Peripheral nerve allograft: how innovation has changed surgical practice
Gregory Buncke
The Buncke Clinic, San Francisco, CA 94114, USA.
Abstract The landscape of available technology and surgical technique has changed over the last several decades, thus leading to changes in the peripheral nerve repair surgical algorithm. Neurorrhaphy is a common procedure; however, it is well recognized that nerve repair should be performed tensionless, thus preventing the ability to perform direct repair with a nerve gap. Historically, nerve gaps were repaired with autograft. However, autograft surgery has been associated with complications such as numbness and chronic pain, which left surgeons searching for alternatives. Nerve allografts were first utilized in the 1800s but failed due to the immune response. In the modern era, they were again utilized in the 1980s, but did not gain popularity because of the need for the use of immunosuppressants. It was evident through the 1990s that continued innovation in peripheral nerve repair was needed, as studies showed that only approximately 50% of patients with nerve gap repair achieved good or excellent outcomes. In the 2000s, the advent of an engineered nerve allograft (Avance? Nerve Graft) changed the landscape of peripheral nerve repair. Early clinical evaluation of Avance showed that adequate sensation was able to be achieved in nerve gaps up to 30 mm, providing an alternative to autografts. As engineered nerve allograft use became more conventional, studies showed 87.3% meaningful recovery in nerve gaps up to 50 mm. Furthermore, recent studies have shown that gaps between 50-70 mm have shown 69% meaningful recovery. While technology and surgical technique continue to improve, these results are promising for large nerve gap repair.
Keywords: Peripheral nerve repair, nerve autograft, nerve allograft, nerve conduit
INTRODUCTION
Hand and wrist injuries occur in 6.6% of emergency room visits in the United States, costing $48.6 billion annually[1]. Peripheral nerve injuries occur in 2.5% of trauma patients[2], with the average number of peripheral nerve procedures at 558,862 annually[3]. The most frequently injured nerves treated within hospitals include ICD-9-CM 955.6, upper extremity digital nerve; ICD-9-CM 955.2, ulnar nerve; ICD-9-CM 955.3, radial nerve; and ICD-9-CM 953.4, the brachial plexus[4]. Peripheral nerve injuries have socioeconomic costs, direct patient costs, and can affect patient quality of life.
The notable socioeconomic costs for the patient include missed work/school due to regular physician appointments, procedures, and hospital visits. These appointments and procedures can result in significant direct costs to the patient, which can be compounded by the loss of wages due to missed work[5]. Additionally, patient quality of life can be impacted by disrupted sleep patterns, social life, extremity function, personal life, professional activities, and mood[6]. Notably, 64% of patients with peripheral nerve injuries have missed at least one month of work or school, and 24% of patients with nerve injuries have missed at least 12 months of work or school[5]. These significant impacts on patients’ economic standing and quality of life highlight the importance of continuing to improve outcomes in the treatment of peripheral nerve injuries.
When a peripheral nerve is injured, the resulting injury may lead to varying disruptions in the peripheral nerve anatomy. These varying injury severities result in different functional impacts, which are related to the anatomical structure of peripheral nerves. The peripheral nerve is composed of several layers of connective and functional tissues that support the electrical impulse propagation and the structure of the nerves.
Peripheral nerves extend from the spinal cord and are comprised of both sensory (afferent) and motor (efferent) nerve fibers[7]. These nerve fibers, called axons, are either myelinated or unmyelinated [Figure 1][7]. Unmyelinated axons are ensheathed individually or in small groups within Schwann cells[7]. The Schwann cells are in contact with only a small section of the axon, which requires several Schwann cells aligned consecutively to cover the length of the axon[7]. Myelinated axons have a similar appearance to unmyelinated axons, as they are surrounded by Schwann cells; however, the Schwann cells have deposited a compacted layer of cytoplasm and cell membrane called the myelin sheath[7]. The myelin sheath serves to insulate the axons and improves nerve impulse conduction between the nodes of Ranvier, which are areas where there are natural interruptions in the myelin sheath[7]. Injuries isolated within the myelin sheath are classified as Seddon’s neurapraxia or Sunderland’s Type 1 [Table 1], which often recovers spontaneously[8,9]. Individual nerve fibers, both myelinated and unmyelinated, are bound together by connective tissues called the endoneurium, perineurium, and epineurium[7].
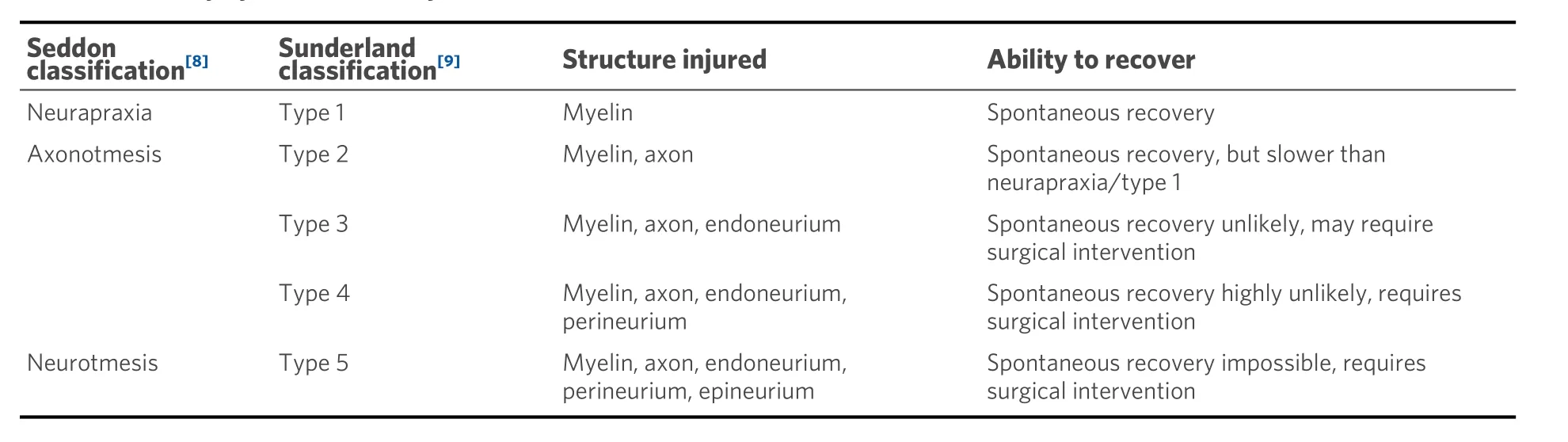
Table 1. Nerve injury classification by Seddon[8] and Sunderland[9]
Each nerve fiber is surrounded by the endoneurium, which is a loose collagenous connective tissue layer[7]. Bundles of endoneurium are contained within fascicles, which are surrounded by a connective tissue layer called the perineurium[7]. The perineurium consists of uniformly organized flattened laminae of fibroblasts with alternating sheets of collagen[7]. The outermost layer of the nerve, the epineurium, is composed of irregularly arranged collagenous tissue that provides elasticity and absorption of mechanical forces[7,10]. Peripheral nerve injuries resulting in a complete discontinuity in the peripheral nerve axon and surrounding connective tissue layers are classified as Seddon neurotmesis and Sunderland type 5.
Subsequent to nerve injury, the nerve proximal to the injury undergoes traumatic degeneration up to the first node of Ranvier, while the nerve distal to the injury undergoes Wallerian degeneration [Figure 2A][11].
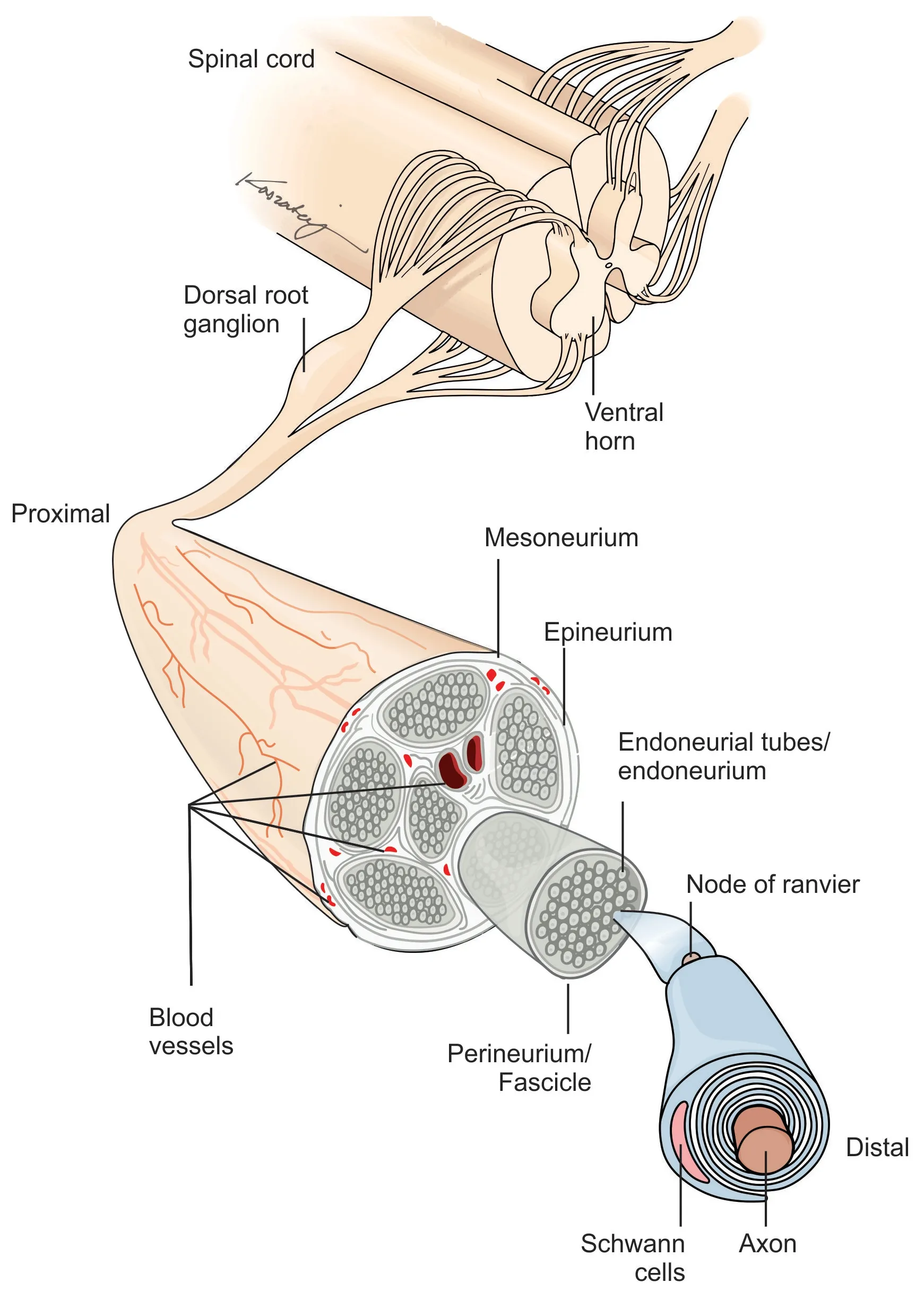
Figure 1. Peripheral nerve structure.
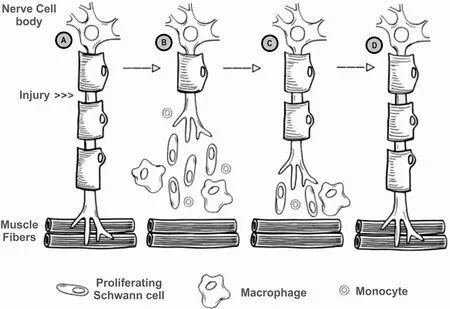
Figure 2. Process of Wallerian degeneration after peripheral nerve injury (A) intact nerve, prior to nerve injury, location of injury noted; (B) post-injury Wallerian degeneration of the nerve distal to the injury, with traumatic degeneration up to the first node of Ranvier in the nerve stump proximal to the injury; (C) axonal sprouting from the nerve proximal to the injury, where the growth cone extends down basal lamina of the endoneurial tubes; and (D) nerve regeneration complete, with connection established with distal target organ.
During Wallerian degeneration, Schwann cells and macrophages degrade myelin in the nerve distal to the injury [Figure 2B][12]. Axonal sprouting occurs from the nerve proximal to the injury within 24 hours of the injury, where the axonal growth cone extends down the basal lamina of the endoneurial tubes [Figure 2C][11]and connects to the distal target organ [Figure 2D]. When a nerve injury classified as Sunderland type IV (axonotmesis) and Sunderland type V (neurotmesis) occurs, surgical intervention is required due to loss of continuity in axons and, at a minimum, the endoneurium and perineurium[8,9]. Surgical treatment is required in 43.5% of patients suffering from peripheral nerve injuries[2], with 56% of patients undergoing direct repair and 44% of patients undergoing nerve gap repair[3]. While direct nerve repair is the historical standard for nerve repair, the nerve ends must be reapproximated without tension at the suture[13]. However, nerve transections may result in a gap between the nerve stumps due to tissue loss from the injury, surgical debridement, or natural retraction of the nerve[14,15].
When a nerve gap exists during peripheral nerve regeneration, the endoneurial tubes of the distal nerve stump cannot be accessed by the leading axonal growth cone from the proximal nerve stump due to the physical distance between the nerve stumps, thus leading to the development of a neuroma[16]. The repair of a transected nerve should be performed such that healthy fascicles are reapproximated in a tensionless manner, as tension has been shown to lead to ischemia and decreased axonal outgrowth[17]. In the case of a nerve gap, repair is often performed via nerve grafting using conduit, autograft, or allograft to span the gap between the nerve stumps [Figure 3][18]. Nerve repair has changed notably over the last several decades due to the introduction of microsurgical techniques and off-the-shelf options for bridging nerve gaps.

Figure 3. Peripheral nerve repair algorithm.
Off-the-shelf options for bridging nerve gaps include extracellular matrix scaffolds that support axonal regeneration, cellular and non-cellular graft additives. Ideally, a nerve scaffold should: be readily available, be biodegradable over a time appropriate for the application, be able to be revascularized, support cell migration, elicit a limited immunogenic response, allow for oxygen and nutrient diffusion, be adjustable for the nerve injury severity, not lead to long-term nerve compression, and support nerve regeneration[19-21]. Furthermore, Porzionatoet al. proposed that the best scaffold for tissue engineering is decellularized extracellular matrix of the same origin as the target tissue[22]. Scaffolds that support axonal regeneration can be engineered from natural or synthetic materials, although natural materials are thought to show improved biocompatibility, decreased toxicity, and better cellular migration[19]. The addition of bioactive factors and cells have also been investigated with the use of nerve scaffolds to stimulate cell migration and provide a preferential substrate for axonal migration[19]. While many of these advancements are not yet clinically available, novel materials for peripheral nerve repair such as engineered nerve allografts and nerve conduits are currently clinically available. The clinical emergence of these materials has inevitably changed the landscape of peripheral nerve repair and the surgical algorithm. This manuscript will explore the advent of nerve conduits and engineered peripheral nerve allografts and how their use has impacted the repair of transected peripheral nerves over time. Furthermore, this manuscript will focus on the clinical application of these technological advancements in extremities, as the most frequently injured nerves are located in the extremities.
HISTORY OF PERIPHERAL NERVE REPAIR
Nerve autograft
Records of peripheral nerve repair date back to the Hippocratic era[23]; however, the techniques and materials used for nerve gap repair have changed through the years [Figure 4]. In the 1800s, various techniques for peripheral nerve repair were described in the literature, including segmental nerve repair using nerve autograft[38], conduits of various materials[38-40], and cellular nerve allografts[41]. Nerve autograft is considered the historical standard for peripheral nerve gap repair when tensionless direct nerve repair cannot be achieved. Nerve autografts can be successfully utilized as single grafts, cabled, interfascicular, or vascularized grafts[38]. Nerve autografts should be chosen from a donor nerve that is considered expendable and would not lead to undesirable defects after graft harvest[38]. The most common autologous nerve graft is harvested from the sural nerve[38,42]; however, other nerve autograft sources may include medial and lateral antebrachial cutaneous nerve, the dorsal antebrachial nerve, the superficial branch of the radial nerve, dorsal branch of the ulnar nerve, lateral femoral cutaneous nerve, and the posterior interosseous nerve[38]. The selection of the nerve autograft is determined by the surgeon with feedback from the patient regarding the anticipated sensory deficit from the graft harvest. Sural nerve resections, such as sural nerve autograft harvest or sural nerve biopsy, have been shown to cause sensory deficits in 92.9% of patients and sensory symptoms (e.g., tingling cold intolerance, paresthesia, dysesthesia, or irritating sensation) in 41.1% of patients[43]. Additionally, sural nerve autograft harvest has been linked to increased incidents of chronic pain, wound infection, wound complications, impact on daily life, post-operative hematoma, and deep vein thrombosis[43]. Due to these complications, alternative bridging materials have been investigated[6].
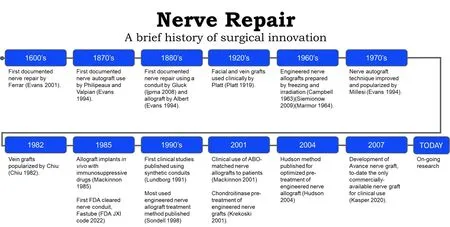
Figure 4. Timeline of surgical innovation for peripheral nerve repair[18,19,24-37].
Nerve conduits
Nerve conduits were an early alternative to nerve autograft, as nerve conduits circumvent nerve autograft harvest related complications, reduce scar tissue invasion within the nerve gap and help prevent axonal escape, which decreases the likelihood of neuroma formation[25]. In the 1920s, fascial and vein grafts were used clinically by Platt[26,44], and further popularized by Chiuet al.[29]in the 1980s[45].
In the 1990s and early 2000s, various synthetic and biologic nerve conduits were proposed for use in the literature. Early results indicated that conduits could be used to bridge nerve gaps less than 3 cm[46]. However, more recent literature suggests that nerve gaps measuring up to 1 cm are the limit for repair with a nerve conduit[47]. This is due to the lack of structural guidance in hollow conduits and the reliance on the formation of a fibrin cable within the conduit to provide axonal guidance across the gap. At longer gap lengths, the fibrin cable does not provide adequate structure for the regenerating axon throughout the regeneration process[48]. In the repair of peripheral nerve gaps beyond 1 cm, a nerve graft should be utilized[47]. While there are various FDA-cleared nerve conduits, a review in 2021 found that these conduits are mostly used in gaps less than 1 cm and exhibit poorer outcomes in longer gap repairs [Table 2][48].
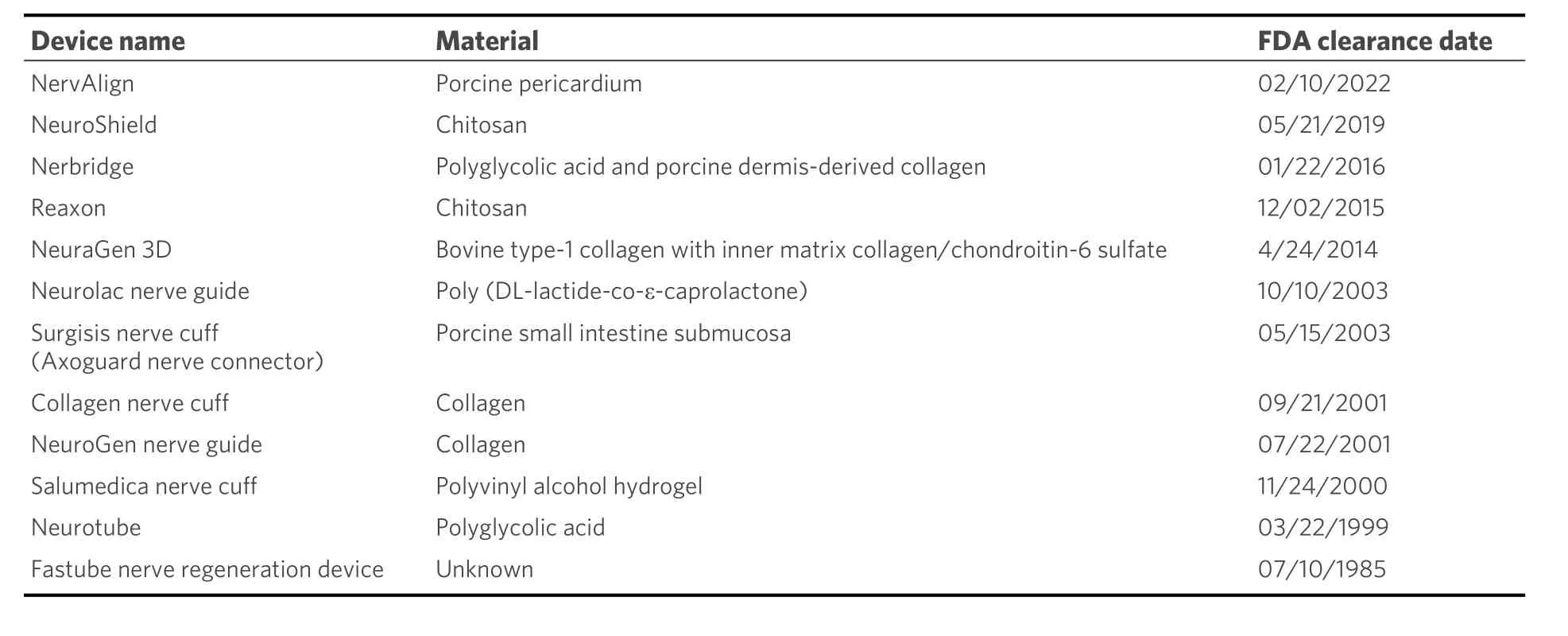
Table 2. Nerve conduits cleared by the FDA[31,49]
Nerve allograft
Nerve allografts were sought as an alternative to autografts, as nerve allografts can be prepared and stored in tissue banks, do not lead to a secondary donor surgical site and provide the proper structural guidance needed for peripheral nerve regeneration[18]. Engineered nerve allografts were first noted in the literature in the 1960s, which were pre-treated by freezing and irradiation[18,27,28]. However, the initial success of engineered nerve allografts faced limitations during early surgical use, as grafts were noted to show delayed axonal outgrowth, elicited an immunologic response and resulted in nerve graft rejection[50-53]. Research to improve nerve allograft outcomes continued, including major histocompatibility complex (MHC) matching, patient immunosuppression and nerve graft processing methods. In 1985, Mackinnonet al. attempted MHC matching in rats and found good regeneration in MHC matched allografts and poor regeneration in the MHC unmatched grafts[30].
In 2001, Mackinnon evaluated the clinical use of donor allograft nerve that was blood-type (ABO) matched to patient recipients[34]. These ABO-matched nerve allografts showed good sensory and motor outcomes in patients[34]. The immunologic response in cellular allogenic nerve grafts was noted to decrease over time as the donor Schwann cells were replaced by host Schwann cells[54], but immunosuppressive treatments, including Cyclosporin-A and tacrolimus (FK506), were still required[18]. Alternative areas of research, such as nerve allograft pre-treatments, were investigated to circumvent the need for immunosuppressives.
Research in engineered nerve allograft pretreatment development has included cryopreservation, lyophilization[55], freeze/thaw cycling[56], cold storage, chemical treatments to extract cellular debris or predegenerate the graft[33,35,36,57,58], and irradiation[27]. The most prolific engineered nerve allograft pre-treatment protocol used in literature was proposed by Sondellet al.[33]in 1998, which used Triton X-100 and sodium deoxycholate solution to chemically lyse cells[59]. This pre-treatment protocol resulted in the removal of the myelin sheath and cells from engineered nerve allografts, resulting in a satisfactory nerve regeneration responsein vivo[33]. Later research was conducted in 2001 by Krekoskiet al. showing that chondroitin sulfate proteoglycan glycosaminoglycan side chains, known to inhibit axonal growth by functional blockade of laminin, could be removed from the nerve by treatment with a chondroitinase ABC enzyme[35]. The methods proposed by Krekoskiet al. showed that chondroitinase pre-treated engineered nerve allografts improved the growth-promoting properties of the nerve allografts and resulted in more axons growing at longer lengths through the chondroitinase treated allografts compared to grafts that were not treated with chondroitinase[35]. Several years later, in 2004, Hudsonet al. proposed an engineered nerve allograft pretreatment protocol using Sulfobetaine-16, Triton X-200, and Sulfobetaine-10 to improve cell lysis while maintaining the extracellular matrix[36]. The Hudsonet al. study showed that engineered nerve allograft pretreatment with mild detergents resulted in axon densities that were comparable to nerve isografts (considered to be the equivalent of nerve autografts in pre-clinical studies)[36]. Furthermore, these Hudson pre-treated engineered nerve allografts also showed 910% more axon density than thermally treated engineered neve allografts and 401% more axon density than the pre-treatment process proposed by Sondell[33]. Pre-treating engineered nerve allografts with both the Hudson pre-treatment and the Krekoski pre-treatment has been found to be the most effective pre-treatment for engineered nerve allografts, when compared among other well-established nerve allograft pre-treatment protocols[59]. Avance?nerve graft was developed using both pre-treatment methods[37]outlined by Hudsonet al.[36]and Krekoskiet al.[35].
The effort to develop an engineered nerve allograft utilizing these two protocols involved over 20 years of research by two research groups, and came to fruition in 2007 when Avance nerve graft was made available as an off-the-shelf engineered nerve allograft for clinical use[37]. To date, Avance nerve graft is the only engineered nerve allograft commercially available in the United States for clinical use. The initial preclinical studies showed that Avance nerve allografts did not show an immunogenic reaction and maintained the native extracellular matrix structure of the nerve, including laminin, a protein critical to neurite outgrowth[60].
CLINICAL USE OF AVANCE NERVE GRAFT
The first clinical report of Avance nerve graft use was published in 2009 and involved 8 patients with 10 sensory nerve repairs[61][Table 3]. The average gap length was 2.23 cm (range 0.5-3 cm), and all patients had sensory improvement by 9 months[61]. Through 2016, several clinical publications demonstrated that adequate sensation was achieved in sensory nerve gap repair using Avance nerve graft in gaps up to 30 mm in the upper and lower extremity, providing an alternative to autografts[64,65,76-79]. Engineered nerve allografts, such as Avance nerve graft, provided advantages over nerve autografts, including circumventing donor-site morbidity, off-the-shelf availability, easy to use, and reduced operative time[61,64,65,76-78]. Further research expanded the gap length and nerve type repaired with Avance nerve graft.
In 2012, Brookset al. published the first results of nerve gaps repaired with Avance nerve graft up to 50 mm in length, which included 76 nerve gaps averaging 22 mm (range, 5-50 mm) in sensory, mixed, and motor nerves located in the upper and lower extremity[62]. Brooks and colleagues showed meaningful recovery in 87.3% of nerve gaps repaired with Avance nerve graft, where meaningful recovery was defined as a return of motor recovery to M3 or greater and sensory recovery to S3 or greater using the Medical Research Council Classification (MRCC) scale[62]. Additionally, there were no significant differences in sensory and motor outcomes between sensory, mixed, or motor nerve repairs[62]. In late 2012, Choet al. published on 51 sensory, mixed, and motor nerve gaps repaired with Avance nerve graft in the upper extremity only[63]. Choet al.and colleagues showed in nerve gaps averaging 23 mm (range, 5-50 mm), 86% of repairs achieved S3 or M4 and above recovery[63]. The adoption of engineered nerve allograft into clinical use and early publications led to the development of an evidence-based algorithm.
In 2012, Ducicet al. discussed that direct repair should be used in nerve gaps less than 5 mm, nerve conduits should be used in gaps 5 mm to 15 mm, engineered nerve allografts should be used in nerve gaps 5 mm to 50 mm, and nerve autografts should be used in nerve gaps 5 mm to greater than 50 mm[47]. This suggested that both engineered nerve allograft and nerve autograft could be used in similar gap sizes. By 2014, it was proposed by Rinkeret al. that engineered nerve allografts, such as Avance nerve graft, were the most significant development in peripheral nerve surgery since the introduction of microsurgery[80].
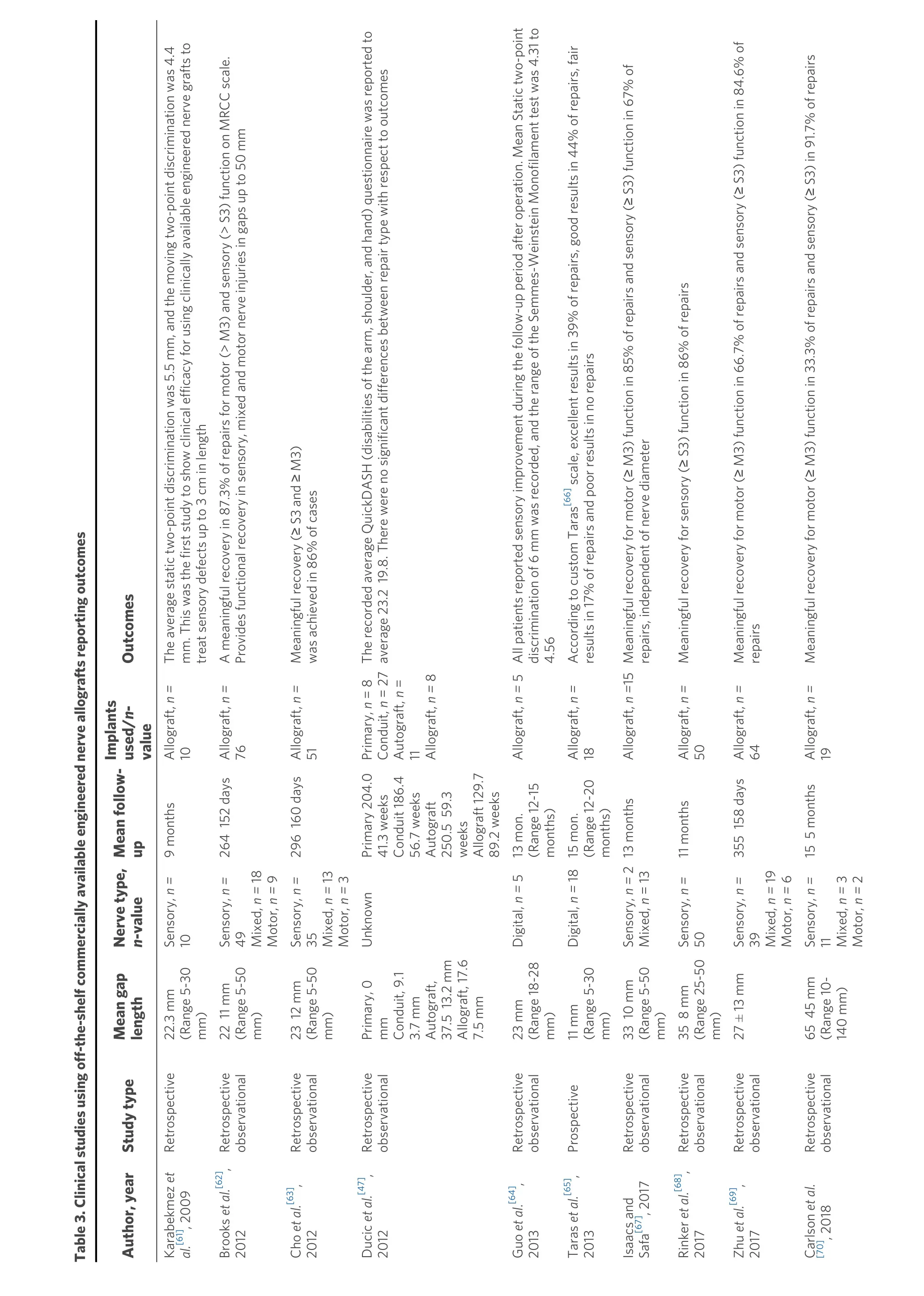
Page 9 of Buncke. PlastAesthetRes 2022;9:38 https://dx.doi.org/10.20517/2347-9264.2022.08 17 Table 3. Clinical studies using off-the-shelf commercially available engineered nerve allografts reporting outcomes Author, year Study type Mean gap length Nerve type, n-value Mean follow- up Implants used/n- value Outcomes Karabekmez etal.[61], 2009 Retrospective 22.3 mm (Range 5-30 mm)Sensory, n=10 9 months Allograft, n =10 The average static two-point discrimination was 5.5 mm, and the moving two-point discrimination was 4.4 mm. This was the first study to show clinical efficacy for using clinically available engineered nerve grafts to treat sensory defects up to 3 cm in length Brooks etal.[62], 2012 Retrospective observational 22 11 mm (Range 5-50 mm)Sensory, n =49 Mixed, n =18 Motor, n =9 264 152 days Allograft, n =76 A meaningful recovery in 87.3% of repairs for motor (> M3) and sensory (> S3) function on MRCC scale. Provides functional recovery in sensory, mixed and motor nerve injuries in gaps up to 50 mm Cho etal.[63], 2012 Retrospective observational 23 12 mm (Range 5-50 mm)Sensory, n=35 Mixed, n =13 Motor, n =3 296 160 days Allograft, n =51 Meaningful recovery (≥ S3 and ≥ M3) was achieved in 86% of cases Ducic etal.[47], 2012 Retrospective observational Primary, 0 mm Conduit, 9.1 3.7 mm Autograft, 37.5 13.2 mm Allograft, 17.6 7.5 mm Unknown Primary 204.0 41.3 weeks Conduit 186.4 56.7 weeks Autograft 250.5 59.3 weeks Allograft 129.7 89.2 weeks Primary,n= 8 Conduit, n= 27 Autograft, n= 11 Allograft, n =8 The recorded average QuickDASH (disabilities of the arm, shoulder, and hand) questionnaire was reported to average 23.2 19.8. There were no significant differences between repair type with respect to outcomes Guo et al.[64], 2013 Retrospective observational 23 mm (Range 18-28 mm)Digital, n =5 13 mon. (Range 12-15 months)Allograft, n =5All patients reported sensory improvement during the follow-up period after operation. Mean Static two-point discrimination of 6 mm was recorded, and the range of the Semmes-Weinstein Monofilament test was 4.31 to 4.56 Taras et al.[65], 2013 Prospective 11 mm (Range 5-30 mm)Digital, n =1815 mon. (Range 12-20 months)Allograft, n =18 According to custom Taras[66] scale, excellent results in 39% of repairs, good results in 44% of repairs, fair results in 17% of repairs and poor results in no repairs Isaacs and Safa[67], 2017 Retrospective observational 33 10 mm (Range 5-50 mm)Sensory, n=2 Mixed, n= 13 13 months Allograft, n =15Meaningful recovery for motor (≥ M3) function in 85% of repairs and sensory (≥ S3) function in 67% of repairs, independent of nerve diameter Rinker etal.[68], 2017 Retrospective observational 35 8 mm (Range 25-50 mm)Sensory, n=50 11 months Allograft, n =50 Meaningful recovery for sensory (≥ S3) function in 86% of repairs Zhu etal.[69], 2017 Retrospective observational 27 ± 13 mm Sensory, n=39 Mixed, n =19 Motor, n =6 355 158 days Allograft, n =64 Meaningful recovery for motor (≥ M3) function in 66.7% of repairs and sensory (≥ S3) function in 84.6% of repairs Carlson etal. [70], 2018 Retrospective observational 65 45 mm (Range 10-140 mm)Sensory, n=11 Mixed, n =3 Motor, n =2 15 5 months Allograft, n =19 Meaningful recovery for motor (≥ M3) function in 33.3% of repairs and sensory (≥ S3) in 91.7% of repairs

Page 10 of Buncke. PlastAesthetRes 2022;9:38 https://dx.doi.org/10.20517/2347-9264.2022.08 17 Nietosvaara etal.[71], 2019 Case series 20-50 mm digital 10-19 months Allograft, n= 3 Resorption of the engineered nerve graft or neuroma proximal to the nerve graft Safa etal.[72], 2019 Retrospective observational 33 ± 17 mm (Range 10-70) mm Mixed and motor, n =22 779 480 daysAllograft, n =22 Meaningful recovery was reported in 73% of repairs. No significant differences were noted between gap lengths or mechanism of injury Leckenby et al. [73], 2020 Retrospective observational 26.6 ± 16.0 mm (Range 8-100 mm)Sensory, n= 110 Mixed, n= 25 417 214 days Allograft, n= 135 Meaningful recovery for motor (≥ M3) function in 36% of repairs and sensory (≥ S3) in 77% of repairs. Inferior prognosis for larger diameter and longer grafts Safa etal.[74], 2020 Retrospective observational 24 15 mm (Range 3-70 mm)Sensory, n= 386 Mixed, n =77 Motor,n=12 417 days (Range 120-3286 days)Allograft, n= 624 Overall meaningful recovery in 82% of repairs in sensory, mixed and motor nerve repairs Peters etal.[75], 2021 Case series Range 60-110 mm Median, n =3 Ulnar, n =2 9-24 months Allograft, n =5Subjects had previously experienced an iatrogenic injury. Subjects showed no clinical sensory or motor recovery and showed significant neuropathic pain ranging between 8 and 10 on a 10-score visual analog scale. Histology showed axonal regeneration stalling mid-graft in 3 of 5 grafts and no axonal regeneration in 2 of 5 grafts MRCC: Medical research council classification.
Furthermore, Rinker noted that most major hand centers had updated their nerve repair algorithm as a result of the introduction of these engineered nerve allografts[80]. Additional clinical evidence focusing on long-gap repair was presented by Rinkeret al.in 2017, where a patient population of 50 digital (sensory) nerve gaps measuring 25 mm to 50 mm repaired with Avance nerve graft showed S3 or greater recovery in 86% of repairs[68]. Use of Avance nerve graft to repair 15 large diameter (4-5 mm in diameter) nerve gaps averaging 33 mm in length (range 5-50 mm) using a single Avance nerve graft was evaluated in 2017, which showed functional recovery of S3 or M3 and better of sensory and motor function in 67% and 85% of repairs[67].
An additional update to the surgical algorithm was proposed in 2017, when Ducicet al. suggested that utilizing a nerve conduit for connector-assisted repair may help overcome difficulties noted in the literature that may impede peripheral nerve regeneration after repair[81]. This connector-assisted repair was proposed for direct repair as well as using with engineered nerve allograft to prevent misalignment of the fascicles during the repair[81]. This algorithm was supported by further clinical evidence in 2018, when Carlsonet al. evaluated 19 sensory, mixed, and motor nerve gaps averaging 65 mm in length (range, 10-140 mm) that were repaired with Avance nerve graft reinforced at the nerve coaptation site with Axoguard? nerve protector[70]. Carlson and colleagues found that 91.7% of repairs showed S3 or better sensory recovery, with 66% meaningful recovery in gap lengths greater than 50 mm[70]. As clinical evidence of the efficacy of Avance nerve graft continued to build, surgeons adapted their surgical algorithm to include the use of Avance nerve graft.
Evidence presented in 2018 by Azouzet al. showed that 70% of hand surgeons used engineered nerve allografts in their surgical practice, as noted by the use of current procedural terminology codes 64910 (nerve repair with synthetic conduit or vein allograft), 64890 (nerve graft, single strand, hand, < 4 cm), 64831 (suture of digital nerve, hand, or foot, 1 nerve), 64911 (nerve repair with autogenous vein graft), 64999 (unlisted procedure nervous system) and 64834 (suture of 1 nerve, hand, common sensory nerve)[82]. Additionally, the surgical algorithm for peripheral nerve repair has continued to be updated, as recently updated algorithms incorporate the use of engineered nerve allograft as well as autograft in gaps up to 7 cm in length[83,84]. The clinical evaluation of 22 nerve repairs using engineered nerve allograft in 2019 showed meaningful motor recovery, as noted by M3 function or above, in 73% of nerve repairs with a median of 33 ± 17 mm (10-70 mm) for nerve graft lengths[72]. Furthermore, motor recovery of M3 or above was reported at 80% for nerve gaps 10-25 mm, 63% for nerve gaps 26-49 mm, and 75% for nerve gaps 50 mm or larger[72]. While this was a small cohort, additional larger studies provided similar evidence. In 2020, Safaet al. reported on meaningful recovery, defined as S3/M3 or greater, in 624 sensory and mixed nerve gap repairs measuring up to 70 mm in length[74]. Safa and colleagues showed that in gap lengths 50-70 mm, repair with Avance nerve graft resulted in 69% meaningful recovery, with no statistical difference between the 50-70 mm, 30-49 mm, and 15-29 mm nerve gap repair groups[74]. Furthermore, there was an overall meaningful recovery of 82% in nerve gaps up to 70 mm in length, which was noted to be comparable to historical data for nerve autograft and exceeding historical literature for conduit[74].
While a plethora of positive clinical evidence has been presented for the use of engineered nerve allografts, additional literature has been published with lower success rates. In 2019, a case series of three patients was presented by Nietosvaara outlining poor results due to engineered nerve graft resorption[71]; however, the failures were attributed to possible infection and host rejection. In 2020, Leckenbyet al. presented suboptimal results for a single-center experience with engineered nerve allograft and found that 77% of patients achieved sensory recovery of S3 or better and 36% of patients achieved motor recovery of M3 or better[73]. However, Leckenbyet al. suggested that outcomes with the engineered nerve grafts were similar to nerve autograft in short nerve gaps[73]. Further discussion by Leckenbyet al.suggested that issues were encountered with increased length and diameter of engineered nerve grafts[73].
When considering the body of evidence for the clinical use of engineered nerve grafts for the repair of peripheral nerve defects, there is overwhelming support for their application. Furthermore, the use of engineered nerve grafts can also circumvent the comorbidities associated with nerve autograft. With these considerations, there is sufficient support for further clinical use of engineered nerve allografts, such as Avance nerve graft.
DISCUSSION
Peripheral nerve repair, much like other surgical repairs, has undergone a drastic transformation in the last 20 years. This transformation has been largely in part due to the development of innovative peripheral nerve repair materials such as nerve conduits and engineered nerve allografts. Nerve conduits have provided a material to improve peripheral nerve repair by providing a protected environment for peripheral nerve regeneration, moving the suture away from the regenerating axons at the proximal nerve stump, and allowing for selective reinnervation of the distal target[81]. While these advances in the application of nerve conduits have been useful, the application of a nerve conduit should be limited to nerve gaps less than 1 cm in length[47]. Longer nerve gaps require the use of a nerve autograft or engineered nerve allograft. While nerve autografts have shown good outcomes in large gaps, the patient comorbidities associated with nerve autograft harvest often include chronic pain, wound infections, wound complications, and sensory deficits[43]. While recovery from some of these complications may occur, sensory deficits have shown variable outcomes, with 0%-11% of adult patients experiencing complete sensory recovery[43]. The variability in recovery of sensory deficits has been suggested to be correlated with the length of the resected nerve segment, where longer nerve segment resections, such as nerve autograft harvest, show larger areas of chronic sensory deficits[43]. The acute and chronic comorbidities related to nerve autograft harvest should be a consideration for the surgical algorithm of surgeons performing peripheral nerve repair. Furthermore, alternatives to nerve autografts should be considered by evaluating evidence-based outcomes. The advent of engineered nerve allografts has provided one such alternative to nerve autografts that circumvent the associated donor-site morbidities associated with nerve autografts and offer promising outcomes.
The development of engineered nerve allografts has required decades of research, but has been proven to achieve successful clinical outcomes. The early use of pre-treated engineered nerve allografts showed limited successful outcomes[50-53]or required immunosuppression[85]. With later advancements in the field, the advent of an optimized nerve allograft pre-treatment method allowed for the successful implantation of engineered nerve allografts without the use of immunosuppressives and with outcomes that are comparable to autograft[62,63]. The development and clinical use of this off-the-shelf engineered nerve allograft, Avance nerve graft, has finally driven changes in the peripheral nerve repair surgical algorithm. Initial clinical research supported the use of engineered nerve allografts in nerve gaps up to 30 mm, with a later expansion of successful clinical use for nerve gap repair up to 50 mm. However, a recent clinical study supports the use of engineered nerve allografts for both sensory and mixed/motor nerve repair in gaps up to 70 mm in length[74]. With this recent publication, the application of engineered nerve allografts can be confidently used clinically in nerve gaps up to 70 mm in length.
The successful outcomes of peripheral nerve repair with engineered nerve allograft are promising; however, limitations exist in the literature, including the lack of randomized controlled clinical trials comparing engineered nerve allograft to autograft. While retrospective clinical trials lack stringent controls seen in randomized clinical trials, the retrospective studies reviewed in this manuscript provide the best comparative data available to date in the repair of peripheral nerve gaps. By evaluating the clinical data chronologically, it is clear that technological advancements in peripheral nerve repair support the use of engineered nerve grafts with increasing gap lengths over time. This method of chronological presentation serves to present the data as it has been shared with the field, ensuring a balanced presentation of meaningful studies to the clinical community. It is expected that technology will continue to improve, thus changing the future surgical algorithm for peripheral nerve repair.
The clinical application of materials and techniques currently in early-phase research will continue to change the landscape of peripheral nerve repair. Some notable early-phase research includes engineered nerve allografts, engineered nerve conduits with and without fillers, and cellular and non-cellular graft additives. Engineered nerve allografts have included various processing techniques, including cold preservation[51], freeze thawing[86], detergents[36], and irradiation[87]. While various engineered nerve allografts have been researched, only Avance has been made available commercially as an off-the-shelf engineered nerve allograft. This limits the ability to evaluate the clinical efficacy of different engineered nerve allografts. Engineered nerve conduits have been investigated using various synthetic and natural materials, which may be either resorbable or non-resorbable.
A recent meta-analysis found that autograft vein conduits, autograft muscle-vein conduits, engineered collagen tubes (e.g., NeuroMatrix, Neuroflex, NeuraGen? nerve guide), and Neurolac? (a bioresorbable synthetic material) were the most studied and best nerve conduit options[88]. New materials for conduit fillers and additives have been explored to enhance conduit efficacy and increase the application length of these materials. Research on new conduit filler materials includes fibrin, laminin, collagen, and synthetic aligned matrices[89]. Furthermore, additives have been investigated with various nerve graft materials, including cellular (e.g., Schwann cells, fibroblasts, and bone stromal cells) and non-cellular (e.g., neurotrophic growth factor, fibroblast growth factor, glial growth factor, ciliary neurotrophic factor, vascular endothelial growth factor, and brain-derived growth factor) components[89]. These additives to luminal fillers have shown beneficial nerve regeneration effects across a nerve gap[89].
Engineered nerve allografts have also been evaluated with the use of additives and cellular enhancements, which showed that enhancement with additives[90]or cells[91]improve regenerative potential. However, these results are only available in the pre-clinical phase of research, as to date there is no FDA cleared commercially available biological additives or cells for the clinical application to engineered nerve conduits or allografts[48]. The ability to use this technology clinically will require additional research and clearance by appropriate regulatory bodies. When available, the clinical use of new conduit materials, luminal fillers, additives or cells will likely continue to expand the surgical algorithm in the future.
CONCLUSION
In conclusion, engineered nerve allografts have impacted the peripheral nerve repair surgical paradigm and should be considered as an alternative to nerve autograft for peripheral nerve repair. The use of engineered nerve allografts, such as Avance nerve graft, show meaningful motor and sensory recovery in nerve gaps up to 70 mm in length. Recent data has provided confidence for the use of clinically available engineered nerve grafts even for repair of longer nerve gaps 50-70 mm in length. Furthermore, the use of engineered nerve allograft as an alternative to autograft circumvents nerve autograft comorbidities, such as sensory deficits and chronic pain. While the surgical algorithm for peripheral nerve repair is ever-changing, additional research and clearance for clinical use by regulatory bodies are required to advance current surgical techniques.
DECLARATIONS
Acknowledgments
The author would like to acknowledge the support of Dr. Anne Engemann and her team for review of this manuscript prior to submission.
Authors’ contributions
The author contributed solely to the article.
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
The author is a paid consultant for Axogen; however, no financial support was provided for the creation of this manuscript.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2022.
 Plastic and Aesthetic Research2022年5期
Plastic and Aesthetic Research2022年5期
- Plastic and Aesthetic Research的其它文章
- Ethical challenges in vascularized composite allotransplantation of the lower extremity: lessons learned from hand transplantation and implications for the future
- Introduction to special issue “Recent Advances in Skin Anti-Aging Agents”
- AUTHOR INSTRUCTIONS
- Current concepts in microsurgical soft tissue reconstruction of lower extremity trauma in a singlevessel extremity
- Soleus muscle flap for reconstruction of lower extremity trauma. Workhorse or glue factory?
- Ehlers-Danlos syndrome: prevalence and outcomes in gender affirming surgery - a single institution experience
