Recent advances on the nitrogen-rich 1,2,4-oxadiazole-azoles-based energetic materials
Wen-Li Co ,Qmr-un-Nis Triq ,Zhi-Min Li ,Jun-Qing Yng ,b,Jin-Guo Zhng ,*
a State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,100081 PR China
b CAS Key Laboratory of Energy Regulation Materials,Shanghai,200032,China
Keywords:1,2,4-Oxadiazole-azoles Triazole Tetrazole Furazan Energetic materials Furoxan
ABSTRACT This review covers recent advances in the synthesis and energetic performance of nitrogen-rich 1,2,4-oxadiazole-azoles-based energetic materials.These materials comprise of 1,2,4-oxadiazole subunit as a key structural motif linked to different nitrogen-rich or nitrogen-oxygen azoles:tetrazole,furazan,furoxan,1,3,4-oxadiazole,pyrazole,and triazole.Particular attention is devoted to the introduction of various energetic groups including nitro,nitramino,azo,azoxy,dinitromethyl,trinitroethyl moieties,and their combination.The physicochemical and available performance parameters including density,decomposition temperature,heat of formation,detonation pressure,detonation velocity,impact sensitivity,and friction sensitivity of typical energetic compounds are also provided and analyzed.Eventually,it was obtained that several screened compounds exhibit superior detonation properties and outstanding insensitivities,which can be classi fied as a new family of high-performance energetic materials.Additionally,1,2,4-oxadiazole-azoles-based energetic materials still have many thorough works to further exploited and studied,expecting to get very promising insensitive high explosives for practical application and industrialization.
1.Introduction
In the modern world,the synthesis of materials that can store and release huge amounts of energy is a top priority due to the community's reliance on explosives,aircraft,mining,and pyrotechnics[1].This has prompted abundant studies designing and storage of energy in materials,as well as the ease of use and the reduction of size while maximizing energy output.Notably,chemical energy storage has a clear bene fit,that is,the energy density could be readily increased by rational design.Due to their wide applicability,many energetic materials(EMs)have been proposed and studied with speci fic properties in relevance to the application.The development of novel energetic materials with enhanced performance and lower sensitivity is a never-ending quest for researchers[2-5].Energetic materials have been one of the most important functional materials in the global race for supremacy in the fields of modern weaponry and equipment for a long time.The obtaining of the desired molecule with suf ficient energy and adequate safety through careful con figuration design and ef ficient synthetic pathways is one of the primary problems in the field of high-energy-density materials(HEDMs).The new compounds not only have high velocity(D>8500 m/s),good thermal stability(T>200C),and low sensitivities(IS>7 J,FS>120 N),but also meet other requirements such as low/no water solubility,green decomposition products,good compatibility,highyield,cost-effective synthesis,batch production,and so on[6,7].
The high nitrogen heterocyclic compounds,particularly N-rich or N/O-rich heterocycles(often referred to as N-rich heterocycles),have always stimulated researchers'interest[8-11].These N-rich heterocycles are widely used in HEDMs,including nontoxic gas generators,low-smoke pyrotechnics,insensitive explosives,and chlorine-free energetic propellants[12-15].Common N-rich heterocycles for the building blocks of HEDMs are mainly focused on the five-and six-membered rings,such as azoles[16-20]and azines[21-23].Due to the perturbation of nitrogen atoms within these N-rich heterocycles,the combination of atomic orbitals is superior to that of a pure carbon ring,resulting in improved molecular stability,high thermal stability,and reduced impact sensitivity.Owing to their planar structure and compactπ-π stacking interactions,N-rich heterocyclic derivatives possess high density,good oxygen balance(OB),high heat of formation(HOF),and improved detonation parameters as compared to conventional explosives(e.g.,2,4,6-trinitrotolune (TNT),2,4,6-triamino-1,3,5-trinitrobenzene(TATB),cyclo-1,3,5-trimethylene-2,4,6-trinitamine(RDX),1,3,5,7-tetranitrotetraazacyclooctane(HMX),and hexanitrohexaazaisowurtzitane(CL-20),Table 1)[24].Except for these building backbones,the introduction of some connected units(such as,-NH-,-NH-NH-,-N=N-)and energetic functionalities(such as,-NH,-NO,-NHNO,-N,-ONO,-C(NO))can boost nitrogen content,HOF,and density,and hence detonation performance. The fore-mentioned suggests that N-rich heterocycles-based high-energy explosives has obvious advantages over typical ring/cage-shaped explosives.As a result,rapid progress has been made in the design and synthesis of N-rich heterocycles in the area of advanced HEDMs.

Table 1 Energetic and physicochemical properties of several well-known primary explosives.
Because of their high nitrogen content,high positive heats of formation,good thermal stability,and energy controllability,azoles(five-membered heterocycles)are often utilized as backbones for the building of HEDMs.According to the number of N/O atoms on the ring,azole can be classi fied as pyrrole,pyrazole,imidazole,triazole,tetrazole,oxadiazole,and the total nitrogen structure-pentazole,which was first synthesized and reported in 2017 by prof.Ming Lu's group[26-28].Great progresses and achievements have been obtained on HEDMs of single pyrazole,triazole,tetrazole,and furazan.Now,N-rich polycyclic and fused ring energetic molecules with extended conjugated plane and delocalizedπ-bond also have been designed and synthesized[29,30].Due to the presence of active oxygen atoms,oxadiazoles are a kind of unique energy unit among azoles.The 1,2,5-oxadiazole(furazan)has the highest HOF in the four oxadiazoles(Fig.1)and then attracts a lot of attention in the synthesis of HEDMs[31-33].Several typical energetic materials were studied based on furazan[34].For example,3,4-bis(4-nitrofurazano-3-yl)furoxan(DNTF)exhibits high density(1.937 g/cm),high detonation velocity(8930 m/s),and good explosion heat(5789 J/g),all of which are superior to HMX and close to CL-20,suggesting that DNTF could be used as an effective liquidphase carrier in melt-cast explosives[35,36].Another compound,4,4-bis(nitro)azofuroxan(DDF)shows an excellent detonation velocity of 10000 m/s(measured)and a high density of 2.02 g/cm[37]comparable to CL-20(2.04 g/cm).What insuf ficient is that DDF has poor thermal stability(T=119C)and high impact sensitivity(IS=2 J).Similarly,other reported furazan-based EMs,such as 3,4-di(nitramino)furazan(T=99C,IS<1 J,FS<5 N)[38],all revealed extremely low practical uses.
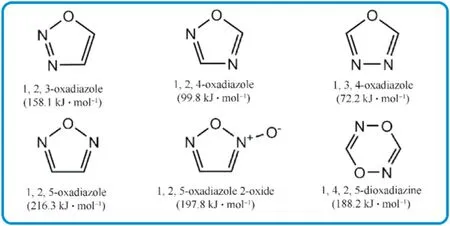
Fig.1.Structural unit and heats of formation of different oxadiazole moieties.
The HOF s of isofurazan(1,3,4-oxadiazole and 1,2,4-oxadiazole,except for 1,2,3-oxadiazole which is unstable[39])are considerably lower than furazan,which have been rarely explored and studied as structural units of EMs.3-Nitro-5-nitramino-1,2,4-oxadiazole with calculated density(1.883 g/cm),theoretical detonation velocity(9095 m/s),and detonation pressure(37.68 GPa)was initially reported in 2012(Fig.2(a))[40].It also exhibits a low impact sensitivity(15 J),indicating that it is a high-performance insensitive energetic single molecule.In 2016,the energetic salts based on 3-nitro-5-nitramino-1,2,4-oxadiazole were described,and the guanidinium salt exhibited excellent performance among them[41].Following that,isofurazan-based energetic materials have gotten a lot of interest and have become a research hotspot in the design and synthesis of energetic materials.Klap¨otke et al.thoroughly examined the derivatives of 5,5-dinitromethyl-3,3-bis(1,2,4-oxadiazole)[31],particularly 5,5-bis(trinitromethyl)-3,3-bi(1,2,4-oxadiazole) and 5,5-bis(fluorodinitro-methyl)-3,3-bi(1,2,4-oxadiazole)[42,43],demonstrating a fine balance between high detonation levels and low sensitivities.Several energetic functions(-NH,-NO,-ONO,-N,-NHNO,dinitromethyl-,trinitromethyl-,fluorodinitromethyl-,azo,azoxy,etc.)can be introduced into isofurazan backbones to prepare promising HEDMs.For instance,potential energetic plasticizing ingredient bis(1,2,4-oxadiazole)bis(methylene)dinitrate[44],single explosive 3,3-bis(5-nitramino-1,2,4-oxadiazole-3-yl)-4,4-azo-furazan with high theoretical detonation velocity over 10000 m/s[45],and heatresistant explosive 5,5-bis(2,4,6-trinitrophenyl)-2,2-bi(1,3,4-oxadiazole)(TKX-55)[46].Their molecular structures and performance characteristics were depicted in Fig.2(b)-(d).Furthermore,the less easily cleaved N-O bonds in a ring,the more thermally stable compounds will be obtained[47].Isofurazan is,therefore,stable than furazan.Combining various azole rings into a single molecule has been proven a powerful method for building skeletons of high-performance energetic materials[48,49],which could synthesize new energetic molecules with desirable characteristics by combining the advantages of different azoles.It is believed that introducing the 1,2,4-oxadiazole ring into different azole rings to generate an extendedπ-conjugated system will improve the thermal stability and the heat of formation of novel energetic molecules.Then there would be a fair balance between high energy and safety.The thermal stability of 4-(5-methyl-1,2,4-oxadiazol-3-yl)-N-(2,2,2trinitroethyl)-1,2,5-oxadiazol-3-amine(Fig.2(f)) [50] appears to increase after one side 2,2,2-trinitroethylamino in 3,4-bis(2,2,2-trinitroethyl-amino)furazan(Fig.2(e))[51]is substituted by 5-methyl-1,2,4-oxadiazole.Meanwhile,sensitivity to impact and friction is greatly reduced.In short,one of the most effective ways in the design and synthesis of HEDMs is to combine 1,2,4-oxadiazole with other high-energy azoles and distinct energetic functionalities.
Although the research period is insuf ficient,some signi ficant breakthroughs have been made in the study of 1,2,4-oxadiazoleazoles-based energetic molecules.Meanwhile,the construction method and mechanism of the 1,2,4-oxadiazole cyclization reaction have been reviewed in detail[52].The synthesis and properties of EMs base on mono-,azo-,and bis-1,2,4-oxadiazoles were also thoroughly examined[53].The design and synthesis of 1,2,4-oxadiazole-azoles-based energetic materials have not been reported systematically,which is necessary for the further study of high-performance EMs.Taking this as the starting point,the achievements of 1,2,4-oxadiazole-azoles that have been designed and synthesized recently are summarized.The N-rich azoles are involved in tetrazole,furazan,furoxan,1,3,4-oxadiazole,pyrazole,and triazole.The physicochemical and energetic performance parameters of typical energetic compounds are also provided,including density(d),decomposition temperature(T),oxygen balance(OB,based on CO),the heat of formation(HOF),detonation pressure(P),detonation velocity(D),impact sensitivity(IS),and friction sensitivity(FS).
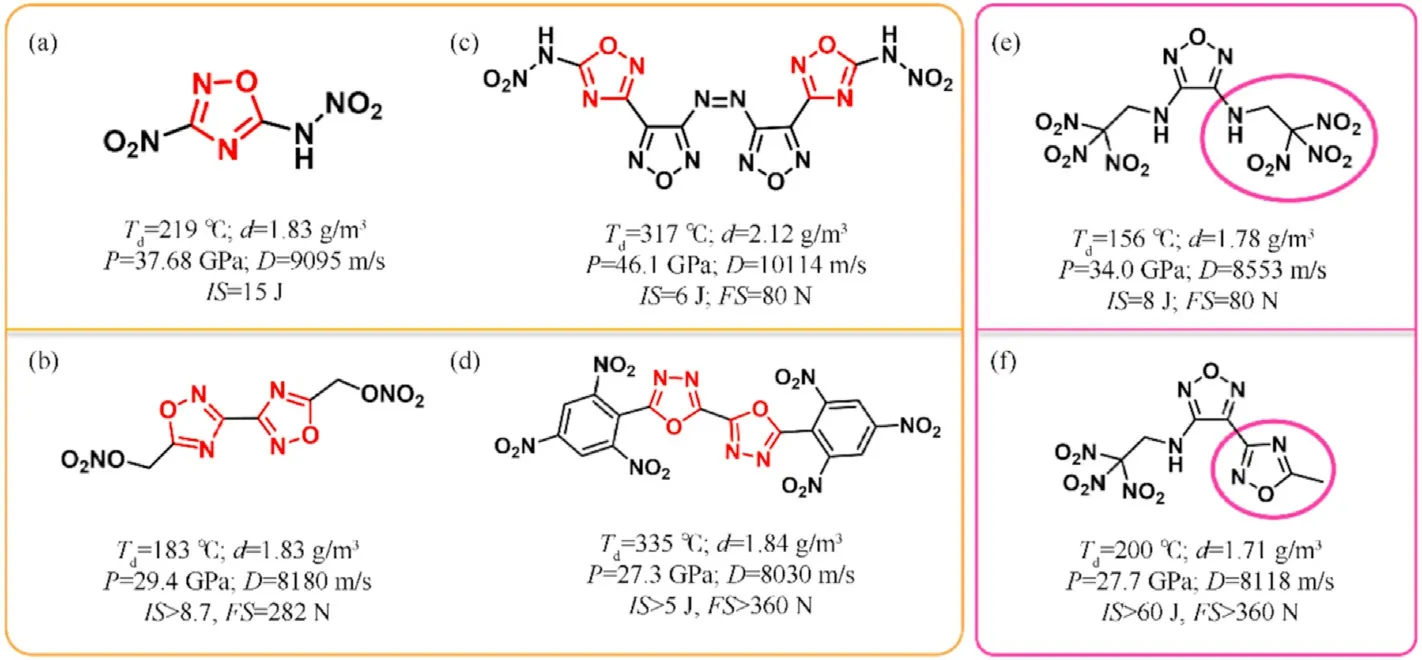
Fig.2.The properties of isofurazan-based energetic materials.
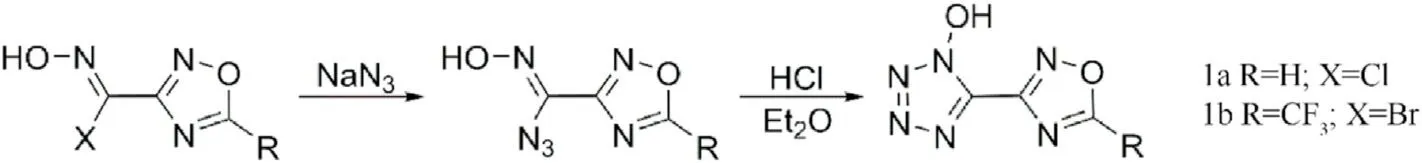
Fig.3.Scheme of synthesis of 1a and 1b.
2. 1,2,4-Oxadiazole-azoles-based energetic materials
2.1. 1,2,4-Oxadiazole-tetrazole-based compounds
1H-tetrazole is a unique energetic unit with high nitrogen content(80%)and high heat of formation(237.07 kJ/mol)[54].The combination of 1,2,4-oxadiazole and 1H-tetrazole encourages the formation of a single molecule with a high heat of formation.The introduction of the hydroxyl substituent results in a higher density,as well as improved oxygen balance and detonation parameters[55].Furthermore,deprotonation of 1H-tetrazole and 1-hydroxytetrazole yields tetrazolate and 1-hydroxytetrazolate,which can be used as the anions in 1,2,4-oxadiazole-tetrazolebased energetic salts.By combining 1,2,4-oxadiazole with tetrazoles,a variety of desired energetics can be obtained.
Up to now,few 1,2,4-oxadiazole-tetrazole-based energetic compounds have been reported.As early as 1989,Andrianov et al.[56]studied the isomerization accompanied by rearrangement of oxime group attacked by nucleophiles and found that isomerization of azidoxime was not accompanied by rearrangement when azidoxime was treated with hydrogen chloride in ethyl ether under the same conditions,but tetrazole obtained by cyclization.5-(1,2,4-oxadiazolyl-3)-l-hydroxytetrazole (1a) and l-hydroxy-5-(5-tri fluoromethyl-1,2,4-oxadiazolyl-3)tetrazole(1b)were synthesized and characterized by IR and NMR(Fig.3).
Six N-rich compounds bearing two nitro-tetrazole rings connected to 1,2,4-oxadiazole rings via C-C and C-N linkages were designed to examine the structure-property relationship between C-C and C-N linkages in the design of energetic materials(Fig.4)[57].With a theoretical approach,the HOFs and crystal densities of the six designed compounds are predicted.All the designed compounds possess solid state HOFs above 830 kJ/mol,densities greater than 1.73 g/cm,detonation velocities above 8300 m/s,and detonation pressures over 30 GPa.Combined with the above calculated results,these compounds are all potential high-energy materials,which can be further synthesized.Namely,the combination of 1,2,4-oxadiazole ring with tetrazole is a promising strategy in the design of HEDMs.
Until 2017,1,2,4-oxadiazole-tetrazole-based energetic compounds were fully examined experimentally.Diaminoglyoxime(DAG),a common starting material,by a one-pot procedure reacted with ethyl chlorooxoacetate in pyridine-chloroform followed by induced cyclization affording the intermediate 3,3-bi(1,2,4-oxadiazole).The target product 5,5'-((3,3-bi(1,2,4-oxadiazole))-5,5-diyl)bis(1H-tetrazol-1-ol)(2)was obtained by amidation,dehydration,diazotization and cyclization(Fig.5)[58].Similarly,ethyl cyanoformate was used as the starting material for the synthesis of 5,5'-(1,2,4-oxadiazole-3,5-diyl)bis(1H-tetrazol-1-ol)(3)(Fig.6)[58].The corresponding hydroxylammonium salt(2a)and ammonium salt(3a)were also prepared by the designated route.Multinuclear NMR,IR,mass spectroscopy,elemental analysis,differential scanning calorimetry(DSC),and sensitivity measurements were used to characterize all the synthesized compounds.X-ray single-crystal diffraction was used to con firm the crystal structure of salts 2a and 3a.The decomposition temperatures of these four compounds are higher than 220C,with 3a showing the highest decomposition temperature of 271C,which is higher than RDX(219C).Compounds 2,2a,and 3a are insensitive not only to electrostatic discharge but also to impact and friction.They could be considered as the candidates for insensitive heat-resistant explosives.2 was used for the preparation of N-rich energetic salts,which contain following counter cations:hydroxylammonium(2a),hydrazinium(2b),dimethylammonium(2c),5-amino-1H-tetrazol-4-ium(2d),and aminoguanidinium(2e)(Fig.5)[59]in order to explore the effect of the counter-ions on packing and crystal density.
Fig.4.Molecular structure of designed 1,2,4-oxadiazole-nitrotetrazole based energetic compounds.

Fig.5.Scheme of synthesis of 2 and 2a-2e.

Fig.6.Scheme of synthesis of 3 and 3a.
The crystal density of salts 2a-e falls into the range of 1.544-1.873 g/cm.The ranking order of crystal density of the 5 compounds are sequenced as 2c<2e<2b<2d<2a.For all five structures,the four rings show coplanar con figuration or only a slight deviation from co-planarity.Dimethylammonium salt(2c)has the lowest crystal density due to the poor steric match of cations and anions(sheet-like packing)and few hydrogen bonds.Ma et al.described four different packing types of energetic molecules in detail[60].Because of the less ef ficient mixing-type packing,aminoguanidinium salt(2e)has a slightly higher density than 2c,despite the remarkable hydrogen bonding.Among the five structures,hydroxylammonium salt(2a)is denser due to ef ficient wavelike packing.The ef ficient steric match of energetic ion and counter-ion will provide ef ficient crystal packing,which will affect the density and detonation performance of the energetic salts,along with signi ficant hydrogen-bonding abilities.However,no information on the detonation performance of these compounds was provided.
Yan et al.[61]developed and synthesized a variety of energetic compounds based on 1,2,4-oxadiazole that integrated the bene fits of a 1,2,4-oxadiazole motif with various energetic functions,following Pagoria's research ideas[58].The 1-hydroxytetrazole motif was added to 1,2,4-oxadiazole to produce 5-(1,2,4-oxadiazolyl-3-yl)-1-hydroxytetrazole(5)(Fig.7).Precursor 1,2,4-oxadiazole-3-chloroxime(4)was formed by four-step reactions including cyclization and chlorination reaction.In addition,by interacting directly with bases in methanol or ethanol,compound 5 was converted into three energetic nitrogen-rich salts(5a-c).
EA,IR,and multinuclear NMR were used to fully characterized the compounds 5 and 5a-c.The structural data for 5 and ammonium salt(5a)were obtained using X-ray single-crystal structural analysis.The physiochemical and energetic properties of these prepared compounds are listed in Table 2.5 melts at 133C and then decomposes at 143C at a heat rate of 5C/min.Energetic salts(5a-c)are decomposed directly with no prior melting,and their thermal decomposition temperatures lie in the range of 141(5c)to 174C(5a).Among these three metal-free salts,the density of hydroxylammonium salt(5c,1.79 g/cm)is the highest.Despite their low-density values,hydrazinium (5b) and hydroxylammonium(5c)salts are superior to commonly used RDX(D=8795 m/s,P=34.9 GPa)in detonation(D=8913-8929 m/s,P=30.4-32.7 GPa)attribute to their high positive heat of formation(372-478 kJ/mol).5b and 5c have a lower impact and friction sensitivity(IS=8 J,FS=96-108 N)than RDX.As a result,salts 5b and 5c could be investigated as suitable RDX substitutes.The impact sensitivity of 5b and 5c are lower than that of RDX(IS=7.4 J)and they also have low sensitivity to friction(FS=96-108 N).Consequently,salts 5b and 5c might be screened as potential alternatives to RDX.It's also worth noting that the neutral compound 5 has the lowest mechanical sensitivity(IS=10 J,FS=120 N),indicating that the synthesis of nitrogen-rich energetic salts could improve the mechanical sensitivity of energetic materials greatly.
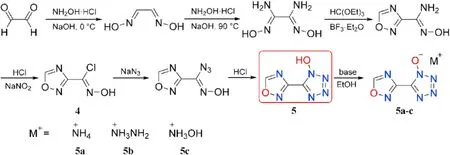
Fig.7.Scheme of synthesis of 5 and 5a-c.
2.2. 1,2,4-Oxadiazole-furazan/furoxan-based compounds
1,2,4-Oxadiazole-furazan/furoxan-based energetic materials have been well-studied in 1,2,4-oxadiazole-azoles-based energetic materials.The introduction of various isofurazan rings into energetic molecules is an excellent method for lowering sensitivity while maintaining a high level of energy[50].As a result,incorporating 1,2,4-oxadiazole as a desensitizing motif into other energetic skeletons is one of the most promising ways to maximize the potential of 1,2,4-oxadiazole-based energetic molecules.By inserting a furazan ring into energetic molecules,the density,heat of formation,and detonation velocity can be increased by approximately 0.06-0.08 g/cm,200 kJ/mol,and 300 m/s,respectively[62].In the design of next-generation insensitive HEDMs,a combination of the 1,2,4-oxadiazole motif and furazan rings was proven to be a promising approach.
Barbieux-Flammang et al.[63]treated cyanoacetic acid with a mixture of nitric and sulfuric acids to produce 3,4-dicyanofuroxan,which was then fragmented by short contact time flash vacuum thermolysis(FVT)to provide metastable cyanogen N-oxide([N≡C-C≡N→O]). And then 4-cyano-3-(3-cyano-1,2,4-oxadiazolyl-5-yl)-furazan-2-oxide(6)and its isomer 3-cyano-4-(3-cyano-1,2,4-oxadiazolyl-5-yl)-furazan-2-oxide (7)were prepared by trimerization of cyanogen N-oxide.4-Cyano-3-(3-cyano-1,2,4-oxadiazol-5-yl)-furazan(8)was obtained by reducing the two isomers 6 and 7 with triethyl phosphite(Fig.8).X-ray crystal structure analysis andC NMR spectroscopy were used for structure elucidation.
Through nitrosation,addition,and thermal dehydration,a valuable precursor 4-amino-3-furazan-carboxamidoxime(AOCA)was synthesized from commercially available malononitrile.AOCA is considered a signi ficant synthetic platform in the design of a series of high-energy-density materials(Fig.9)[58,64,65].
Condensation of AOCA with diethyl oxalate yielded 4,4'-((5,5-bi(1,2,4-oxadiazole))-3,3-diyl)bis(furazan-3-amine) (9). Subsequently, 3,3-bis-(4-nitrofurazan-3-yl)-5,5-bi(1,2,4-oxadiazole)(10)was produced through oxidation of amino groups.In a reaction with AOCA,cyanogen bromide(BrCN)can be used as the electrophilic agent,followed by oxidation to produce 3-(4-nitro-furazan-3-yl)-1,2,4-oxadiazol-5-amine(11).Under different conditions,4,4'-(1,2,4-oxadiazole-3,5-diyl)bis-(furazan-3-amine) (12) was synthesized from ACOA or 4-aminofurazan-3-carbonitrile(ACOD)and used as a building block in the synthesis of energetic neutral compounds 4-(3-(4-nitro-furazan-3-yl)-1,2,4-oxadiazol-5-yl)-furazan-3-amine (13), 4,4'-(1,2,4-oxadiazole-3,5-diyl)bis(3-nitrofurazan)(14),and N,N'-((1,2,4-oxadiazole-3,5-diyl)bis(furazan-4,3-diyl))dinitramide(15).
Compound 10 is a thermally stable(T=295C)and dense(1.94 g/cm)energetic materials with a high positive enthalpy of formation(891 kJ/mol)and low sensitivity(IS=15 J,FS=250 N).Similarly,11 and 13 are insensitive energetic compound(IS=36 J,FS=360 N).Compound 13 has a low melting point of 92-93C,making it suitable for use as a substitute for TNT or other explosives in melt-castable systems,as well as a component for insensitive enhanced blast explosives or insensitive propellants.In addition,14 as a zero-hydrogen explosive,exhibits lower melt point(60-62C)than TNT(80C),higher density(1.91 g/cm)than RDX(1.82 g/cm),and less insensitive to impact(IS=10 J)than HMX(IS=148 N).Thus,14 can be considered as a substitute for TNT,the addition of some zero-valence metal in it produces thermal-baric explosive(TBX)and by mixing with other explosives leads to the synthesis of high-energy plasticizer.The critical physicochemical and energetic properties for 10,11,and 14 are shown in Table 3.
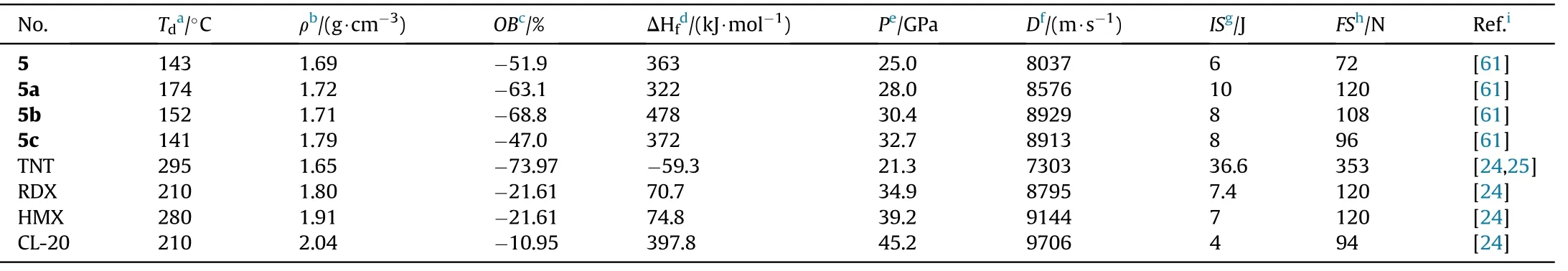
Table 2 Energetic and physicochemical properties of 5 and its salts 5a-c.
Tsyshevsky et al.[66-68]proposed a completely new and effective strategy named“Comprehensive End-to-End Design”as new guiding principles to design novel high-performance energetic materials based on a comprehensive analysis of the structureproperty-function correlations of existing oxadiazole-based energetic materials, combined with multiscale computational modeling.Their group also came up with several helpful and practical empirical equations that could be used to calculate and predict explosive properties based on the chemical composition and structure of the materials under investigation.
Compound 15 was converted into the corresponding ammonium and hydrazinium salts(15a-b).The precursor 3-(4-aminofurazan-3-yl)-1,2,4-oxadiazol-5-amine (AFOA) was prepared by cyclization of AOCA with BrCN.Wei et al.[65]synthesized and employed dinitramino compound as a building block in the preparation of several energetic salts(16a-g)either by direct acidbase neutralization or metathesis procedure(Figs.9 and 10).
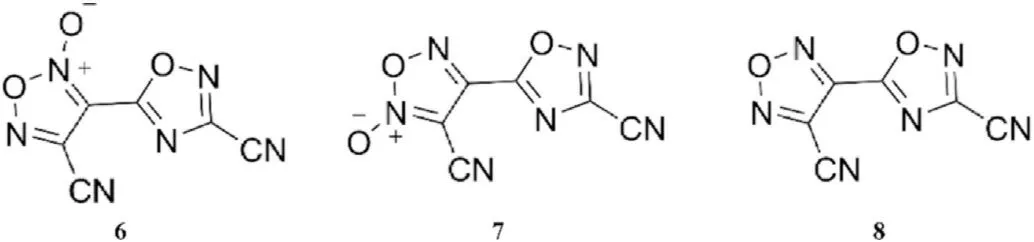
Fig.8.Scheme of molecular structure of trimers 6 and 7 of cyanogen N-oxide and their reduced product 8.
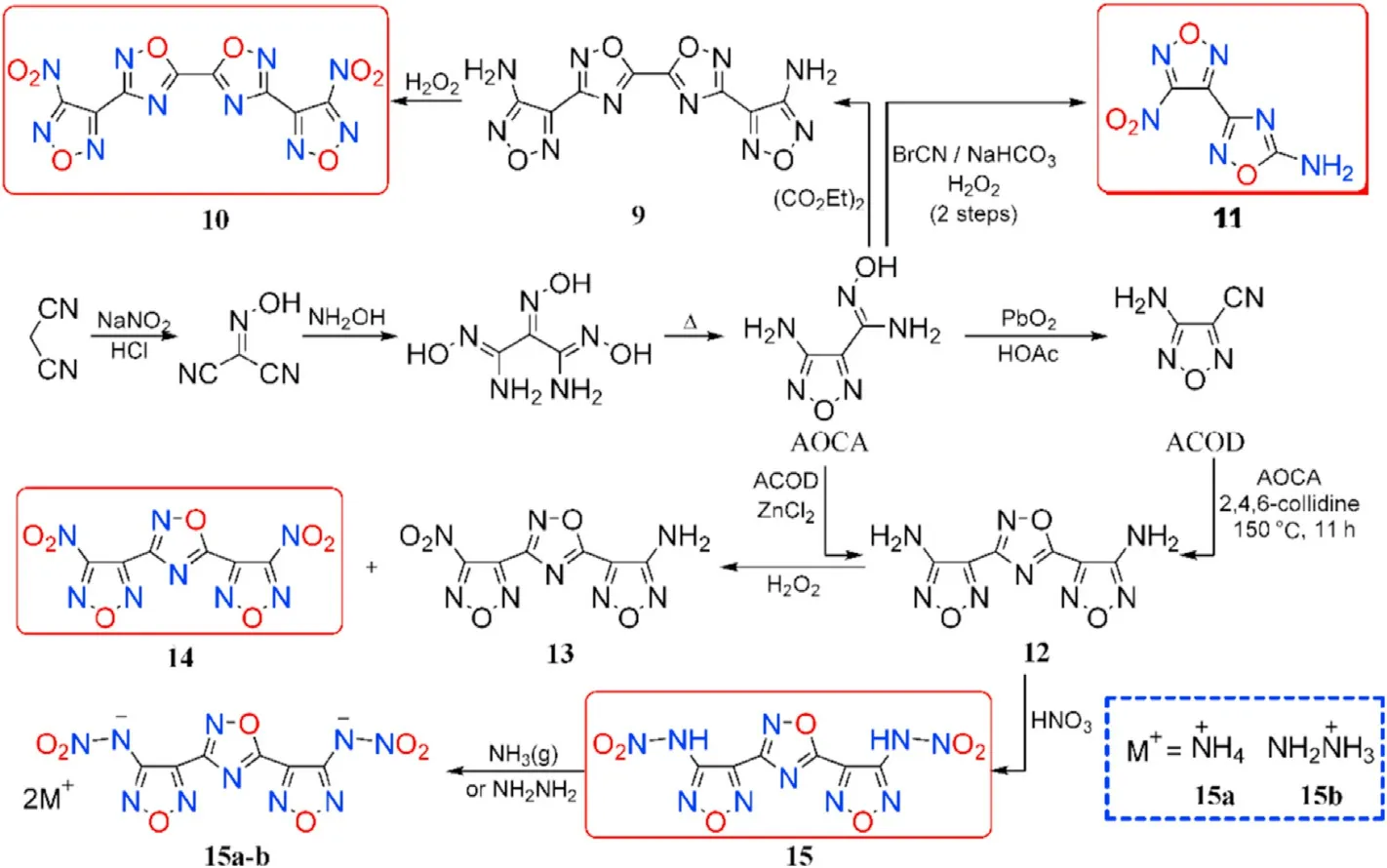
Fig.9.Scheme of synthetic route of neutral compounds 9-15 and salts 15a-b.
As shown in Table 3,all nitrogen-rich energetic salts 15a-b and 16a-g have good thermal stabilities(172-269C),acceptable densities (1.67-1.85 g/cm),and high heats of formation(154.9-894.3 kJ/mol).The calculated detonation velocities range from 8102 m/s(16a)to 9046 m/s(16c),while the detonation pressures range from 25.0(16d)to 37.4 GPa(16c).The measured sensitivities of each of the salts towards impact(IS=14-26 J)and friction(FS=120-240 N)demonstrated that all compounds presented less sensitivity than RDX(IS=7.4 J,FS=120 N).Among them,the hydroxylammonium salt(16c)exhibits outstanding performance,with a high density(1.85 g/cm),acceptable HOF(250.8 kJ/mol),perfect oxygen balance(0),excellent detonation parameters(37.4 GPa,9046 m/s),and low sensitivities(IS=16 J,FS=240 N)superior to RDX.It could be used as a new green ingredient in secondary explosives or propellants.Among the following salts,triaminoguanidinium salt(16g)has the best detonation performance(D=8653 m/s,P=28.0 GPa)and the highest positive heat of formation(894.3 kJ/mol).
Amidoxime AOCA was also served as a starting material for the four bi-heterocyclic skeletons made by combining 1,2,4-oxadiazole and aminofurazan.The azo derivatives 17,18,20,and 21 were obtained by oxidizing the amino group in the furazan ring with KMnOin HCl at different concentrations.Compound 18 was directly nitrated with AcO/HNOin acetic acid affording nitramine-substituted product 19(Fig.11)[45].The energetic characteristics were discussed and shown in Table 4.The five neutral compounds(17-21)have excellent thermal stability(>240C),making them excellent heat-resistant explosives.The highest decomposition temperature was observed for 19(317C).It's worth noting that 19 has a remarkable density(2.12 g/cm)and a high heat of formation(1038.0 kJ/mol),which could be attributed to the presence of the nitramino groups.In consequence,19 is the most promising HEDMs with extraordinary detonation pressure(46.1 GPa)and velocity(10114 m/s)exceeded the value of HMX(D=9144 m/s,P=39.2 GPa)and CL-20(D=9706 m/s,P=45.2 GPa).The con flict between high energetic performance and safety exists in compounds 19(IS=6 J,FS=80 N),which have slightly lower mechanical sensitivity than CL-20 (IS= 4 J,FS=48 N).
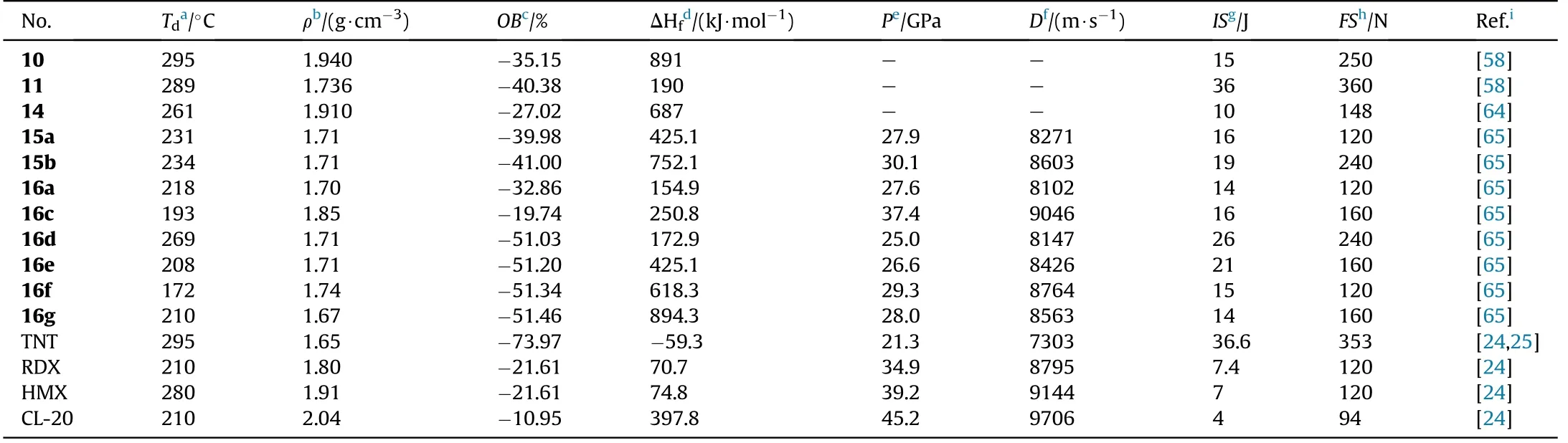
Table 3 Energetic and physicochemical properties of 10-16,15a-b,and 16a-g.
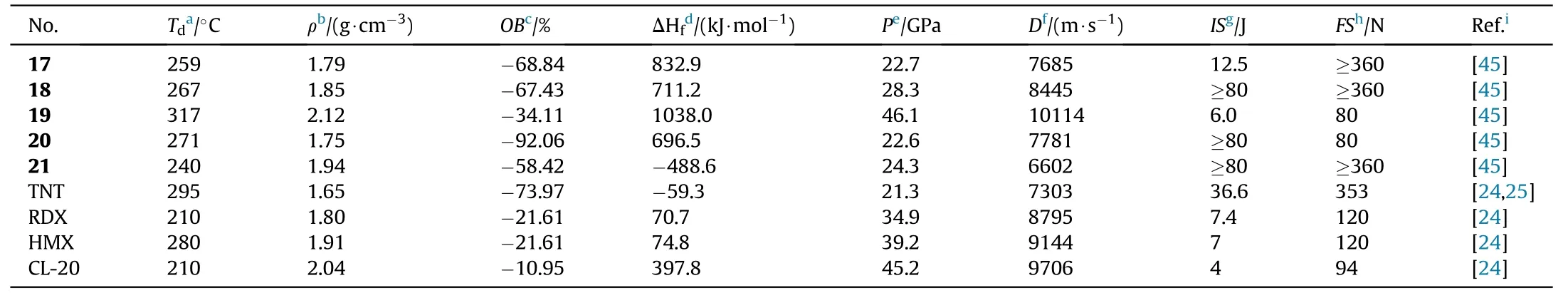
Table 4 Energetic and physicochemical properties of 17-21.
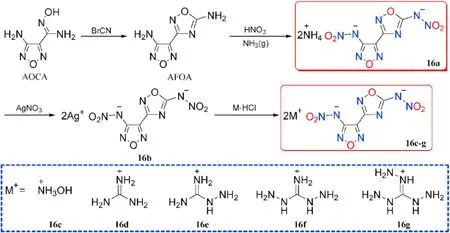
Fig.10.Scheme of synthetic route of energetic salts 16a-g.
Azo group[-N=N-]can be reduced to[-NH-NH-]and oxidized to azoxy group[-N=N(O)-],resulting in a wide range of energetic molecules.Sun et al.[69]synthesized 1,2,4-oxadiazolefurazan-based compounds 22-26 connected by three different linkages based on 3-(4-aminofurazan-3-yl)-1,2,4-oxadiazol-5-amine(AFOA,Fig.12)to explore the effect of the azo group(-N=N-)in energetic materials.The nitrogen-rich salts 23a-c,24a,and 26a-d formed from the neutralization of 23,24,and 26,respectively.All of the compounds were thoroughly characterized,and the energetic performance particularly thermal characteristics,was scienti fically analyzed.
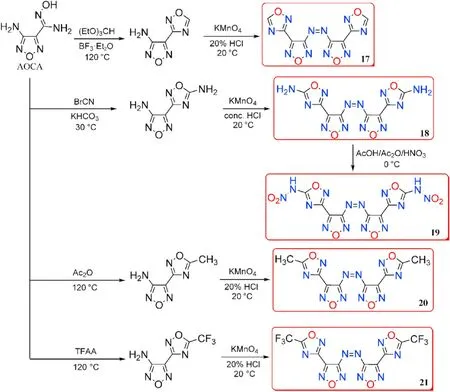
Fig.11.Scheme of synthesis of energetic compounds 17-21.
As shown in Table 5,all compounds exhibit high densities(1.72-1.95 g/cm), high positive heat of formation(289.3-1378.9 kJ/mol),and excellent detonation performance(D=8013-9042 m/s,P=24.5-35.0 GPa).They are also thermally stable(T=204-322C),and totally insensitive(IS>40 J,FS>360 N).Compound 23 decomposes at 322C,which is similar to the heat-resistant explosive TATB (330C)[70],and is signi ficantly higher than the reduction product 24(271C)and oxidation product 26(307C).Salts 23a-c(T=239-318C)with a-N=N-group exhibit greater thermal stability than azoxy[-N=N(O)-]salts 26a-d(204-227C)and-NH-NH-connected salt 24a(219C),which is consistent with Qu et al.[45]As a result,an azo-bridge can markedly improve thermal stability.It was also shown that adding coordinated oxygen[-N=N(O)-]to azo-based EMs could reduce their thermal stability.Meanwhile,the heat of formation of 23(529.7 kJ/mol)is greater than that of 24(289.3 kJ/mol)and 26(493.4 kJ/mol),indicating that the azo group can boost the heat of formation of energetic materials signi ficantly.Lin et al.[71]developed a series of energetically insensitive nitrogen-rich organic salts(23d-k)based on the thermal stability and high positive HOF of 23.Signi ficantly,azoxy-linked 26 has better detonation properties(8844 m/s,34.4 GPa)than 23(8507 m/s,30.7 GPa)and 24(8209 m/s,27.9 GPa)due to its higher oxygen balance and density.When the same cations are involved,their corresponding salts comply with the same tendency(26a>23a,26d>24a).
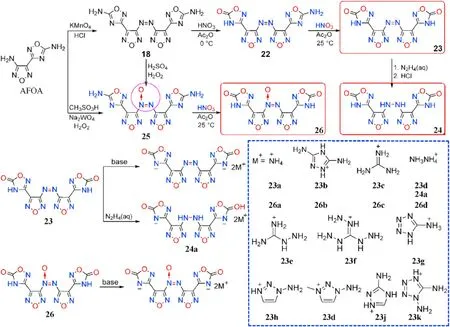
Fig.12.Scheme of synthesis of energetic compounds 22-26 and nitrogen-rich salts.
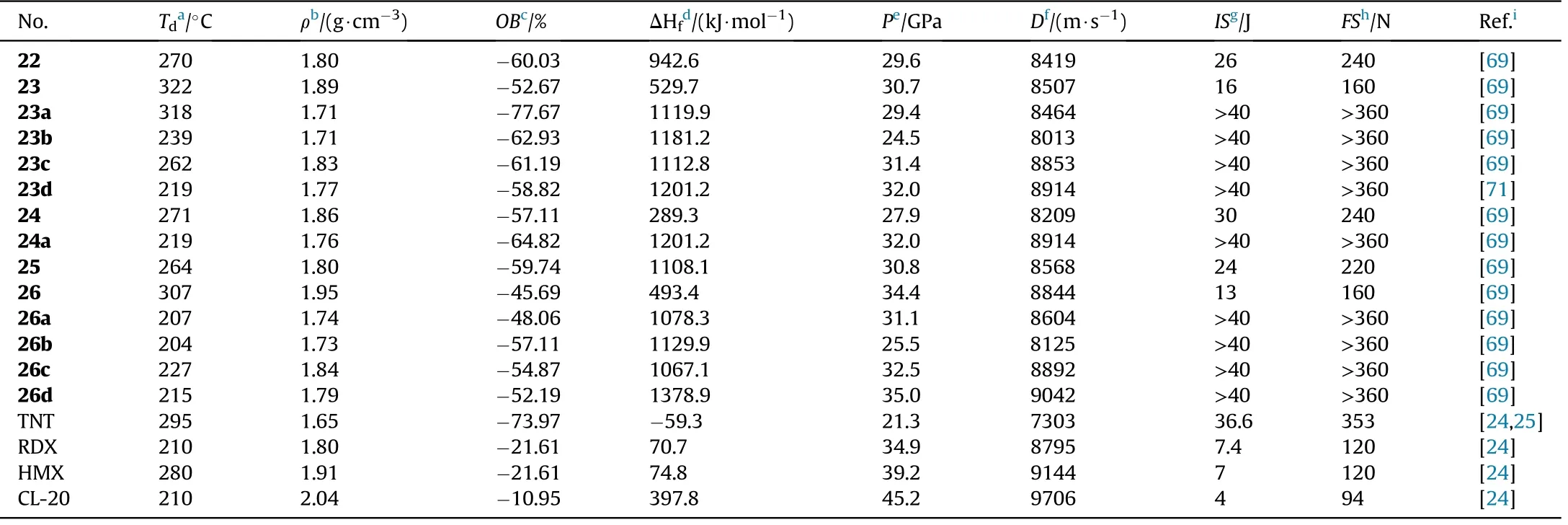
Table 5 Energetic and physicochemical properties of 22-26 and their selected corresponding salts.
In addition to the three linked moieties indicated above,different N-alkyl linkages can be used as fine connected subunits in the synthesis of 1,2,4-oxadiazole-furazan-based energetic compounds.To introduce the methylene linkage,the amino group in 4-(1,2,4-oxadiazol-3-yl)furazan-3-amine was treated with aqueous formaldehyde solution,yielding the precursor of 27.The nitro group in 3-nitro-4-(1,2,4-oxadiazol-3-yl)furazan can be nucleophilically substituted with ethylene diamine,resulting in the precursor of 29.27 and 29 with-N(NO)-(CH)-N(NO)-(n=1or 2)bridges were prepared by nitration of the NH-fragments at the two precursors in a mixture of acetic anhydride and 100%HNO,which perfectly solved the high acidity of the NH-group.It should be noted that when the 1,2,4-oxadiazole ring is treated with hydrazine hydrate,product 28 and 30 are formed(Fig.13)[72].
The four neutral derivatives synthesized 27-30 have moderate thermal stabilities(138-175C),acceptable densities(1.60-1.77 g/cm),high enthalpies of formation(586.2-810.7 kJ/mol),high detonation velocities(7914-8185 m/s),and low sensitivities to mechanical stimuli(IS>27 J,FS>240 N,Table 6).The combination of these performance data suggests that these compounds could be employed as replacements for TNT as new insensitive energetic explosives.The performance of compound 28 and 30 after the destruction of 1,2,4-oxadiazole ring was much lower than that of 27 and 29.For instance,the density,decomposition temperature,and detonation properties of 29(d=1.75 g/cm,T=175C,D=8185 m/s,P=27.9 GPa)are all signi ficantly higher than those of 30(d=1.60 g/cm,T=145C,D=7914 m/s,P=23.3 GPa).These findings indicate that the 1,2,4-oxadiazole ring is an important motif in the development of high-performance energetic materials.
At a high temperature(180C),AOCA reacts with acetamide to produce the key intermediate 3-amino-4-(5-methyl-1,2,4-oxadiazol-3-yl)furazan(AMOF).The mannich reaction of AMOF with electron de ficient trinitroethylethanol yields trinitroethylamino derivatives 31,which are then nitrated with a mixture of 100%HNOand acetic anhydride to yield N-nitrated 32.AMOF was also used as a starting reactant in the direct nitration of 33 using 100%nitric acid.After that,acid-base neutralization of 33 produced a library of salts 33a-i(Fig.14)[50].
Thermal stabilities(159-246C)and moderately high densities(1.63-1.78 g/cm)were observed in all synthesized compounds(Table 7).Except for compound 32,they are all insensitive to impact(IS>37.8 J)and friction(FS>360 N)due to the bicyclic structure's compact packing of planar geometric con figurations.The hydroxylammonium salt(33a)seemed to have the best detonation performance(D=8545 m/s,P=30.2 GPa),which was better than the analogous neutral molecule 33(7810 m/s,24.1 GPa)and comparable to RDX.
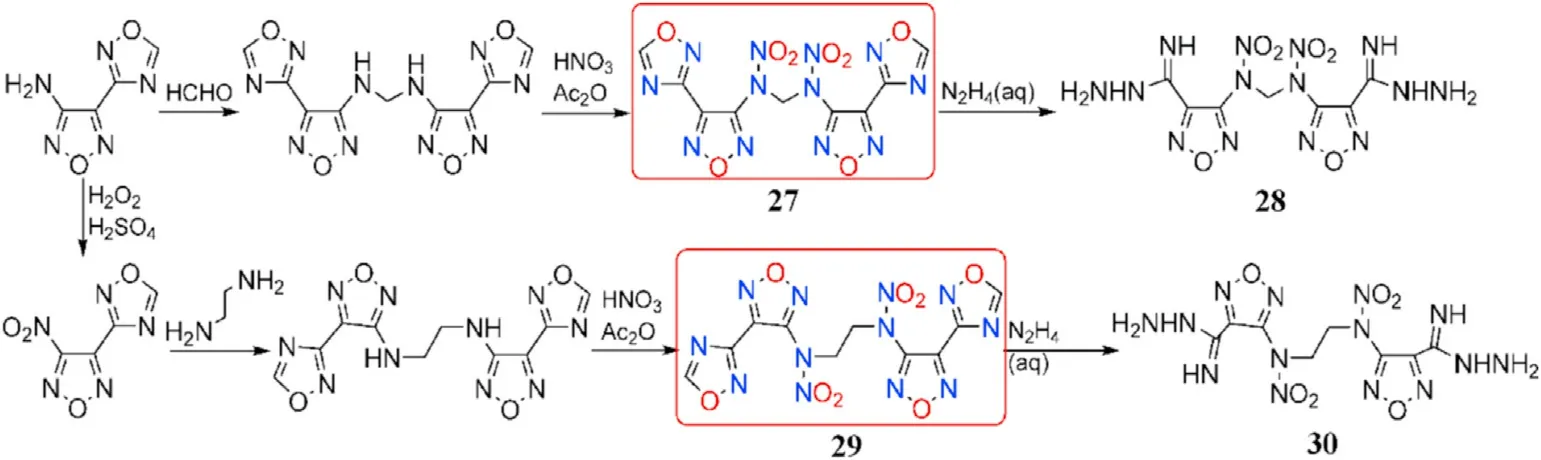
Fig.13.Scheme of synthesis of energetic compounds 27-30.
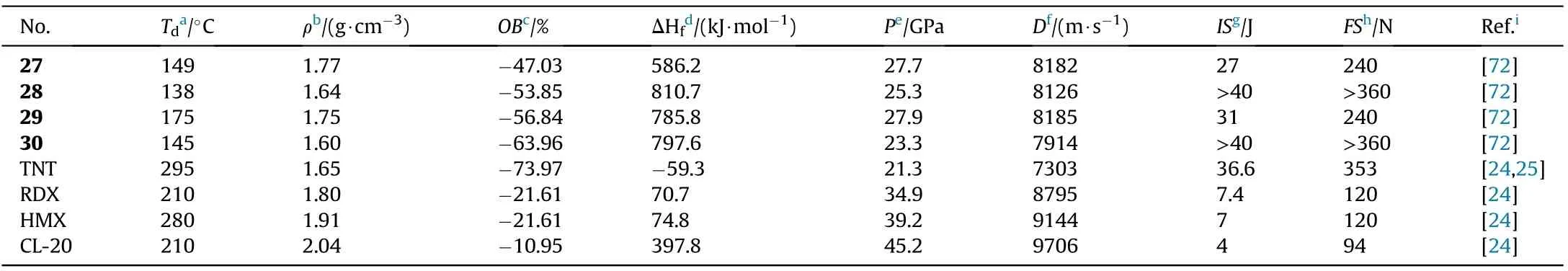
Table 6 Energetic and physicochemical properties of 27-30.
Based on the obtained compound 9,it was nitrated with fuming HNOto produce N,N-([5,5-bi(1,2,4-oxadiazole)]-3,3-diylbis(furazan-3,4-diyl))dinitramide(34),which was metathesized with various bases to yield a variety of energetic salts(34a-l)[73].Wang et al.[74]provided the five salts 34m-q.Compound 9 can be used in the mannich reaction to produce 35 when combined with trinitroethanol.Both NH groups in 35 were ef ficiently nitrated to form 36 under the same conditions as Zhang et al.[72](Fig.15).Salts 34a-q are thermally stable(187-303C),whereas 34 has the lowest decomposition temperature of 84C(Table 8).The two metal salts have greater thermal stabilities(274C and 303C)than organic salts(187-263C).The twenty compounds have densities ranging from 1.668 g/cmto 1.973 g/cm,although only five organic compounds(34,34b,34c,35,and 36)have the density near to RDX(1.82 g/cm).The highest heat of formation was calculated for the di(4-amino-1,2,4-triazolium)salt(34g,1345.2 kJ/mol),followed by the di(5-amino-1,2,4-triazolium)salt(34h,1087.9 kJ/mol)and di(3,5-diamino-1,2,4-triazolium)salt(34d,1014.6 kJ/mol).As for the explosive performance of energetic molecules 34 and 34a-q,with an estimated detonation velocity of 9030 m/s and a detonation pressure of 36.0 GPa,hydroxylammonium salt(34c)is the most potential alternative for RDX.The subsequent explosive experiment also con firmed this fact that 34c is indeed more powerful than RDX.Furthermore,the neutral compound 34 and 35 as well as the dihydrazinium salt(34b),had comparable detonation characteristics(D:8793,9062,and 8836 m/s,P:33.3,37.2,and 33.0 GPa,respectively)to RDX.More notably,36 signi fies thermally stable(T=151.8C),dense energetic materials(1.93 g/cm)with greater detonation capability(9550 m/s,41.9 GPa)than RDX and HMX.It is,however,highly sensitive to impact(IS=4.5 J),similar to CL-20(IS=4 J),yet insensitive to friction(FS=120 N),similar to RDX(FS=120 N).As a result,these compounds 34,34b-c,and 36 are attractive as eco-friendly and useful energetic compositions.
The optimal reaction conditions for the cyclocondensation of amidoxime group to the 1,2,4-oxadiazole ring were explored by He et al.[75].The expensive methyl malonyl chloride was replaced by dimethyl malonate and potassium carbonate was removed.The unique inexpensive AOCA cyclocondensation process yielded 4-amino-3-(5-acetic acid methyl ester-1,2,4-oxadiazolyl)-furazan(37),which after nitration with mixed acids and base treatment yielded energetic organic salts 38a-c(Fig.16)[75].Thus,in a 1,2,4-oxadiazole-furazan-based compound,the energetic functions nitramine and dinitromethyl were successfully introduced.The densities of salts 38a-c(1.804-1.862 g/cm,Table 8)are higher than RDX(1.82 g/cm).Especially,detonation properties of salts,namely dihydrazinium 38b(D=9028 m/s,P=38.0 GPa)and hydroxylammonium salt 38c(D=9064 m/s,P=36.0 GPa),similar to HMX.The prepared salts(38b-c)show relatively low sensitivities towards mechanical stimuli(IS=19-22 J,FS>360 N).These results indicate a good balance of excellent detonation characteristics and adequate sensitivities,making them ideal secondary explosive choices.
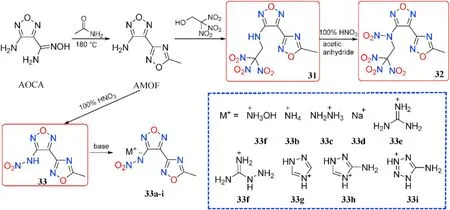
Fig.14.Scheme of synthesis of energetic compounds 31-33 and salts 33a-i.
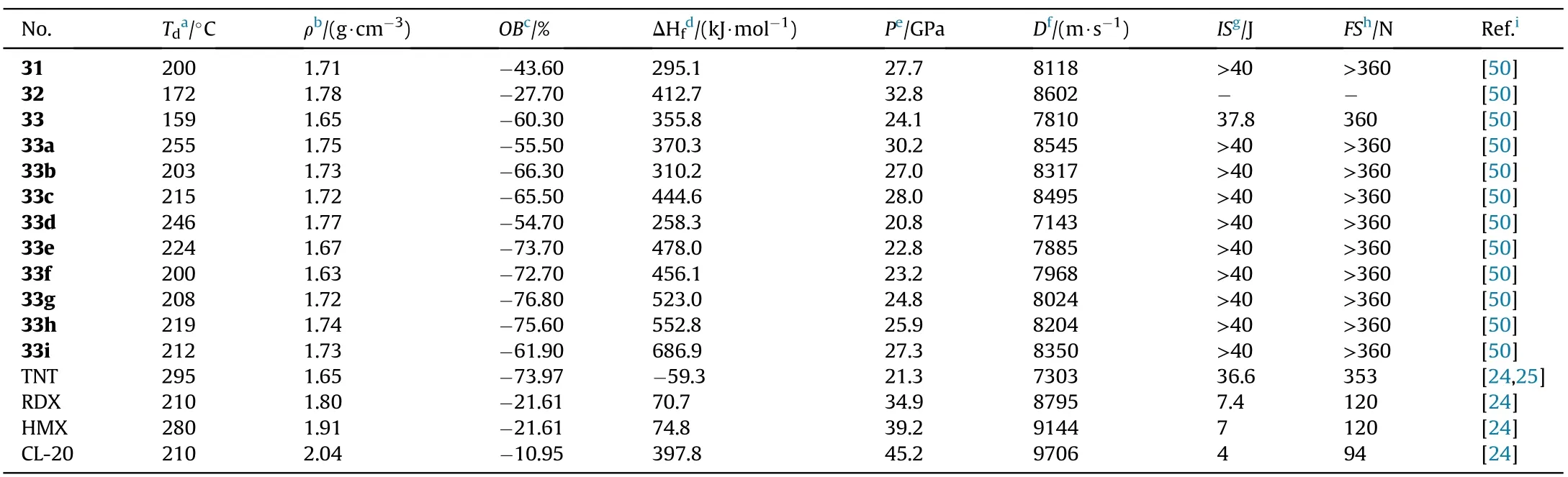
Table 7 Energetic and physicochemical properties of 31-33 and salts 33a-i.
In the synthesis of 1,2,4-oxadiazole-based insensitive energetic materials,Wang et al.developed a new easy and ef ficient approach for the construction of 1,2,4-oxadiazole ring.The original toxic and hazardous reagents were replaced with nontoxic acetamide,KMnOand KCOin the preparation of 23 and 39.The steps were shortened,the procedures were simpli fied,and the yields were increased.The 39 is chosen as the starting materials for a serious of derivatives 40-42.The nitrogen-rich salts 40a-c and 42a-c formed from the neutralizing of 40 and 42,respectively(Fig.17)[76].Except for 40(127.25C),all of the synthesized compounds are thermally stable(161.83-315.75C,Table 9).The densities of the four neutral compounds(1.80-1.89 g/cm)are higher than the six salts(1.61-1.71 g/cm).The most promising compound for practical use to replace RDX is 41,which has a high density(1.89 g/cm),acceptable sensitivity(8 J,240 N),and good detonation pressure(35.81 GPa)and velocity(8892 m/s).
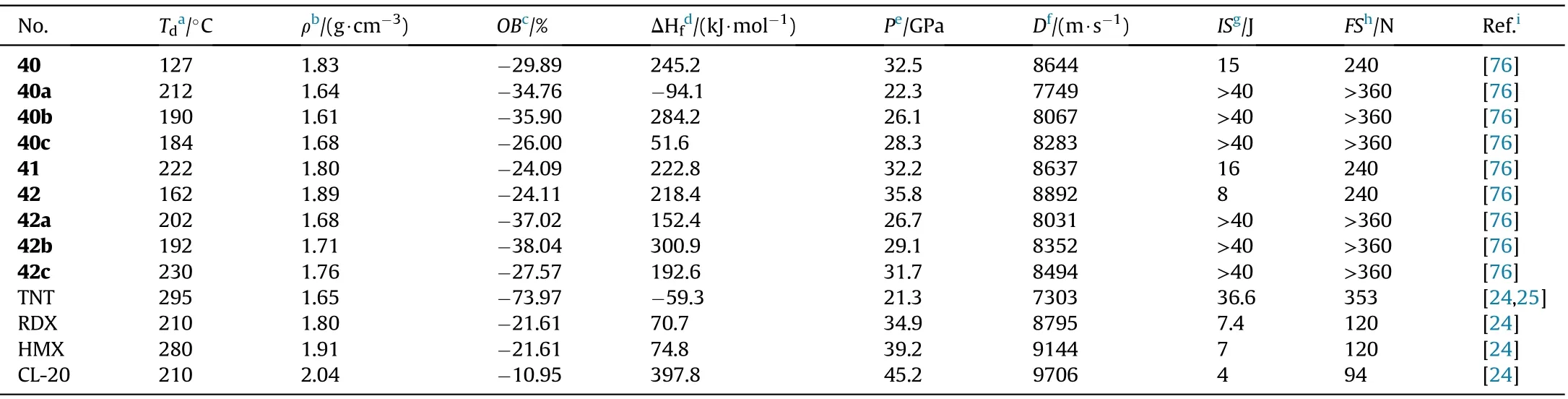
Table 9 Energetic and physicochemical properties of 40-42 and salts 40a-c and 42a-c.
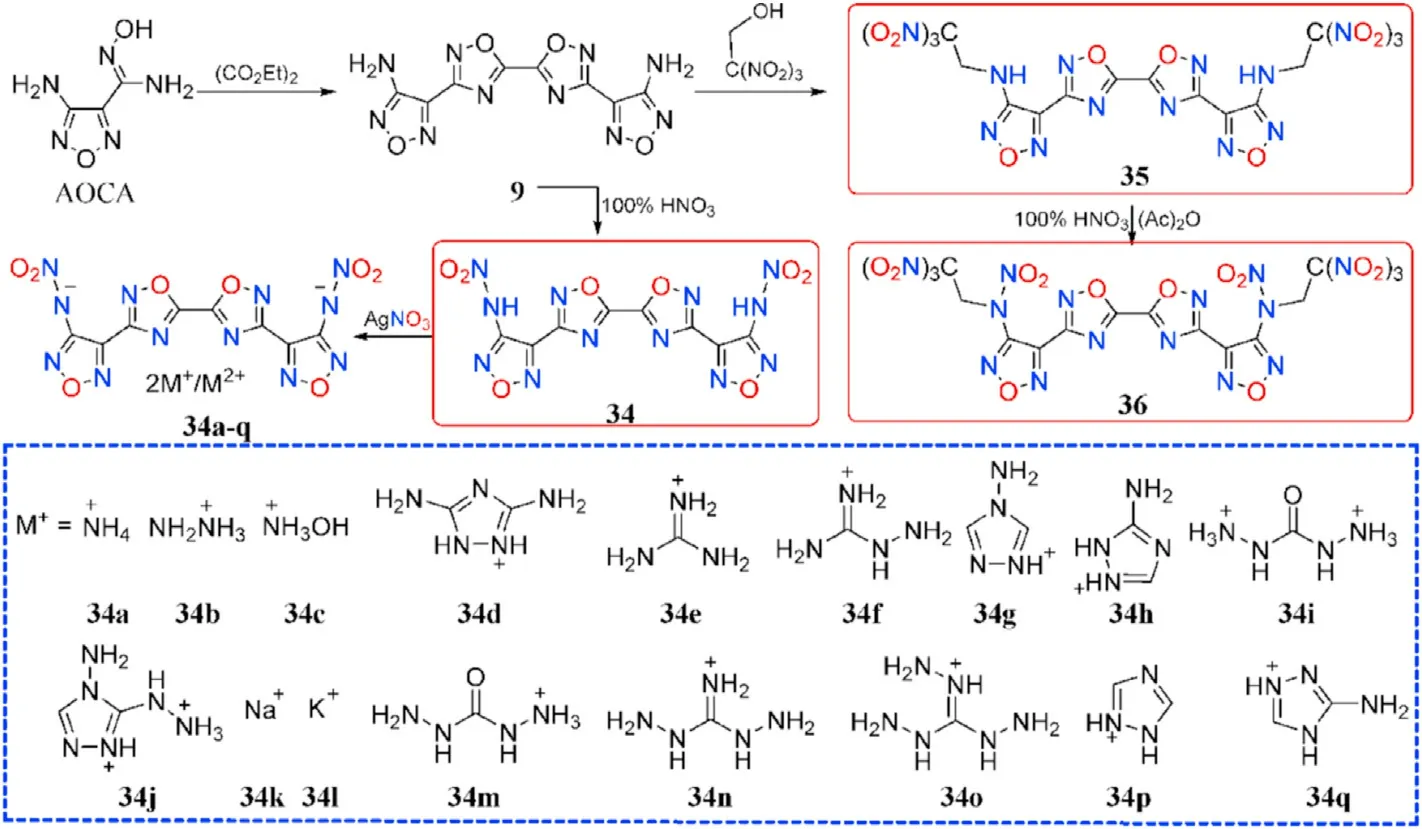
Fig.15.Scheme of synthesis of energetic compounds 34-36 and 34a-q.
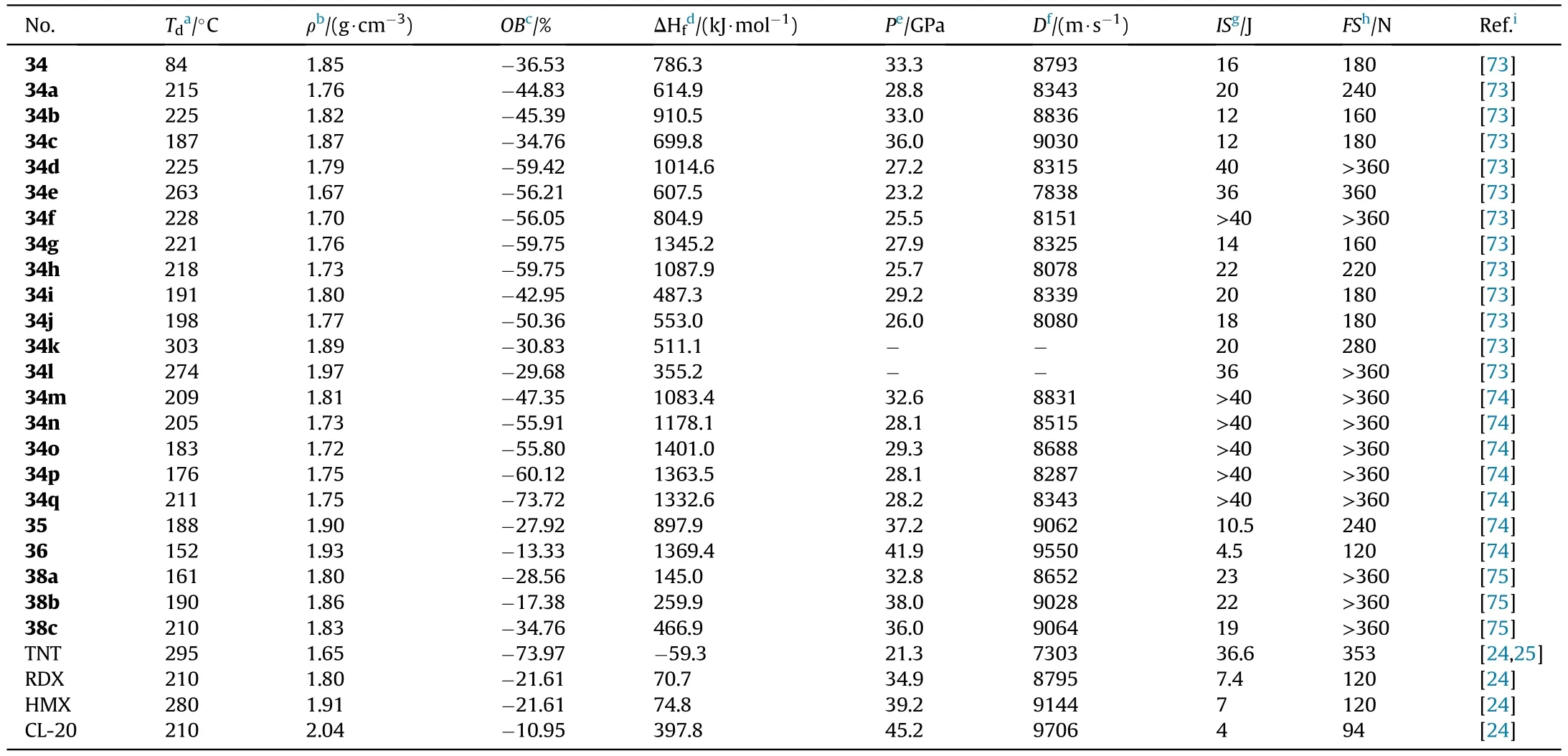
Table 8 Energetic and physicochemical properties of 34-36 and salts 34a-q and 38a-c.
According to studies,nitrogen-rich energetic salts will have greater thermal stability and detonation performance than the precursors before salt production,implying that salt formation is a promising method for obtaining next-generation energetic materials[73,77-79].Given this,Wang et al.[80]treated 3-(4-aminofurazan-3-yl)-1,2,4-oxadiazol-5-amine(AFOA)with fuming HNOto produce N-(4-(5-amino-1,2,4-oxadiazol-3-yl)-furazan-3-yl)nitramide(43),a simple metathesis of which was used to produce series of energetic salts(43a-f)using nitrogen-rich cations carefully selected(Fig.18).Single-crystal X-ray diffraction was used to con firm the identities of all seven compounds.The intrinsic structure-property relationships were methodically and completely investigated using crystal data and theoretical calculations.
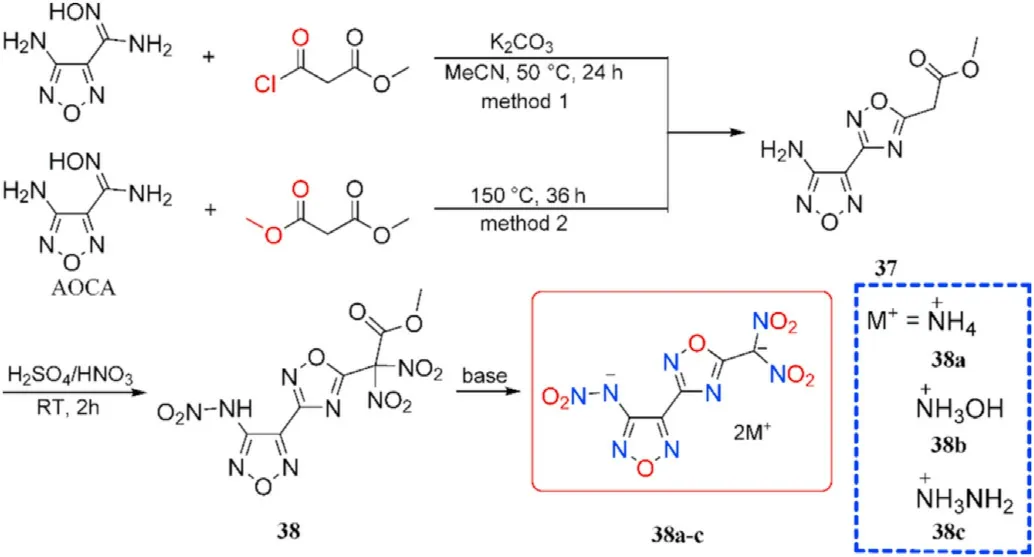
Fig.16.Scheme of synthesis of energetic compounds 37-38 and 38a-c.
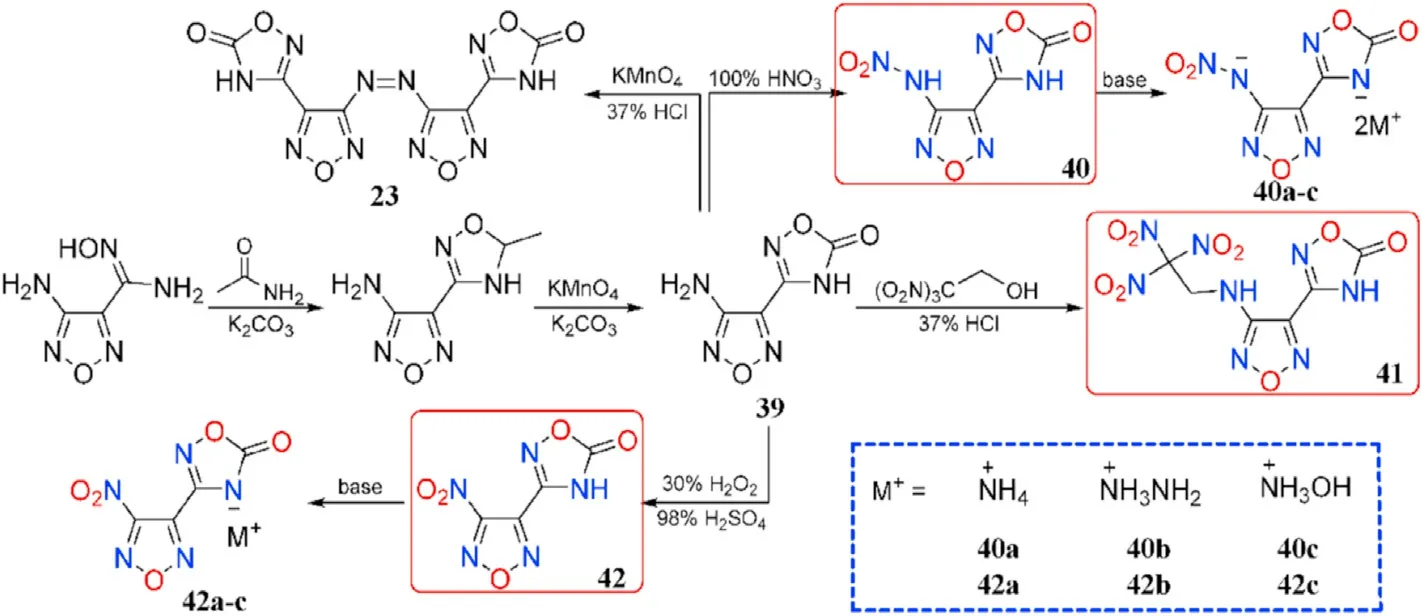
Fig.17.Scheme of synthesis of energetic compounds 39-42,40a-c,and 42a-c.
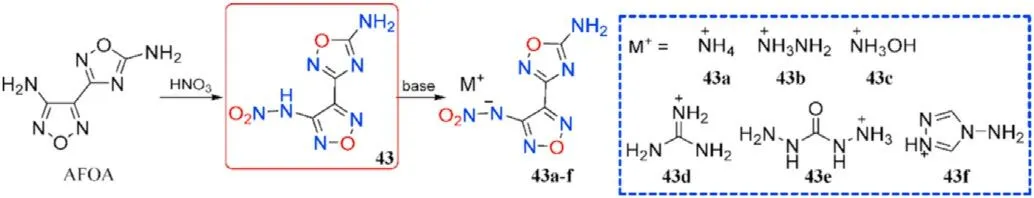
Fig.18.Scheme of synthesis of energetic compounds 43 and 43a-f.
As shown in Table 10,all the salts 43a-f(175-235C)have higher thermal stabilities than the neutral precursor 43(111C).In comparison to 43(D=8398 m/s,P=29.7 GPa,IS=25 J,FS=240 N),salts 43a-c and 43e present excellent energetic performance(D=8602-8838 m/s,P=30.0-33.3 GPa)and pleasant mechanical sensitivities(IS>40 J,FS>360 N),which allow them to be used as an environmentally friendly and worthy energetic constituent.As a result,salt formation is a cost-effective and practical method for designing and synthesizing novel energetic materials with desired qualities such as high positive heat of formation,brilliant detonation properties,and acceptable safety.
The nitramine motif was added to N-rich heterocycle scaffolds,which resulted in an increase in density and oxygen balance in the target energetic materials.Amidoxime AOCA undergoes cyclization to the precursor [3-(4-Amino-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazol-5-yl]-methylene acetate(44)when it is treated with 2-chloro-2-oxoethyl acetate.The acetate group of 44 cannot be converted directly to the nitrate ester subunit,but it can be nitrated to 45 and then treated with aqueous ammonia to generate ammonium salt(46).Oxidation,hydrolysis,and nitration techniques were used to obtain structures 47-50.Compound 50 was quickly converted to silver salt(50a)by treatment with suf f icient silver nitrate,and metathesis of silver salt(50a)with ammonium chloride,hydroxylamine hydrochloride,and biguanide hydrochloride afforded three energetic nitrogen-rich organic salts(50b-d,Fig.19)[81].The characteristics of the desired compounds 48,50,and 50b-d were thoroughly examined(Table 11).Apart from the free acid 50(1.83 g/cm),the hydroxylammonium salt(50c)had the highest density(1.82 g/cm)among the three energetic organic salts,whereas the biguanide salt(50d)had the lowest(1.75 g/cm).Except for 50(T=60C,IS=10 J),all compounds are thermally stable(>135C)and insensitive to impact(IS>35 J)and friction(FS>360 N),with the azo-based derivative 48 having the highest decomposition temperature of 200C.The f ive compounds exhibit detonation velocities ranging from 7809 to 8822 m/s and detonation pressures ranging from 27.2 to 35.2 GPa.Among them,hydroxylammonium salt(50c,D=8822 m/s,P=35.2 GPa)has slightly better energetic performance than RDX,making it a benef icial and green high-performance energetic molecule.
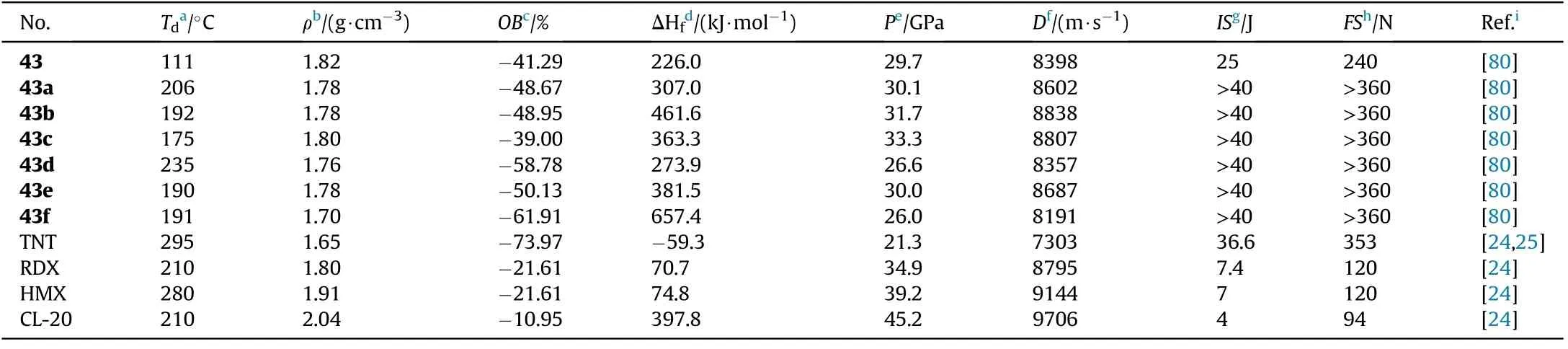
Table 10 Energetic and physicochemical properties of 43 and salts 43a-f.
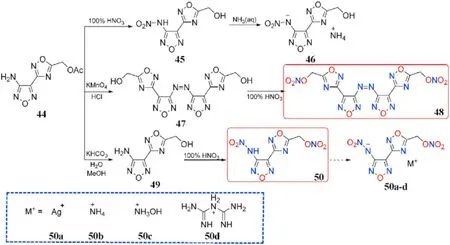
Fig.19.Scheme of synthesis of energetic compounds 44-50 and salts 50a-d.
With the exception of hydroxyl and nitrate ester group,another energetic azide group was also introduced to the AOCA to synthesize compound 3-Amino-4-(5-methyleneazide-1,2,4-oxadiazol-3-yl)furazan (51).Then the 3-nitroamino-4-(5-methyleneazide-1,2,4-oxadiazol-3-yl)furazan silver salt(52a)was prepared by nitri fication and metathetical reaction.Salt 52a was used to react with nitrogen-rich hydrochloride to prepare four energetic salts 52b-e.Besides,the amino group in 51 can be easily oxidized with HO/HSOsystem to obtain 3-nitro-4-(5-methylene azide-1,2,4-oxadiazol-3-yl)furazan(53,Fig.20)[82].As shown in Table 11,hydroxylamine salt(52c)presented an excellent detonation velocity and detonation pressure of 9090 m/s and 35.76 GPa,respectively,which is superior than RDX.The oxidized product 54 existed as a liquid at room temperature and exhibited acceptable detonation performance,which could be used as a liquid explosive or propellant component.Compounds 51 possessed favorable melting point(83.2C)and decomposition temperature(182.9C),indicating that it may be competitive for application in molten-cast explosives to replace TNT.
Through N-oxidation,furoxan,also known as 1,2,5-oxadiazole-2-oxide,adds a coordination oxygen atom to the nitrogen atom in the furazan ring.The two active oxygen atoms that comprise the furoxan ring has a“hidden”nitro group that not only boosts the oxygen content(37.2%)and oxygen balance,but also increases the densities and detonation velocities of energetic materials[62,83].
Fershtat et al.[84]improved the reaction conditions for synthesizing the 1,2,4-oxadiazole ring from the amidoxime group in 2015.The raw materials for the initial amidoximes were 3-methyl-4-cyanofuroxan and 3,4-dicyanofuroxan.The reaction conditions for preparing 4-(1,2,4-oxadiazol-3-yl)-3-methylfuroxan(54)in 86%yield were condensation of starting amidoxime(N-Hydroxy-4-methyl-furazan-3-carboximidamide 5-oxide)and excess trimethyl orthoformate at 20C in 1 min catalyzed by Lewis acid scandium tri flate(Sc(OTf),10 mol.%)in the absence of a solvent.The double cyclization reaction of bisamidoxime was performed under the same conditions to obtain 3,4-bis(1,2,4-oxadiazol-3-yl)furoxan(55,method 1 in Fig.21),which yielded 82%-44%higher than Yan et al.[61]procedure.With a melt point of 84-86C,compound 55 appeared to be a melt-castable material.
Johnson et al.[85]again optimized the reaction conditions in the preparation of 55 in 2019.When the expensive catalyst Sc(OTf)was replaced with the cost-effective boron tri fluoride diethyl etherate(BF·EtO),triethyl orthoformate were used as a reactant,at room temperature bisamidoxime could be converted to the target 55 in a higher yield(97%,method 2 in Fig.21).This reaction protocol should be suitable for industrial scale-up.In comparison to the above values 98C provided by Yan et al.[61]and 84-86C reported by Fershtat et al.[84],a different melt point(114.3-117.8C)of 55 was observed.Furthermore,55 exhibits high density(1.79 g/cm),high heat of formation(357 kJ/mol),good detonation performance(D=7577 m/s,P=24.1 GPa)as well as low sensitivities(IS>34.7 J,FS>360 N)(Table 12),outperforming the traditional melt-castable explosive TNT (d = 1.65 g/cm,HOF=-59.3 kJ/mol,D=6950 m/s,P=20.5 GPa,IS=15 J,FS>240 N,respectively).The Electro-Static Discharge(ESD)of 55(ESD>0.25 J)was also determined and revealed a low electrostatic sensitivity than TNT(ESD=0.25 J).As a result,55 could be used in casting operations as a potential melt-castable eutectic ingredient mixed with other energetic materials.
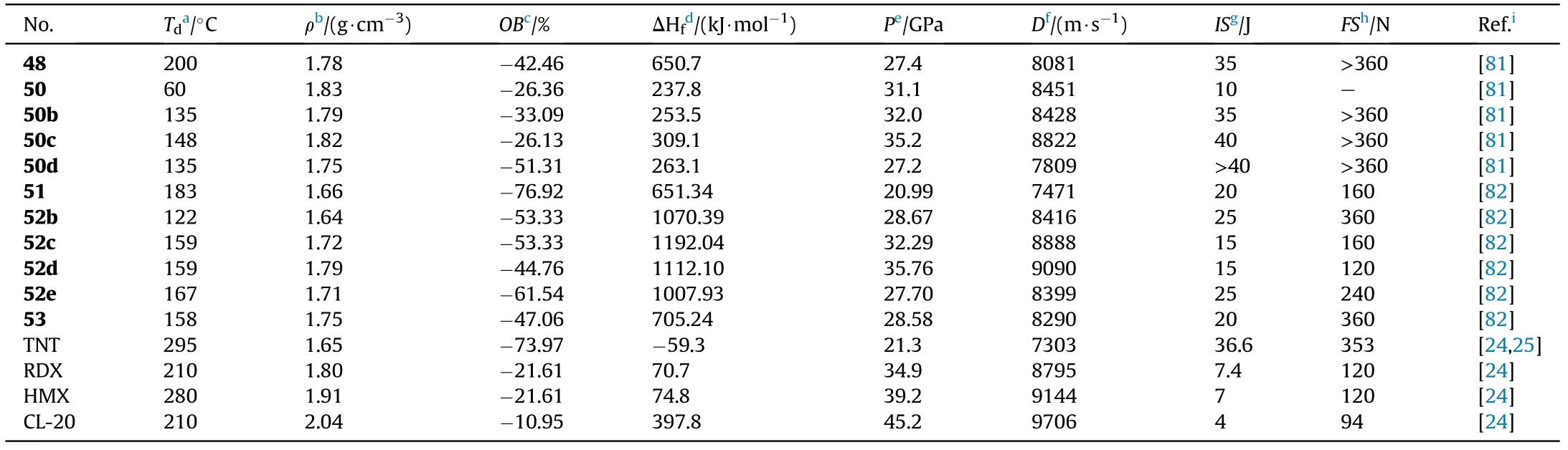
Table 11 Energetic and physicochemical properties of 48-54,salts 50b-d and 52b-e.
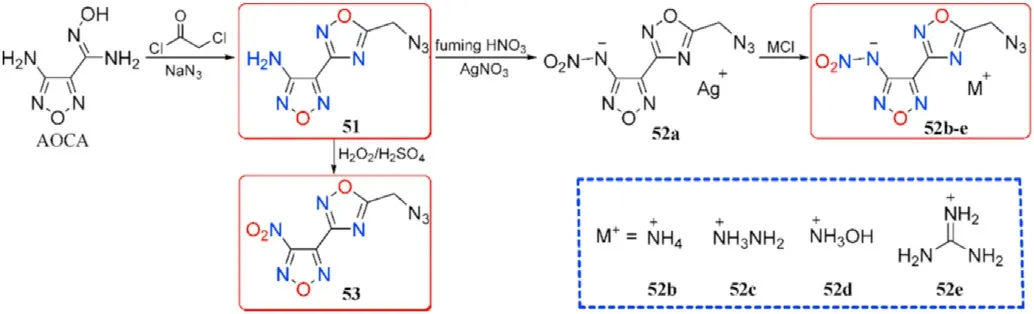
Fig.20.Scheme of synthesis of energetic compounds 51-53 and salts 52a-e.
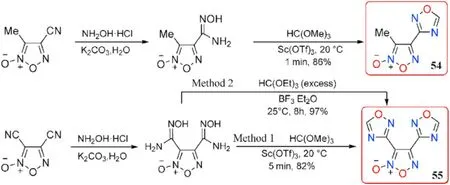
Fig.21.Scheme of synthesis of energetic compounds 54-55.
The mono-amidoxime was produced by the chemoselective nucleophilic addition of 3,4-dicyanofuroxan with hydroxylamine and subsequent cyclization into 4-(5-amino-1,2,4-oxadiazol-3-yl)-3-cyano-furoxan(56),which was then transformed into two types of 1,2,4-oxadiazole-furoxan-based energetic structures(57-58)by introducing different energetic motifs such as nitroamino,tetrazole,or dinitromethyl,respectively.Their corresponding energetic nitrogen-rich salts(57a-e and 58a-b)were also obtained(Fig.22)[86].Note that the potassium ion in salts 58a-b from dipotassium salt 58 still remained.
As presented in Table 12,the energetic salts(57a-e)have higher thermal stability(152-234C)than the neutral compound 57,which decompose at 121.2C.Compounds 57 and 57c are dense energetic materials(d=1.84-1.86 g/cm)with excellent detonation performance(D=8639-8977 m/s,P=31.8-35.3 GPa)and adequate sensitivities(IS=11-13 J,FS=160-360 N)comparable to RDX.Although the density of dihydrazinium salt(57b,1.74 g/cm)does not exceed 1.80 g/cm,the heat of formation is incredibly high(702.9 kJ/mol).As a result,it has a comparable detonation velocity(D=8713 m/s)to RDX.Dipotassium salt(58)beats lead azide(Pb(N))by signi ficantly in oxygen balance(12.2)and detonation velocity(8076 m/s)and have an excellent density(2.14 g/cm)and thermal stability(186.4C).It's worth noting that 58 has a high sensitivity to impact(IS=3 J)but a low sensitivity to friction(FS=60 N),making it potentially safer to handle than Pb(N).Compound 58 has the characteristics and performance properties that make it a viable green candidate for replacing toxic lead-based primary explosives.
According to Hermann et al.[87],the reactivity of the amino group bonded to 1,2,4-oxadiazole ring is not high enough in some circumstances,owing to the exceptionally poor solubility of 1,2,4-oxadiazole-based derivatives in ordinary organic solvents.Based on it,the desired polycyclic heterocyclic structures comprising both 1,2,4-oxadiazole and furazan or furoxan backbones 60 and 62 were synthesized[88,89].The initial diamines AAOF and AFOA were prepared through two different routes started from the same primer reactant malononitrile.In the first step,the amino group at the furoxan ring in AAOF was oxidized to nitro,yielding 4-nitro-3-(5-amino-1,2,4-oxadiazol-3-yl)furoxan (59).The other amino group at 1,2,4-oxadiazole ring in compound 59 was further transformed to azo derivative bis(4-nitro-1,2,5-oxadiazole-2-oxid-3-yl)-azo-1,2,4-oxadiazole(60)by direct oxidative coupling reaction under the condition of KMnOand HCl.The synthesis procedures of 61 and 62 are similar to those of 59 and 60(Fig.23).The important properties of compounds 59-62 were listed in Table 13.
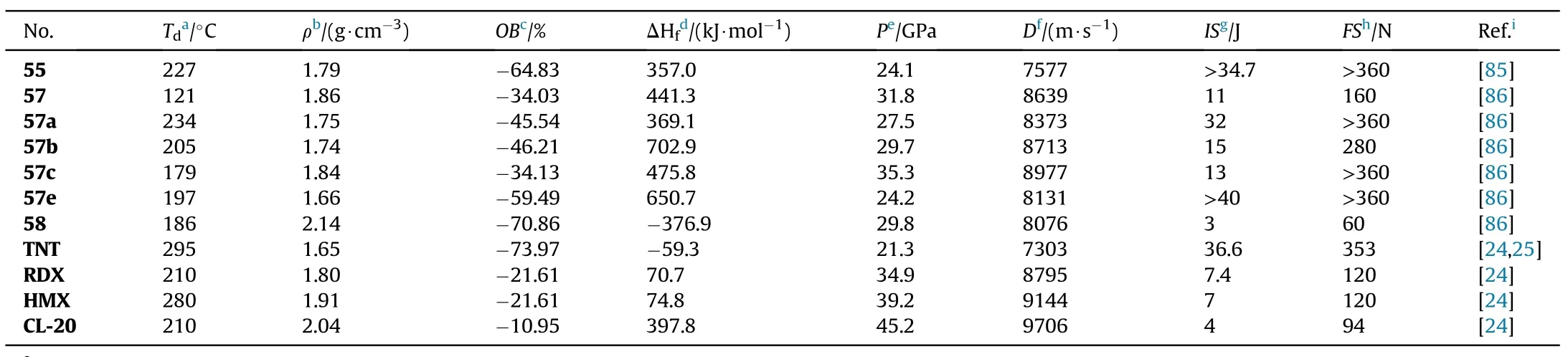
Table 12 Energetic and physicochemical properties of 55-58 and salts 57a-e.
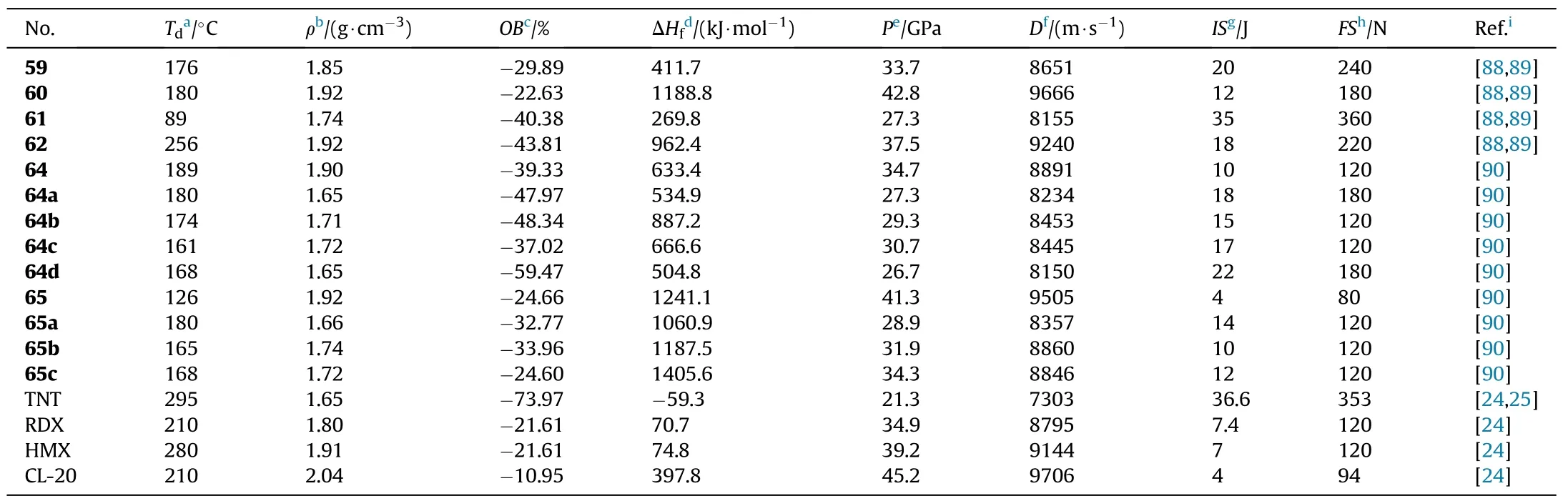
Table 13 Energetic and physicochemical properties of 59-65 and salts 64a-d and 65a-c.
By comparison,it can be found that the thermal stabilities,densities,heats of formation,and detonation parameters of azo-1,2,4-oxadiazole-derived energetic molecules 60 and 62 are higher than corresponding compounds(59 and 61).The prepared 59 and 60,as expected,have superior densities,higher heats of formation,and better detonation performance than 61 and 62,which lack Noxide moieties in furazan ring.These four compounds are all insensitive towards impact(IS=12-35 J)and friction(FS>180 N).As a result,all synthesized compounds,with an exception of 61,have better combination properties than the benchmark secondary explosive RDX.Compound 60 was not only exhibiting excellent detonation performance(D=9666 m/s,P=42.8 GPa)comparable to CL-20(D=9706 m/s,P=45.2 GPa),but it also has a lower sensitivity(IS=12 J,FS=180 N)than HMX(IS=7.4 J,FS=120 N).These positive contributions highlight its potential applications as an environmentally friendly insensitive energetic material for RDX and HMX replacement.
The amino group at the furoxan ring in AAOF can also be chemoselectively inserted in the oxidative coupling,yielding 3,3-bis(5-amino-(1,2,4-oxadiazol-3-yl)-4,4-azofuroxan (63), which was then nitrated to two distinct products 3,3-bis(1,2,4-oxadiazol-5(4H)-one)-3-yl)-4,4-azofuroxan(64)and 3,3-bis(5-nitramino-1,2,4-oxadiazole-3-yl)-4,4-azofuroxan(65)using different nitrating reagents.A series of energetic salts(64a-d and 65a-c)were obtained by neutralization with various nitrogen-rich basic amines(Fig.24)[90].
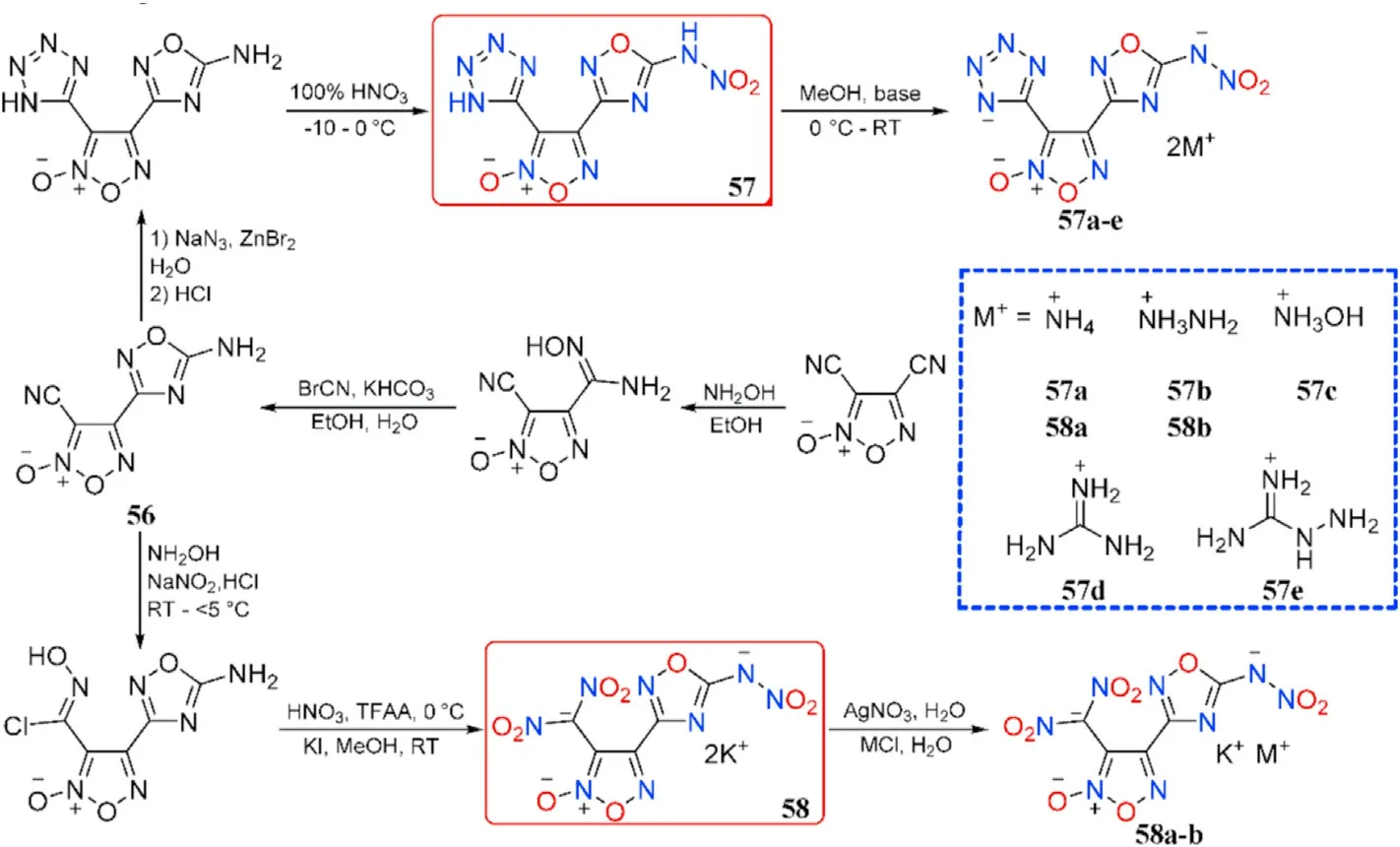
Fig.22.Scheme of preparation of energetic compounds 56-58,salts 57a-e and 58a-b.
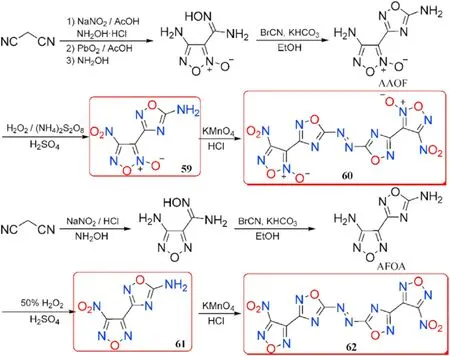
Fig.23.Scheme of Preparation of energetic neutral compounds 59-62.
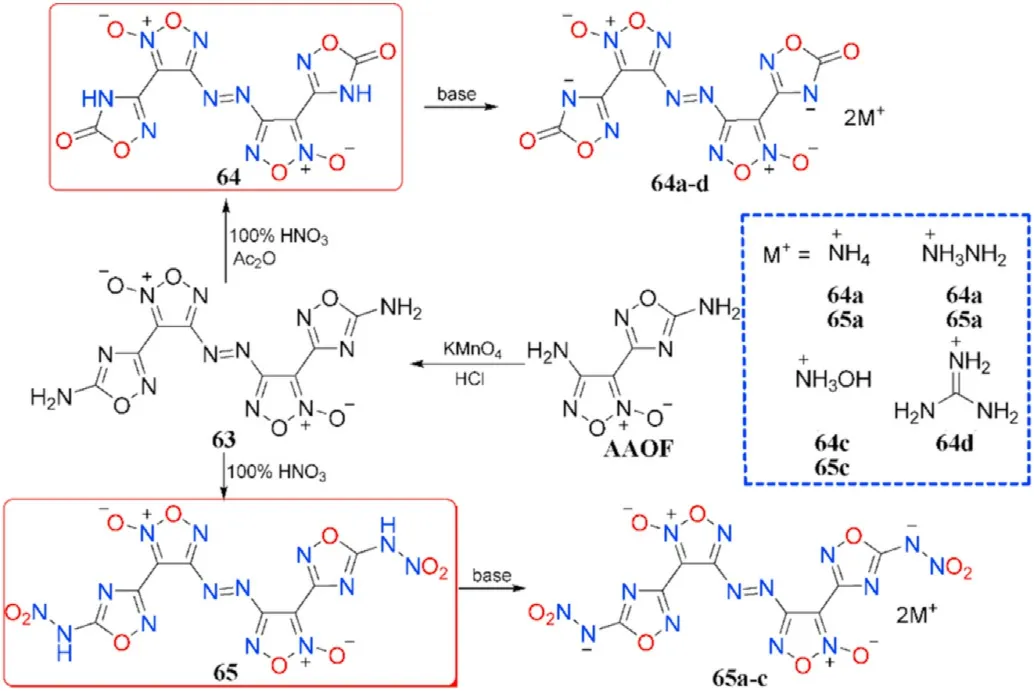
Fig.24.Scheme of preparation of energetic compounds 63-65,64a-d and 65a-c.
All the energetic materials presented have strong properties such as high density(1.65-1.92 g/cm),positive heats of formation(504.79-1405.62 kJ/mol),excellent detonation performance(D=8150-9505 m/s,P=26.7-41.3 GPa),and low sensitivities(IS>4 J,FS>80 N)(Table 13).Among them,65 has the highest density of 1.92 g/cm,highest detonation properties(D=9505 m/s,P=41.3 GPa)and acceptable sensitivities(IS=4 J,FS=80 N).Furthermore,compounds 64 and 65b-c also show good detonation performance(D=8846-8891 m/s,P=31.9-34.7 GPa),making them amenable for high-energy density materials.
2.3. 1,2,4-Oxadiazole-1,3,4-oxadiazole-based compounds
Among the three stable oxadiazoles(Fig.1)and,3,4-oxadiazole has the lowest heat of formation.the reaction conditions for the insertion of 2,5-substituted energetic functionalities will be quite rigorous because the carbon atoms at the 2,5-positions of the 1,3,4-oxadiazole ring are very unreactive.Accordingly,1,3,4-oxadiazolebased derivatives have been studied rarely as a component in energetic materials. Nonetheless, previously described 1,3,4-oxadiazole-based energetic materials have strong thermal stabilities and low sensitivities[91-95].For example,the well-known heat-resistant explosive TKX-55[46].Consequently,the combination of 1,3,4-oxadiazole with 1,2,4-oxadiazole could provide appealing frameworks for designing and synthesizing energetic materials that achieve a good balance between high performance and sensitivity.
Using comprehensive density functional theory(DFT)calculations,two 1,2,4-oxadiazole-1,3,4-oxadiazole and two bi(1,2,4-oxadiazole)-based structures were designed in combination with common nitro/nitramine groups,and their structure-property relationships were examined(Fig.25)[96].The results reveal that compounds 66-69 have satisfactory calculated detonation performances,such as high heats of detonation(5756-6095 J/g,excellent detonation velocities(8390-8840 m/s)and detonation pressures(31.65-36.23 GPa).The detonation properties of 66 and 69,except for explosion heat,outperform the commonly used high explosive RDX,implying that 1,3,4-oxadiazole could be a potential building block for developing novel HEDMs with balanced detonation properties and stabilities.
To date,only a few valuable and powerful compounds incorporating 1,2,4-oxadiazole and 1,3,4-oxadiazole rings were experimentally synthesized.Treatment of glyoxime with hydroxylamine hydrochloride in sodium hydroxide solution converts it to diaminoglyoxime cyclization yields the double-ring molecule diethyl[3,3-bi(1,2,4-oxadiazole)]-5,5-dicarboxylate;hydrazinolysis of the latter gives [3,3-bi(1,2,4-oxadiazole)]-5,5-dicarbohydrazide,which is then treated with BrCN to afford the precursor containing four oxadiazole rings named 5,5'-([3,3-bi(1,2,4-oxadiazole)]-5,5-diyl)bis(1,3,4-oxadiazol-2-amine),followed by nitration of both amino groups yields the desired product dinitramine 70.Compound 70 was directly neutralized with three nitrogen-rich bases affording insensitive energetic salts(70a-c,Fig.26)[97].
As shown in Table 14,neutral product 70 has the highest density of 1.90 g/cm,which is comparable to HMX(1.91 g/cm),resulting in the highest detonation characteristics(D = 8789 m/s,P=32.7 GPa)among the four compounds.The three nitrogen-rich energetic salts(70a-c)are also dense materials(1.79-1.84 g/cm)with excellent detonation performance,among them,hydroxylammonium salt(70b)has the highest detonation pressure(31.7 GPa)whereas hydrazinium salt(70c)exhibits the highest detonation velocity(8648 m/s).Only 70b has an onset decomposition temperature below 180C in terms of thermal stabilities.These compounds have sensitivities that are equivalent to or lower than RDX,particularly 70,which has an impact sensitivity of 10 J and a friction sensitivity of 110 N.These 1,2,4-oxadiazole-1,3,4-oxadiazole-based novel derivatives have an acceptable impact and friction sensitivities,as well as promising detonation properties and high thermal stability,making them suitable candidates for high-performance new insensitive energetic materials.
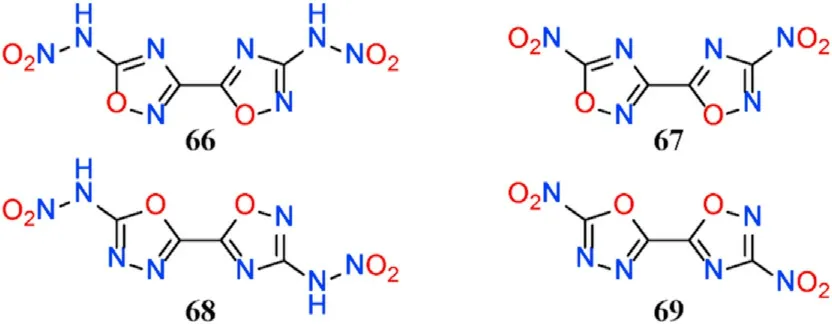
Fig.25.Molecular structures of compounds 66-69.
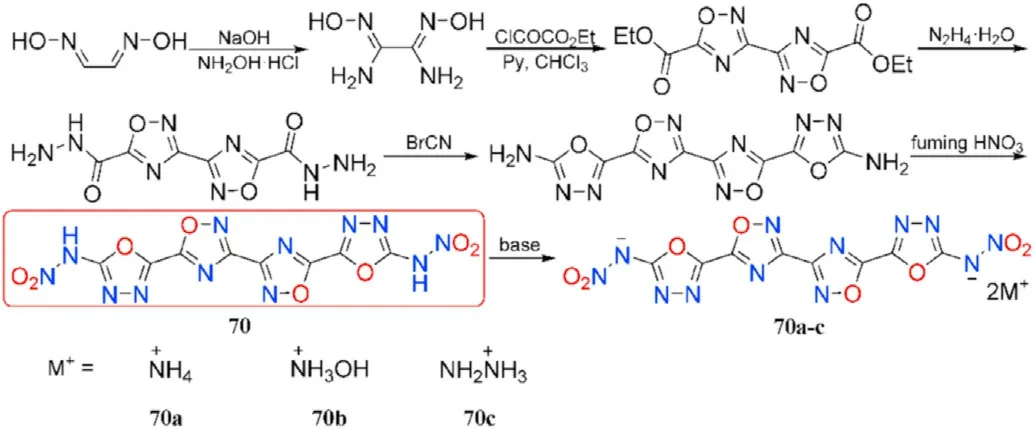
Fig.26.Scheme of preparation of energetic molecules 70 and its salts 70a-c.
2.4. 1,2,4-Oxadiazole-pyrazole-based compounds
Pyrazole has been considered as a favorable building block for energetic materials based on the good thermal stabilities and low sensitivities exhibited by existing pyrazole energetic compounds[98-100].To explore the difference between C-nitro and N-nitro groups on energetic characteristics,Yan et al.[101]synthesized several representatives of 1,2,4-oxadiazole-pyrazole-based molecules incorporating additional C-nitro and N-nitro groups through several transformations of 1H-pyrazole-3-carbonyl chloride.The cyclization of 1H-pyrazole-3-carbonyl chloride with N-hydroxy-1H-pyrazole-3-carboximidamide or DAG produces 3,5-di(1H-pyrazole-3-yl)-1,2,4-oxadiazole(71)and 5,5-di(1H-pyrazol-3-yl)-3,3-bi(1,2,4-oxadiazole)(72),respectively,which are then nitrated in acetic anhydride with an excess of fuming nitric acid and thermally rearrangement in benzonitrile at 140C to give 3,5-bis(3-nitro-1Hpyrazol-5-yl)-1,2,4-oxadiazole(73)and 5,5-bis(3-nitro-1H-pyrazol-5-yl)-3,3-bi(1,2,4-oxadiazole)(74),respectively.The finished product of tetra-C-nitro derivatives 3,5-bis(3,4-dinitro-1H-pyrazol-5-yl)-1,2,4-oxadiazole(75)and 5,5-bis(3,4-dinitro-1H-pyrazol-5-yl)-3,3-bi(1,2,4-oxadiazole)(76)was carried out at 50C for 8 h using concentrated HSO/fuming HNO(1:1).Similarly,when 71 and 72 were treated with HSO/fuming HNO,they are ef ficiently converted to di-C-nitro derivatives 3,5-bis(4-nitro-1H-pyrazol-3-yl)-1,2,4-oxadiazole (77)and 5,5-bis(4-nitro-1H-pyrazol-3-yl)-3,3-bi(1,2,4-oxadiazole)(78),which are then selectively nitrated at the pyrazole ring nitrogen by acetic anhydride and fuming nitric acid at room temperature to yield di-C-di-N-nitro derivatives 3,5-bis(1,4-dinitro-1H-pyrazol-3-yl)-1,2,4-oxadiazole (79)and 5,5-bis(1,4-dinitro-1H-pyrazol-3-yl)-3,3-bi(1,2,4-oxadia-zole) (80),respectively(Fig.27).
The critical properties of compounds(75-80)were thoroughly investigated to better understand the effect of C-nitro and N-nitro groups on energetic properties(Table 15).In comparison to 77 and 78,the densities of compounds 75,76,79,and 80 increased signi ficantly after the addition of the other two nitro groups,whether C-nitro or N-nitro.Furthermore,the tetra-C-nitro derivatives 75 and 76 exhibit higher densities(1.84 and 1.86 g/cm)than the di-C-di-N-nitro derivatives 79 and 80(1.82 and 1.83 g/cm),indicating that C-nitro groups contribute more positively to density than N-nitro groups.The decomposition temperatures of the 6 energetic compounds ranged from 156C(79)to 317C(78).The di-C-di-N-nitro derivatives 79 and 80 present the most excellent detonation performances(D=8904 and 8867 m/s,P=35.1 and 34.5 GPa,respectively)among them,which are superior to the values of RDX.Tetra-C-nitro derivatives 75 and 76 show higher thermal stabilities(272-274C)and lower sensitivities(IS>31 J,FS>360 N)than 79 and 80,as well as comparable detonation properties(D=8685-8741 m/s,P=33.4-34.0 GPa)to RDX,suggesting that they could be used as secondary explosives.The di-CNOderivatives 77 and 78 have the highest decomposition temperatures of 314C and 317C,respectively,as well as the lowest sensitivities(IS>40 J,FS>360 N)and good detonation performance(P=25.2-26.4 GPa,D=7991-8027 m/s)among compounds 75-80.It could be noted that compounds 77 and 78 have better explosive properties than the heat resistant explosive HNS and can be considered the next generation of insensitive heat resistant energetic materials.

Table 14 Energetic and physicochemical properties of 70 and salts 70a-c.
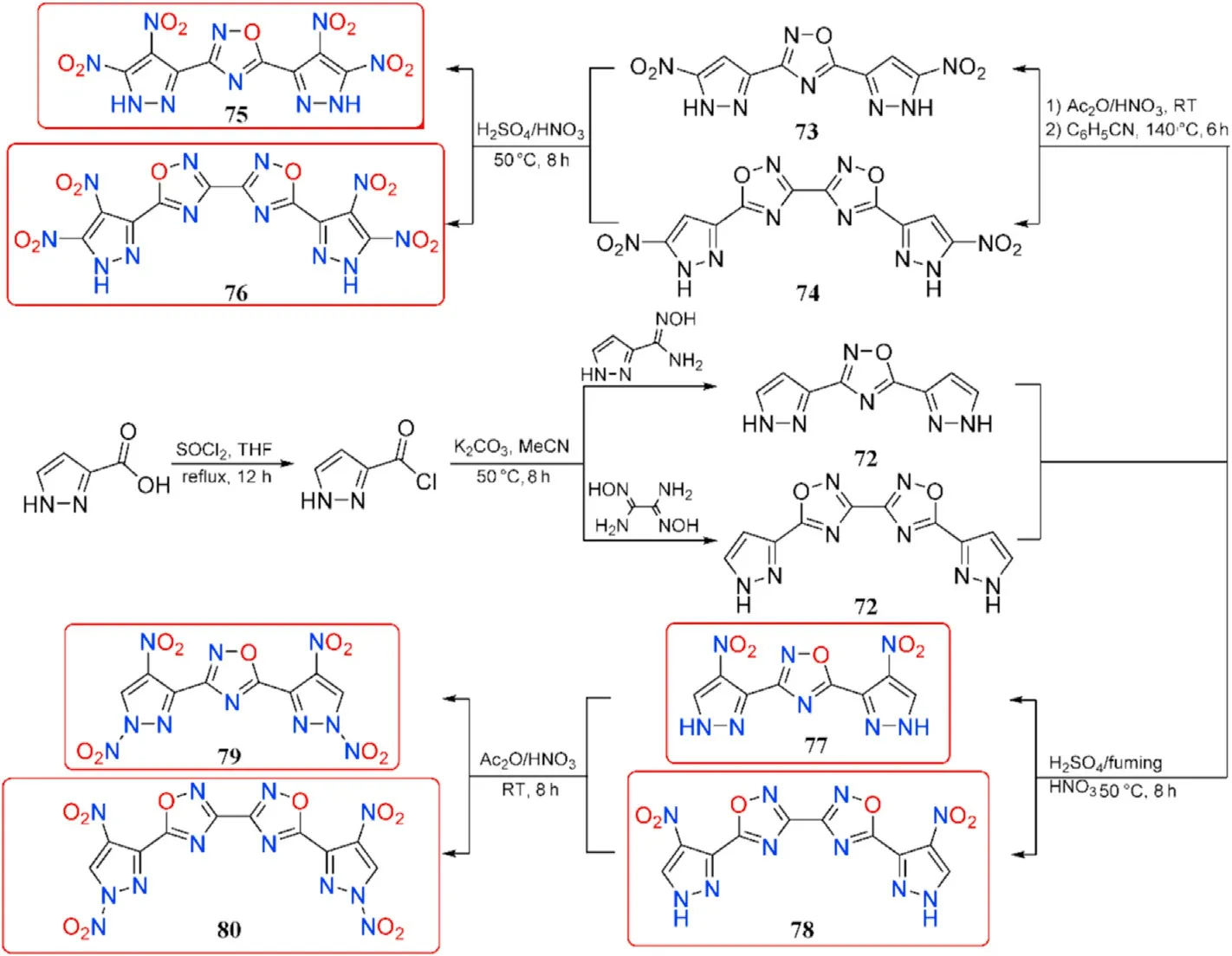
Fig.27.Scheme of preparation of energetic molecules 71-80.
2.5. 1,2,4-Oxadiazole-triazole-based compounds
Various 1,2,4-oxadiazole-triazole compounds have been reported,mainly in the areas of pesticides,although there have been few publications in the field of energetic materials.In 2020,a biheterocyclic assemblies of 1,2,4-oxadiazole and 1,2,3-triazole were designed and synthesized.The precursor in the synthesis of 3-(5-Amino-1H-1,2,3-triazol-4-yl)-1,2,4-oxadiazol-5-amine(81)is 2,2,2-trichloro-N-(4-(5-(trichloromethyl)-1,2,4-oxadiazol-3-yl)-
1H-1,2,3-triazol-5-yl)acetamide,which was produced using a tandem addition/cyclization protocol from 5-Amino-1H-1,2,3-triazole-4-carbonitrile.Compound 81 was then nitrated with various nitration reagents and methods to yield the single-nitrated and whole-nitrated oily products 82 and 84,which were then neutralization with several bases to yield the two series of energetic salts 82a-c and 84a-d,respectively(Fig.28)[102].Compound 81 and its derivatives were comprehensively characterized using XRD,IR,EA,multinuclear NMR spectroscopy,and DSC.The important properties of the six organic energetic salts are investigated(Table 16).
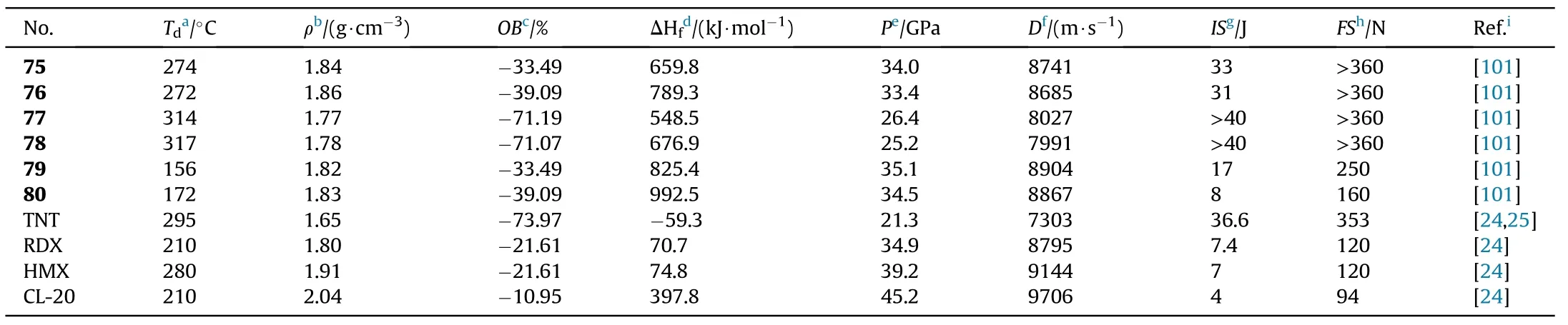
Table 15 Energetic and physicochemical properties of 75-80.
The decomposition temperatures of the six salts ranged from 165 to 213C,with salt 82b having the highest decomposition temperature of 213C.The dihydroxylammonium salt(84c,1.81 g/cm)showed the highest density of the six energetic salts,while the dihydrazinium salt(84a,1.66 g/cm)has the lowest.Salts 84a(1.66 g/cm)and 84b(1.68 g/cm)have lower densities than their corresponding salts 82a(1.73 g/cm)and 82b(1.70 g/cm),which could be due to inef ficient packing.All energetic salts have high heats of formation(174.3-505.0 kJ/mol)and acceptable impact(IS=6-22 J)and friction(FS=120-324 N)sensitivities.The densities and calculated heats of formation were used to predict the detonation properties of the six salts.Compound 84c has the highest detonation heat(Q=5787 J/g)and detonation performance(D=8682 m/s,P=33.6 GPa),which is comparable to RDX(Q=6306 J/g,D=8795 m/s,P=34.9 GPa).As can be seen,the single-nitrated energetic salts(82a-c)have higher thermal stability than the corresponding whole-nitrated energetic salts(84a-c).On the other hand,salts 84a-c show a better detonation performance than salts 82a-c due to the higher oxygen balance of wholenitrated products.

Fig.28.Scheme of preparation of energetic molecules 81-84 and their salts.
Similarly,3-(5-amino-1H-1,2,4-triazol-3-yl)-1,2,4-oxadiazol-5-amine(85),which contains a new bicyclic coplanar backbone with two amino groups was designed by our group(Fig.29)[103].Its two nitrated derivatives 86 and 87 and their nitrogen-rich energetic salts were also synthesized.Owing to a coplanar conjugated con figuration and extensive hydrogen bond interactions,most of the compounds have good thermal stability and detonation performance(Table 16).Among them,87 and 87c exhibit high detonation properties(D=9087 and 9135 m/s,P=35.9 and 35.3 GPa)comparable to RDX(8795 m/s,34.9 GPa)and low sensitivity(IS=20 and 16 J;FS=252 and 288 J)safer than RDX and HMX.In addition,salts 86c and 87b also possess acceptable detonation parameters,with detonation velocities of 8787 and 9018 m/s,and detonation pressures of 30.5 and 31.5 GPa,respectively.The TGDSC-MS-FTIR simultaneous analysis technique was employed to detect the main decomposition gaseous products,indicating that the NH,HO,N,NO,CO,and NO were included during their thermal decomposition.The positive results of these triazoleoxadiazole-based energetic materials indicate a potential design method for high-performance energetic materials.The synthesis and application of these series of compounds are still under exploration.
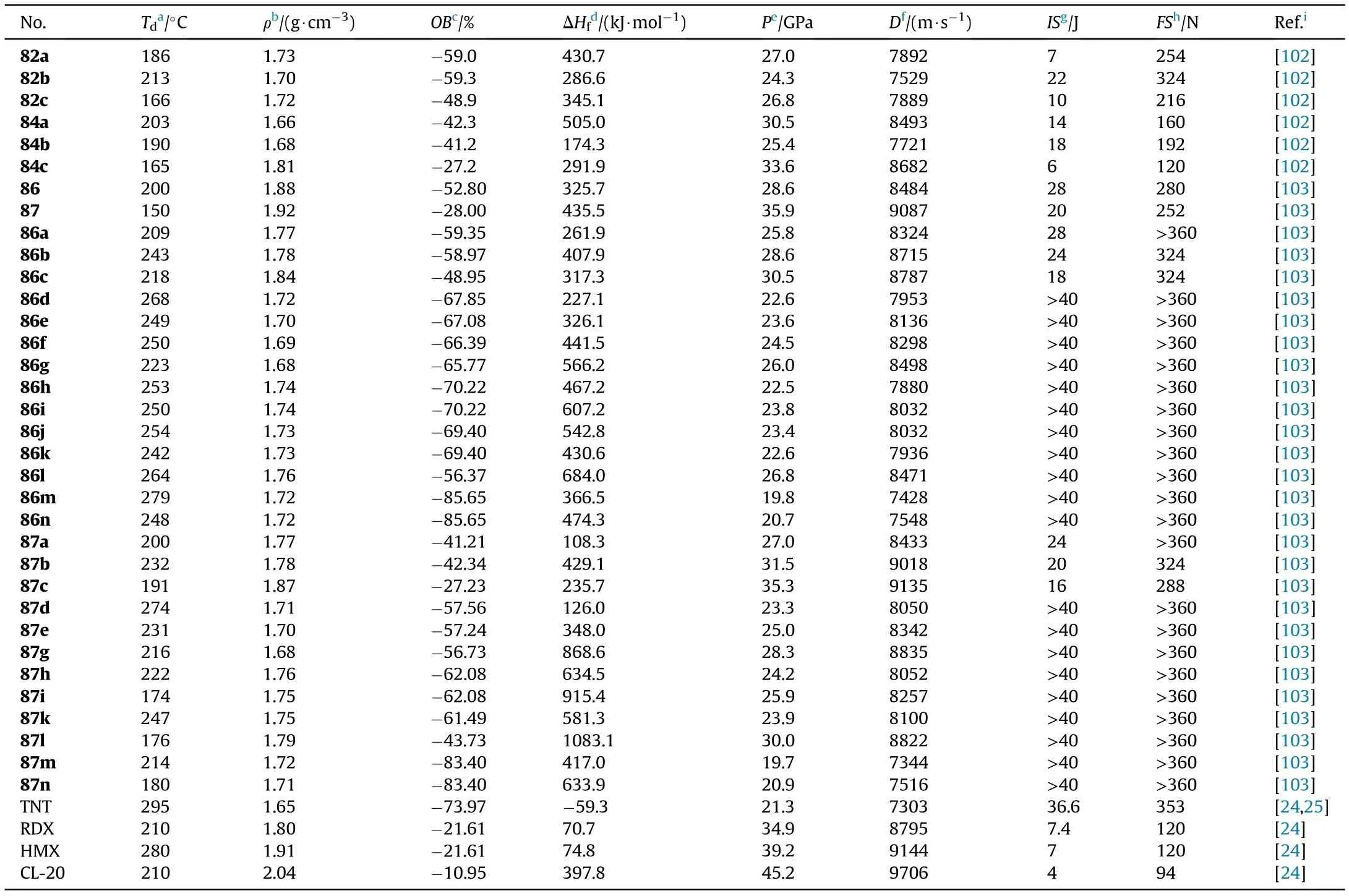
Table 16 Energetic and physicochemical properties of compounds 86,87,salts 82a-c,84a-c,86a-n and 87a-n.
Apart from the above-mentioned literature,there has been no more extensive research based on 1,2,4-oxadiazole linked triazoles(1,2,3-triazole or 1,2,4-triazole)in the field of energetic materials.However,by linking 1,2,4-triazole with furazan,a variety of polycyclic heterocyclic energetic molecules have been developed and reported,particularly 3,4-bis(1-nitro-1,2,4-triazole-3-yl)furoxan(BNTAF)[104],provide a new approach to construct energetic backbones and expand the research on the molecular design of energetic materials to a new level.Prof.Shreeve's group reported a set of 1,2,4-triazole-furazan-based compounds(Fig.30)[105-108]having high densities above 1.80 g/cm and meet the performance indicators of HEDMs,no matter neutral molecules or energetic salts,and could be considered as an alternative for HEDMs.As a result,the combination of the oxygen-containing 1,2,4-oxadiazole ring with triazoles is a new trend in the synthesis of energetic materials.
3.Conclusion and perspective
In summary,the 1,2,4-oxadiazole ring has a slightly lower heat of formation than the 1,2,5-oxadiazole ring,but it exhibits a good balance between energy and stability.The reported 1,2,4-oxadiazole-azoles-based energetic materials were summarized and examined in this review,which includes synthesis methods,density,HOF,detonation properties,and insensitivities.Some criteria for new HEDMs have been established to determine whether the newly obtained compounds can be used as potential substitutes for commonly used energetic compounds like TNT,RDX,HMX,or CL-20.The results show that high-performance energetic materials can be obtained by combining the 1,2,4-oxadiazole with other different high-energy azoles(such as,tetrazole,furazan,furoxan,1,3,4-oxadiazole,pyrazole,and triazole)and then chemically modifying these 1,2,4-oxadiazole-azoles by various energetic substituents(including nitro,nitramino,azo,azoxy,dinitromethyl,and trinitroethyl).The resulting energetic compounds obtained by this method can effectively achieve a good balance between high detonation performance and low sensitivity.More importantly,several 1,2,4-oxadiazole-azoles-based energetic molecules discussed in this review exhibit favorable overall performance than commonly used explosives.More concretely,among all gathered energetic compounds herein,some of them exhibit similar or even better detonation properties compared to RDX(5,5b,5c,16,16g,18,
23c,26,26b,26d,33a,34,34b,34c,35,41,43a,43b,43c,43e,50c,57,57c,59,62,64,65b,65c,70,75,76,79,80,81c,87,and 87c)and HMX(19,36,38b,38c,50c,60,and 65).When considering thermal stabilities and the sensitivities of the compounds,5b,5c,16,23c,26,26b,26d,34c,41,43c,57c,62,65c,79,and 80 could be possible candidates to replace RDX and 38b,38c,and 60 may be possible alternatives to replace HMX.Besides that,compounds 2,2a,3a,77,and 78,especially 23,could be possible candidates for insensitive heat-resistant energetic materials to replace TATB or HNS.Compared to TNT,13,14,and 55 are promising melt-castable eutectic ingredients.Dipotassium salt 58 might be a prospective green primary explosive to replace toxic lead-based primary explosives(Fig.s1).These results provide con firmatory evidence that the 1,2,4-oxadiazole ring is indeed an ef ficient and ideal moiety for the design and synthesis of novel advanced energetic materials with balanced energy and safety properties.
Some remarkable progress in the studies of 1,2,4-oxadiazoleazoles-based energetic compounds have been obtained,but it is mainly focused on 1,2,4-oxadiazole-furazan-based compounds due to the short research time.There are still many works in 1,2,4-oxadiazole linked other N-rich heterocycles,such as,tetrazole Noxide,triazole,1,3,4-oxadiazole,pyrazole,and other fused rings,which need to be further exploited and fully studied.Furthermore,a 1,2,4-oxadiazole-azoles-based molecule with a large“planar”conjugated system can be designed fromthe molecular level,which is expected to obtain the target energetic molecule with a more compact structure,higher energy level,and lower mechanical sensitivities.Then,the potential prospect of these compounds with planar con figuration in military and civilian purposes such as environmentally-friendly gas generators,insensitive high explosive(IHE),smoke-free pyrotechnics,and energetic propellants with low signature(chlorine-free)will be further explored and investigated in the future.Based on the above idea,our group designed and synthesized a set of energetic molecules by linking 1,2,4-oxadiazole with 1,2,4-triazole which present excellent properties and are being collated as soon as possible for publication.Besides that,with the continuous progress of synthesis technology and equipment of energetic materials,1,2,4-oxadiazole-azoles-based energetic materials still have a prosperous future.It is expected to get very promising 1,2,4-oxadiazole-azoles-based insensitive high explosives for practical application and industrialization.
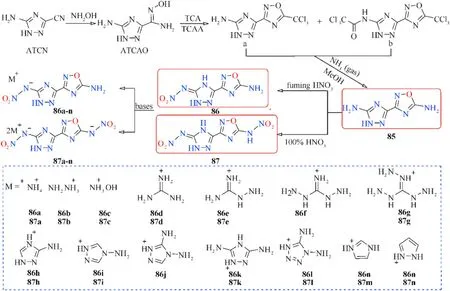
Fig.29.Scheme of preparation of energetic molecules 85-87 and their nitrogen-rich salts.
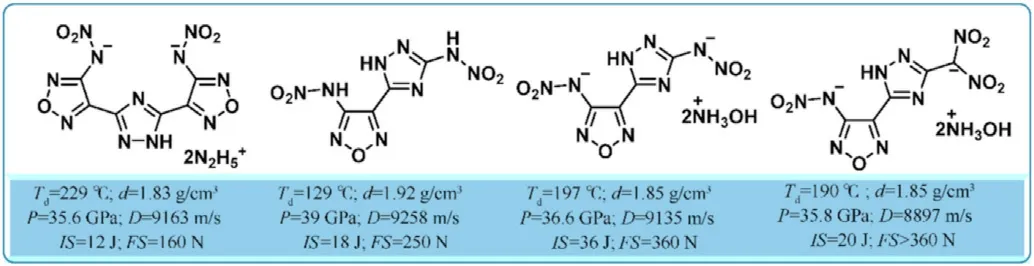
Fig.30.The properties of several 1,2,4-triazole-furazan-based energetic materials.
4.Caution
Readers are reminded that the information given in this review is intended to cover the progress of recent research on 1,2,4-oxadiazole-azoles-based energetic materials.Most of these molecules collected are nevertheless energetic materials that might explode during preparing,handling or manipulating!Please handle these materials by experienced personnel and with care!Precautionary measures are mandatory and protective equipment like safety glasses,face shields,leather coats,Kevlar gloves,and ear protectors is highly recommended.The authors strongly suggest that the original references be consulted for detailed safety information.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to in fluence the work reported in this paper.
We are thankful to the NSAF(No.U1830134),NSFC(No.21905023 and 22175025),State Key Laboratory of Explosion Science and Technology(No.YBKT21-02),and Open Research Fund Program of CAS Key Laboratory of Energy Regulation Materials(No.ORFP2020-01)for their generous financial support.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dt.2021.12.002.
- Defence Technology的其它文章
- High explosive unexploded ordnance neutralization-Tallboy air bomb case study
- Dynamics and rebound behavior analysis of flexible tethered satellite system in deployment and station-keeping phases
- Finite element analysis of functionally graded sandwich plates with porosity via a new hyperbolic shear deformation theory
- Investigation on the penetration of jacketed rods with striking velocities of 0.9-3.3 km/s into semi-in finite targets
- The effect of strain rate on compressive behavior and failure mechanism of CMDB propellant
- Adaptive target and jamming recognition for the pulse doppler radar fuze based on a time-frequency joint feature and an online-updated naive bayesian classi fier with minimal risk

