Catalytic Hydrothermal Liquefaction of Algae for Production of Bio-oil with Solid Superacid CatalystSO42?/ZrO2
LIU Mengfan, YU Xin, YU Xiaofan, ZHAO Yongnian, FENG Lijuan, LI Xianguo, and YAO Shuo
Catalytic Hydrothermal Liquefaction of Algae for Production of Bio-oil with Solid Superacid CatalystSO42?/ZrO2
LIU Mengfan, YU Xin, YU Xiaofan, ZHAO Yongnian, FENG Lijuan, LI Xianguo, and YAO Shuo*
Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China
Solid superacid SO42?/ZrO2as heterogeneous catalyst was prepared to upgrade the bio-oil in the progress of hydrothermal liquefaction (HTL) for the represented algaeofandThe solid superacid catalyst could obviously adjust the composition of the bio-oil and improve the higher heating values (HHVs). The catalytic performance could be regulated by adjusting the acid amount and acid strength of SO42?/ZrO2. Furthermore, it was explored the catalytic effects of SO42?/ZrO2by the HTL for algae major model components, including polysaccharides, proteins, lipids, binary mixture and ternary mixture. The results showed that the introducing of SO42?/ZrO2catalyst could increase the yields of bio-oil from proteins and lipids, and avoid the Maillard reaction between polysaccharides and proteins. Moreover, a possible reaction pathway and mechanisms has proposed for the formation of bio-oils from HTL of algae catalyzed by SO42?/ZrO2based on the systematic research of the producing bio-oil from major model components.
bio-oil; heterogeneous catalyst; solid superacid catalyst; hydrothermal liquefaction; algae major model components
1 Introduction
Bio-oil from renewable biomass sources is the most promising replacement for the exhaustibility fossil fuels. Algae biomass, which own the characteristics of fast growth rate, low requirements on growth environment, inhibition of water pollution and greenhouse effect, are considered as sustainable development and environmental-friendly feedstock for bio-oil production (Biswas, 2017; Wang, 2017; Sharma, 2021).The mainly approaches to produce bio-oil from algae are tran- sesterification conversion, biochemical conversion and thermochemical method (Ambaye, 2021). Compared with above methods, hydrothermal liquefaction (HTL) of algae belonging to the thermochemical method features priority for the following aspects: i) it is an economical process especially for the wet algae feedstock since it avoids the dewatering and drying steps (Saber, 2016; Sheng, 2018; Marzbali, 2021); ii) the components of algae can be fully utilized; iii) the oil products generally possess higher energy content and better stability properties than pyrolysis oil (López Barreiro, 2013; Hu, 2017; Xu, 2020);iv) the decomposition of biochemical compositions into aque-ous-phase, heavy oil and light oil products requires quite low activation energy (Kim, 2013; Vo, 2017a, 2017b; SundarRajan., 2021).
In order to increase the yield and quality of bio-oil, the solvent water in HTL is heated and pressurized to a subcritical state, which has excellent solubility to biological macromolecules such as proteins and polysaccharides, and can promote the progress of acid-catalyzed reaction (Zhu, 2017; Xu, 2020). It has been found that the ionic product constant (w) of water increases from 10?14to 10?12when the temperature increases from 25 to 350℃ (Toor, 2011). The resulting high levels of H+and OH?can promote the acid or base-catalyzed of HTL process (Cheng, 2017; Galadima and Muraza, 2018). In addition, the introducing of catalysts is an effective strategy to increase bio-oil yield and improve the quality. The application of various homogeneous and heterogeneous catalysts in algal HTL has attracted extensive attention (Gai, 2015;Saber, 2016). Yang(2017) introduced sulfuric acid and acetic acid as the homogeneous catalysts in HTL and the bio-oil yield has been obviously increased. However, heterogeneous catalyst has received extensive attention for the advantages with environment-friendly, convenient recovery and cyclic utilization,.(Carrero, 2015; Navalon, 2016). Solid acid catalyst is a typical heterogeneous catalyst, which is widely used in alkylation, esterification, isomerization, HTL,. In order to achieve prominent catalytic effect, it should focus on the acidity and stability of the solid acid catalyst. Relevant research efforts have confirmed that increasing the acidic of catalyst can improve the fluidity and reduce the boiling point of bio-oil (Yang, 2017). Accordingly, solid superacid which provide strong acidity can be regarded as a recommendable kind of heterogeneous catalyst. SO42?/ZrO2with super acidity have been seen as a representative solid superacid for the advantages of easy preparation, high hydrothermal stability and strong activity. Therefore, the use of solid superacid catalyst SO42?/ZrO2in HTL of algae exhibits broad application prospects.
The biochemical composition of algae is also an important factor to influence the product yield and quality. Biller(2011) preliminarily reported the related effects using soya oil and microalgae. However, it is still worth further exploring other model compounds such as polysaccharides and proteins. In addition, few studies have an insight into the effects of catalysts on the distribution and properties of HTL product for algae major model components (Peng, 2018). Considering comprehensively,with high polysaccharideandwith high proteins and lipids were selected as representative algae to produce bio-oil by HTL.
Herein, a series of solid superacid SO42?/ZrO2were prepared by calcining at different temperature to explore the effect of the catalyst acidity on its catalytic performance. Subsequently, it investigated the optimum temperature and dosage of the prepared catalyst on bio-oil production for the selected algae from HTL. Furthermore, it has been conducted the same performance for main components of algae including polysaccharides, proteins and lipids. The analysis of bio-oil product by means of higher heating values (HHVs), thermal gravimetric analysis (TGA) and gas chromatography-mass spectrometry (GC- MS) was conductive to approve the effects of each biochemical composition for bio-oil and the formation pathways.The recycling of the SO42?/ZrO2catalyst used in the HTL confirmed the catalyst still exhibits good catalytic activity after 4 cycles. In this study, it firstly proposed the formation pathways of bio-oil from algae and model com- pounds with the solid superacid SO42?/ZrO2as heterogeneous catalyst. The results could provide a fundamental basis for the screening of catalyst in the HTL of algae.
2 Msterials and Methods
2.1 Materials
Crude polysaccharides, proteins anddry powder were purchased from Shanxi Pioneer Biotech Co., Ltd. Oleic acid and glycerol were mixed in a molar ratio of 1:3 representing a model lipid material since lipids are easily hydrolyzed to fatty acids and glycerol under hydrothermal conditions., which was collected from Stone old man area in Qingdao, China, was used as algae liquefaction raw materials. All chemicals and reagents are analytical grade without treatment. The proximate analysis of moisture, ash, volatile matter, fixed carbon and ultimate analysis of C, H, N of the same batch of algae have been previously reported by our group (Zhao, 2017).
2.2 Preparation of Catalyst
SO42?/ZrO2catalyst was prepared by precipitation-im- pregnation. Zirconium oxychloride solution at a concentration of 0.125gmL?1was prepared and the added ammonium hydroxide drop by drop to adjust the pH value to 9 with rapidly stirring. After aging for 12h at room temperature, the precipitate was filtered and washed with deionized water until chlorine-free. The filter cake was dried at 110℃ for 24h and then grind. The resulting powder was immersed in 1 mol L?1dilute sulfuric acid for 12h and filtered. The resulting solid was dried at 110℃ for 2h and then calcined for 4h.
2.3 Characterization of Catalyst
The method of back titration is selected to determine the total acid amount of SO42?/ZrO2catalyst (Yang, 2015). 0.1g calcined SO42?/ZrO2catalyst was added to 20 mL 0.1molL?1sodium hydroxide standard solution and reacted for 1h. The liquid was obtained by centrifugation and the remaining sodium hydroxide solution was titrated with hydrochloric acid standard solution calibrated by NaHCO3. The formula for quantity of total acid amount of SO42?/ZrO2catalyst is as follows:
[H+] (mmolg?1) = (NaOH×NaOH?HCl×HCl)/. (1)
SO42?/ZrO2catalystwas characterized by D8-Advance X-ray diffractometer (XRD) from Bruker Company, Ger- many. Test conditions: anode target Cu target, diffraction wavelength 1.5406?, operating voltage 40kV, current 40mA, NaI scintillation counter, step length 0.05?step?1, scanning speed 3?min?1, scan angle 2=10?–70?. The sur- face morphology and elements of SO42?/ZrO2were observed by scanning electron microscopy (SEM, Zeiss Su- pra 55) and energy X-ray dispersive spectrometer (EDS, X-MAX-20), respectively.
2.4 Apparatus and Experimental Procedure
The HTL process was performed in a stainless steel autoclave (CJK-(0.2)), equipped with an electrically heated furnace, a magnetic stirrer and a temperature controller. In a typical run, the autoclave was charged with suitable polysaccharides, protein powder, and oleic acid- glycerol mixtures or algaeThe ratio of material to liquid is 0.1gmL?1. The autoclave was sealed and purged with nitrogen for 5min before heated from 200 to 300℃ with 20℃ intervals and held for 10min at each temperature. Then autoclave was cooled down to room temperature. The gas products produced by HTL were released from the reactor and then the reactor was opened. Liquid products were separated by a series of filtration and extraction procedures.
Dichloromethane (CH2Cl2) was added after filtering out the residue to extract crude oil phase. The CH2Cl2phase was evaporated at 35℃ under reduced pressure to remove the solvent, and the remaining liquid product is defined as bio-oil. The aqueous phase was vacuum evaporated at 60℃ to remove water and the residue was defined as water-soluble organics (WSOs). The residue was dried at 110℃ for 12h and then weighed. Product yield was calculated by the following equations:
y=m/m×100%, (2)
y=m/m×100%, (3)
y=m/m×100%, (4)
y=100%?y?y?y, (5)
wherey,y,yandycorrespond to the yield of bio-oil, residue, water-soluble organics and gas, respectively.m,m,mandmrefer to the mass of bio-oil, residue, water-soluble organics and feed, respectively.
2.5 Analysis of Bio-oil
Higher heating values (HHVs) of bio-oils were analyzed by using the ZR-3R combustion heat experimental device produced by Nanjing Dosuke Technology Development Co., Ltd. with 0.001℃ temperature difference re- solution, and the temperature resolution is 0.01℃.
Gas chromatography-mass spectrometry (GC-MS) ana- lyses of bio-oils were carried out using an Agilent 7890A/5975C (Santa Clara, CA, US) with a DB-5 column (30m×250μm×0.25μm). The carrier gas was helium with a flow rate of 1.5mLmin?1. 1mL of CH3OH solution of the sample (0.1–0.2gmL?1) was injected into the column. The injector was set at split mode with an inlet temperature of 280℃ and the diversion flow was 20mLmin?1. The GC-MS oven temperature was held at 40℃ for 3min and then raised to 300℃ with a heating rate of 10℃min?1. Compounds were identified by means of the National Institute of Standards and Technology (NIST) library of mass spectra.
Thermal gravimetric analysis (TGA) of bio-oils was performed in nitrogen atmosphere. Samples were purged to a constant weight at room temperature and then heated to 900℃ with a heating rate of 15℃min?1. The gas flow rate was 30mLmin?1.
2.6 Reusability of Catalyst
The catalyst was used for successive batches. During each cycle, the residue obtained by HTL of.were collected and white powder was obtained after calcined at 550℃ for 4h. Then the white powder was added into the HTL of.for the next cycle. The yields and HHVs of bio-oil were measured according to the assay described in the previous paragraph.
3 Results and Discussion
3.1 Characterization of Solid Superacid Catalyst SO42?/ZrO2
Acid amount is an important property of acid catalyst, while the influence of total acid amount on the catalytic performance of SO42?/ZrO2catalyst is less studied. The calcination temperature has a particularly prominent effect on its acid amount. Therefore, it was measured the total acid amount of SO42?/ZrO2catalyst calcined at different temperature (Fig.1a). The total acid amount of SO42?/ZrO2catalyst fluctuates slightly but maintains above 2.5mmolg?1when the calcination temperature ranges from 200 to 400℃. With the further increase of calcination temperature, the total acid amount of SO42?/ ZrO2catalyst shows a decreasing trend until it is close to zero at 800℃. The decrease of acid amount can be ascribed to the reduction of total hydroxyl content on the surface, which is influenced by the surface hydroxyl density and surface area of solid acid catalyst with the calcination temperature increases (Qin, 2004).

Fig.1 Total acid amount (a) and XRD patterns (b) of SO42?/ZrO2 catalyst calcined at different temperatures.
Acid strength is another fundamental property of solid acids. The acid strength of SO42?/ZrO2catalyst calcined at 400, 550 and 600℃ had been studied by our group (Zhao, 2017). The proportion of strong acidity of SO42?/ ZrO2catalyst calcined at 550℃ was more than 60% which is higher than 400 and 600℃. Herein, we further explore the essential reasons by XRD, which indicates the difference of acid strength can be ascribed to the different crystalline state of SO42?/ZrO2catalyst at different calcination temperature. Since sulfate trends to combine with materials with tetragonal crystal system, the SO42?/ZrO2catalyst with tetragonal crystal system possesses strong acidity and shows better catalytic performance than other types. As the XRD patterns in Fig.1b, the broad peak indicates the solid superacid catalyst SO42?/ZrO2calcined at 400℃ was amorphous. Therefore, the lower calcination temperature is not conducive to the formation of surface acid sites for SO42?/ZrO2catalyst. The peaks of tetragonal crystals appeared with the calcination temperature rising to 550℃, indicating that more tetragonal crystals appeared in the SO42?/ZrO2catalyst and the surface acidity enhanced. In SO42?/ZrO2solid superacid, sulfate acts in a double coordination form with metal ions on the surface of metal oxide. The formation of SO42?/ZrO2solid superacid center is mainly due to the coordination adsorption of SO42?on the surface of metal oxide. However, the intensity of the peaks of tetragonal crystals decreased when the calcination temperature further increased to 600℃, which indicates that an excessively high calcination temperature would have an adverse effect on the formation of tetragonal crystals and reduced the acidity of SO42?/ZrO2catalyst. Overall, it can further conclude that 550℃ is the optimum calcination temperature for the preparation of SO42?/ZrO2catalyst. Fig.2 shows the surface morphology and elements of SO42?/ZrO2catalyst, which has a smooth surface, indicating that the material supported on the surface has not been shed by high temperature (Wang, 2009). Sulfur was detected in the EDS spectrum, suggesting that the sulfate ions had been successfully supported on ZrO2.

Fig.2 SEM images and EDS spectrum of SO42?/ZrO2 catalyst.
3.2 Effect of Temperature and SO42?/ZrO2 Catalyst Dosage on Bio-oil Yield from HTL of Algae
The HTL of algae is an extremely complex process that involves interaction among polysaccharides, proteins and lipids. In order to understand the effect of different biochemical composition of algae on the yield of bio-oil obtained from HTL,with high polysaccharide andwithhigh proteins and lipids are selected as representative algae.
In order to explore the optimum temperature for the HTL ofand, the experiment without catalysts were conducted firstly at different liquefaction temperature and the product distribution results were shown in Fig.3a. The optimum liquefaction temperatures forandare 280 and 300℃ and the corresponding maximum yield of bio-oil are 12.63% and 23.39%, respectively (Fig.3b). The yields of residue fromHTL are higher than those ofat different temperatures.The optimum temperature and maximum bio-oil yield forare both lower than those for, which indicated that algae with higher proteins and lipids content required higher liquefaction temperature and higher bio-oil yield and lower residue yield can be obtained.

Fig.3 Product yields from HTL of Enteromorpha prolifera (E) and Chlorella vulgaris (C) versus liquefaction temperature (a) and the dosage of SO42?/ZrO2 (b).
Keeping the optimum reaction temperature and adding SO42?/ZrO2catalyst, the bio-oil yield produced byfluctuates slightly compared to which was catalytic with ZrO2carrier or no catalyst, and maintains at about 10% and the yield of residue is almost constant. Nevertheless, the yield of bio-oil forincreases firstly and then decreases with the increase of SO42?/ZrO2catalyst dosage compared to which was catalytic with ZrO2carrier or no catalyst. It indicates that excessive use of SO42?/ZrO2catalyst would inhibit the formation of bio-oil. The residue yields ofare higher than those ofat different SO42?/ZrO2dosage. As a result, SO42?/ ZrO2catalyst is more effective for the HTL of algae with higher proteins and lipids content, which can significantly increase the bio-oil yield and decrease the residue yield.
3.3 Effect of Temperature and SO42?/ZrO2 Catalyst Dosage on Product Yields from HTL of Model Compounds of Algal Main Components
To further explore the influence of main composition of algae including polysaccharides, proteins and lipids on the yield and composition of bio-oil and understand the bio-oil formation pathway, the similar experimental were performed to study the effect of temperature and addition of SO42?/ZrO2catalyst on product distribution obtained from HTL of model compounds of algal main components.
HTL experiments were then conducted for three major model components at different temperatures (Fig.4a). The yield of bio-oil from lipids is significant higher than that of polysaccharides and proteins, since polysaccharides are freely soluble in water at room temperature and easily de- composed into water-soluble polar small molecules under the subcritical hydrothermal environment. Hence, polysaccharides had little contribution to the formation of bio- oil. In virtue of the corresponding optimal yield, 200, 300 and 280℃ were respectively selected as the optimal liquefaction temperature for polysaccharides, proteins and lipids.
In addition, it is also investigated the effect of SO42?/ ZrO2catalyst dosage on the HTL for three major model components at the corresponding optimal liquefaction temperature (Fig.4b). It can conclude that WSOs and gas products are the main products for polysaccharides in HTL. The bio-oil yield does not affect by the amount of SO42?/ZrO2catalyst, while the residue yield increases with SO42?/ZrO2catalyst dosage. This is due to the hydrolysis of polysaccharides in a subcritical water environment to produce 5-Hydroxymethylfurfural (5-HMF) which is prone to polymerize in an acidic environment to generate a coke-like substance. For proteins and lipids, only bio-oil yields are the subject of discussion. The bio-oil yields of proteins and lipids reach the highest when the amount of SO42?/ZrO2catalyst is 5% and 9%, respectively. By comparing the performance of three major model components in HTL, it is further verified that the contribution of main components of algae to bio-oil is lipids>proteins>polysaccharides.
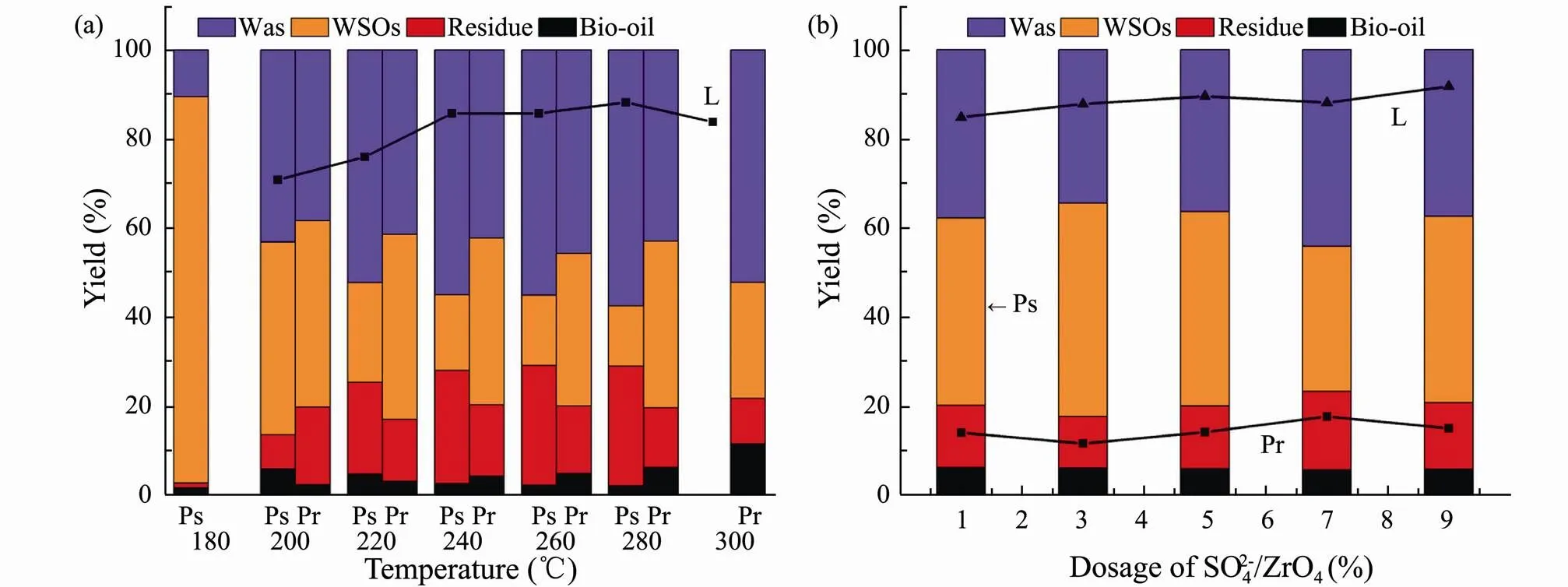
Fig.4 Product yields from HTL of polysaccharides (Ps), proteins (Pr) and lipids (L) versus liquefaction temperature (a) and the dosage of SO42?/ZrO2 (b).
3.4 Effect of Temperature and SO42?/ZrO2 Catalyst Dosage on Bio-oil Yield from HTL of Binary Mixtures
The effects of reaction between polysaccharides and proteins on the HTL of algae can be roughly judged by the bio-oil yield distribution and bio-oil composition. A series of different proportions of polysaccharide and protein mixtures are hydrothermally liquefied at 200, 250 and 300℃, respectively (Fig.5a). When liquefaction tem- perature is 200℃, the increase of polysaccharides content contributes to the formation of bio-oil. However, the yield of bio-oil shows a decreasing trend in varying degrees with the increase of polysaccharides content at liquefaction temperature of 250 and 300℃. This is consistent with the higher bio-oil yield of proteins HTL at high temperatures and the higher bio-oil yield of polysaccharides HTL at low temperatures.
The influence of SO42?/ZrO2catalyst dosage with different proportion of polysaccharides-proteins binary mix- tures on bio-oil yield is evaluated by the experimental bio-oil yield (solid line) and theoretical bio-oil yield (dash line) at the optimum liquefaction temperature of 300℃. As seen in Fig.5b, the bio-oil yield slightly increases with the addition of SO42?/ZrO2catalyst for the binary mixtures with 20% and 40% polysaccharide, while the yield of bio-oil decreases slightly with polysaccharides content of 80%. In addition, the experimental bio-oil yields with polysaccharides content of 80% are closer to the theoretical bio-oil yields comparing with the polysaccharide content of 20% and 40%.
HTL experiments are conducted for equal mass proportion of binary mixtures for polysaccharides-lipids and proteins-lipids, and the yields of bio-oil varying with liquefaction temperature are shown in Fig.5c. The yield of bio-oil from polysaccharides-lipids decreases with liquefaction temperature increasing which is opposite of the result of lipids HTL alone. It is speculated that the increase of liquefaction temperature is not conducive to polysaccharide HTL and even cause side reactions of polysaccharides and lipids, which led to the decrease of bio-oil yield. The similar results have also been found by Yang’s group (Yang., 2015, 2019). On the contrary, the yield of bio-oil from proteins-lipids increases with the increase of liquefaction temperature and reaches the highest at 300℃.

Fig.5 Bio-oil yields from HTL of polysaccharides-proteins versus polysaccharides content (a) and the dosage of SO42?/ZrO2 (b). Bio-oil yields from HTL of polysaccharides-lipids and proteins-lipids binary mixtures versus liquefaction temperature (c) and the dosage of SO42?/ZrO2 (d). Solid line, test bio-oil yield; dash line, theoretical bio-oil yield.
HTL experiments with varying amount of SO42?/ZrO2catalyst are conducted on equal mass proportion of binary mixtures for polysaccharides-lipids and proteins-lipids (Fig.5d). The yield of bio-oil from proteins-lipids HTL reached the highest with 5% SO42?/ZrO2catalyst addition. On the contrary, the yield of bio-oil decreases for polysaccharides-lipids HTL with the addition of SO42?/ZrO2catalyst.
3.5 Effect of Temperature and SO42?/ZrO2 Catalyst Dosage on Bio-oil Yield from HTL of Ternary Mixtures Simulated Algae
In order to make use of HTL results of model compounds more accurately to infer the actual liquefaction process of algae, three major components were mixed to simulate algae. Simulatingandwere performed by mixing polysaccharides, proteins and lipids at a mass fraction of 80%, 18% and 2%, 10%, 60% and 30%, respectively. HTL experiments are conducted at different liquefaction temperatures and different addition of SO42?/ZrO2catalyst. The optimal bio-oil yield of simulatedis at 220℃ shown in Fig.6a. The trend of bio-oil yield varying with liquefaction temperature of simulatedis different with the real one for the complexity of algae structure. With respect to simulating, the bio-oil yield is significantly higher than that of simulatingat the optimal liquefaction temperature of 300℃, which is similar to the HTL results of real algae. This indicates that higher liquefaction temperature can promote HTL of algae with higher protein and lipids content and increase the yield of bio-oil.
The results of bio-oil distribution from simulated and real algae varying with the dosage of SO42?/ZrO2catalyst are shown in Fig.6b. For the simulation of, the yield of bio-oil hardly changes with the amount of SO42?/ZrO2catalyst. The possible reason is that polysaccharides occupy a larger proportion in the ternary mixtures and the products distribution results are similar to that of polysaccharides HTL. Besides, as for the simulated, the yield of bio-oil first increases and then decreases with the increase of SO42?/ZrO2catalyst dosage.
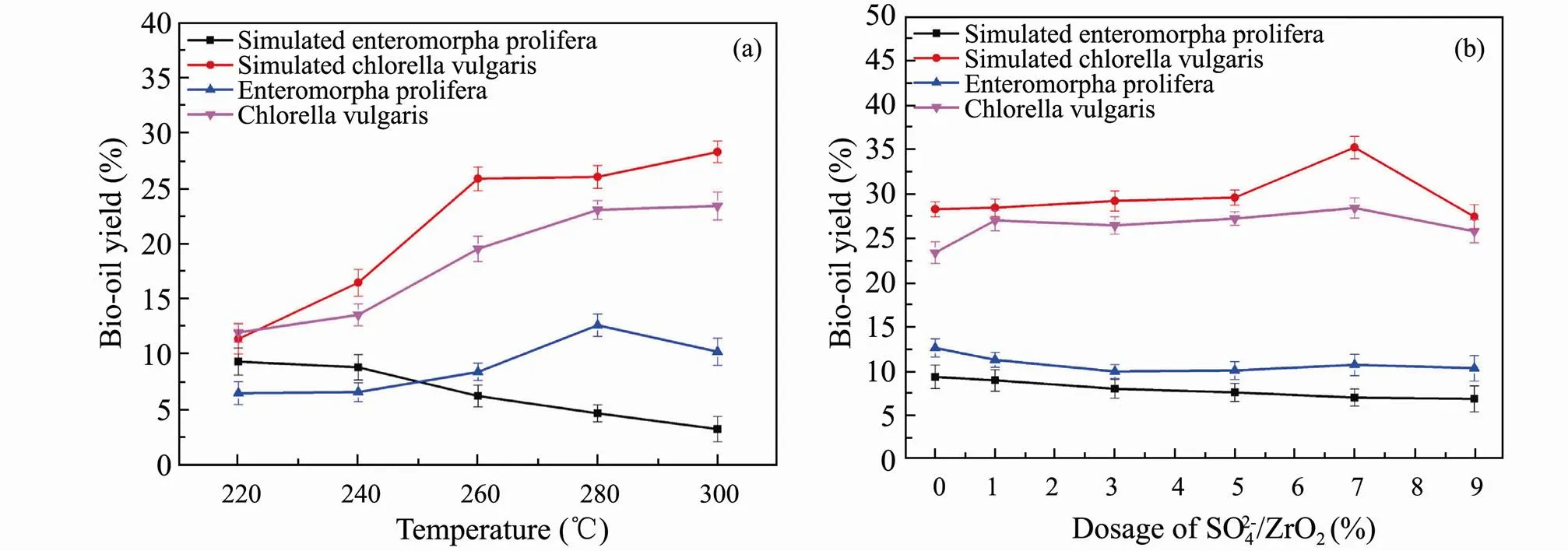
Fig.6 Bio-oil yields from HTL of ternary mixtures and real algae versus liquefaction temperature (a) and the dosage of SO42?/ZrO2 (b).
3.6 Recycling of SO42?/ZrO2 Catalyst
The reusability of the SO42?/ZrO2catalyst was studied by successive batches. As shown in Fig.7, the SO42?/ZrO2catalyst still exhibits 92.06% of its initial conversion after 4 cycles. From the XRD patterns of SO42?/ZrO2catalyst after each circle (Fig.8), the tetragonal peak of SO42?/ ZrO2catalyst is substantially the same as original SO42?/ ZrO2catalyst after calcination, indicating the good cyclic regeneration performance. Therefore, the solid superacid SO42?/ZrO2with high hydrothermal stability is considered to be a kind of good heterogeneous catalyst for regeneration recycling.
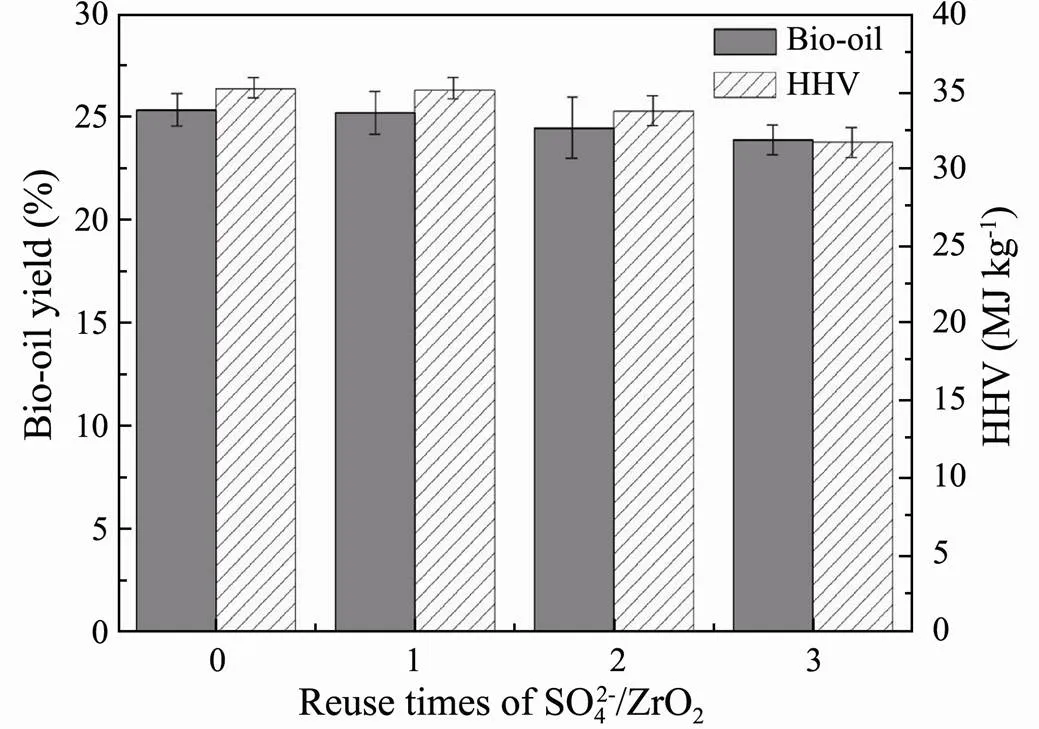
Fig.7 Bio-oil yields and HHVs from HTL of Chlorella vul- garis varying with cycling times of SO42?/ZrO2 catalyst.

Fig.8 XRD pattern versus reuse times of SO42?/ZrO2 catalyst: without reuse (a); reused once (b); reused twice (c); reused three time (d).
3.7 Elemental Analysis of Bio-oils
Elemental analysis results with HHVs of bio-oils produced from three major model compounds are shown in Table 1. It shows that hydrogen content for catalytic conditions of bio-oil increases significantly and carbon content decreases obviously, which resulted in an increase in the H/C ratio and HHVs of the bio-oil obtained in the presence of SO42?/ZrO2catalyst. High H/C ratios cause low molecular weights, which associates with TGA analysis, indicating that the light component proportion increases greatly when SO42?/ZrO2catalyst are employed. By using GC-MS, it can further conclude that the addition of SO42?/ZrO2catalyst actually promotes the decarboxylation of amino acids and causes higher H/C ratios, which is an efficient way to increase HHVs.

Table 1 Elemental analysis of bio-oil from HTL of algal major components
Note:?calculated by difference.
3.8 TGA of Bio-oils
Although thermal decomposition may occur during the heating process, TGA provides an estimate of the boiling point range of bio-oil (Yuan, 2010;Nazari, 2015; Yang, 2017). In this study, it carried out TGA of bio-oils obtained from catalytic and non-catalytic HTL of three major model compounds (Fig.9). The estimated boiling point distributions of bio-oils are shown in Table 2, which can be roughly divided into three temperature intervals. The results show that the addition of SO42?/ ZrO2catalystslightly increases the content of light component for bio-oil obtained from polysaccharides and lipids. It also proves that SO42?/ZrO2catalyst possesses strongly acidity and the acid condition is beneficial to the cracking reaction to get smaller molecular weight organics. Among three model compounds, the highest content of light components in bio-oil is obtained from lipids, which reaches 73.68% with SO42?/ZrO2catalyst.
For bio-oil from HTL of polysaccharides, the medium component proportions decrease greatly and mainly convert into light components in the presence of SO42?/ZrO2catalyst. It indicates that the SO42?/ZrO2catalyst promotes algae to split into more small organic molecules and significantly narrow the boiling point range of bio-oil of polysaccharides.
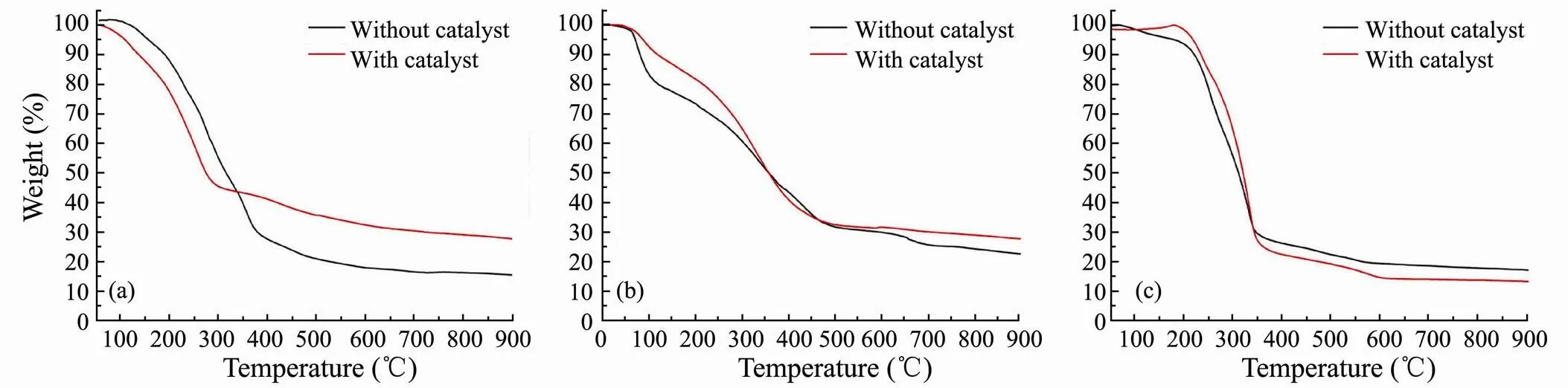
Fig.9 TGA curves of bio-oils obtained from HTL of polysaccharides (a), proteins (b) and lipids (c).

Table 2 Boiling point distributions of bio-oil estimated by TGA
3.9 GC-MS Analysis of Bio-oils and General Reaction Network
Compared with bio-oil, the composition of gas and aqueous phase products from HTL of algal major components is relatively simple. The gas phase products are mainly composed of CO2, H2and hydrocarbons. The largest proportion of gas is CO2from HTL of polysaccharides and proteins analyzed by GC. The main components in the aqueous phase formed by HTL of polysaccharides are acids and alcohols, while that from HTL of proteins is Nitrogen-containing compounds such as pyrazine, pipe- razine and pyrrole (Yang, 2017). Herein, we emphatically analyze the composition of bio-oil from HTL of algal major components.
During the HTL, the biochemical composition of bio- oil is mainly influenced by catalyst. Therefore, the chemi- cal composition of bio-oil obtained with and without SO42?/ZrO2catalyst was analyzed at the same time by GC-MS. The total ion chromatogram (TIC) of bio-oil obtained is shown in Fig.10. Although SO42?/ZrO2catalysthas little effect on the yield of bio-oil, it significantly changed the composition of bio-oil from HTL of polysaccharides. Organic products of polysaccharides liquefaction are mainly divided into five categories: cyclic ketones, furfural, furans, hydrocarbons and phenols (Table 3). Among these organic products, 5-HMF has the largest proportion without the catalyst, while the content of 5-HMF decreased and the content of furan increased significantly in the presence of SO42?/ZrO2catalyst. It is speculated that the addition of SO42?/ZrO2catalyst promotes the degradation of 5-HMF into furans in the HTL process. To some extent, furans are inclined to polymerize to undesired coke-like substances, which led to an increase in the yield of residue with the addition of SO42?/ZrO2catalystimproves. Parsa(2017) reported that furfurals or furans are more unstable under alkali condition or neutral conditions. They tended to decompose by dehydration and partial degradation of furfural or furans to produce phenolic compounds. In this study, due to the strongly acidity of SO42?/ZrO2catalyst, the hydrolysis of furfural or furans to phenols is inhibited, resulting in a decrease in phenolic substances. Cyclic ketones can be formed by dehydration, isomerization, and cyclization of sugars and reducing sugars. The formation of cyclic ketones is inhibitd by SO42?/ZrO2catalyst. The way of bio-oil deoxidation during HTL is generally achi- eved by removing CO2or water molecules. The removal of oxygen in the form of water is inhibited, but the formation of CO2is promoted, and the H/C in bio-oil is improved, so the HHVs of bio-oil are promoted in the presence of SO42?/ZrO2catalyst.

Fig.10 TIC spectraof bio-oil obtained from HTL of polysaccharides (a), proteins (b), lipids (c) and polysaccharides-pro- teins (d).

Table 3 Major chemical compositions of bio-oils obtained from HTL of polysaccharides
Major bio-oils components from HTL of proteins can be divided into ketones, phenols, amines, amides, alcohol and various N-containing heterocyclic compounds (Table 4). The ketones in bio-oil are formed by the process of intermolecular condensation, hydrolysis and isomerization of amino acids produced by proteins. The results show that N-containing heterocyclic compounds posses- ses a larger proportion without catalyst and the total content decreases with the addition of catalyst, which proves that SO42?/ZrO2catalyst promotes the denitrification reaction. In addition, the reduction of amine compounds indicates that SO42?/ZrO2catalyst may inhibit the decarboxylation reaction of amino acid, resulting in the decrease of released CO2, H/C and HHVs of bio-oil. The substantial increase of alcohols indicates the addition of SO42?/ZrO2catalyst promoted the deamination reaction of amino acids, while the main product carboxylic acids further reduced to alcohols. The hydrocarbon substance increases significantly, which is possibly due to SO42?/ ZrO2catalyst promote the decarboxylation of amino acids to generate alkanes and alkenes.
Lipids are the mixtures of glycerol and oleic acid in which glycerol is generally dissolved in the aqueous phase without being converted to oil phase products. The oleic acid is mainly reconstituted by HTL to form four kinds of fatty acids (Table 5). The addition of SO42?/ZrO2catalyst promoted the formation of linoleic acid, palmitic acid and-Hexadecenoic acid but inhibited the formation of 6-Octadecenoic acid. In addition, a small amount of hydrocarbons was detected, which mainly derived from polysaccharides and proteins. The total content of fatty acid decreased, which is possibly due to the further decarboxylation of fatty acid to form hydrocarbon in the presence of SO42?/ZrO2catalyst.

Table 4 Major chemical compositions of bio-oils obtained from HTL of proteins

Table 5 Major chemical compositions of bio-oils obtained from HTL of lipids
The resulting bio-oil from HTL of polysaccharides- proteins binary mixtures is mainly composed of N-con- taining heterocyclic compounds, cyclic ketones, amides, phenols, alcohols and other substances (Table 6). Among them, N-containing heterocyclic compounds such as pyra- zines and pyrroles have a large proportion. Yang(2017) concluded that these N-containing heterocyclic compounds were formed by Maillard reactions between polysaccharides and proteins degradation products. Gai(2015) reported that a part of N-containing heterocyclic compounds were formed by the reaction of protein- produced ammonia and polysaccharide-produced cyclic oxygen compounds. By introducing SO42?/ZrO2catalyst, the pyrazines content in the bio-oil decreases which indicates that the catalyst might inhibit the above two reactions to increase the bio-oil yield. In addition, the content of cyclic ketones in the bio-oil also decreases with the addition of SO42?/ZrO2catalyst, since the cyclic ketones are the product of Maillard reaction in which are inhibited by the catalyst. However, the contents of phenols and al- cohols increases obviously, indicating the SO42?/ZrO2catalyst promoted the deamination of amino acids and the generation of alcohols reduced from carboxylic acids. The resulting alcohols can reduce the viscosity of bio-oil to make it exhibits good fluidity.

Table 6 Major chemical compositions of bio-oils from HTL of polysaccharides-proteins
()
()

Category CompoundArea (%) No catalystWith catalyst Pyrroles3-Ethyl-2,4,5-trimethyl-1H-pyrrole3.084.01 N-Ethylpyrrole2.231.97 2-Methyl-3,4-diethyl-1H-pyrrole1.532.77 2-Ethyl-3,4,5-trimethyl-1H-pyrrole4.220.84 N-Acetylpyrrolidine–3.13 2-Ethyl-3,4,5-trimethyl-1H-pyrrole–1.26 Phenols2,3,5,6-Tetramethylhydroquinone2.333.38 3-Hydroxymethyl-5-methoxyphenol1.432.64 3-Ethoxy-4-methoxybenzaldehyde3.163.01 5-Methoxy-2-(methoxymethyl)phenol2.854.69 Alcohols2-Ethylhexanol–10.47 3-Methoxybenzyl alcohol1.73– AmidesN-Methylsuccinimide4.632.41 Hexadecanamide6.880.41
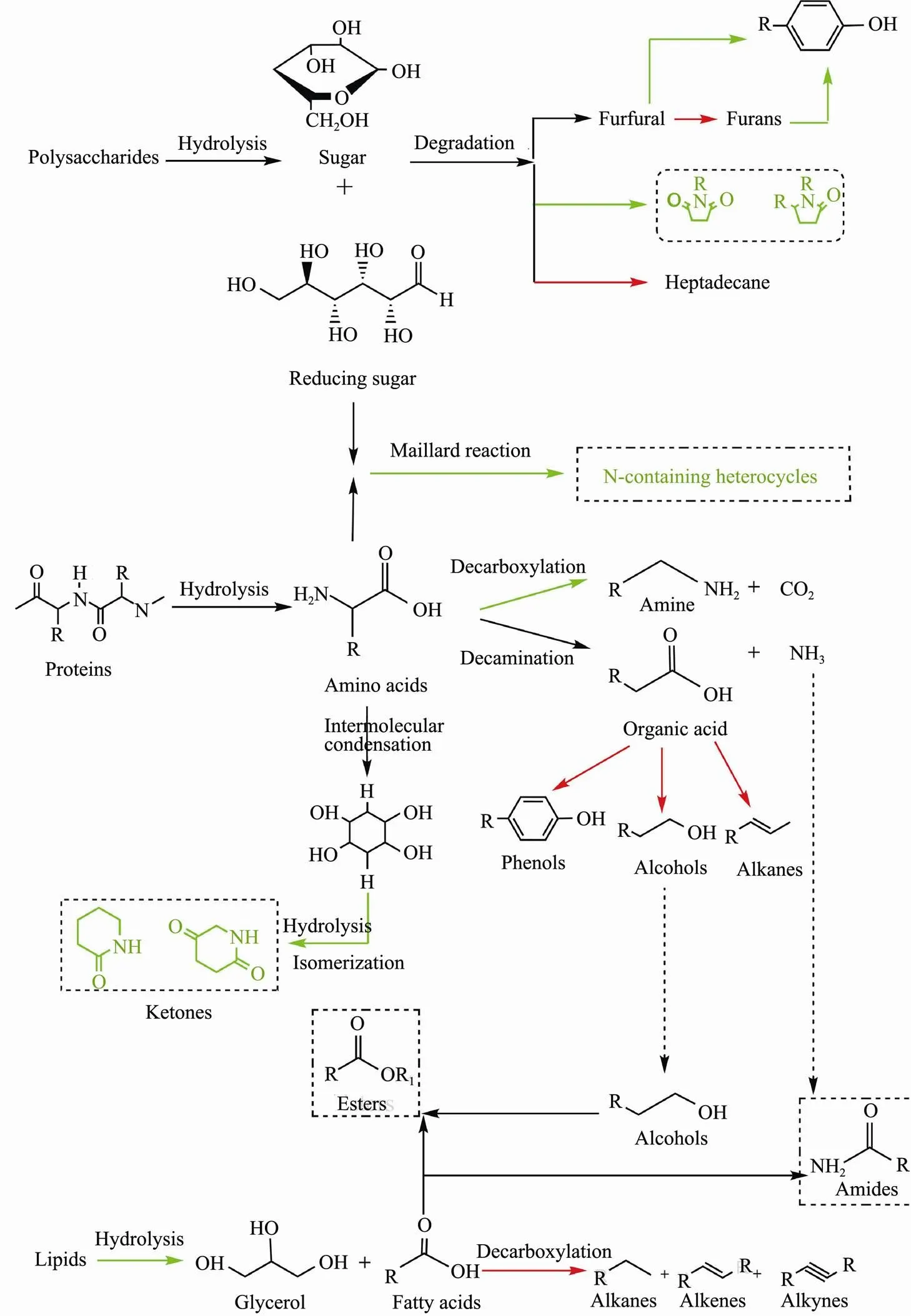
Fig.11 Formation pathways of bio-oils from HTL of algae three major model components in the presence of SO42?/ZrO2 catalyst. Red and blue represents the promoted and inhibited process by the solid acid catalyst.
Overall, the addition of SO42?/ZrO2catalyst can significantly regulate the composition and content of bio-oil and affect the degradation and interaction pathway of polysaccharides, proteins and lipids (Fig.11). During the HTL process, the reaction pathways can be divided into the following steps: 1) at low temperature stage, the main algae components polysaccharides, proteins and lipids hydrolyzed into small molecules; 2) with the increasing of temperature, the small molecules were further degrad- ed, dehydrated, decarboxylated and deaminated to decompose into active intermediates; 3) as temperature continues to rise, Maillard reaction, amidation reaction, esterification reaction were tended to occur between the active intermediates (Galadima and Muraza, 2018; Wang, 2018; Marzbali, 2021;).The coordination of sulfate radical with metal atoms on the oxide surface provides activity for the SO42?/ ZrO2solid acid (Li, 2018). The addition of solid acids has an important influence on macromolecular hydrolysis during the whole process of producing bio-oil, and controls the further reaction rate of bio-oil formation process, which is beneficial to accelerate the formation of bio-oil from cellular components (Yang., 2017).
4 Conclusions
Solid superacid SO42?/ZrO2has been prepared by precipitation-impregnation method and the acid amount showed a decreasing trend as the calcination temperature increased. The prepared SO42?/ZrO2can been seen as excellent catalyst in the HTL ofand, which promote the increasing of light component proportion and the HHVs, while inhibited the Maillard reaction between polysaccharides and proteins to upgrade the bio-oil producing. Based on the analysis of the properties of bio-oil produced by the major model components, it has put forward the formation path- ways of bio-oils from HTL of real algae in the presence of solid superacid catalyst SO42?/ZrO2.
Acknowledgements
This research was supported by the Shandong Provincial Natural Science Foundation, China (No. ZR2019BB 033) and the Fundamental Research Funds for the Central Universities of Ocean University of China (No. 201813 031).
Ambaye, T. G., Vaccari, M., Bonilla-Petriciolet, A., Prasad, S., van Hullebusch, E. D., and Rtimi, S., 2021. Emerging tech- nologies for biofuel production: A critical review on recent progress, challenges and perspectives.,290: 112627, DOI:10.1016/j.jenvman. 2021.112627.
Biller, P., Riley, R., and Ross, A. B., 2011. Catalytic hydrothermal processing of microalgae: Decomposition and upgrading of lipids., 102 (7): 4841-4848, DOI: 10.1016/j.biortech.2010.12.113.
Biswas, B., Kumar, A. A., Bisht, Y., Singh, R., Kumar, J., and Bhaskar, T., 2017. Effects of temperature and solvent on hydrothermal liquefaction ofalgae., 242: 344-350, DOI: 10.1016/j. biortech.2017.03.045.
Carrero, A., Vicente, G., Rodríguez, R., Peso, G. L. D., and Santos, C., 2015. Synthesis of fatty acids methyl esters (FAMEs) frommicroalga using heterogeneous acid catalysts., 97: 119- 124, DOI: 10.1016/j.bej.2015.02.003.
Cheng, F., Cui, Z., Chen, L., Jarvis, J., Paz, N., Schaub, T.,., 2017. Hydrothermal liquefaction of high- and low-lipid algae: Bio-crude oil chemistry., 206: 278-292, DOI: 10.1016/j.apenergy.2017.08.105.
Gai, C., Zhang, Y. H., Chen, W. T., Zhang, P., and Dong, Y. P., 2015. An investigation of reaction pathways of hydrothermal liquefaction usingand, 96: 330-339, DOI: 10.1016/j.enconman.2015.02.056.
Galadima, A., and Muraza, O., 2018. Hydrothermal liquefaction of algae and bio-oil upgrading into liquid fuels: Role of heterogeneous catalysts., 81: 1037-1048, DOI: 10.1016/j.rser.2017.07.034.
Hu, Y. L., Feng, S. H., Yuan, Z. S., and Xu, C. B., 2017. Investigation of aqueous phase recycling for improving bio-crude oil yield in hydrothermal liquefaction of algae., 239: 151-159, DOI: 10.1016/j.biortech.2017.05. 033.
Kim, S. S., Ly, H. V., Kim, J., and Choi, J. H., 2013. Thermogravimetric characteristics and pyrolysis kinetics of algasp. biomass., 139: 242-248, DOI: 10.1016/j.biortech.2013.03.192.
Li, L., Yan, B., Li, H. X., Yu, S. T., Liu, S. W., Yu, H. L.,., 2018. SO42?/ZrO2as catalyst for upgrading of pyrolysis oil by esterification., 226: 190-194, DOI: 10.1016/j.fuel.2018. 04.006.
López Barreiro, D., Zamalloa, C., Boon, N., Vyverman, W., Ronsse, F., Brilman, W.,., 2013. Influence of strain-specific parameters on hydrothermal liquefaction of microalgae., 146: 463-471, DOI: 10.1016/j.bior- tech.2013.07.123.
Marzbali, M. H., Kundu, S., Halder, P., Patel, S., Hakeem, I. G., Paz-Ferreiro, J.,., 2021. Wet organic waste treatmenthydrothermal processing: A critical review., 279: 130557, DOI: 10.1016/j.chemosphere.2021.130557.
Navalon, S., Dhakshinamoorthy, A., Alvaro, M., and Garcia, H., 2016. Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts., 312: 99-148, DOI: 10.1016/j.ccr.2015.12.005.
Nazari, L., Yuan, Z., Souzanchi, S., Ray, M. B., and Xu, C. B., 2015. Hydrothermal liquefaction of woody biomass in hot- compressed water: Catalyst screening and comprehensive characterization of bio-crude oils., 162: 74-83, DOI: 10. 1016/j.fuel.2015.08.055.
Parsa, M., Jalilzadeh, H., Pazoki, M., Ghasemzadeh, R., and Abduli M. A., 2017. Hydrothermal liquefaction ofandmacro-algae for biocrude production., 56: 234-244, DOI: 10. 1016/j.biortech.2017.10.059.
Peng, Y. B., Xu, Y. F., Dearn, K. D., Geng, J., and Hu, X. G., 2018. Noveltribo-catalysis for improved tribological properties of bio-oil model compound., 212: 546-553, DOI: 10.1016/j.fuel.2017.10.080.
Qin, J. L., Yu, D. H., and Chen, J. D., 2004. Effect of calcination temperature on surface acidity of TiO2/SiO2., 18 (6): 472-474, DOI: 10.16084/j.cnki.issn 1001-3555.2004.06.014.
Saber, M., Golzary, A., Hosseinpour, M., Takahashi, F., and Yoshikawa, K., 2016. Catalytic hydrothermal liquefaction of microalgae using nanocatalyst., 183: 566-576, DOI: 10.1016/j.apenergy.2016.09.017.
Saber, M., Nakhshiniev, B., and Yoshikawa, K., 2016. A review of production and upgrading of algal bio-oil., 58: 918-930, DOI: 10.1016/j. rser.2015.12.342.
Sharma, N., Jaiswal, K. K., Kumar, V., Vlaskin, M. S., Nanda, M., Rautela, I.,., 2021. Effect of catalyst and temperature on the quality and productivity of HTL bio-oil from micro- algae: A review., 174: 810-822, DOI:10. 1016/j.renene.2021.04.147.
Sheng, L. L., Wang, X., and Yang, X. Y., 2018. Prediction model of biocrude yield and nitrogen heterocyclic compounds analysis by hydrothermal liquefaction of microalgae with model compounds., 247: 14-20, DOI: 10.1016/j.biortech.2017.08.011.
SundarRajan, P., Gopinath, K. P., Arun, J., GracePavithra, K., Adithya Joseph, A., and Manasa, S., 2021.Insights into valuing the aqueous phase derived from hydrothermal liquefaction., 144: 111019,DOI:10.1016/j.rser.2021.111019.
Toor, S. S., Rosendahl, L., and Rudolf A., 2011. Hydrothermal liquefaction of biomass: A review of subcritical water technologies., 36 (5): 2328-2342, DOI: 10.1016/j.energy. 2011.03.013.
Vo, T. K., Kim, S., Ly, H. V., Lee, E. Y., Lee, C. G., and Kim, J., 2017a. A general reaction network and kinetic model of the hydrothermal liquefaction of microalgaesp., 241: 610-619, DOI: 10.1016/j.biortech. 2017.05.186.
Vo, T. K., Ly, H. V., Lee, O. K., Lee, E. Y., Kim, C. H., Seo, J. E.,., 2017b. Pyrolysis characteristics and kinetics of microalgalsp. KRS101., 118: 369-376, DOI: 10.1016/j.energy.2016.12.040.
Wang, B., Zhu, J. P., and Ma, H. Z., 2009. Desulfurization from thiophene by SO42?/ZrO2catalytic oxidation at room temperature and atmospheric pressure., 164 (1): 256-264, DOI: 10.1016/j.jhazmat.2008. 08.003.
Wang, W. J., Yu, Q., Meng, H., Han, W., Li, J., and Zhang, J. L., 2018. Catalytic liquefaction of municipal sewage sludge over transition metal catalysts in ethanol-water co-solvent., 249: 361-367, DOI: 10.1016/j.biortech. 2017.09.205.
Wang, X., Tang, X. H., and Yang, X. Y., 2017. Pyrolysis mechanism of microalgaesp. based on model compounds and their interaction., 140: 203-210, DOI: 10.1016/j.enconman.2017. 02.058.
Xu, Y. F., Hu, Y. H., Peng, Y. B., Yao, L. L., Dong, Y. H., Yang, B. X.,., 2020. Catalytic pyrolysis and liquefaction behavior of microalgae for bio-oil production., 300: 122665, DOI: 10.1016/j.biortech.2019.122 665.
Xu, Y. F., Liu, K., Hu, Y. H., Dong, Y. H., and Yao, L. L., 2020. Experimental investigation and comparison of bio-oil from hybrid microalgaesuper/subcritical liquefaction., 279: 118412, DOI: 10.1016/j.fuel.2020.118412.
Yang, W. C., Li, X. G., Li, Z. H., Huang, F. B., Sun, X. M., and Feng, L. J., 2015. Application of solid acid SO42?/Fe2O3in thermal liquefaction of., 31 (5): 1075-1081, DOI: 10.3969/j.issn.1001-8719.2015.05.007.
Yang, W. C., Li, X. G., Li, Z. H., Tong, C. H., and Feng, L. J., 2015. Understanding low-lipid algae hydrothermal liquefaction characteristics and pathways through hydrothermal liquefaction of algal major components: Crude polysaccharides, crude proteins and their binary mixtures., 196: 99-108, DOI: 10.1016/j.biortech.2015.07.020.
Yang, W. C., Li, X. G., Zhang, D. H., and Feng, L. J., 2017. Catalytic upgrading of bio-oil in hydrothermal liquefaction of algae major model components over liquid acids., 154: 336-343, DOI: 10.1016/j. enconman.2017.11.018.
Yang, W. C., Wang, Z. W., Han, J. B., Song, S., Zhang, Y., and Gong, W. M., 2019. The role of polysaccharides and proteins in bio-oil production during the hydrothermal liquefaction of algae species., 9: 41962-41969, DOI: 10.1039/ c9ra07150d.
Yuan, Z., Cheng, S., Leitch, M., and Xu, C. B., 2010. Hydrolytic degradation of alkaline lignin in hot-compressed water and ethanol., 101 (23): 9308-9313, DOI: 10.1016/j.biortech.2010.06.140.
Zhao, Y. N., Feng, L. J., Liu, S. S., Li, D., and Yang, W. C., 2017. Application of solid acid SO42?/ZrO2in ethanol thermal liquefaction ofand., 33 (3): 447-455, DOI: 10.3969/j.issn.1001- 8719.2017.03.008.
Zhu, Z. B., Si, B. C., Lu, J. W., Watson, J., Zhang, Y. H., and Liu, Z. D., 2017. Elemental migration and characterization of products during hydrothermal liquefaction of cornstalk., 24: 9-16, DOI: 10.1016/j.biortech.2017. 06.085.
June 30, 2021;
April 11, 2022;
April 28, 2022
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
. E-mail: yaoshuo@ouc.edu.cn
(Edited by Ji Dechun)
 Journal of Ocean University of China2022年5期
Journal of Ocean University of China2022年5期
- Journal of Ocean University of China的其它文章
- Stress Analysis of Wire Strands by Mesoscale Mechanics
- Effect of Temperature on the Carbon, Nitrogen, and Phosphorus Nutrient Budgets of Steelhead Trout (Oncorhynchus mykiss) with Different Sizes
- Impact of Evaporation Duct on Electromagnetic Wave Propagation During a Typhoon
- A New α-Cyclopiazonic Acid Alkaloid Identified from the Weizhou Island Coral-Derived Fungus Aspergillus flavus GXIMD 02503
- Design of Copper Oxide Nanosheets-Loaded Zeolite with Efficient Inhibition of Marine Bacteria
- 3-D Marine CSEM Modeling in General Anisotropic Media by Using an Adaptive Finite Element Approach Based on the Vector-Scalar Potential
