Functionalized Metal-organic Frameworks for White Light Emission and Highly Sensitive Sensing of PA and Fe3+/Al3+①
HUANG Jing-Jing ZHAO Dan YIN Guo-Jie
(Luoyang Institute of Science and Technology, Luoyang 471023, China)
ABSTRACT Three isomorphic Ln-MOFs [Ln(bcbob)(H2O)(DMF)] (Ln = Tb for 1, Eu for 2, Gd for 3; DMF= N,N-dimethylformamide) have been constructed from a semi-rigid V-shaped organic linker 3,5-bis((4′-carboxylbenzyl)oxy)benzoilate acid (H3bcbob) under solvothermal conditions. X-ray single-crystal diffraction analysis reveals that they exhibit a two-dimensional (2D) layered structure. Compounds 1~3 show the characteristic green and red emissions of Ln3+ and blue emission arising from the organic ligand, respectively. Based on their photoluminescence properties, the white-light emitting materials 4 and 5 with longer fluorescence lifetime (ms grade)and higher quantum yield (e.g. 40.61% for 4) are fabricated. Remarkably, 1 exhibits good sensing ability on nitroaromatic compounds, especially for picric acid (PA). In addition, 1 is still a highly selective sensing material for Fe3+ and Al3+.
Keywords: lanthanide organic frameworks, aromatic carboxylic acid, fluorescence, doping, chemical sensingdiiron; DOI: 10.14102/j.cnki.0254-5861.2011-3304
1 INTRODUCTION
In recent years, the government has been concerned about environmental matter such as water, air and industrial-waste pollution, etc. due to the hazards they pose to human health.To our knowledge, nitroaromatic compounds are widely utilized in industries for various chemical synthesis and explosive materials. But their notorious environmental pollution and explosiveness nature make them high dangerous to living things and human life. Among these explosives, picric acid (PA) has been generally used in the fields of medicines,explosives, paper making and textile. However, it is high toxic and may cause male infertility, anemia and other severe health problems[1,2]. Meanwhile, iron plays a key role in the metabolic process for organisms. A shortage of iron element or an excess would also cause serious health problems to humans[3-5]. The traditional methods for pollution-detection require complicated instruments and are high-cost. Accordingly, it is extremely important and urgent to develop novel methods that are highly efficient and can be applied to a variety of pollutants.
Metal-organic frameworks (MOFs), as a very important class of multifunctional inorganic-organic hybrid materials,have received tremendous attention due to their structural diversity[6,7], and the potential applications in gas storage and separation[8-12], fluorescent sensors[13-19], drug delivery[20-24]and catalysis[25-30]. Among diversity applications, the luminescent lanthanide-based MOFs (Ln-MOFs) play an important role in sensing and detecting due to their distinguishing advantages as large Stokes shifts, high luminescence efficiency, narrow emission peaks, high color purity and relative long fluorescent lifetime. However, the direct excitation of lanthanide luminescence materials was restrained by the forbidden 4f-4ftransition. The luminescence performance of most Ln-MOFs is usually sensitized based on specific energy transfer from organic chromophores bearingπ-conjugated centers to Ln3+sites, which are known as “antenna effect”[31,32]. In recent decades, aromatic carboxylate ligands have been broadly applied in the synthesis of Ln-MOFs, because they exhibit unique optical properties and interesting structural features.
Besides, Ln-MOFs offer a platform for the development of functional luminescent materials, in particular those with white-light emission. Considering that Ln-MOFs are usually isomorphic and the spatial regularity of their construction units in such structures provides the probability of incorporating distinct Ln3+into one framework without damage to the original framework structures, these mixed Ln-MOFs can simultaneously generate the emission colors of different Ln3+. And finally, white-light emission can be achieved by precise modulation of the relative amount of different Ln3+and by varying the excitation wavelength[33-35].
As a semi-rigid V-shaped ligand, 3,5-bis((4′-carboxylbenzyl)oxy)benzoilate acid (H3bcbob, Scheme 1) endowed with three carboxylic groups has diverse bridging modes to construct multi-dimensional MOFs. Previously, H3bcbob, 1,2-bis(4-pyridyl)-ethene (bpe) and 1,2-bis(4-piperidyl)-propane(bpp) were used in our group to construct several fascinating frameworks. Herein, via utilizing H3bcbob as a bridging ligand, three isomorphous Ln-MOFs [Ln(bcbob)(H2O)(DMF)](Ln = Tb for 1, Eu for 2, Gd for 3) with a 2D layered structure are firstly prepared under solvothermal conditions.Based on their photoluminescence properties, we successfully fabricate two white-light emitting materials 4 and 5 by precise modulation of the relative amount of distinct Ln3+and varying the rational excitation wavelength. Remarkably,1 exhibits good sensing ability on nitroaromatic compound,especially for picric acid (PA). In addition, 1 is still a highly selective sensing material for Fe3+and Al3+.
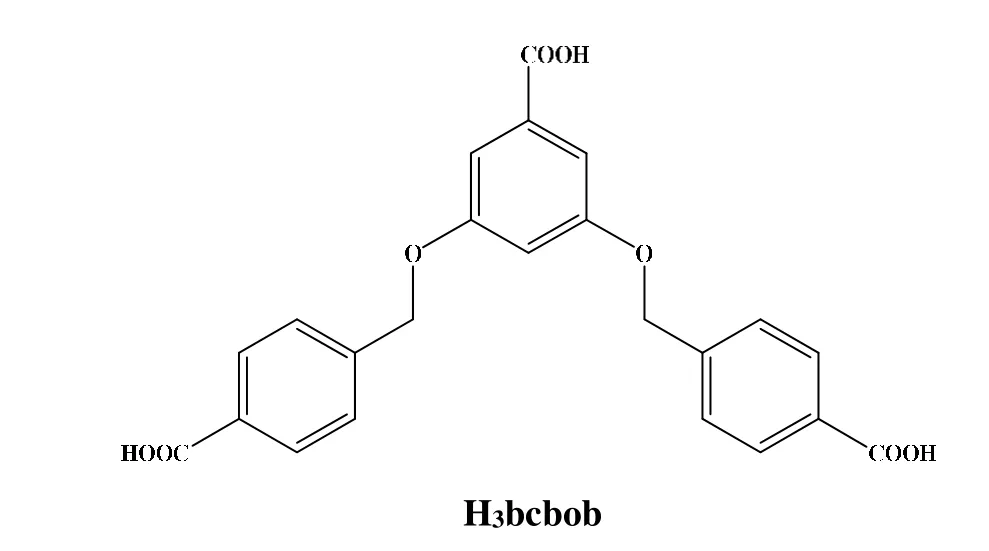
Scheme 1. Molecular structure of H3bcbob
2 EXPERIMENTAL
2. 1 Common materials and general methods
The materials and solvents used were purchased from specific merchant, and further purification procedures were not performed. Infrared (IR) spectrum test was recorded on a Perkin Elmer Spectrum 1 spectrophotometer (4000~400 cm-1)via utilizing a powder material on a KBr salver. Powder X-ray diffraction (PXRD) data were smoothly collected on a Rigaku/max-2550 diffractometer with Cu-Kαradiation (λ=1.5418 ?). Elemental analysis (C, H and N) was performed on a Pekin-Elmer 2400LS II elemental analyzer. Thermogravimetric (TG) behavior was investigated on a Pekin-Elmer TGA-7 instrument with a heating rate of 10oC·min-1in air. Fluorescence spectra were recorded on a LS 55 fluorescence/phosphorescence spectrophotometer at 298 K. Commission International de l’Eclairage (CIE) color coordinates were calculated on the basis of international CIE standards.Fluorescence lifetime and quantum yield were measured on an Edinburgh Instrument FLS920 steady-state transient fluorescence spectrometer at 298 K.
2. 2 Synthesis of the title complex
[Tb(bcbob)(H2O)(DMF)] 1 A mixture of Tb(NO3)3·6H2O(23 mg, 0.05 mmol) with H3bcbob (21 mg, 0.05 mmol) and DMF (6 mL) (acidified to pH = 4 by 6M HNO3) was heated to 160oC for 3 days, and subsequently cooled to room temperature. The obtained colorless columnar crystals were collected, washed with DMF, and dried in air. Yield:ca.42%based on Tb(III). Anal. Calcd. for C26H24NO10Tb 1: C, 46.65;H, 3.61; N, 2.09. Found: C, 46.69; H, 3.59; N, 2.66%. IR(cm-1): 1660 (m), 1593 (s), 1539 (m), 1423 (s), 1375 (s),1150 (s), 1051 (s), 864 (m), 836 (m), 784 (s), 715 (w), 673(w), 640(w), 598 (w).
[Eu(bcbob)(H2O)(DMF)] 2 The colorless crystals 2 in column were obtained from a similar self-assembly to that of 1 except Eu(NO3)3·6H2O (22 mg, 0.05 mmol) in place of Tb(NO3)3·6H2O. Yield:ca.40% based on Eu(III). Anal.Calcd. for C26H24NO10Eu 2: C, 46.77; H, 3.62; N, 2.10.Found: C, 46.63; H, 3.67; N, 2.53%. IR (cm-1): 1664 (m),1589 (s), 1537 (m), 1419 (s), 1372 (s), 1148 (s), 1047 (s),862 (m), 834 (m), 782 (s), 713 (w), 670 (w), 638(w), 595 (w).[Gd(bcbob)(H2O)(DMF)] 3 The colorless crystals of 3 in column were acquired from a similar synthetic strategy to that of 1 except Gd(NO3)3·6H2O (22 mg, 0.05 mmol) in place of Tb(NO3)3·6H2O. Yield:ca.36% based on Gd(III).Anal. Calcd. for C26H24NO10Gd 3: C, 47.17; H, 3.65; N, 2.11.Found: C, 46.94; H, 3.48; N, 2.43%. IR (cm-1): 1662 (m),1591 (s), 1539 (m), 1420 (s), 1374 (s), 1149 (s), 1049 (s),864 (m), 835 (m), 783 (s), 714 (w), 672 (w), 639 (w), 597 (w).
[Tb0.2Eu0.35Gd0.45(bcbob)(H2O)(DMF)] 4 The colorless columnar single crystals 4 were gained from a similar building methods to that of 1 except Tb(NO3)3·6H2O,Eu(NO3)3·6H2O and Gd(NO3)3·6H2O (0.05 mmol, molar ratio: 0.2:0.35:0.45) in place of Tb(NO3)3·6H2O. Yield:ca.35% based on H3bcbob. IR (cm-1): 1661 (w), 1595 (s), 1538(w), 1418 (s), 1371 (m), 1301 (w), 1145 (s), 1047 (s), 864(w), 835 (m), 783 (s), 716 (w), 669 (w), 635 (w), 597 (w).[Tb0.5Eu0.5(bcbob)(H2O)(DMF)] 5 The colorless crystals of 5 in column were acquired successfully from a similar construction methods to that of 1 except Tb(NO3)3·6H2O and Eu(NO3)3·6H2O (0.05 mmol, molar ratio: 0.5:0.5) in place of Tb(NO3)3·6H2O. Yield:ca.39% based on H3bcbob. IR(cm-1): 1666 (w), 1598 (s), 1540 (w), 1419 (s), 1372 (m),1300 (w), 1146 (s), 1045 (s), 860 (w), 838 (m), 784 (s), 718(w), 673 (w), 632 (w), 600 (w).
2. 3 X-ray structure determination
The powder X-ray single-crystal diffraction analysis reveals that 1-3 are isomorphic with each other. Compounds 2 and 3 have poor crystal quality and are unsuitable for singlecrystal X-ray diffraction test. Table 1 provides the crystal data of complex 1. The data were collected smoothly with Mo-Kαradiation (λ= 0.71073 ?) on a Bruker APEX-II CCD diffractometer. Meanwhile, with the SHELXTL program, the structure of this complex was solved by direct methods[33]. In refinement, the non-hydrogen atoms were assigned anisotropic displacement parameters in the refinement. And the whole hydrogen atoms on the benzene rings were also treated rationally by using a riding model. In addition, the hydrogen atoms on the coordinated H2O molecules were not located. The structure was then refined onF2using SHELXL-97[36]. The CCDC number is 1840267 for 1. Crystal data for C26H24NO10Tb (Mr= 669.38 g/mol): monoclinic system, space groupC2/m,a= 13.8958(6),b= 23.9513(11),c= 8.6270(9) ?,β= 103.164(1)°,V= 2795.8(3) ?3,Z= 4,T= 273 K,μ(Mo-Kα) = 2.584 mm-1,Dc= 1.590 g/cm3, 35601 reflections measured (5.4≤2θ≤55°), 3288 unique (Rint= 0.145,Rsigma=0.0417) which were used in all calculations. The finalR=0.040 (I> 2σ(I)) andwR= 0.1103 (all data).
3 RESULTS AND DISCUSSION
3. 1 Structural description
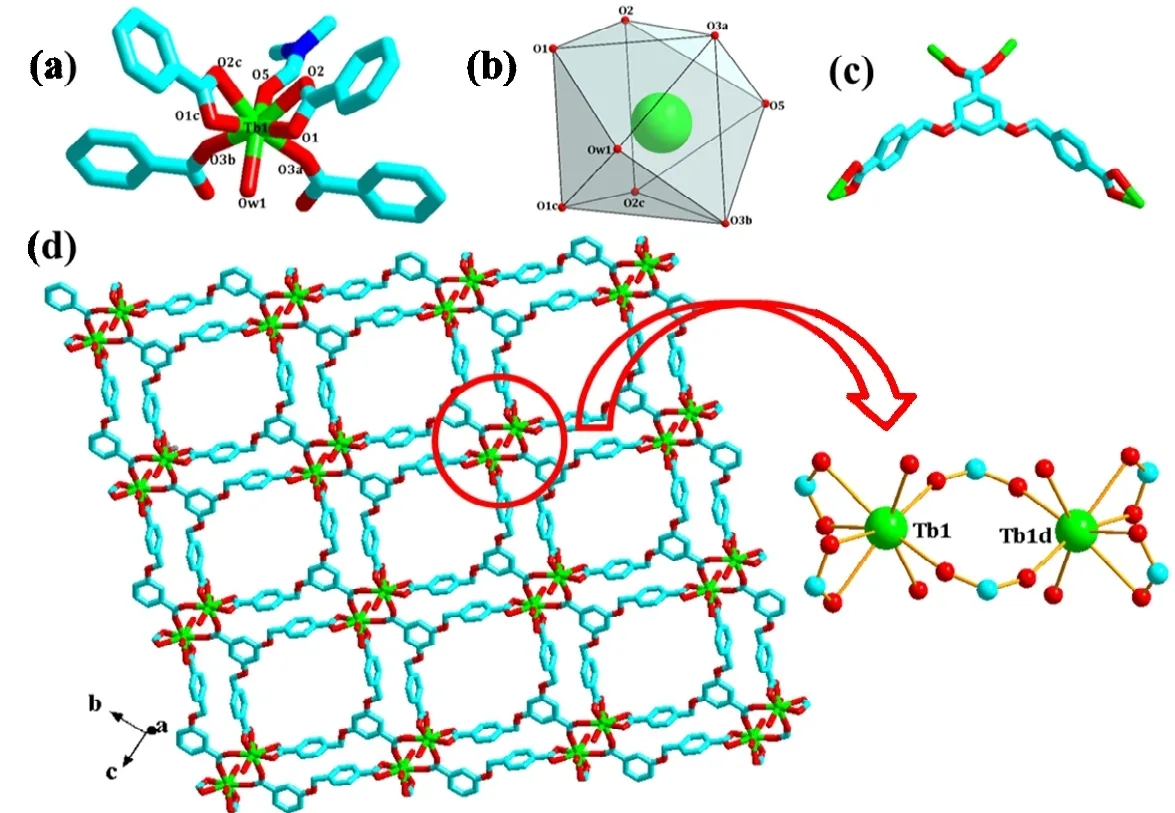
Fig. 1. Coordination environment around Tb1 (a), schematic representation of distorted TbO8 distorted tricapped triangular prismatic coordination polyhedron (b), coordination modes of bcbob (c) and 2D network (only O atoms in DMF and H2O are shown)in compound 1 (a: -x+1/2, -y+1/2, -z; b: -x+1/2, y-1/2, -z; c: x, -y, z; d: 1-x, y, 1-z)
X-ray single-crystal diffraction determination displays that 1 is a bcbob3--extended 2D Tb3+coordination polymer. It crystallizes in space groupC2/m, and the asymmetric unit is composed of a half Tb3+ion, a half bcbob molecule, a half DMF molecule, and a half coordinated water molecule. As Fig. 1a shows, Tb1 with a distorted tricapped triangular prismatic geometry (Fig. 1b) is coordinated by six carboxylato O atoms (O(1), O(2), O(1c), O(2c), O(3a), O(3b)), one water molecule (O(w1)), and one DMF molecule (O(5)). The Tb1-O distances span a wide range from 2.287(8) to 2.484(9) ?.The bcbob3-ions connect four Tb3+cations by acting as a hexadentate ligand through its three carboxyl groups based on two distinct coordination modes, μ2-η1:η1bis-monodentate bridging method and μ1-η1:η1chelating way (see Fig. 1c).Bridged by bcbob3-, 1 self-assembles into a 2D layered network (Fig. 1d). The molecular stricter of 1 is basically planar,and the three carboxylate groups are also coplanar with their corresponding aromatic rings, diverging in lateral directions.But, the carboxyphenyl rings at 3- and 5-positions are nearly perpendicular to the middle one (the dihedral angle between these planes is 86.2o). Two Tb3+generate a dual-core building block, Tb2(COO)6(H2O)2(DMF)2, with the Tb···Tb distance of 5.0366(17) ?. Then the adjacent binuclear units are bridged by flexible tripodal ligands, thus leading to a 2D network.
3. 2 Characterization
Fig. S1 presents the experimental and simulated powder XRD patterns of 1~5. The experimental powder XRD pattern for 1~5 are in accord with the simulated one generated on the basis of structural data, confirming that the as-synthesized product is in pure phase and 1~5 are isomorphic.
The TG analyses of 1~3 were performed to investigate the thermal stability. As shown in Fig. S2, the curves indicate that three compounds have good thermal stability and exhibit similar thermal behavior due to their isomorphous nature.Hence, only 1 was taken as a representative example for discussion. Compound 1 underwent three steps of weight loss.A minor weight loss for the first step should be assigned to the removal of one coordinated water molecule (calcd.: 2.7%;found: 2.6%). The continuous weight loss in the temperature of 190~270oC corresponds to the loss of one DMF molecule(calcd.: 13.6%; found: 13.1%). In the third step, the organic molecule departed, and Tb3+combined synchronously with O2into Tb2O3(calcd.: 27.3%; found: 27.9%).
3. 3 Photoluminescence properties
The fluorescence properties of compounds 1~3 and H3bcbob ligand were investigated in the solid state at 298 K.As shown in Fig. 2, once excited (λex= 365 nm), the emission of H3bcbob ligand exhibits a broad peak and with a maximum intensity at 425 nm maybe because of the bcbobcentered electronic transition (π→ π* or n → π*). Under excitation at 315 nm, 1 exhibits characteristic emission band of Tb3+at 489, 545, 586 and 620 nm, corresponding to the5D4→7FJ(J= 6-3). And the hypersensitive peak at 544 nm determines the green-light emission of 1. For the emission of 2 (λex= 315 nm), the five peaks at 579, 591, 613, 652, and 699 nm should be assigned to the5D0→7FJ(J= 0-4) transition of the Eu3+ion. The red light of 2 is dominated by the emission at 613 nm. Upon excitation at 360 nm, compound 3 exhibits a blue light emission with a wide band centered at 428 nm, which is quite similar to that of free H3bcbob ligand,except for a slight red shift. Since the energy of the lowest excited states of Gd3+6P7/2is too high to accept the energy of the ligand, the characteristics emission peak of 4f-4ftransition at 311 nm is not observable. Therefore, the emission peak of 3 at 433 nm should be attributed to the internal ligandπ*→πcharge transfer.

Fig. 2. Solid-state photoluminescence spectra of 1 (a), 2 (b), 3 (c) and H3bcbob (d)
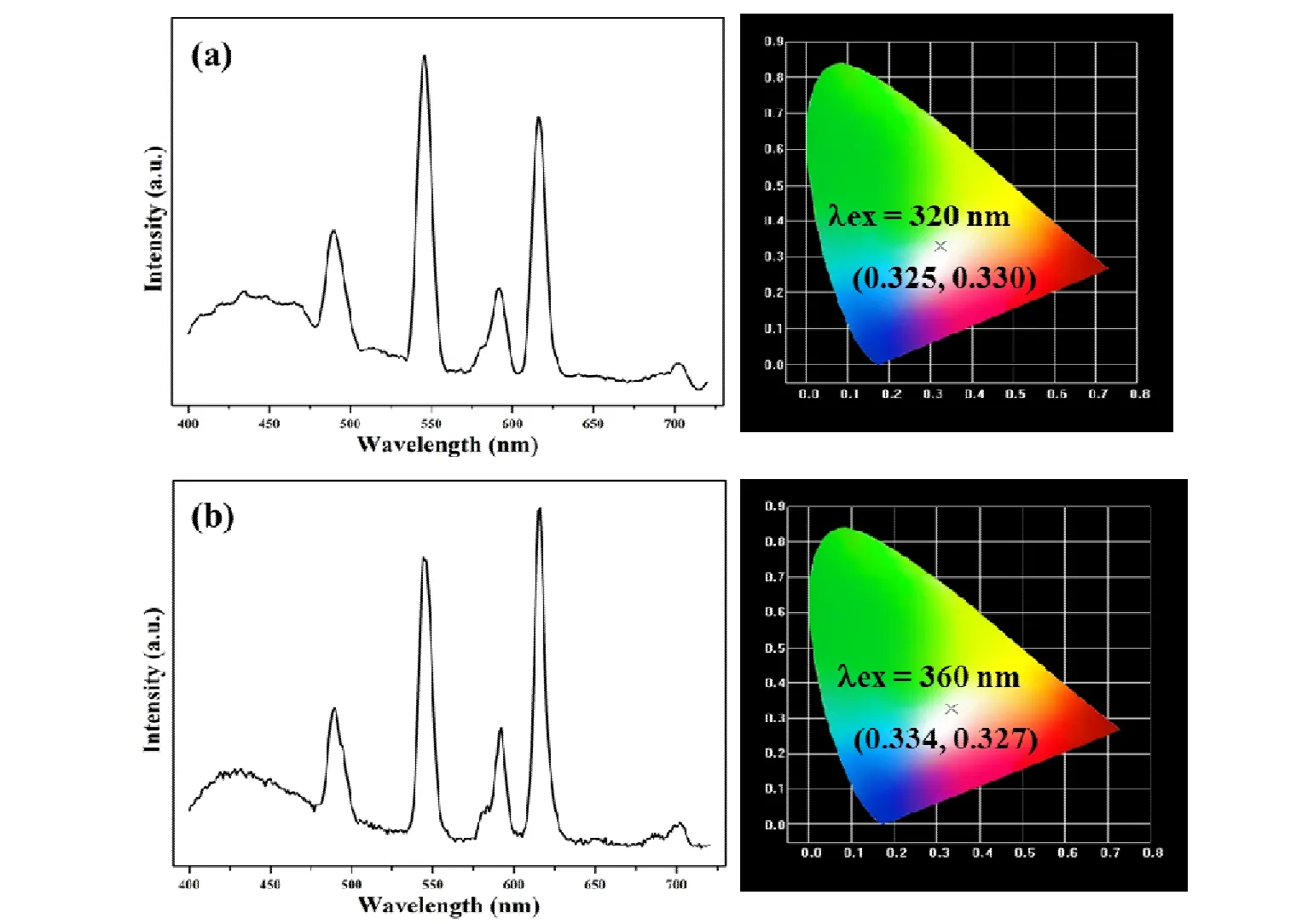
Fig. 3. Solid-state emission spectra and CIE chromaticity diagram for 4 (a) and 5 (b) excited at 360 nm
Compounds 1~3 emit RGB primary color, and they are isomorphic. Therefore, it is expected to obtain white-light photoluminescence by doping different Ln3+into the identical framework. By precisely adjusting the ratio of Tb3+/Eu3+/Gd3+, and finding suitable excitation wavelengths, whitelight emitting compound 4 [Tb0.2Eu0.35Gd0.45(bcbob)(H2O)-(DMF)] was successfully obtained. When excited at 320 nm,the corresponding CIE chromaticity coordinates (0.325,0.330) are very close to those of the pure white light (0.333,0.333), suggesting that 4 is a white-light emitting material(Fig. 3a). The quantum yield for 4 is 40.61%. Powder XRD analysis proves that 4 is isomorphic with 1~3 (Fig. S1).
Owing to its blue light emission, H3bcbob ligand can be used as blue light sources, so the materials possessing whitelight emitting characteristic might be acquired by doping Tb3+and Eu3+into one framework. When the conditions are appropriate, the combination of the “antenna effect” and the ligand luminescence can achieve white light emission. By carefully optimizing the molar ratio of Tb3+and Eu3+, we successfully designed and constructed white-light emitting compound 5 [Tb0.5Eu0.5(bcbob)(H2O)(DMF)]. When excited at 360 nm, only part of the energy transfers from the ligand to the excited state of the Ln3+. The remaining energy ensures itself to emit the blue light. The solid-state emission spectra and CIE chromaticity diagrams are shown in Fig. 3b. The corresponding CIE coordinate (0.334,0.327) at 360 nm is close to the coordinate for pure white light with the quantum yield to be13.46%. The Powder XRD analysis proves that 5 is isomorphic with 1~3 (Fig. S1).
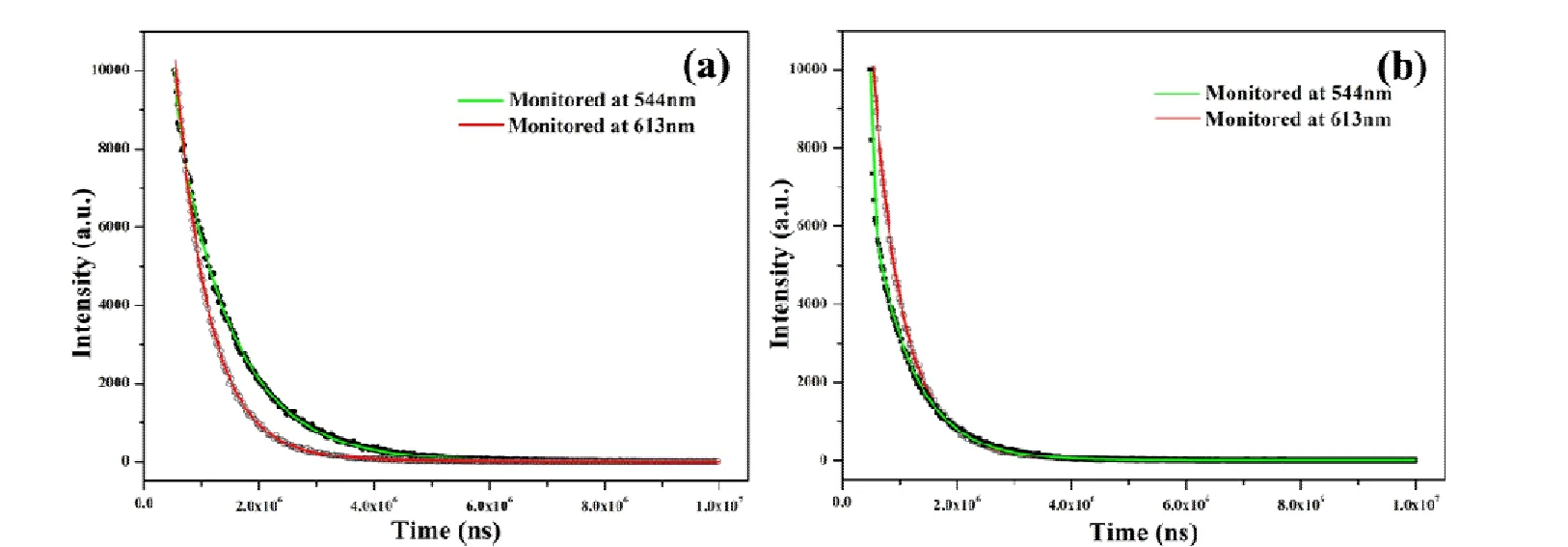
Fig. 4. Luminescence decay profiles of 4 (a) and 5 (b)
The efficient luminescence emission decay tests were executed for doped 4 and 5 at 298 K. And the decay curves are well fitted into a double exponential function: I = I0+A1exp(-t/τ1) + A2exp(-t/τ2), in which τ1and τ2are construed as the luminescence lifetimes. A summary about the detailed fitting results is displayed in Table S1. In Fig. 4, the PL decay curves of 4 and 5 are recorded at 298 K accompanied by the emission monitored by the5D4→7F5transition at 544 nm and the5D0→7F2transition at 613 nm (λex= 320 nm for 4 andλex= 360 nm for 5).
3. 4 Detection of nitro aromatics
Based on the photoluminescence properties of 1, nitroaromatic compounds such as nitrobenzene (NB), 4-nitrotolune(4-NT), 2,4-dinitrotolune (2,4-DNT), 2,4-dinitrophenol (2,4-DNP) and picric acid (PA) were used for investigating the sensing properties of compound 1. Before measurements were executed carefully, stable suspension was prepared with the steps as follows: a ground powder sample of 1 (3 mg) was dispersed in DMF solution of different quencher (3 mL, 100 μM), and then treated by ultrasonication for 30 min.As shown in Fig. 5a, the fluorescence spectrum of each suspension is similar to the spectrum in pure DMF, except that the emission intensity shows different degree of reduction.The decreasing order of quenching effect is PA > 2,4-DNP >2,4-DNT > NB > 4-NT. Especially for PA, the quenching efficiency is as high as 80% (Fig. 5b). This indicates that 1 can detect trace amounts of nitro pollutants. Compound 1 is stable in these nitro aromatics, which is confirmed by the XRD pattern (Fig. S3).

Fig. 5. Photoluminescence behaviors of 1 in the presence of nitroaromatic compounds with a concentration of 100 μM (a), photoluminescence quench percentage (b); Emission spectra of 1 with gradually increased PA (Inserted plot showing a fluorescence change after adding PA) (c), and Stern-Volmer plot of Io/I vs the concentration of PA (Inserted plot being Stern-Volmer plot at lower concentrations)
To investigate in depth the quenching efficiency of the PA materials, the emission behaviors of suspensions of 1 with different PA concentration were measured. The powder 1 (3 mg) was dispersed into 3 mL DMF to generate a suspension.And then PA was gradually added into this suspension. With the increases of PA concentration, the fluorescence intensity of the suspension gradually decreases (Fig. 5c). When the PA concentration is 6 μM, the fluorescence intensity of 1 decreases by 19%, while the PA concentration is 100 μM, and the fluorescence intensity of 1 reduces by 92%. Fig. 5d gives the Stern-Volmer plot of relative fluorescence intensity (Io/I)vsPA concentration, whereIoandIrepresent the fluorescence intensity of 1 before and after adding PA. The fitting curve at low concentration field is a good linear (R2= 0.999).The calculatedKsvvalue is 3.6 × 104M-1, which is equivalent to the data in the reported literature. The fluorescence quenching caused by PA should be the process of the interaction between the electron donor and acceptor. H3bcbob is an electron-rich ligand that can act as an electron donor.Nitro compounds are typical electron-deficient materials and are potential electron acceptors. When 1 contacts PA, the energy transfer path in 1 will change. The energy from H3bcbob is transferred to PA, instead of being transferred to the Tb3+. Namely, PA destroys the "antenna effect" of the ligand, resulting in fluorescence quenching. In addition,there is aπ-conjugated structure in 1, so a fluorescent signal can be rapidly given once encountering PA.
3. 5 Detection of metal ions

Fig. 6. Photoluminescence behaviors of 1 in various M(NO3)x solutions with a concentration of 100 μM (Insert shows the fluorescence change after adding Fe3+ and Al3+ under the UV lamp (a), photoluminescence quench percentage (b))
In addition, we also investigate the sensing ability of 1 for various metal ions by the same method. The grounded powder samples (3 mg) were dispersed in DMF solutions (3 mL)with different metal ions, and the fluorescence intensity of each suspension was measured after 30 min of ultrasonication. As shown in Fig. 6, the fluorescence spectra show that various metal ions have different effects on the luminescence of 1. When dispersed in different metal ions, the fluorescence intensity of 1 at 544 nm does not decrease significantly except Al3+and Fe3+solutions. However, the fluorescence spectra of 1 in Al3+and Fe3+solutions are obviously different. Fe3+has a great quenching effect on luminescence intensity (quenching efficiency is 82%); Al3+quenches the emission of Tb3+and enhances the emission of the ligand,giving rise to a blue light. This suggests that 1 is has high selectivity for Fe3+and Al3+.
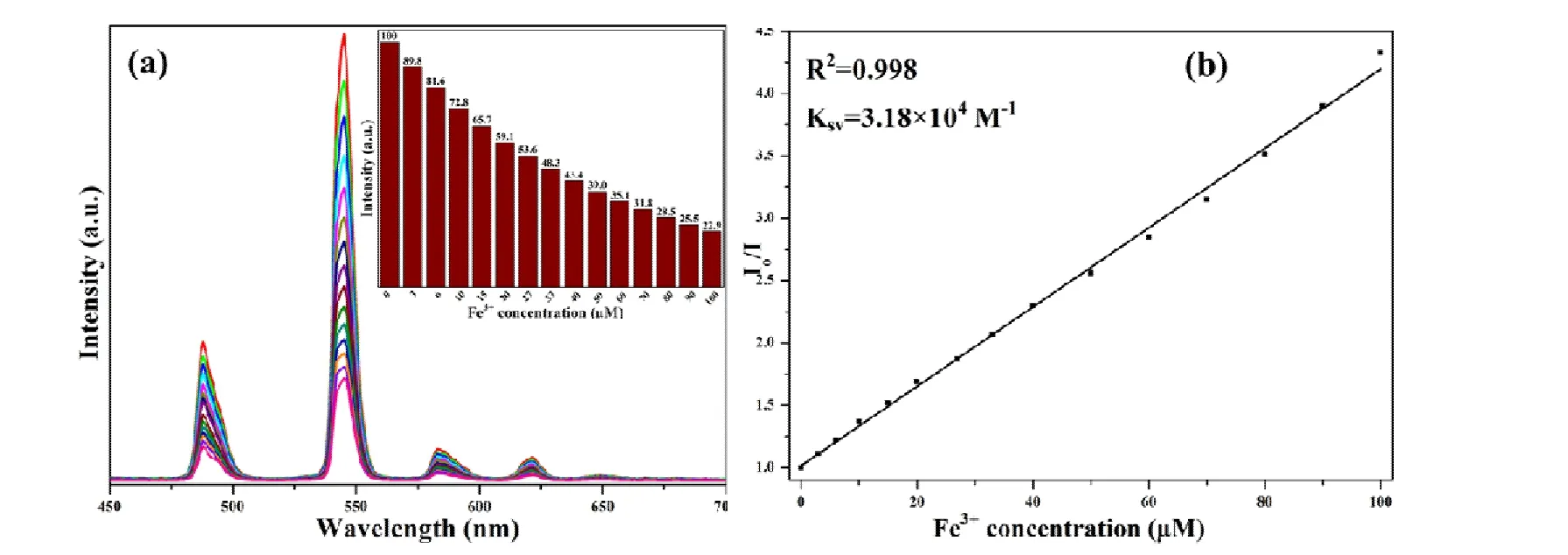
Fig. 7. Emission spectra of 1 with gradually increased Fe3+ (a) and Stern-Volmer plot of Io/I vs concentration of Fe3+ (b)
In order to better understand the fluorescence response of 1 to Fe3+and Al3+, the fluorescence change of the suspension was measured with increasing the ion concentration. The powder samples were ground and immersed in different concentrations of Fe3+or Al3+, and then their luminescence spectra were recorded. As demonstrated in Fig. 7, with the increase of Fe3+concentration, the emission intensity of suspension decreases gradually. Fig. 7b gives the fitting cure of the Stern-Volmer plot, which is in good agreement with the linear equation (R2= 0.998). The calculatedKsvvalue is 3.18 × 104M-1, which is equivalent to the data reported[37-39].As shown in Fig. 8a, with the increase of Al3+concentration,the luminescence intensity of 1 at 544 nm gradually decreases and the emission of the ligand gradually increases.When the concentration is 100 mM, the luminescence of Tb3+is nearly completely quenched and only the emission of the ligand can be observed. In order to observe this process more directly, we take pictures of the suspension with corresponding concentration under UV light irradiation at 365 nm, finding that the suspension changes from green to bright blue (Fig. 8b). Simultaneously, we discover that the framework of 1 dissolves gradually with increasing the Al3+concentration. When the Al3+concentration reaches 0.1 mM, the solution is completely clear. According to the previous literatures, the quenching effect on luminescence MOFs by metal ions may be attributed to the following factors: i) interactions between metal ions and organic ligands[40-41]; 2) destruction of the crystal structure by the metal ions[42]; 3) ion exchange between the central metal ions of the framework and the targeted cations[43-44]. The powder XRD patterns of 1 in different metal ion solutions disclosed that Fe3+did not destroy the framework of the compound (Fig. S4). We speculate that the fluorescence quenching caused by Fe3+should be the interaction between and the O atom in the structure.The fluorescence quenching caused by Al3+should be the collapse of the framework of 1.

Fig. 8. Emission spectra of 1 in the presence of different concentration of Al3+ (a) and the corresponding photographs for 1 in the presence of different concentrations of Al3+ under UV-light (b)
4 CONCLUSION
In summary, using flexible ligand 3,5-bis((4′-carboxylbenzyl)oxy)benzoilate acid (H3bcbob) as linker, three 2D isomorphic Ln-MOFs 1-3 have been constructed under solvothermal conditions. Based on their photoluminescence properties, two white-light emitting materials 4 and 5 were fabricated. Compounds 4 and 5 possess longer fluorescence lifetime and higher quantum yields, which should be due to the largerπ-conjugated structure of bcbob3-. Systematical fluorescence quenching studies show that 1 can highly sensitively sense the different nitroaromatic compounds, especially for PA (Ksv= 3.6 × 104M-1). The fluorescence quenching of 1 should be related to the electron deficient nature for PA. Due to their electron deficient nature, the intrinsic energy transferring path is changed and the “antenna effect”is prohibited, so the quenching phenomenon occurs. In addition, 1 is a highly selectively sensing material for Fe3+(Ksv=3.18 × 104M-1) and Al3+(enhance the ligand luminescence).
- 結構化學的其它文章
- Two Polynuclear Fe Complexes with Boat-like Core:Syntheses, Structures and Magnetic Properties①
- A Robust Heterometallic Cd(II)/Ba(II)-Organic Framework with Exposed Amino Group and Active Sites Exhibiting Excellent CO2/CH4 and C2H2/CH4 Separation①
- Synthesis, Crystal Structure, Spectroscopy and Hirshfeld Analysis of 4,6-Diamino-2-cyclopropylaminopyrimidine-5-carbonitrile with Different Solvents: N,N-dimethylformamide, Methanol and Water①
- Syntheses, Crystal Structures and DNA-Binding Properties of Zn(II) and Mn(II) Complexes Based on Imidazole Derivatives and Carboxylic Acid
- CoMFA Study on Anti-proliferative Activity of Fluoroquinolone Amide Derivatives①
- Synthesis and Properties of Dinuclear Europium(III)Complex Containing 2-Benzoylbenzoic Acid①

