Structural and Electronic Properties of Lutetium Doped Germanium Clusters LuGen(+/0/-) (n = 6~19):A Density Functional Theory Investigation①
YANG Zho-Feng CAO Zhen-Zhu Aziz U Rehmn YANG Ju-Ci,b②
a (School of Chemical Engineering, Inner Mongolia University of Technology, and Inner Mongolia Key Laboratory of Theoretical and Computational Chemistry Simulation, Hohhot 010051, China)
b (School of Energy and Power Engineering, Inner Mongolia University of Technology, Hohhot 010051, China)
ABSTRACT Structural growth mechanism, energetics, and electronic properties of cationic, neutral, and anionic lutetium doped germanium cluster LuGen(+/0/-) (n = 6~19) were comprehensively studied by the ABCluster unbiased global search technique with a hybrid density functional theory approach. Compared to the experimental PES, the anion evolution of structure can be clearly defined as four-phase: from the adsorbed to the link structure, then to the half cage motif, and finally to the endohedral structure. The results revealed that the LuGe16- as Frank-Kasper structure with high symmetry of Td can greatly enhance the stabilities. Doped structures have shown thermodynamic stability and appropriate energy gap. These materials are suitable semiconductors. Various approaches, including quasi-spherical geometry with closed-shell model, aromaticity, UV-Vis spectra, density of states (DOS) and partial density of states (PDOS) were applied to further support the results.
Keywords: lutetium doped germanium clusters, the ground state structure, density functional theory,electronic property; DOI: 10.14102/j.cnki.0254-5861.2011-3305
1 INTRODUCTION
In the semiconductor industry, germanium-based lowdimensional nanomaterials with high performance are gradually becoming potential candidates for non-silicon transistors[1,2]. How to develop new materials with highperformance properties has been the primary challenge for both theoretical and experimental scientists over the last decades. It has been widely used in a variety of crucial high-tech fields, for example, using germanium nanoparticles as a light-absorbing layer for solar cell[3,4], as doping center of luminescent material application to LED device[5,6], as a high performance broadband photo detector because of having excellent band gap[7], and playing an important role in solid state batteries[8].
Binary germanium cluster, a class of excellent self-assembled nanomaterials, doped by transition metal (TM) or rare earth (RE) metal, have unique salient properties in the field of structural, electronic, magnetic, and optical aspects. As a suitable building block, stability is an essential factor determined by both geometric configurations and electronic structure[9,10]. In the early study of transition metal doped germanium cluster by Kumar and Kawazoe, the researchers performed DFT calculation to explore series of M@Gen(n=14~16; M = Ti, Zr, Hf, Fe, Ru, Os) cluster and further comparatively analyzed the different growth behavior between M@Genand M@Sinincluding several possible cage structures of Frank-Kasper, fullerene-like, capped decahedral and cubic model[11,12]. From here on, transition metal doped germanium cluster with various elements has been studied[13-20]. On another side of rare earth doped germanium cluster, Tang with his coworker studied the ground state geometry, energy gap, and optical gap of Ge12M (M = Sc~Ni) by using density functional theory, and concluded that Ge12M clusters having magnetic moments from 1 to 5 μBwere regarded as potential magnetic materials with tunable magnetic properties[21]. Borshch’s group using density func-tional theory took the examination of ScGen-(n= 6~16)cluster for optimization of spatial structure and electronic spectra comparably studied with experimental results[22]. Qin et al. combine genetic algorithm with first-principles calculation aiming to find out the lowest-energy structure of GenM+/0(n= 9, 10; M = Si, Li, Mg, Al, Fe, Mn, Pb, Au, Ag, Yb, Pm,and Dy)[23]. In 2012, Nakajima’s group utilized photoelectron spectroscopy to investigate a series of MGen(M = Sc, Ti, V, Y,Zr, Nb, Lu, Hf, and Ta). The results pointed that Ge16cage with larger cavity to encapsulate RE and TM atom formed anionic core shell structure, for which electronic and geometric closings are uniformly satisfied[24]. Thereby, the RE and TM based germanium nanomaterials possess a number of great potential applications for next generation devices. That’s why it is necessary to further study comprehensively.
In this article, on the basis of Nakajima’s previous experimental data[24], we performed the growth behavior of LuGen(+/0/-)(n= 6~19) and simulated photoelectron spectroscopy of LuGen-(n= 6~19) cluster, focusing on the electronic structure of anionic LuGe16-as super atom with Frank-Kasper stable motif[25]. Another way to say is that the goal of this research is to present the theoretical investigation of the effect of Lu (lutetium) metal doping on germanium clusters, while taking predicted optical and electronic properties into account for potential application to optoelectronic materials.
2 COMPUTATIONAL DETAILS
The initial structures are originated from: (1) By using the ABCluster unbiased global search technique, which can be seen as a random generator[26,27]. When it was combined with Gaussian software package, more than 400 initial guessed geometries for each LuGen(+/0/-)(n= 6~19) were generated and optimized with the TPSSh functional combined with 6-31G for Ge atom and ECP60WMB for Lu (lutetium) atom by Gaussian 09 software package[28,29]. The energy change threshold as convergence condition is set to 10-6hartree. (2)The substitutional structure schemes were adopted, in which a Lu atom was substituted for a Ge in the most stable structure of Gen+1cluster. Subsequently, the low-lying isomers are further optimized by DFT-TPSSh functional with all-electron cc-pVTZ basis set for Ge atom and ECP28MWB basis set for Lu[30,31]. Vibrational frequency analyses were performed to confirm those isomers are located at true minima on the potential energy surface. The single point energy calculations of the selected isomers were conducted by DFT-TPSSh functional with aug-cc-pVTZ basis set for Ge and ECP28MWB basis set for Lu. The PES spectra of LuGen-(n= 6~19) were calculated by means of Koopmans’ theorem[32,33]. The density of states (DOS) and partial density of states (PDOS) of LuGe16-have been obtained by ViennaAb initioSimulation Package (VASP)[34-37], with Perdew-Burke-Ernzerhof generalized gradient approximation (PBE-GGA) functional[38]. The projector augmented wave (PAW) was set to explore the inert core electron[39,40]. To prevent interaction between adjacent clusters, the 40 × 40 × 40 ? edge lengths cubic cells with periodic boundary condition were taken into account. The plane wave cut-off energy was set up to 500 eV. The structures, isosurface maps, PES spectra and orbitals were created by visualization software of VMD and Multiwfn[41,42].
3 RESULTS AND DISCUSSION
3. 1 Ground state structure of LuGen(+/0/-) (n = 6~19)
The anionic, neutral and cationic clusters with stable geometries and point group are shown in Figs. 1~3, and the corresponding low-lying isomers with different energies are added in Figs. S1~S3, respectively. For anionic clusters,their ground states are predicted to be a singlet. Forn= 6, the ground state structure is a pentagonal bipyramid withCssymmetry which is the same as pure Ge7ground state structure where Lu atom replaces the top vertex of pyramid of the Ge atom[43]. Forn= 7, the ground state structure is a bi-capped tetragonal bipyramid withC2vsymmetry where two capped Ge atoms symmetrically adsorbed on its two faces. Forn= 8, both 8a1 and 8a2 isomers compete for the ground state structure with each other owing to the fact that both of them are degenerated in energy (the energy difference is only 0.02 eV). 8a1 and 8a2 are both bi-capped pentagonal bipyramids, and the difference lies in the two capped Ge atom adsorption sites. The 9a geometry withCssymmetry is based on 8a2 structure by adding a Ge atom. Forn= 10~12,the 10a is a link structure where Lu atom works as a linker to link two orthogonal Ge5trigonal bipyramid (TBP), that for 11a is adding one Ge at 10a one side trigonal bipyramid to form two paralleled TBP and one Ge capped TBP, that for 12a is adding one Ge atom on each bilateral side of 10a. Forn= 13, the Lu atom is located in the half cage center. The 13a1 and 13a2 are degenerated due to close energy difference of 0.04 eV. The 13a2 is hexagonal antiprism withC6vsymmetry where adsorbed one Ge atom on the hexagon surface and the 13a1 can be viewed that one Ge vertex of hexagonal antiprism of 13a2 moves to the triangular surface. Forn= 14, the 14a is seen as adding one Ge atom on 13a2 with slight distortion. Forn= 15~17, all 15a, 16a and 17a structures are derived from 16a of Frank-Kasper structure withTdhigh-symmetry where the Lu atom locates at the cage center. The 15a is viewed as 16a by reducing one Ge atom from the bottom triangle and 17a is also viewed as 16a by adding one Ge atom on the bottom triangle.Both 15a and 17a have some extended distortion. Forn= 18~19, these are all cage structures with Lu atom embedded in the center. 18a comes from the cage skeleton of 16a by adding two Ge atoms, with one in the middle layer and the other in the bottom. 19a consists of a six-membered ring symmetrical on both sides where each face capped one Ge atom, and a fivemembered ring is at the middle symmetry plane to form an endohedral structure.
For neutral clusters, their ground states are calculated to be a doublet. Forn= 6, it is the same as that anionic structure of pentagonal bipyramid. The difference is on the Lu atom that replaces the Ge atom site. Forn= 7 and 8, there are one and two Ge atom capped LuGe5pentagonal bi-pyramids, respectively. Forn= 9, its structure is equal to the pure Ge10cluster in which the Lu atom replaces a Ge atom on the top site. Forn= 10 and 11, the 10n is a tetra-capped pentagonal bipyramid and the 11n is on that structure to add one Ge atom. Forn=12, the 12n is regarded as two sub-clusters of capped trigonal bipyramid linked by Lu atom. Forn= 13 and 14, both are link-structure with Lu as the linker. In such structures, one side is the same as capped tetragonal anti- prism and another side is four Ge atoms forming a trigonal pyramid for 13n.Five Ge atoms form a trigonal bipyramid for 14n. Forn= 15,the 15n resembles to the 18n structure by removing Ge capped on each side at the ring face and one Ge atom on the symmetry plane. Forn= 16, the 16n is distorted Frank-Kasper, and forn= 17 and 19, such structures are the same as that of the anionic one discussed above. Forn= 18, the 18n cage skeleton is consistent with two five-membered rings as mirror symmetry where two Ge atoms are capped on each side at the ring face and the remaining four Ge atoms, in order to form rhombus, are coplanar on the symmetry plane.
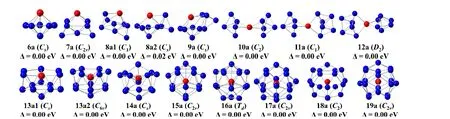
Fig. 1. The lowest-energy structures of LuGen- (n = 6~19) with point group.The blue and red balls represent the Ge and Lu atoms, respectively
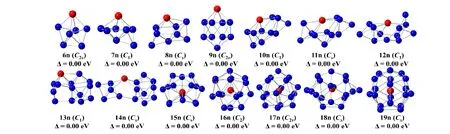
Fig. 2. The lowest-energy structures of LuGen (n = 6~19) with point group. The blue and red balls represent the Ge and Lu atoms, respectively
For cationic clusters, their ground states are evaluated to be singlet. Forn= 6 and 7, the 6c is like 6n. And 7c is on that structure by adding one Ge atom. Forn= 8 and 9, the 8c is a three Ge atom capped tetragonal bipyramid and 9c is based on 8c for more than one Ge atom. Forn= 10, 10c is the same as the Ge11pure cluster[44]. Forn= 11~15, all of them are seen as link structures where Lu atom serves as the linker.11c is two Ge5trigonal bipyramids which have jointly shared one Ge atom that is coplanar with Lu linker at the plane of symmetry. 12c is divided into one part of trigonal pyramid(Ge3) and another part of tetragonal antiprism (Ge8) linked by Lu at the center. 13c is based on 12c by adding one Ge atom at the tetragonal antiprism top site and 14c is based on 13c with the addition of one Ge atom at the trigonal pyramid site.15c is two capped tetragonal antiprisms which share three Ge atoms on the central symmetry plane and Lu atom is on the center of that geometry. Forn= 16 and 17, they are analogous to those of the corresponding neutral. Forn= 18 and 19, 18c is built as Lu of center atom around two symmetrical sixmembered rings and five-membered rings, with the remaining Ge atoms situated at three vertices of the triangle. 19c is the geometry for further epitaxial growth of 18c.

Fig. 3. The lowest-energy structures of LuGen+ (n = 6~19) with point group.The blue and red balls represent the Ge and Lu atoms, respectively
In summary, from the above description, it can be concluded that: (1) The LuGen(+/0/-)(n= 6~19) growth to be cage structure are clearly exhibited whenn= 16. (2) The anion growth system has a distinct half cage phase. However, the cation and neutral cluster growth are not. (3) The energy surface of LuGen(+/0/-)is so flat, which indicates distinguishing ground state geometries in the experiment needs more attention.
3. 2 PES spectra of LuGen- (n = 6~19)
The photoelectron spectra of different ground state isomers of LuGen-(n= 6~19) determined theoretically and compared with the experimental spectra are shown in Fig. 4. For LuGe6-,the four peaks (x, a~c) are located at 2.90, 3.31, 3.98 and 4.84 eV which are highly consistent with experimental ones(X, A~C) at 2.95, 3.33, 4.02 and 4.82 eV. For LuGe7-, the five peaks (x, a~d) are placed at 2.72, 3.15, 3.77, 4.31 and 4.53 eV, in good agreement with the experimental data (X,A~D) of 2.70, 2.98, 3.45, 4.23 and 4.53 eV. For LuGe8-, two isomers of 8a1 and 8a2 have similar energy whose photoelectron spectra are shown. For 8a1, the six main peaks (x,a~e) reside at 2.83, 3.30, 3.63, 4.08, 4.57 and 4.80 eV, and also for 8a2, the six main peaks (x′, a′~e′) are found at 3.05,3.45, 3.68, 4.20, 4.60 and 5.01 eV, compared with the experimental spectra of six peaks (X, A~E) of 3.06, 3.30,3.71, 4.20, 4.61 and 5.03 eV. The conclusion can be deduced that such two types of isomers are coexisting because of the similar structures of two capped pentagonal bipyramids and close energy. For LuGe9-, the three peaks (x, a, b) of 3.16,3.89 and 4.72 eV of the theoretical spectra are in excellent agreement with experimental spectra (X, A, B) of 3.23, 3.89 and 4.72 eV. For LuGe10-, the three peaks (x, a, b) positioned at 3.79, 4.31 and 4.78 eV are corresponding experimental value (X, A, B) of 4.10, 4.52 and 4.98 eV. The theoretical result has a certain red shift compared with the experimental one. For LuGe11-, the simulated spectra of three peaks marked as x, a and b are 3.60, 4.19 and 4.68 eV. In comparison with the experimental X, A and B of 3.85, 4.21 and 4.73 eV, the peaks of a and b agree well with A and B. For LuGe12-, the peaks (x, a~c) situated at 3.47, 4.35, 4.77 and 5.05 eV contrast with the experimental values (X, A~C) of 3.67, 4.51,4.94 and 5.34 eV. The calculation result has a small deviation from the experimental one. For LuGe13-, the 13a1 and 13a2 have been presented as similar total energy isomers. The 13a1 and 13a2 have three peaks placed at 3.38, 3.80, 4.27 eV and 3.54, 3.99, 4.87 eV correlated to the experimental peaks of 3.59, 4.03 and 4.83 eV. Owing to similar half cage structures, combined with PES, 13a1 and 13a2 are the coexisting structures. For LuGe14-, three distinct peaks (x, a, b) lie in 3.63, 4.12 and 5.11 eV, which coincide with three experimental peaks (X, A, B) of 3.68, 4.13 and 5.14 eV. For LuGe15-, simulated peaks are placed at 3.93, 4.66 and 5.23 eV against experimental peaks of 3.98, 4.69 and 5.25 eV. For LuGe16-, due to stable Frank-Kasper structure, the two peaks of x and a conform with X and A at 3.96 and 4.99 eV. In much the same as LuGe16-, the LuGe17-of simulated and experimental PES exhibits four obvious peaks of 3.47 (3.47),4.03 (4.08), 4.67 (4.70) and 5.23 (5.29) eV. For LuGe18-, its simulated PES spectra have four distinct peaks (x, a, b, c) at 3.68, 4.16, 4.93 and 5.44 eV, respectively. Except for the first peak, the last three peaks (a, b, c) reproduce good experimental peaks (A, B, C) of 4.16, 4.93 and 5.44 eV[24]. The adjacent isomers 17a and 19a have a small bump in the low energy region of the experimental PES spectra[24], so we infer that 18a should also have this peak. But it is not observed in the experimental PES spectra[24]. This conclusion needs to be further verified by experiment. For LuGe19-, the three peaks(x, a, b) of 3.76, 4.15 and 5.33 eV are analogous to the experimental peaks (X, A, B) of 3.67, 4.15 and 5.33 eV.

Fig. 4. Simulated and experimental PES spectra of LuGen- (n = 6~19) clusters
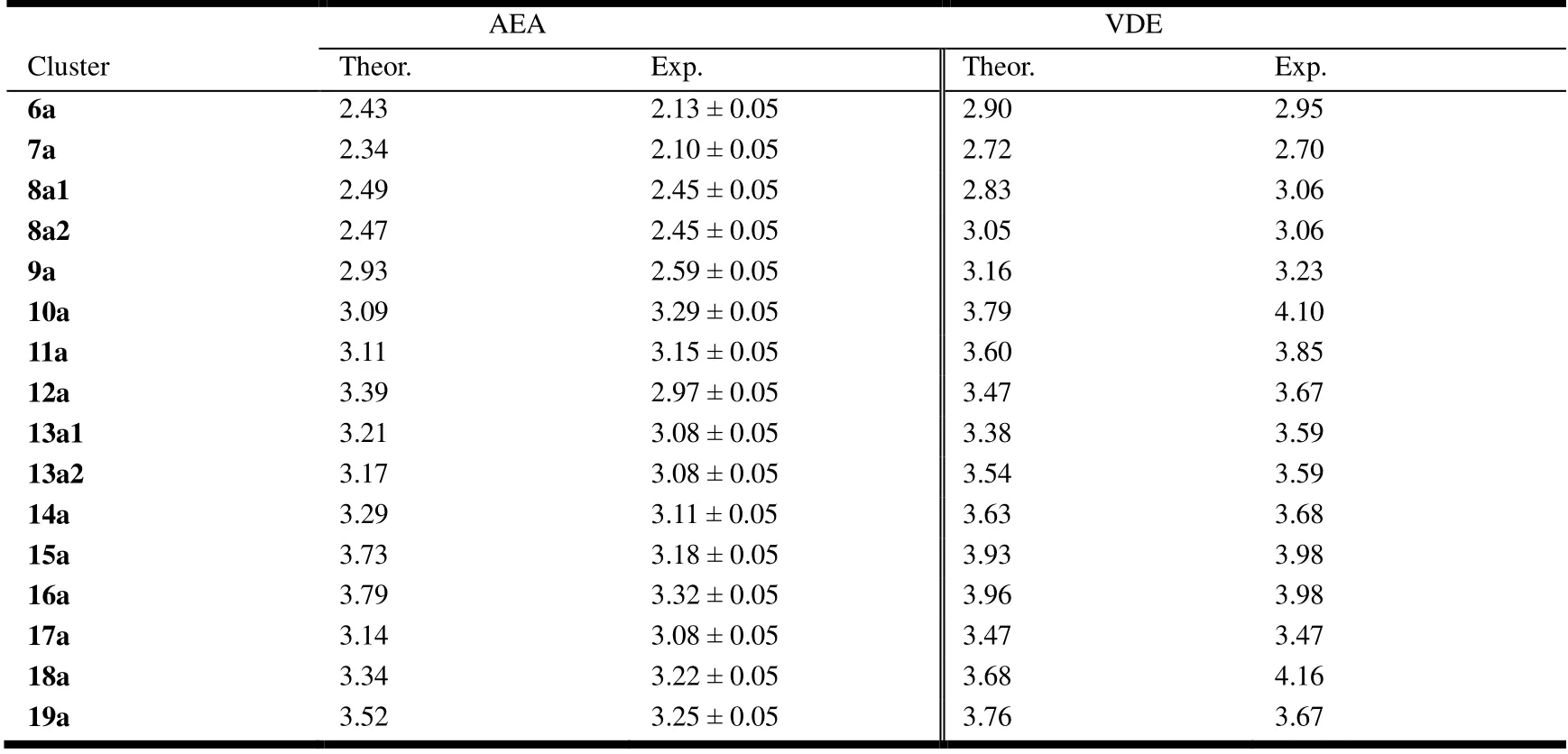
Table 1. Theoretical and Experimental AEA, and VDE of LuGen (n = 6~19)
Theoretical calculation of adiabatic electron affinities(AEAs) and the first vertical detachment energies (VDEs) of LuGenclusters as well as experimental dataare collected in Table 1. The calculated values of AEA and VDE are derived from AEA =E(optimized neutral) -E(optimized anion) and VDE =E(neutral at optimized anion geometry) -E(optimized anion). From Table 1, for both AEA and VDE, the experimental values of 8a2, 13a2, 14a and 17a are in good agreement with the theoretical ones with a minor difference less than 0.20 eV. The largest deviation of AEA is for LuGe15,which is off by 0.55 eV. Although PES is a powerful experimental technique for measuring AEAs and, in principle,is the most accurate scheme to determine the AEAs of the corresponding neutral species, it is difficult to determine accurate AEAs if the recorded PES shows a featureless long and very rounded tail with no clear onset. The PES of LuGe15-may be one such example[24]. In this case, theoretical calculation is necessary to help determine accurate AEAs,especially for excellent agreement between the theoretically simulated and experimental PES spectra. Therefore, we dare to predict the AEA of LuGe15is 3.73 eV rather than 3.18 eV.The theoretical VDE values of 6a, 7a, 9a, 12a, 15a, 16a and 19a are all in accord with their experimental ones with energy difference less than 0.20 eV. Besides, the calculated and experimental VDE of 10a, 11a, 12a and 18a do not match well. However, the AEA of simulation and experiment are consistent, which proves the credibility of the results from the other side. In summary, the simulated AEA and VDE outcomes of 8a2 and 13a2 are highly in line with corresponding experimental values, further proving that 8a1, 8a2 and 13a1,13a2 are coexistence systems. By the way, LuGe16-as the most stable Frank-Kasper structure has the highest value in AEA and VDE no matter for theoretical and experimental results.
3. 3 Ionization potential
Ionization potential (IP), including vertical ionization potential (VIP) and adiabatic ionization potential (AIP), is the important parameter in both physical and chemical properties.The VIP is described as the difference of total energies where the equation follows VIP =E(cation at optimized neutral geometry) -E(optimized neutral) and AIP defined as the difference of total energies calculated by AIP =E(optimized cation) -E(optimized neutral). The calculation results of VIP and AIP of LuGen(n= 6~19) are all gathered in Table 2,with no experimental data for comparison. For the sake of observation, point line chart is also presented in Fig. 5, in which the highest value of VIP for LuGe15is 7.08 eV and that of AIP for LuGe16is 6.20 eV. On the contrary, the lowest values of VIP and AIP are 5.85 eV for LuGe6and .61 eV for LuGe11. On the whole of VIP and AIP curves, the three humps are obviously displayed inn= 7, 12 and 15 for VIP and four humps inn= 7, 9, 12 and 16 for AIP.

Fig. 5. Ionization potential of LuGen (n = 6~19) clusters

3. 4 Stabilities
To investigate the relative stabilities of the lowest energy structure of LuGen(+/0/-)(n= 6~19), the average bonding energy (ABE) and second energy difference (Δ2E) are calculated by equations (1.1)~(1.3) in whichEis the total energies related to atom or compound, and HOMO-LUMO gap (Egap) is derived from the energy of the lowest unoccupied molecular orbital minus the highest occupied molecular orbital. All are exhibited in Fig. 6.
From Fig. 6a, it is clear to see that the ABE values of the neutral cluster are all lower than the others, which indicates the cation and anion are more stable because both of them have closed-shell electronic configuration which has increased their stabilities. The ABE value of LuGe16-is 3.54 which is higher than the other anionic and neutral clusters. The reason can be described that not only LuGe16-is Frank-Kasper endohedral motif with highTdsymmetry, but also has the closed-shell electronic configuration as super atom with ultra-stability. Owing to LuGe16-, the neutral and cationic LuGe16are also more stable than the other ones of the same type because both those structures are derived from Frank-Kasper structure with different degrees of distortion.
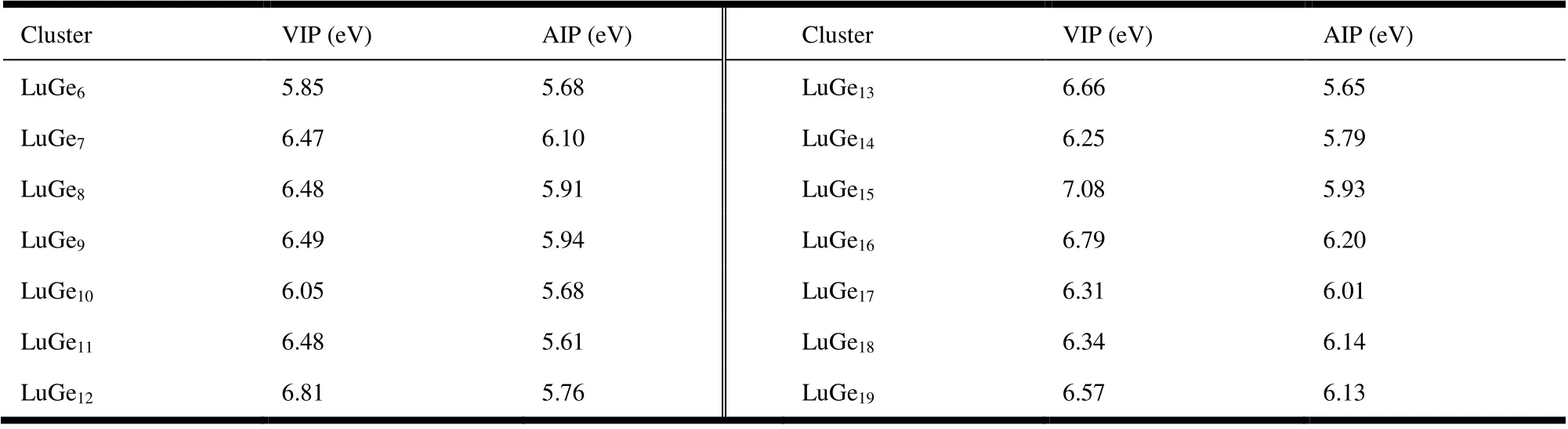
Table 2. Vertical Ionization Potential (VIP) and Adiabatic Ionization Potential (AIP) of LuGen (n = 6~19)
The second-order difference in energy of LuGen(+/0/-)(n=6~19) is shown in Fig. 6b, which is to evaluate the relative stability of such cluster and its two directly adjacent ones. For anion, the three peaks of anionic type are located at LuGe9-,LuGe12-and LuGe16-. For the neutral, the obvious four peaks are situated at LuGe9, LuGe11, LuGe13and LuGe16. For cation,LuGe8+, LuGe11+LuGe13+and LuGe17+have four peaks. That means such clusters are more stable than the adjacent ones.
TheEgapis an important physical parameter for semiconductors to evaluate not only the chemical reactivity but also the optical properties. As can be seen in Fig. 6c, the apparent two peaks of LuGe10-and LuGe16-are about 2.41 and 2.55 eV.As far as we know, such energy gap value is larger enough for the luminescent host from ground states to excited states[45].Besides the proper energy gap for luminescent materials, that is also suitable for catalysis material. As photo catalyst, it is very important for visible light response, so that the materials with adjustable energy gaps can be achieved[46]. As the LuGe19+has the lowest energy gap of 1.06 eV to LuGe16-of 2.55 eV, it can fit within the visible light range. In summary, these clusters as an excellent building block are potentially applied to multifunctional nanomaterials.
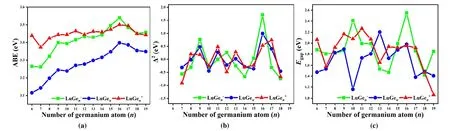
Fig. 6. Graphical representation of (a) Size dependence of the average bond energy (ABE), (b) Second energy difference (Δ2E) and(c) HOMO-LUMO energy gap (Egap) of the ground state LuGen(+/0/-) (n = 6~19)
3. 5 Iso-chemical shielding surface of LuGe16-
To further understand the stability of LuGe16-Frank-Kasper structure, the iso-chemical shielding surface (ICSS)based on the real-space function which is similarly related to the nucleus-independent chemical shift (NICS) is calculated by gauge-independent atomic orbital (GIAO) method, and the results are analyzed by Multiwfn code[47,48]. Compared to NICS, the ICSS can exhibit the entire three-dimensional space of chemical shielding against the external magnetic field as well as different isosurfaces drawn at different isovalues to clearly reveal the shielding or deshielding effect from the delocalized electron. From Fig. 7a, the red region is the shielding area with isovalue of 0.05 ppm and the blue region is deshielding area with isovalue of 0.05 ppm. Due to the high symmetry of the LuGe16-cluster, the shielding areas are exhibited by three protruding red areas, which indicates that the inner region of the cluster has strong magnetic shielding. Owing to the electron lone pair existence, it presents high electron delocalization and aromaticity. Fig. 7b displays the ICSS value from the distance of 0.4 to 3.0 ?,which reveals that the shielding value of the inner cage is larger than that of the outer surface. The maximum shielding value about 83.97 ppm is located at a distance of 1 ? from the center. In conclusion, the measurement of ICSS can be used for the aromatic properties of the LuGe16-cluster, which shows the key factors of its stability.

Fig. 7. ICSS of LuGe16- cluster. (a) Isosurface of ICSS with isovalue of 0.05 ppm (red region) and -0.05 ppm (blue region),(b) ICSS curve map of magnetic shielding value with distance from center
3. 6 Chemical bonding analysis and density of states of the LuGe16- cluster
In order to gain insight and understand the better thermosdynamic and chemical stability of the LuGe16-cluster with Frank-Kasper structure, the adaptive natural density partitioning (AdNDP) method, based on the natural bond orbital(NBO) developed by Zubarev and Boldyrev, is used to study the cluster as a multicenter bonding system[49]. For example,nc-2e is denoted as n from the range 1 to the number of atoms in the cluster system. According to Fig. 8, the LuGe16-cluster of chemical bonding of 68 valence electrons withTdsymmetry can be split into three parts: lone pair, 2c-2e, and 4c-2e. The Ge atom, which is located on each of the four triad axes, has a lone pair. Besides the four Ge atoms on the triad axes, the remaining Ge12cage is attributed to 18 2c-2e localized Ge-Geσbonds possessing 1.87~1.89 electrons in each bond. The last ones are categorized into 12 delocalized 4c-2eσbonds connecting the cage core atom of Lu to the outer Frank-Kasper Ge16skeleton, which can stabilize the entire structure and reveals the full encapsulated LuGe16-endohedral cluster.
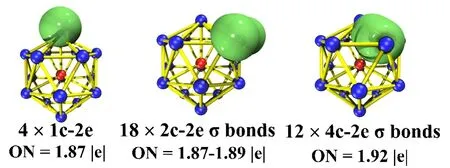
Fig. 8. AdNDP analysis of LuGe16- cluster. ON is the occupation number
For more clarity regarding the unique electronic structure of LuGe16-cluster, the calculated density of states and partial density of states are analyzed and shown in Fig. 9. It can be clearly seen that 6s, 5d, 5p, and 5sorbitals of Lu combined with 4sand 4porbitals of Ge contribute to the main density of states of LuGe16-cluster. The 4forbitals are isolated at-3~-4 eV region. Near the Fermi level (dot line), the main contribution is from 5dorbital of Lu and 4porbital of Ge. In other words, thed-orbital of Lu is mainly participating in hybridization with Ge orbital to stabilize the cluster. Moreover, the bottom part of the conduction band mainly consists of 5dorbitals of Lu, and 4s, 4pand 4dorbitals of Ge. In the context of the AdNDP and DOS analyses, the orbital sequence of LuGe16-can be represented as 1S21P61D101F142S21G182P62D10, a jellium model for 68 valence electrons.
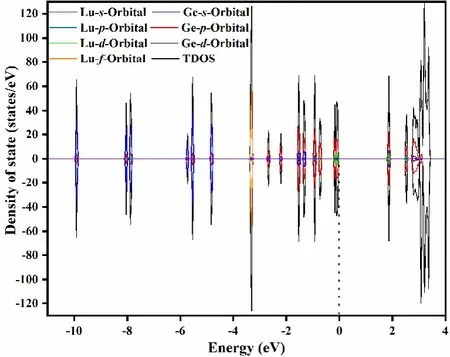
Fig. 9. Calculated and labelled total density of states (TDOS) and partial density of states (PDOS) of most stable LuGe16- cluster
3. 7 UV-Vis spectra of LuGe16- cluster
The UV-Vis spectra are an essential parameter for luminescent and photocatalytic materials. The most stable LuGe16-cluster is selected as a model structure due to its significant stability and appropriate energy gap. Timedependent DFT (TDDFT) calculations are performed at the level of TPSSh/aug-cc-pVTZ for Ge and ECP28WMB for Lu in order to analyze transitions in 120 excited states which are enough to describe. The curves are arranged by Gaussian broadening function with the full width at half maximum(FWHM) about 0.20 eV. Fig. 10 shows that the four peaks are positioned at 334, 369, 408 and 540 nm. The peak of 334 nm has a maximum absorption intensity and its 95% contribution can be decomposed into three parts ofS0→S115with contribution of 8%,S0→S107with contribution of 72% andS0→S104with contribution of 15%. The peak of 369 nm with 92% comes from theS0→S74. The peak of 408 nm mainly has 12% contribution ofS0→S58and 69% ofS0→S35. The last peak of 540 nm is comprised of 46% contribution ofS0→S6and 53% contribution ofS0→S12. The absorption band is in the range of 300~600 nm and most of it falls in the visible light region, which can be excited by natural light.Meanwhile, the strong absorption bands are situated in the blue and near-ultraviolet regions. In other words, the LuGen16-cluster with high stability can be further explored as possible optoelectronic material.
4 CONCLUSION

Fig. 10. Calculated UV-Vis spectra of LuGe16- cluster. Solid and dotted lines represent the absorption curve and oscillator strength, respectively
The growth behavior, thermodynamic stabilities, chemical activities, and electronic properties of cationic, neutral, and anionic lutetium doped germanium cluster LuGen(+/0/-)(n=6~19) are calculated by the ABCluster unbiased global search technique with a TPSSh-hybrid density functional theory. In terms of experimental PES spectra, AEA and VDE compared with the simulations, the growth pattern of anionic LuGen-can be depicted from adsorbed (n= 6~9) to the link structure (n= 10 ~ 12), then to half cage (n= 13~14),finally to the cage-like motif (n= 15~19). For the neutral and cationic LuGenclusters, the growth characteristics are less evident than anions. The neutral LuGenforms the link structure whenn= 12~14 and whenn= 15, those can be formed as cage-like structures. For cations, the link structure begins atn= 11 and lasts untiln= 15, then creates cage-like motif. The stability analysis shows the great performance for LuGe16-, and the reason can be described not only by its unique Frank-Kasper framework with highTdsymmetry without any imaginary frequency, but also by the specific electronic configurations. To further support that discovery,the aromaticity, density of states and AdNDP analysis are set.The ICSS results show the LuGe16-possesses the aromatic nature with a max value of 83.97 ppm. Furthermore, the 3Disosurface of ICSS also proves the strong magnetic shielding effect in the cage inner. The AdNDP results exhibit that the orbital sequence 1S21P61D101F142S21G182P62D10matches 68 electrons forming as a closed-shell superatomic model.Existence of lone pair increases the delocalization of LuGe16-,which is also beneficial for the cluster stability. Finally, the density of states presents the 5dorbitals of Lu hybridized with 4porbital of Ge to further stabilize the cluster of LuGe16-. Owing to the excellent stability and proper energy gap of LuGe16-and others, the lutetium doped germanium clusters are possible candidates to fabricate a number of assembled optoelectronic materials.
- 結(jié)構(gòu)化學(xué)的其它文章
- Two Polynuclear Fe Complexes with Boat-like Core:Syntheses, Structures and Magnetic Properties①
- A Robust Heterometallic Cd(II)/Ba(II)-Organic Framework with Exposed Amino Group and Active Sites Exhibiting Excellent CO2/CH4 and C2H2/CH4 Separation①
- Synthesis, Crystal Structure, Spectroscopy and Hirshfeld Analysis of 4,6-Diamino-2-cyclopropylaminopyrimidine-5-carbonitrile with Different Solvents: N,N-dimethylformamide, Methanol and Water①
- Syntheses, Crystal Structures and DNA-Binding Properties of Zn(II) and Mn(II) Complexes Based on Imidazole Derivatives and Carboxylic Acid
- CoMFA Study on Anti-proliferative Activity of Fluoroquinolone Amide Derivatives①
- Synthesis and Properties of Dinuclear Europium(III)Complex Containing 2-Benzoylbenzoic Acid①

