One-step synthesis of FeO(OH) nanoparticles by electric explosion of iron wire underwater
Ho Yin ,Xin Go ,Peng-wn Chen*
a Institute of Systems Engineering,China Academy of Engineering Physics,Mianyang,Sichuan,612900,China
b State Key Laboratory of Explosion Science and Technology,Beijing Institute of Technology,Beijing,100081,China
Keywords:Electric wire explosion Plasma tunnel Nanoparticles FeO(OH)
ABSTRACT In this study,we investigated electric explosion of iron wire in distilled water with different energy input adjusted by charging voltage.The as-prepared samples were characterized by X-ray diffraction (XRD),scanning electron microscopy (SEM),transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS),showing the presence of iron and multiple iron-based compounds oxides with contents influenced by the experimental conditions.In particular,pure FeO(OH) nanoparticles were obtained using electric explosion of iron wire with energy input of 1125 J at charging voltage of 15 kV.Analysis of discharge current and resistive voltage data indicate that the high energy input induced by strong plasma discharge at high charging voltage is a key factor to form FeO(OH).This study presents a one-step method to synthesize FeO(OH) nanoparticles using electric explosion of iron wire.
1.Introduction
Nanoparticles (NP) typically features diameters below 100 nm taking into account their surrounding interfacial layers [1].Owing to the size effects,NPs possess various unique properties,such as quantum confinement,plasmonic effects,high catalytic efficiency,high reactivity,etc.[1-5].Therefore,investigation on synthesis and applications of NPs has been a central focus of materials science since 1970s[6-8].These and later studies demonstrated influence of structures and morphologies of NPs on their properties and applications in electronics,optics,energy storage,catalysis,medicine,etc [1,9].
Iron or iron-based compound nanoparticles possess various outstanding properties such as high surface area,high reactivity and strong reducibility.Moreover,iron oxides and hydroxides are used for decontamination of harmful pollutants [9-12] as well as catalysts[13],electrode materials[14],for bio-medical applications[15,16]and as gas sensors[17,18].However,the toxicity of free iron inhibits the applications of iron nanomaterials [19],examples of which are mutagenicity induced by Fenton reaction[20]and auxoaction of microorganism proliferation [21].Therefore,inert and non-toxic iron oxides and hydroxides attracts more attention of researchers.
Among above iron-based compounds,FeO(OH) can be applied as pigments[22],as precursors for high-quality magnetic materials[22,23] and as ion exchangers [24] owing to the active functional hydroxyl groups [25].Moreover,FeO(OH) is also effective as photocatalysts for cleaning water [24,25] because of its efficient UV light absorption and narrow energy gap(around 2.2 eV)[26-28].In addition,FeO(OH) can be utilized as one component of functional composite materials,including new magnetic sorbents [29],new composite electrodes[30,31]and photocatalysts[32].Besides,Liou et al.[33] reveals the high performance of FeO(OH) on catalytic degradation of explosives,indicating its high potential application on safety engineering and environmental protection fields.
Various methods have been reported to prepare FeO(OH)nanoparticles,including solution-oxidation methods [22],hydrothermal synthesis [34-36],photosynthetic microorganismmediated synthesis [37],microwave-assisted synthesis [31,38,39],hydrolysis method [40],irradiation reduction and oxidation method [41],etc.Using solution-oxidation method,Ni et al.[22]obtained α-FeO(OH) nano-rods by purifying the precipitates from the solution containing iron sulfate and sodium acetate ions.Hydrothermal synthesis was used to prepare β-FeO(OH)nano-rod by precipitating (at certain pH values and temperatures) solutions of FeCl[34] and FeCl[18].In photosynthetic microorganismmediated synthesis,multiple microorganisms in sterile Bold's basal medium with iron salts at 20.0C are exposed to luminescence,leading to the synthesis of β-FeO(OH) as part of the physiological,chemical and photosynthesis processes[37].With respect to microwave-assisted synthesis,the solution of iron salts (e.g.FeClwith HCl) was heated using microwave oven to induce the reaction for the FeOOH synthesis [31,38].Kasparis et al.[40]employed hydrolysis method to obtainβ-FeO(OH)nanoellipsoids,in which the mixed solution of FeCland polyethyleneimine was heated using oil bath treatment at 80C for 2 h under magnetic stirring(500 rpm).Besides,Jurkin et al.[41]reported the synthesis of δ-FeOOH nanosheets through irradiation reduction and oxidation method,in which Fe(OH)was obtained by the γ-irradiation treatment to FeClsolution with 2-propanol and diethylaminoethyl-dextran hydrochloride at temperature lower than 25C,then dried and oxidized in air to produce δ-FeOOH nanosheets.
Electric wire explosion refers to the phenomenon that,in vacuum or a certain medium,when a strong current produced by the discharge of a capacitor passes through a wire,the wire is evaporated in burst with bright flash by joule heating [42].This phenomenon is accompanied by phase transitions of the wire material and water,non-ideal plasma formation and generation of strong shock waves and light radiation fluxes[43].The ultra-hot explosion products scatter out rapidly along with shockwave,and then cools down in medium to form nanoparticles [44].Thus,the whole process of electric wire explosion consists of two general processes:joule heating process and explosion process in nano-or microseconds [45].Besides,when the energy input is much higher than the wire sublimation energy,the explosion products break down to form strong plasma tunnel during explosion process[42,43],which also influences on the formation of nanoparticles.
Note that physical techniques provide 8% of all nanoparticles production [46].Above those techniques,electric wire explosion has been regarded as the most promising method for nanopowder production [47],and particularly for those with high batch size requested in biomedical applications[48,49].This method has been used to synthesize various nanoparticles including metal nanoparticles [42,50,51],metal compound nanoparticles [52,53],composite nanomaterials [54] and multiple carbon nanomaterials.Y?lmaz et al.[51] prepared Co nanoparticles by exploding cobalt wire in nitrogen.Krishnan et al.[52] synthesized one dimensional CuO nanoparticles by electric explosion of copper wire in distilled water.Safronov et al.[48] prepared iron oxides nanopowder for biophysical applications using iron wire explosion in air.Tanaka et al.[53] recovered tungsten carbide nanoparticles by electric explosion of tungsten wire in liquid paraffin.Gao et al.[54] produced Fe/FeO/graphene nanocomposite using electric explosion of iron wire in graphene oxide suspension.Graphite stick was used to prepare graphene materials by electric wire explosion method in distilled water [45].Furthermore,electric wire explosion can be also applied to prepare nanofluids and colloid featuring high stability [55,56].However,the synthesis of FeO(OH) nanoparticle has been rarely reported through electric wire explosion.
In this work,we demonstrate a one-step route to prepare FeO(OH) nanoparticles via electric iron wire explosion in distilled water as oxygen-poor medium.
2.Experimental procedure
For the recovery of as-prepared samples,a stainless-steel electric explosion chamber with inner diameter of 100 mm and depth of 80 mm (see Fig.1) was utilized for the experiments.Two pure iron electrodes with diameter of 5 mm wrapped with insulation blocks were located on top of the chamber lid.Before the assembly of chamber,400 ml distilled water was poured into the chamber as the electric explosion medium.Then,iron wire (99.95% pure,0.2 mm in diameter and 100 mm long) was fixed to the two electrodes and immersed into distilled water during the fix of lid.Subsequently,the electrodes were connected to a 10-μF capacitor with charging voltage in range of 5-15 kV for pulsed discharge.After the pulsed discharge,the dark suspension was collected,separated and dried using a vacuum freeze dryer for further characterization.
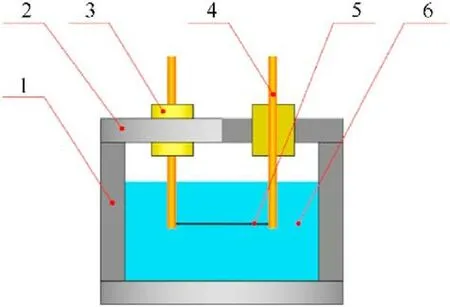
Fig.1. Diagram of electric explosion experiment using iron wire.1 -stainless steel chamber,2 -lid,3 -insulation blocks,4 -iron electrodes,5 -iron wire,6 -distilled water.
X-ray diffraction patterns were collected by X pert pro MPD using Cu Kα with k=0.15406 nm with working voltage and current at 40 kV and 200 mA,respectively.The corresponding scanning step size 2θ was 0.0330.Sample morphologies were analyzed using field emission scanning electron microscopy(FE-SEM,Hitachi S-4800) at 10 kV accelerating voltage and high resolution transmission electron microscopy (TEM,FEI Tecnai G2 F20 S-Twin) at 200 kV accelerating voltage.The elemental composition and chemical bonding states of as-prepared samples were probed by Xray photoelectron spectroscopy (XPS) analysis on a Thermo ESCALAB 250 Xi spectrometer using monochromatic AlK(1486.6 eV)Xray sources.The curve fitting of XPS spectra was carried out using Shirley background correlation with Gaussian-Lorentzian peak shape.
3.Results and discussion
Experimental conditions of electric explosion of iron wires are listed in Table 1,including the condition of charging voltages with corresponding stored energy.Corresponding formed phases of recovered samples are also listed in Table 1 based on the multiple characterization results.
XRD patterns showed the presence of multiple phases of recovered samples,including Fe,FeO,FeO,α-FeO(OH),γ-FeO(OH)(see Fig.2),which are also listed in Table 2.The XRD results imply that the increase of charging voltage is conducive to the formationof iron-based compounds.When the charging voltage is 5 kV with Eof 125 J,the sample consists of iron and FeO formed from the chemical reaction between ultra-hot iron and water during cooling down process[57].When charging voltage is higher(10 kV with Eof 500 J),the recovered samples contains 5 different phases,such as Fe,FeO,Fe3O4,α-FeO(OH) and γ-FeO(OH).The further increase of charging voltage (15 kV with Eof 1125 J) leads to the only two FeO(OH)phases in the recovered sample.Moreover,the contents of above phases were estimated using adiabatic approximation method and listed in Table 2.These results suggest that phase contents are influenced by experimental conditions.The increase of charging voltage decease the content of iron phase from 43.2%to 0%and increase the iron-based compounds content in the recovered samples.These results are in accordance approximately with the previous studies [54,58].
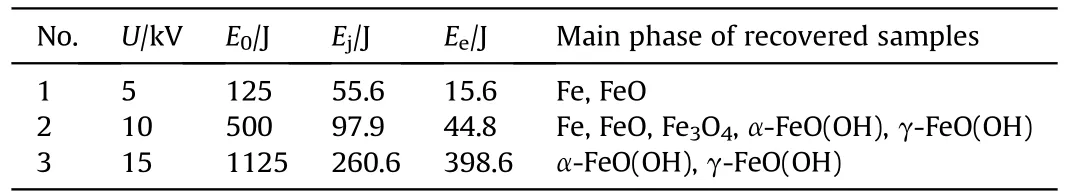
Table 1 Experimental conditions of electric wire explosion experiments with the phases of corresponding recovered samples.a
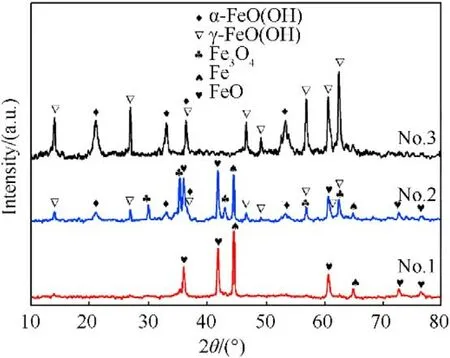
Fig.2. XRD patterns of the samples recovered at different charging voltages.
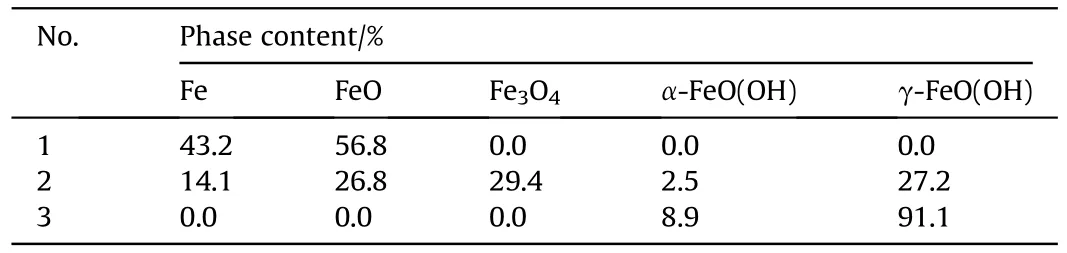
Table 2 Phase contents of Nos.1-3 samples recovered from electric explosion experiments.
Typical SEM image(Fig.3)showed the presence of 10-300 nm spherical nanoparticles with smooth surface in the recovered samples,in which the spherical morphology is induced by the shape of liquid droplets during cooling down process[42,52,57,59].Fig.4 presents typical TEM and high resolution TEM (HRTEM) images of the recovered samples,further confirming their spherical morphology in size of 10-300 nm.In addition,HRTEM images reveal the lattice distances of recovered nanoparticles confirming phases identified by XRD,indicating that higher charging voltage leads to more phases of iron-based compound in recovered samples.In No.1 sample(Fig.4b),the measured lattice distance values are 0.202 and 0.215 nm corresponding to Fe and FeO phases.In No.2 sample (Fig.4d),measured lattice distance values are 0.202,0.253,0.169,0.296,0.270 and 0.330 nm,respectively,indicating the presence of Fe,FeO,α-FeO(OH)and γ-FeO(OH).Fig.4f reveals the lattice distance values of 0.250,0.330 and 0.340 nm,suggesting the presence of α-FeO(OH) and γ-FeO(OH) in No.3 sample.Furthermore,TEM results indicate the nano-twin defect in formed nanoparticles,which is due to the fast non-equilibrium quenching nature of the whole process [55,59].These nano-defects are also reported in the previous investigation on electric wire explosion in a liquid medium [52,59].
Fig.5 presents the XPS spectra of Nos.1-3 samples.Fig.5a,c and 5e show the O1s spectra of each sample,revealing two bands appearing at 530.1 eV and 531.5 eV,assigned to Oand OH[60-63],respectively.Furthermore,the intensities of two O1s bands indicate that the increase of charging voltage is conducive to the formation of OH,especially when the charging voltage is 15 kV.With respect to the Fe2p spectra of Nos.1-3 samples (see Fig.5b,d and 5f),the bands assigned to Fe(719.8 eV),Fe(710.7 and 723.4 eV),Fe(712.7 and 725.5 eV) [60-62,64,65] are observed.When the charging voltage is 5 kV,the Fe2p spectrum presents Feband and Feband,indicating the presence of iron and FeO in No.1 sample.When the charging voltage is higher(10 kV),the Fe2p spectrum indicates the presence of Feband and Feband,suggesting the presence of multiple iron oxides phases in No.2 sample.The further increase of charging voltage (15 kV)leads to only Feband in the Fe2p spectrum of No.3 sample.Besides,mole ratio contents of above ions were calculated based on these XPS data(see Table 3).The moles ratio of OH,Oand Fein No.3 sample is close to 1:1:1,showing the presence of FeO(OH).The phase content results are in agreement with XRD results,approximately,considering the semi-quantitative estimation of XPS characterization and the non-uniform distribution of the components in the samples.
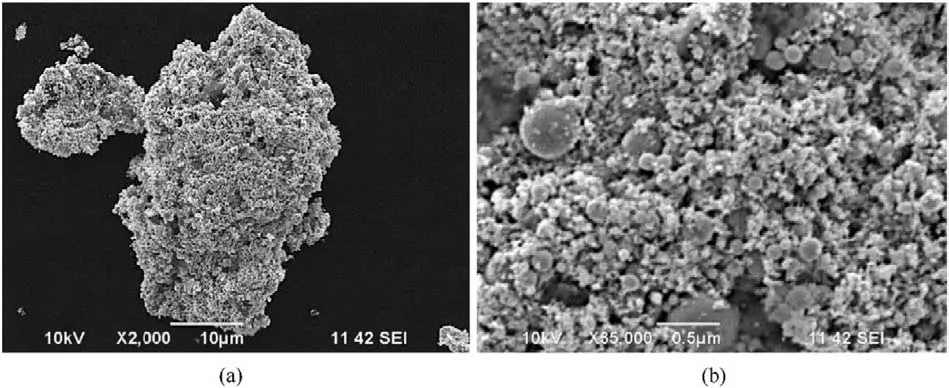
Fig.3. Typical SEM image and higher-magnification SEM image of sample No.3.
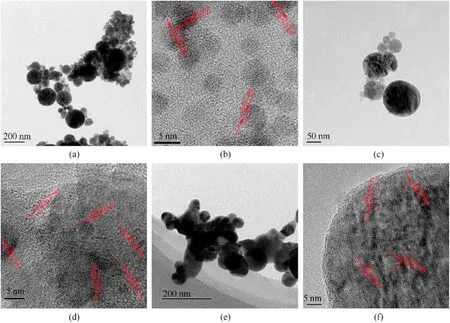
Fig.4. Regular and high resolution TEM images of sample No.1 ((a) and (b),respectively),sample No.2 ((c) and (d),respectively) and sample No.3 ((e) and (f),respectively).
Discharge current and resistive voltage waveforms were recorded during pulsed discharge(see Fig.6),showing the time-resolved phase transitions of the iron wire during pulsed discharge (e.g.melt,vaporization,plasma formation,etc.) [42,51,54].The first current peaks appearing in all current waveforms are observed at 0-8 μs,indicating the joule heating process,in which iron wire starts to melt and vaporized with the rapid increase of temperature and pressure.During the ascending stage of current waveforms(6-8 μs),the corresponding voltage waveforms increase dramatically due to the sharp resistance increase of the iron wire induced by its intense vaporization.Note that,at charging voltage of 15 kV,a second current peak which is stronger and wider comes up as the rapid drop of discharge voltage,indicating the arc-discharge (second discharge) with strong plasma tunnel [42,51,54].Under this condition,the excessive energy input enhances the activity of explosion products and induces the formation of Fe,OH,O·OH,etc,which are conducive to the formation of pure FeO(OH).
Essentially,the energy input is the critical influence of experimental condition on the formed phases in the samples can be also analyzed in terms of energy.The charging voltage,only alternate condition in this investigation,affects stored energy(E)and pulsed discharge behavior.Thus,Eis in range of 125-1125 J based on the following equation:

where C is the capacitance and U is the charging voltage.Discharge energy during the joule heating and during explosion processes can be calculated by

where tand tare the beginning and end of the corresponding process,and i and uare recorded discharge current and resistive voltage,respectively.Above calculation results are listed in Table 1.Moreover,the iron wire is with average mass of 0.025 g and sublimation energy(E)of 186.6 J(sublimation energy of iron is 7480 J/g [42]).
Note that the energy input of No.1 experiment (5 kV) is 71.2 J(E+E),less than E,suggesting the presence of large amount of iron droplets in the explosion products.Under oxygen-poor medium,such as distilled water,partial iron droplets and vapors react with HO to form FeO nanoparticles.With respect to No.2 experiment (charging voltage of 10 kV),the energy input is higher(142.7 J) (E+E),implying higher activity of explosion products.With the energy input after explosion,the ultra-hot iron droplets and vapors react with HO to form more iron-based compound nanoparticles.When the charging voltage is 15 kV,Eof No.3 experiment is greater than E.The strong overheat effect leads to the formation of highly active iron atoms.Furthermore,Evalue of No.3 experiment is 398.6 J,indicating the formation of strong plasma tunnel during explosion consisting of multiple ions with higher activity,such as Fe,·OH and OH,etc.This situation is conducive to the formation of FeO(OH).Thus,the excessive energy input induced the synthesis of pure FeO(OH) in No.3 sample.Furthermore,the huge energy input (E) of strong plasma tunnel leads to ionization of HO to form plenty of hydroxyl ions for the synthesis of FeO(OH).In addition,the strong plasma tunnel leads to higher ambient temperatures,which is similar to the conditions of hydrothermal synthesis methods to synthesize oxyhydroxides,inhibiting formation of iron oxides favoring FeO(OH) in No.3 sample.
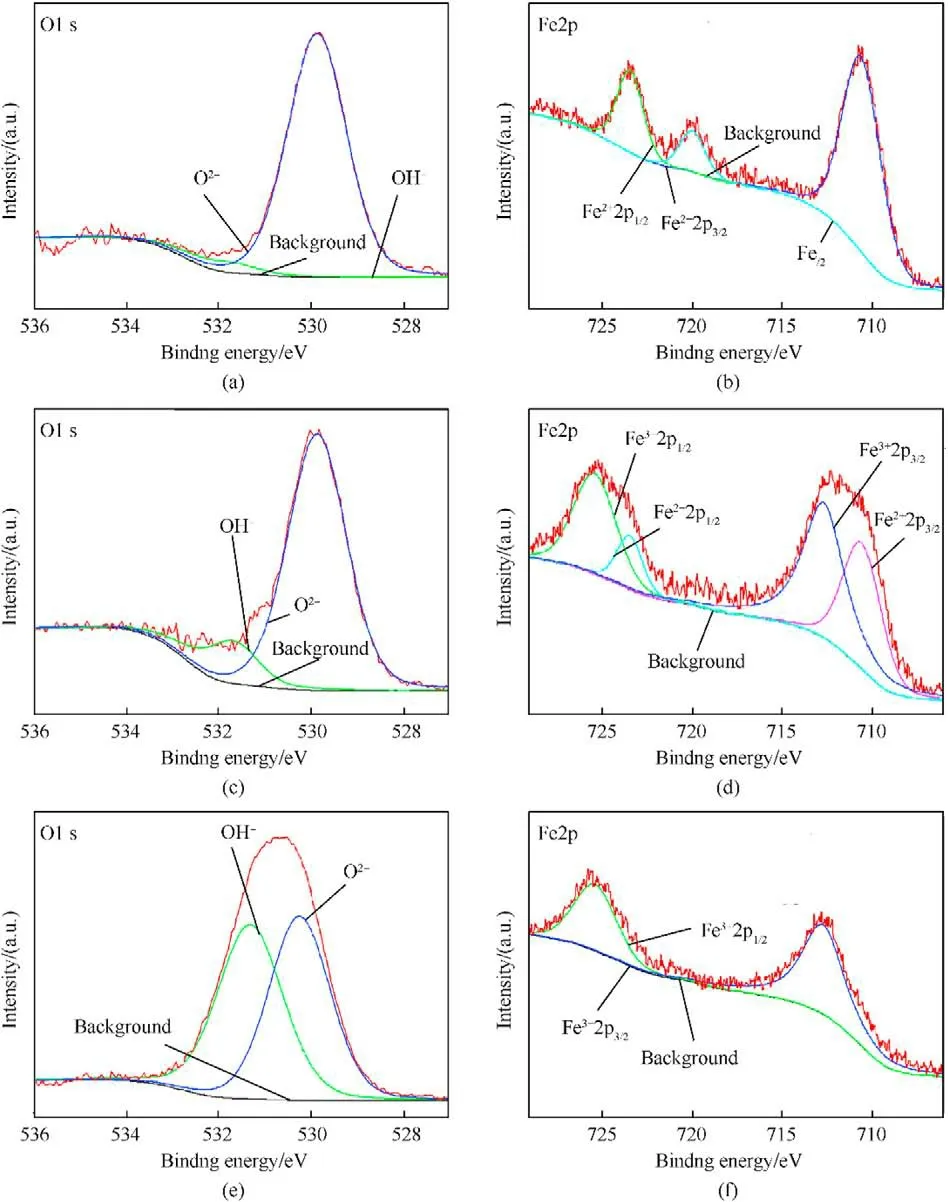
Fig.5. O1s and Fe2p XPS spectra of sample No.1 ((a) and (b),respectively),of sample No.2 ((c) and (d),respectively) and of sample No.3 ((e) and (f),respectively).
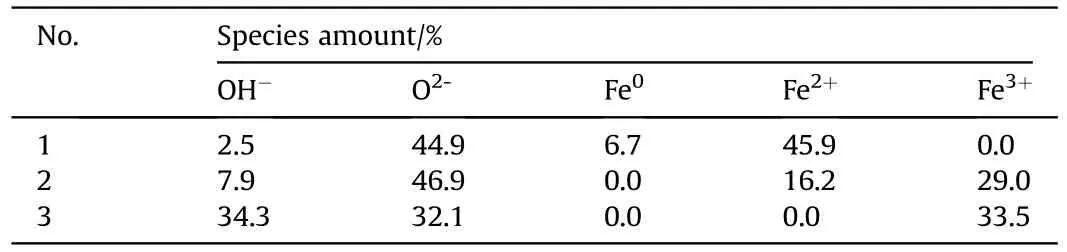
Table 3 Phases contents obtained using XPS data of three different samples.
Thus,based on the above results,the formation mechanism of FeO(OH) using electric wire explosion can be proposed.During electric explosion process,the iron wire melted and vaporized to form ultra-hot iron droplets and vapors with high temperature and pressure,leading to the subsequent explosion with chemical reaction to form iron-based compounds.When the charging voltage is high enough(15 kV),the explosion products formed during pulsed discharge breaks down and induce a strong arc-discharge.Consequently,the excessive energy input during this process generate more ·OH and OH,etc,and further enhance the activity of production.Under this condition,the as-prepared sample is pure FeO(OH).At low charging voltages(5 kV and 10 kV),the majority of energy input is injected into iron wire before explosion.Then,the formed iron droplets and vapor burst into distilled water and generates shock wave with no arc-discharge.Consequently,the lower activity of explosion products reacts with HO molecules during cooling down process,leading to formation of mixed multiple iron-based nanoparticles,such as Fe and FeO nanoparticles(5 kV)and FeO,FeOand FeO(OH)nanoparticles(10 kV).Our study also indicates that the energy input,delicately adjusted by charging voltage,is the critical factor to form FeO(OH) in distilled water medium.Moreover,the increase of energy input enhance the activity of explosion product,leading to higher compound content and higher oxygen degree of recovered samples.
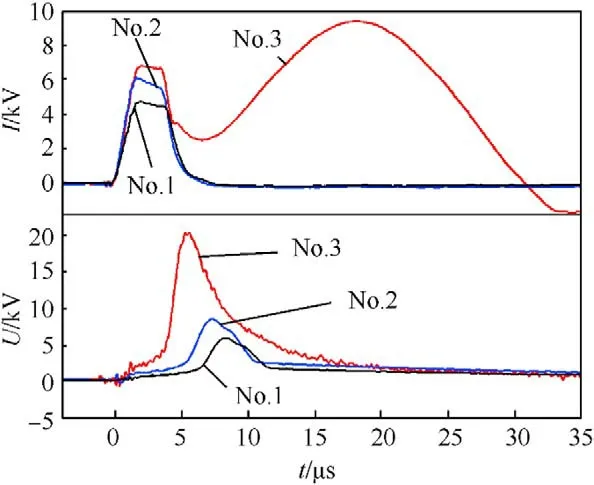
Fig.6. Typical discharge current and resistive voltage waveforms of Nos.1-3 samples.
Moreover,in this study,the production suspension of No.3 experiment after 20 times pulsed discharge of iron wires was dried using vacuum frozen drier and measured to be 0.341 g.The results show that the yield of FeO(OH) nanoparticles is approximately 68.2% due to the mass loss during recovery and other sample treatment processes.Thus,it can be a potential industrial production process to produce FeO(OH)nanoparticles,once the system is equipped with necessary mechanical and automatic facilities,such as PLC controller,automatic wire supply roll,etc.
4.Conclusion
In our study,we demonstrated an approach to produce FeO(OH)nanoparticles by electric explosion of iron wire at 15 kV charging voltage.Furthermore,high energy input during strong arcdischarge is the critical factor to form pure FeO(OH) nanoparticles via electric explosion of iron wire.Moreover,changing charging voltage is an efficient way to enhance energy input and induce arcdischarge.The increase of energy input adjusted by charging voltage increases the iron-based compound content of recovered samples.At charging voltage of 15 kV,the energy input is high enough to form pure FeO(OH) nanopowder.
This research was supported by National Natural Science Foundation of China (Grant No.11702283).
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Defence Technology的其它文章
- Defence Technology
- A study on the surface overpressure distribution and formation of a double curvature liner under a two-point initiation
- Performances and direct writing of CL-20 based ultraviolet curing explosive ink
- Driving force coordinated control of an 8×8 in-wheel motor drive vehicle with tire-road friction coefficient identification
- Monitoring and Prediction of the Vibration Intensity of Seismic Waves Induced in Underwater Rock by Underwater Drilling and Blasting
- Effects of Mg/PTFE pyrotechnic compositions on reignition characteristics of base bleed propellants and heating mechanism

