Synergistic influences of titanium,boron,and oxygen on large-size single-crystal diamond growth at high pressure and high temperature
Guang-Tong Zhou(周廣通) Yu-Hu Mu(穆玉虎) Yuan-Wen Song(宋元文) Zhuang-Fei Zhang(張壯飛)Yue-Wen Zhang(張躍文) Wei-Xia Shen(沈維霞) Qian-Qian Wang(王倩倩) Biao Wan(萬彪)Chao Fang(房超) Liang-Chao Chen(陳良超) Ya-Dong Li(李亞東) and Xiao-Peng Jia(賈曉鵬)
1Key Laboratory of Material Physics of Ministry of Education,School of Physics and Microelectronics,Zhengzhou University,Zhengzhou 450001,China
2College of Electronical Information Engineering,Yangtze Normal University,Chongqing 408100,China
Keywords: high pressure and high temperature(HPHT),diamond,B2O3,Ti
1. Introduction
Diamond is a material with many excellent properties,including mechanical, thermal, and optical properties, and also has great potential application in the field of electricity.[1]Although pure diamond is a good insulator,it can also have useful electrical characteristics after having been doped with appropriate element. Therefore, the research on the growth and doping mechanism of semiconductor diamond is of great significance in expanding its scientific application. In previous researches of boron-doped diamond (BDD), most of studies demonstrated that high pressure and high temperature(HPHT)or chemical vapor deposition(CVD)method can be adopted,adding boron atoms during diamond growth can make diamond possess the p-type semiconductor conductivity,and the synthesis of BDD electrodes on different substrates and shapes can reach a promising developmental stage.[2–4]
Compared with the research of BDD, the research of boron and oxygen co-doped diamond is rare. In 2016,Xiaoet al.[5]reported the phase diagram distribution of diamond crystals into which B2O3had been added and the effect of B2O3addition on diamond growth and surface morphology. This study focused on the doping of boron into crystals,and further explored the oxygen from B2O3. In 2017,our research group undertook the innovative development synthesis and characterisation of HPHT large single-crystal diamonds under the simultaneous influence of oxygen and hydrogen. The effect of oxygen on the growth of the large-scale synthetic diamond was studied and it was found that the oxygen caused the diamond surface defects to change markedly.[6]In 2018, Liuet al. reported that the boron–oxygen complex defects (B3O and B4O) in BDD surfaces (1 μm–1.5 μm) had low formation energy and shallow donor energy levels,which resulted in the diamond displaying n-type semiconductor characteristics,thus providing a new strategy for producing n-type diamondbased devices.[7]In 2020,Prikhodkoet al. reported the splitting of infrared absorption lines caused by the isotopic content of boron acceptors embedded in semiconducting diamond by doping B2O3at HPHT.[8]The existing consensus of diamond manufacturers shows that oxygen in the synthesis chamber is generally considered to be detrimental to the growth of large-size high pure diamond, and it is commonly avoided as much as possible.[9]However,oxygen has been found to have a significant influence on the performance of diamond semiconductors, changing it from p-type to n-type. In terms of a breakthrough in the preparation of high-quality semiconductor diamond,oxygen may be a key doping element. Therefore,it is meaningful to study and characterise the effect of boron,oxygen, and boron–oxygen co-doping on diamond growth in the preparation of high-quality semiconductor diamond,especially n-type diamond. Additionally,as one of the most abundant elements on the earth,characterising the influence of oxygen on diamond growth will increase the understanding of the formation mechanism of oxygen-containing natural diamond,which is also beneficial to the fields of geophysics and geology.
Thus,in this work,an important element titanium element is introduced into the synthesis cavity, which is in contrast to the traditional research on boron–oxygen-doped diamond.The B2O3is added into an FeNi–C system and an FeNi–Ti–C system to synthesise large single-crystal diamonds. The synergistic influences of boron,oxygen,and titanium on diamond morphology, impurities, and the formation of boron–oxygen complexes are investigated in detail. The role that titanium plays in the growing of boron- or oxygen-doped diamond is also explored.
2. Material and methods
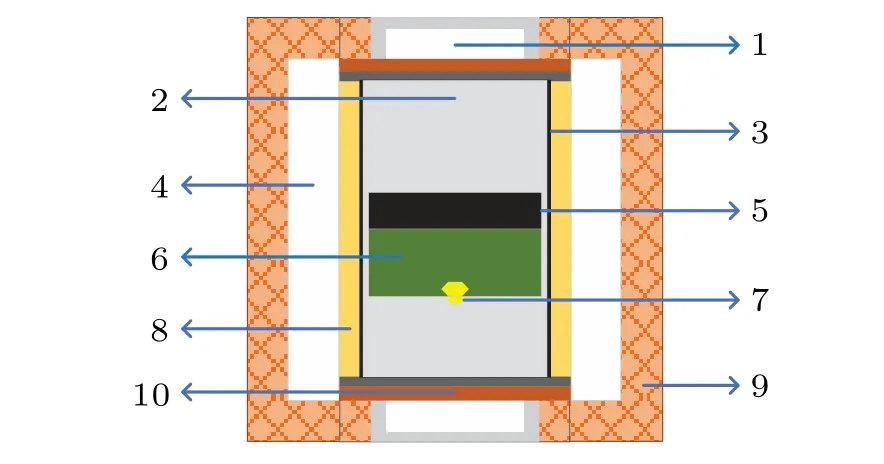
The B2O3-doped diamond synthesis experiments were carried out, respectively, in an FeNi–C system and an FeNi–Ti–C system by the temperature gradient method (TGM) at high pressure and high temperature (HPHT) using a Chinatype large volume cubic high-pressure apparatus (CHPA;SPD-6×1400). The sample assembly is shown in Fig. 1.The high-quality seed crystal used in the synthesis experiment was 0.9 mm–1.0 mm in size,and the{111}plane of the seed crystal was used as the crystal growth plane. The high-purity graphite power (99.9 wt%) was used as the carbon source,FeNi alloy was used as the growth solvent, high-purity titanium (Ti) sheet (99.99 wt%) was added into the solvent and high-purity boron oxide(B2O3)powder(99.99 wt%)was used as the additive.
The graphite and B2O3powders at a specific proportion were placed into a mixer and uniformly mixed. Following mixing, the powder was pressed into bars in equal size,which were then assembled in a synthetic cavity. Prior to the synthesis experiment,to avoid influencing the experimental results by the moisture adsorbed by the sample assembly materials, each sample assembly block was placed in a drying oven at 120°C for one hour, then removed and placed in the high-pressure apparatus for the synthesis experiment.The synthesis conditions were as follows: pressures from 5.5 GPa–5.7 GPa and temperatures from 1300°C–1500°C.A platinum-rhodium(Pt-30%RH/Pt-6%)thermocouple was used to measure the temperature and its joint was near the crystal sample. The experimental pressure was calibrated based on the graphite–diamond phase transition.
After the experiment, the residual solvents on the samples were removed with HNO3and the graphite was removed from the surface of the crystals by a hot mixed solution of H2SO4and HNO3(3:1, v/v). Prior to being tested, the diamonds were ultrasonically rinsed with absolute alcohol for 30 min to remove the residual impurities from the diamond surface.The synthetic diamonds were characterized by optical microscopy (OM), x-ray photoelectron spectroscopy (XPS),Fourier-transform infrared spectroscopy (FTIR), and Raman spectroscopy (Raman). The FTIR was performed using a VERTEX 70-V vacuum Fourier micro-infrared spectrometer in a spectral range of 600 cm-1–4000 cm-1with a spectral resolution of 2 cm-1. Raman spectrum was measured using a Renishaw InVia Raman spectrometer at room temperature,with an excitation curve wavelength of 532 nm and a spectral resolution of 0.5 cm-1in a spectral range of 500 cm-1–2100 cm-1.
3. Results and discussion
3.1. Effect of B2O3 on diamond growth conditions
Diamond crystals are synthesised with the addition of B2O3into FeNi–C and FeNi–Ti–C systems under the HPHT conditions of 5.5 GPa–5.7 GPa and 1300°C–1500°C. The results are summarised in Table 1.
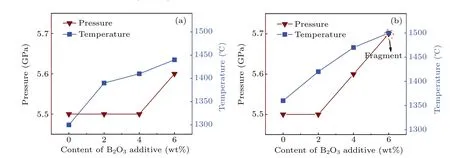
Figure 2 shows that the pressure(P)and temperature(T)vary with B2O3content in the diamond synthesis process for the two systems.It can be found that thePandTincrease with the augment of B2O3content greatly. Specifically, the pressure increases from 5.5 GPa to 5.7 GPa and the temperature increases from 1300°C to 1440°C in FeNi–C system when the B2O3content increases from 0 to 6 wt%. Such a growth trend also appears in the FeNi–Ti–C system. It can be noted that thePandTrequired for crystal growth under the same doping amount increase with the addition of Ti increasing.
Table 1 and figure 2 show that as the content of doped B2O3increases, the conditions for synthesising the diamond crystal increase in both the FeNi–C system and the FeNi–Ti–C system. It is speculated that this is because when the B2O3content increases,the metal catalyst activity is affected to different degrees and increased energy is required for the conversion of graphite into diamond.[10–13]Thus,theP–Tconditions for diamond synthesis increase.
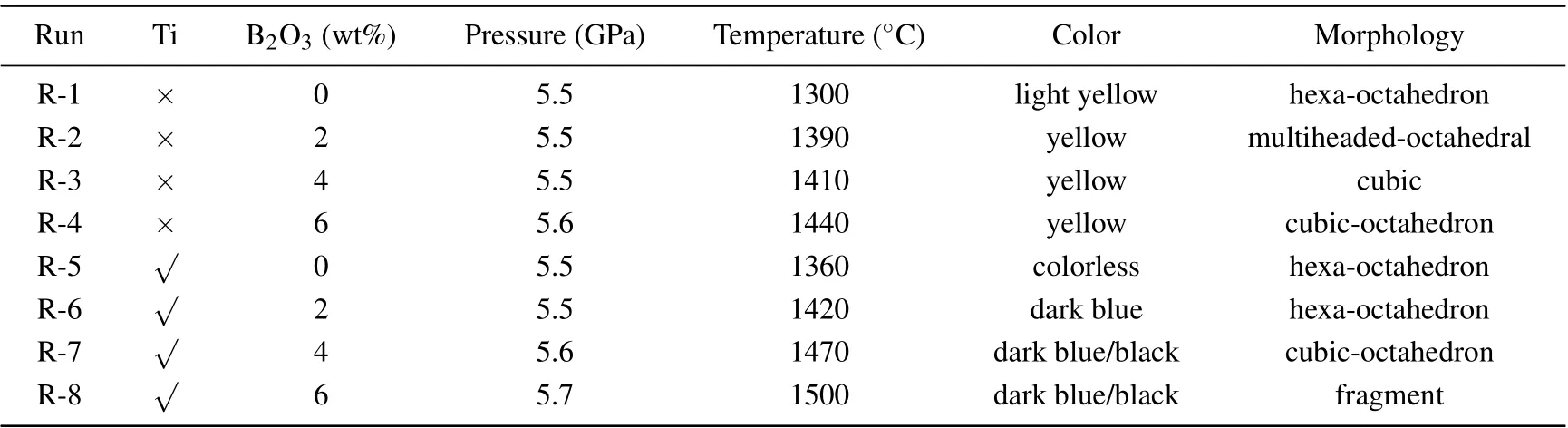
Table 1. Experimental results of diamond crystal synthesis with B2O3 added into FeNi–C and FeNi–Ti–C systems.
3.2. Effect of B2O3 on diamond color and quality
Figure 3 shows optical images of the synthetic diamonds.Figures 3(a)–3(d) show the optical images of crystal growth with 0,2,4,and 6 wt%B2O3additive in the FeNi–C system,respectively. Figures 3(e)–3(h) display the optical images of crystal growth with 0, 2, 4, and 6 wt% B2O3additive in the FeNi–Ti–C system,respectively.
It can be seen from Figs.3(a)–3(d)that with B2O3addition increasing, the shape and surface integrity of the crystal grown in the FeNi–C system change from a complete and regular hexa-octahedron without doping to an octahedron with irregular shape and defective surface,and the inclusion in the crystal also increases markedly. However, the crystal color does not change obviously and remains yellow.
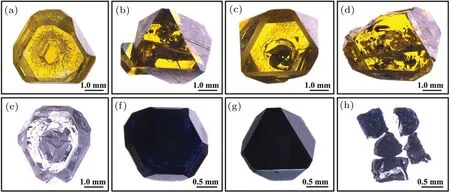
Fig.3. Optical images of diamond crystals synthesised with B2O3 additive, showing[(a)–(d)]growth from FeNi–C system with 0, 2, 4, and 6 wt%B2O3 additive,respectively,and[(e)–(h)]growth from FeNi–Ti–C system with 0,2,4,and 6 wt%B2O3 additive,respectively.
When Ti is added to the solvent and thus forms a system,the crystal changes from yellow color to almost no color at 0 wt%B2O3(Fig.3(e)). When the content of B2O3increases to 2 wt%, the crystal becomes dark blue and emits faint light under the illumination of intense light. When the amount of B2O3is more than 2 wt%, the crystal turns black and almost completely opaque, and the crystal surface is still relatively complete but large defects are found to exist at the bottom of the crystal. At a B2O3content of 6 wt%,a complete diamond crystal cannot be synthesised: only some fine and irregular broken diamond crystals are obtained. From the crystal color and quality,it can be seen that the crystal changes greatly after Ti has been added into the FeNi–C system: boron atoms readily enter into the crystal in the presence of Ti in comparison with the scenario in the absence of Ti.
3.3. Effect of B2O3 on infrared characterisation of diamond
The FTIR is used to study the nitrogen content and internal chemical bond structure of the crystals prepared with different B2O3doping content. Figure 4(a)shows the IR spectra of the crystals synthesised in the FeNi–C system, with curvesa–dcorresponding to B2O3doping content of 0, 2,4, and 6 wt%, respectively. Figure 4(a) contains strong absorption peaks at 1130 cm-1and 1344 cm-1in each spectral curve, which are characteristic of diamond C-centre nitrogen(isolated substitutional nitrogen atoms).[14]Additionally, no boron-related peaks are observed.
According to previously reported equation,[15,16]the nitrogen concentration in diamond is calculated, with the calculated results having an uncertainty less than 5%.[17]Figure 4(b)shows the nitrogen content of each crystal synthesised in the FeNi–C system. It can be seen that with the B2O3doping content gradually increasing, the nitrogen content in the crystal hardly changes. Therefore, the addition of B2O3into the system does not significantly influence the nitrogen concentration in the diamond.
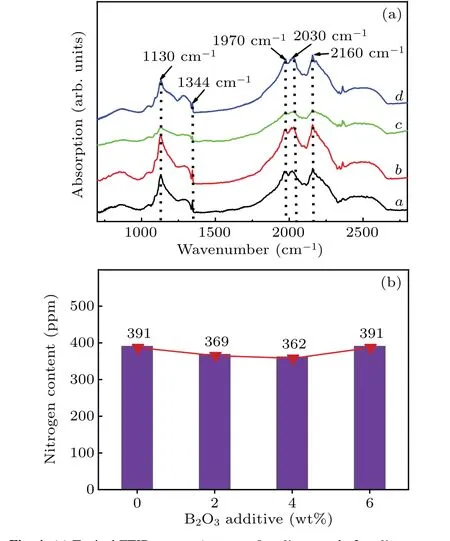
Figure 5 shows the IR spectra of the crystals synthesised in the FeNi–Ti–C system, with B2O3doping content of 0,2, 4, and 6 wt% corresponding to curvese–h, respectively.There is no nitrogen peak in any of the samples after Ti atoms have been added into the solvent, which means that the nitrogen content in these crystals is less than 1 ppm; Ti atoms act as a nitrogen acceptor.[18]The absorption peaks appearing at 2460 cm-1and 2800 cm-1are related to boron and can be attributed to the electronic transition from the ground state to the first and second excited state of neutral boron acceptor,respectively;[19–21]there are two weak boron peaks in the samples without B2O3doping (Fig. 5(e)). This is caused by the low level of boron impurities in the carbon source.[18,22]With B2O3content being 2 wt%, the 2460-cm-1and 2800-cm-1absorption peaks are found to turn significantly more intense and sharper. At the same time,a new absorption peak appearing at 1290 cm-1is observed (Fig. 5(f)), which indicates the presence of uncompensated boron in the diamond structure.The appearance of these peaks proves that boron atoms enter into the diamond after Ti atoms have been added into the system. At 4-wt% B2O3, only a strong peak at 1290 cm-1is present, which corresponds to a single phonon absorption peak of boron impurity in diamond.[23,24]As this is related to the vibration energy of boron atom impurities in the crystal,it indicates that there exist many boron impurities in the crystal at this doping content.When the doping content of B2O3in the system is increased to 6 wt%(Fig.5(h)),the starting point of photoionization continuation shifts toward the low wavenumber (low energy ).[25,26]Therefore, the boron content in the crystal gradually increases with the augment of B2O3doping content.
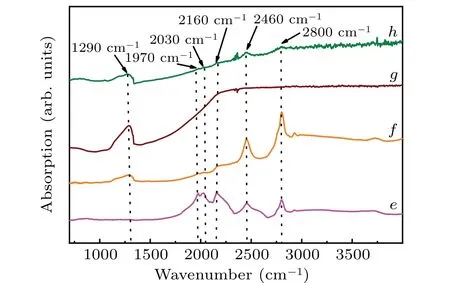
In summary,the IR spectra show that when Ti atoms are added into the metal catalyst system, not only can the nitrogen atoms in the cavity be removed,but also the boron source in B2O3is released and doped into the diamond crystal, thus realizing the growth of diamond with a high concentration.
3.4. Effect of B2O3 on Raman characterisation of diamond
Raman spectrum is used to characterise the internal residual stress and chemical structure of the synthesised crystals.Curvesa–din Fig. 6(a) correspond to B2O3doping content of 0, 2, 4, and 6 wt% in the FeNi–C system and curvese–hin Fig. 6(b) correspond to B2O3doping content of 0, 2, 4,and 6 wt% respectively in the FeNi–Ti–C system. It can be seen from Fig. 6 that each of all samples shows a strong and narrow characteristic diamond peak(close to 1332 cm-1)and no extra peaks, indicating that the synthesised diamond crystals have all a single sp3structure, which also proves that the synthesised diamond crystals are all of high quality.[27]Additionally, the full width at half maximum (FWHM) shows a narrowing trend,and the position of the Raman peak does not change significantly in Fig.6(a),indicating that the quality of the diamond is less affected by B2O3doping in the FeNi–C system.
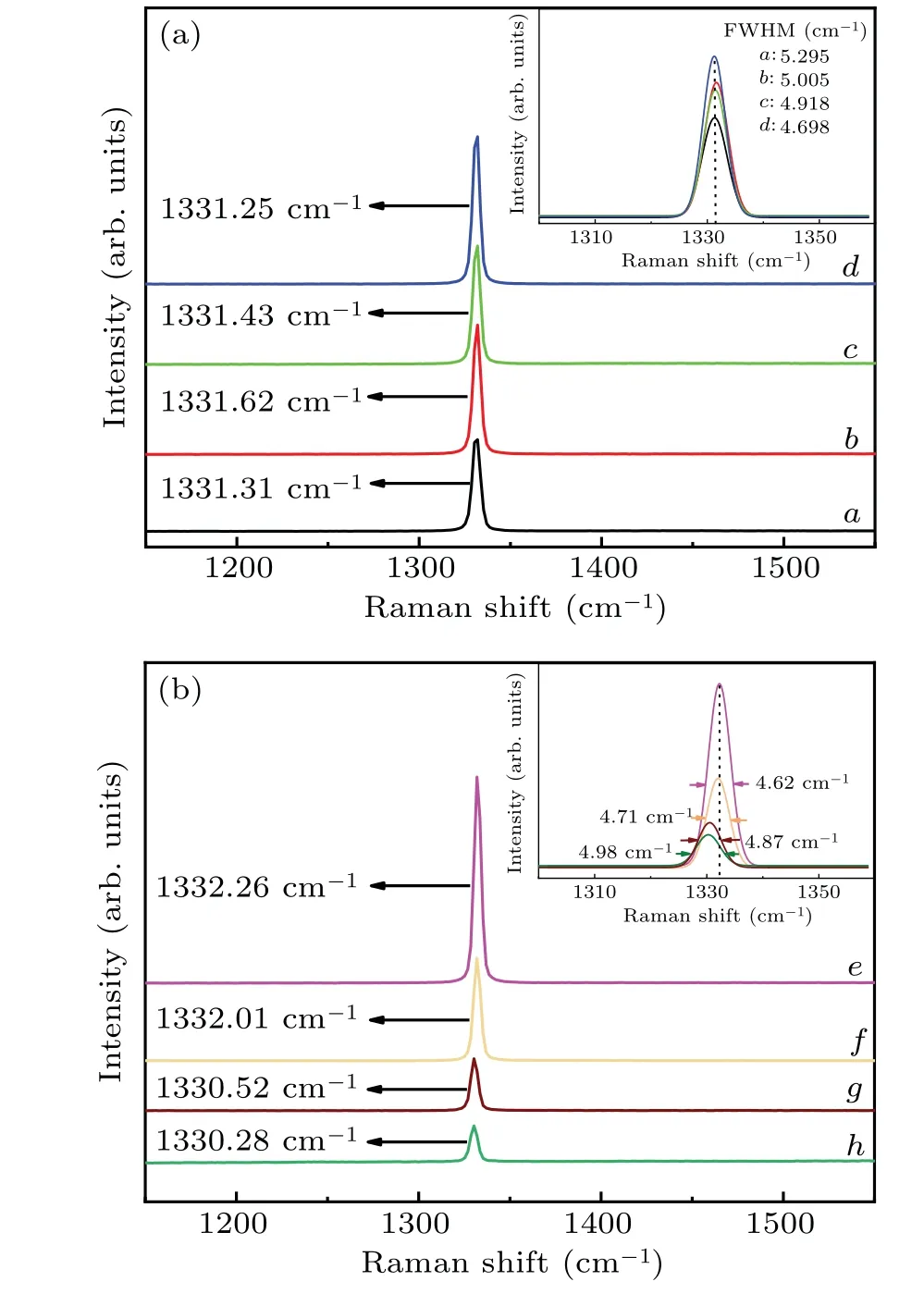
In contrast, as can be seen from Fig. 6(b), as the B2O3doping content increases in the FeNi–Ti–C system the position of the Raman peak is gradually redshifted from 1332.26 cm-1when the doping content is 0 wt%to 1330.28 cm-1when the doping content is 6 wt%. Additionally, the the FWHM gradually shifts from 4.62 cm-1to 4.98 cm-1at 0 wt%to 6 wt%B2O3content, respectively. This indicates that as the doping content increases, more and more boron or oxygen impurities enter into the diamond lattice, thus resulting in a regular decrease in crystallinity, an increase in internal stress and a deterioration in quality.
Boron impurities in diamond crystals result in the expansion of the diamond lattice. Therefore, as the concentration of boron impurities in the crystal increases,the internal stress change leads the Raman peak of diamond to shift toward lower wavenumbers.
3.5. Effect of B2O3 on XPS characterisation of diamond
The XPS is used to study the bonding on the surface and the bonding inside the crystal doped with 4-wt%B2O3in the FeNi–Ti–C system. The test is divided into two parts. First,the crystal surface is characterised after it has been cleaned.Second, the crystal is cut by laser parallel to and 0.80 mm away from the{111}diamond surface and analysed by XPS at 50 nm away from the cut surface. The results are shown in Fig.7.
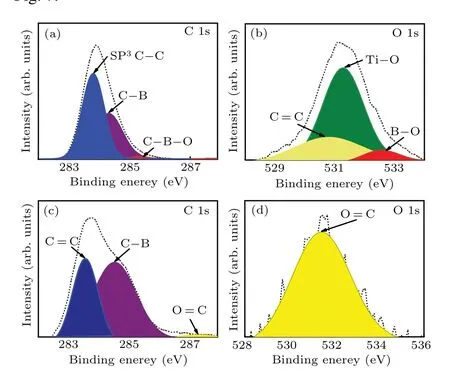
Figures 7(a)and 7(b)show the spectra of the diamond surface,and figures 7(c)and 7(d)show the interior of the diamond after having been cut. The peaks at 285.4 eV and 532.6 eV in Figs. 7(a) and 7(b) correspond to C–B–O and B–O bonds,respectively.[28,29]These results indicate that the boron atoms on the surface of diamond formed B–O complexes with oxygen atoms. Additionally,the carbon atoms are bonded to oxygen atoms at 287.3 eV and 531.4 eV as shown in Figs.7(c)and 7(d),[30,31]indicating that the oxygen atoms successfully enter into the diamond lattice, with 4.0-wt% B2O3content added into the FeNi–Ti–C system. However, there is no peak corresponding to the boron–oxygen combination in Fig.7(c)nor in Fig.7(d), showing that the concentration of boron–oxygen complexes in the crystal is lower than on the crystal surface.It is speculated that this is due to the low boron atom concentration and oxygen atom concentration and the mismatch between their atom radii in diamond.
3.6. Mode of action of titanium in growth chamber
Two experiments are performed to explore the mechanism of boron entering into the diamond crystals in the FeNi–C and FeNi–Ti–C systems. Graphite(Gr)power and Ti powder are mixed uniformly with B2O3powder, with both the mass ratio between Gr and B2O3and the mass ratio between Ti and B2O3being 1:3, respectively, placed in a tube furnace and maintained at 1400°C for 1 h under a vacuum argon atmosphere. The obtained samples are characterized by Raman spectroscopy and compared with those from pure B2O3and pure boron powder as control groups, and their results are shown in Fig.8.
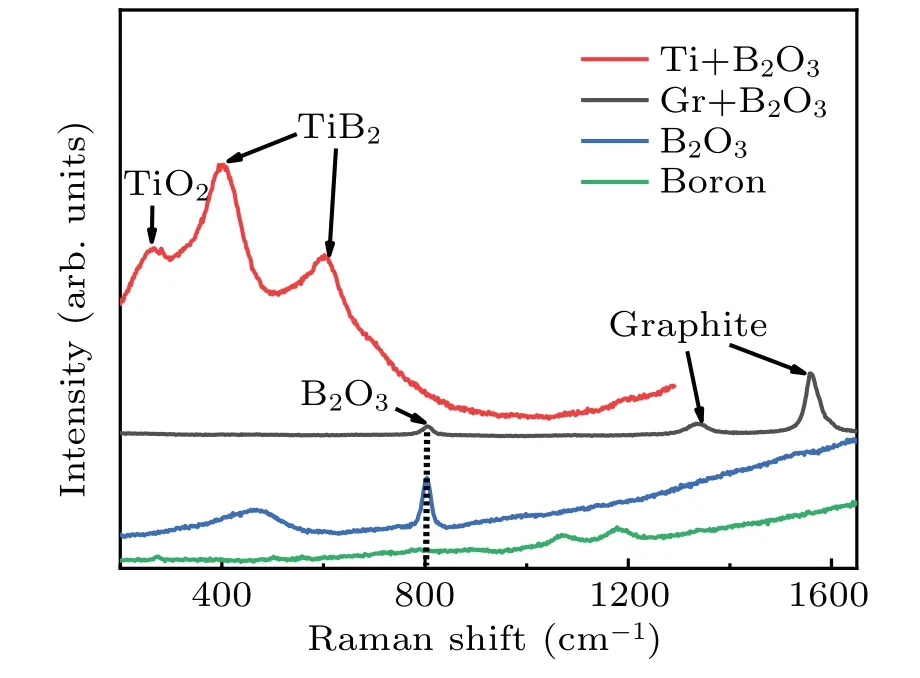
From Fig.8,it can be seen that there is no boron-related peak when B2O3powder was mixed with graphite. However,according to previous research, B2O3will react with carbon to produce carbon oxide (COx) and boron.[32,33]The carbon oxide will poison the metal catalyst, so theP–Tconditions for crystal growth will increase in Fig. 2(a). The absence of a boron-related peak in Fig.8 is because so few boron atoms are generated that they cannot be detected. Another experimental results show that there exist strong peaks of TiO2and TiB2, and it can be seen from Fig.7(b)that the Ti–O bond is also reflected in the XPS spectrum at 530.8 eV.[34]Because the experiments are all carried out under vacuum,it is speculated that the oxygen in TiO2come from B2O3,[35]and the TiB2is produced by the reaction between Ti and boron, and its occurrence also attests that boron is partially decomposed in the cavity. To sum up, Ti and B2O3can undergo substitution to generate elemental boron and oxygen,then boron atoms enter into the crystal when the growth chamber is oxygen-enriched.
4. Conclusions
In this work, large single-crystal diamonds are synthesised by adding different concentrations of B2O3into a solvent–carbon system. The results show that with B2O3content increasing, the carbon oxide and oxygen in the growth chamber increasePandT. The B2O3does not affect the nitrogen content in the diamond and no obvious boron atoms enter into the crystal when there are no Ti atoms in the cavity. By adding Ti into the FeNi–C system,the boron source in B2O3is released. As the B2O3concentration increases a large number of boron atoms enter into the diamond and the color of the crystal changes from almost no color to black color. Furthermore,the boron–oxygen complexes are found to be on the surface of the crystal, and the oxygen-containing bonds also appear in the diamond. These results show that this method can be used to synthesise diamond with boron–oxygen composite defects, and that elemental oxygen in B2O3can successfully enter into the diamond lattice.
The experimental results indicate that Ti reacted with B2O3to form boron and oxygen, creating an oxygen-rich environment in the synthesis chamber and producing boroncontaining diamond.
Acknowledgements
Project supported by the National Natural Science Foundation of China(Grant Nos.11804305,12004341,11704340,and 12004342),the Key Research Project of Higher Education Institution of Henan Province,China(Grant No.19A140006),the Scientific and Technological Project in Henan Province,China (Grant No. 202102210198), the Natural Science Foundation of Chongqing, China (Grant No. cstc2019jcyjmsxmX0391),and the Science and Technology Research Program of Chongqing Municipal Education Commission,China(Grant No.KJQN201901405).
- Chinese Physics B的其它文章
- Switchable terahertz polarization converter based on VO2 metamaterial
- Data-driven parity-time-symmetric vector rogue wave solutions of multi-component nonlinear Schr¨odinger equation
- Neutron activation cross section data library
- Multi-phase field simulation of competitive grain growth for directional solidification
- A novel similarity measure for mining missing links in long-path networks
- Effects of electrical stress on the characteristics and defect behaviors in GaN-based near-ultraviolet light emitting diodes