishizu, A., Kobayashi, H., Ikeda, E.,., 2013. Regulation of gly- colysis by Pdk functions as a metabolic checkpoint for cell cy-cle quiescence in hematopoietic stem cells., 12 (1): 49-61.
Tomita, H., Yokooji, Y., Ishibashi, T., Imanaka, T., and Atomi, H., 2014. An archaeal glutamate decarboxylase homolog func- tions as an aspartate decarboxylase and is involved in β-ala- nine and coenzyme a biosynthesis., 196 (6): 1222-1230.
Trakatellis, A. C., and Schwartz, G., 1970. Biosynthesis of insu- lin in anglerfish islets., 225 (5232): 548-549.
Urbano, J. M., Torgler, C. N., Molnar, C., Tepass, U., Lopez-Va- rea, A., Brown, N. H.,., 2009.laminins act as key regulators of basement membrane assembly and morpho- genesis., 136 (24): 4165-4176.
Vallari, D. S., Jackowski, S., and Rock, C. O., 1987. Regulation of pantothenate kinase by coenzyme a and its thioesters., 262 (6): 2468-2471.
Velí?ek, J., Kubec, R., and Cejpek, K., 2006. Biosynthesis of food constituents: Amino acids: 4. Non-protein amino acids–A re- view., 24 (3): 93-109.
Webb, T., 2000. A literature review of studies on fish egg mor- tality: Implications for the estimation of spawning stock bio- mass by the annual egg production method (funded under MAFF project code MF0426: Fish egg development and mor- tality studies in the Irish Sea).Lowestoft,CEFAS,37pp.
Wei, X. M., Xu, J., Yang, J. M., Liu, X. Q., Zhang, R. R., Wang, W.,., 2015. Involvement of a Serpin serine protease in- hibitor () from molluscin antibac-terial response., 42 (1): 79-87.
Wei, X. M., Zhao, T. Y., Ai, K. K., Li, H. Y., Jiang, X., Li, C.,., 2018. Role of scavenger receptor fromas a co-receptor of Toll-like receptor in initiation of TLR-NF-κB signaling during anti-bacterial response., 84: 14-27.
Wirth, K. G., Wutz, G., Kudo, N. R., Desdouets, C., Zetterberg, A., Taghybeeglu, S.,., 2006. Separase: A universal triggerfor sister chromatid disjunction but not chromosome cycle pro- gression., 172 (6): 847-860.
Xu, R., and Zheng, X. D., 2020. Hemocytes transcriptomes re- veal metabolism changes and detoxification mechanisms in response to ammonia stress in., 29 (9): 1441-1452.
Yamamoto, H., Saito, M., Goto, T., Ueshima, K., Ishida, M., Ha- yashi, S.,., 2019. Heme oxygenase-1 prevents glucocor- ticoid and hypoxia-induced apoptosis and necrosis of osteo- cyte-like cells., 52 (3): 173-180.
Yang, F., Wang, Q. P., Wang, M. H., He, K., and Pan, Y. C., 2012. Associations between gene polymorphisms in two crucial me- tabolic pathways and growth traits in pigs., 57 (21): 2733-2740.
Yepiz-Plascencia, G., and Martínez-Quintana, J. A., 2012. Glucose and other hexoses transporters in marine invertebrates: A mini review., 15 (5): 16.
Yokoi, K., Nakajima, Y., Matsuoka, H., Shinkai, Y., Ishihara, T., Maeda, Y.,., 2020. Impact of,andgene variations on severe drug-related toxicity in cancer pa- tients., 111 (9): 3359-3366.
Zheng, C., Odstrcil, E. A., Tu, B. P., and Mcknight, S. L., 2007. Restriction of DNA replication to the reductive phase of the metabolic cycle protects genome integrity., 316 (5833): 1916-1919.
March 30, 2021;
May 17, 2021;
September 21, 2021
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
. E-mail: fywzxm1228@163.com
E-mail: ladderup@126.com
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年5期
Journal of Ocean University of China2022年5期
- Journal of Ocean University of China的其它文章
- Stress Analysis of Wire Strands by Mesoscale Mechanics
- Effect of Temperature on the Carbon, Nitrogen, and Phosphorus Nutrient Budgets of Steelhead Trout (Oncorhynchus mykiss) with Different Sizes
- Impact of Evaporation Duct on Electromagnetic Wave Propagation During a Typhoon
- A New α-Cyclopiazonic Acid Alkaloid Identified from the Weizhou Island Coral-Derived Fungus Aspergillus flavus GXIMD 02503
- Design of Copper Oxide Nanosheets-Loaded Zeolite with Efficient Inhibition of Marine Bacteria
- 3-D Marine CSEM Modeling in General Anisotropic Media by Using an Adaptive Finite Element Approach Based on the Vector-Scalar Potential
